Abstract
Among adults, wakefulness and rapid eye movement (REM) sleep, compared to non-REM sleep, require higher overall brain metabolism, but in neonates analogous data are not available. Behavioral states with higher metabolic demand could increase vulnerability to hypoperfusion or hypoxia in the compromised neonatal brain. Using cerebral oximetry (near-infrared spectroscopy), and simultaneous polysomnography, we evaluated whether brain oxygen metabolism varies by sleep-wake state among critically ill newborns. For each of ten infants, sleep-wake cycling was detectable and cerebral oximetry varied (p<0.0001) across behavioral states, but the patterns differed among subjects. We conclude that cerebral oxygen metabolism varies with sleep-wake states in high-risk newborns. The direction and degree of these changes are variable and subject-specific in this initial sample, but could reflect or affect brain injury and vulnerability.
Keywords: neonatal, near-infrared spectroscopy, polysomnography
Introduction
In adults, rapid eye movement sleep requires higher overall brain metabolism than non-rapid eye movement sleep1, but in neonates analogous data are not available. Neonates spend about one-third of their existence in active (rapid eye movement) sleep, and higher metabolic demand associated with this state might increase vulnerability to hypoperfusion or hypoxia. Neonates with encephalopathy can exhibit sleep-wake cycling, but remain at risk for adverse neurodevelopmental outcomes2. With advanced neuromonitoring, customized interventions could potentially be designed to modify this risk.
Near-infrared spectroscopy provides bedside measures of tissue oxygen metabolism. The device produces two wavelengths of infrared light (730 and 810nm) using one light-emitting diode and two detectors. The data returned to the detectors reflect deoxyhemoglobin and total hemoglobin, which have different absorption spectra. From these measurements, a regional oxygenation value (rSO2) is calculated3.
We used cerebral oximetry, measured by near-infrared spectroscopy, and simultaneous polysomnography to evaluate whether brain oxygen metabolism varies by sleep-wake state among critically ill newborn infants.
Methods
The University of Michigan Institutional Review Board approved this study. The subjects’ parents provided written informed consent. Newborn infants (gestational age ≥35 weeks), assessed clinically as being at risk for seizures, were recruited prospectively. Infants with severely abnormal electroencephalogram patterns that would preclude detection of sleep-wake cycling were excluded.
Near-infrared spectroscopy from bilateral parietal-occipital scalp sensors was recorded in synchrony with polysomnography. The parietal regions were selected because they are over the cerebrovascular watershed zone, and therefore at risk for ischemic injury. To discern any confounding effect of changes in systemic perfusion, a third sensor was placed on the thigh. Continuous cerebral and systemic rSO2 were measured every 5 seconds using near-infrared spectroscopy (Somanetics INVOS 5100C, Troy, MI). Fractional tissue oxygen extraction (FTOE) was calculated using SaO2 (recorded from the patient’s pulse oximeter) and rSO2 (FTOE = [SaO2 − rSO2]/ SaO2).
Polysomnography was recorded using conventional electroencephalography, extraoculograms, chin surface electromyogram, chest and abdominal excursion (inductance plethysmography), nasal pressure, nasal/oral airflow (thermocouples), snoring sensor, oxygen saturation, transcutaneous carbon dioxide, electrocardiogram, bilateral anterior tibialis surface electromyogram, and digital video. A sleep technologist remained at the bedsides to record detailed behavioral observations. All polysomnograms were scored offline by the same experienced registered polysomnographic technologist, reviewed by a second such individual, and then interpreted by two of the investigators (RAS and RDC).
rSO2 and fractional tissue oxygen extraction were analyzed during polysomnography-defined wakefulness, quiet sleep, and active sleep (defined according to standard guidelines)4. Transitional and indeterminate behavioral states were excluded from these analyses. For each 30-second polysomnogram epoch, rSO2 and SaO2 measurements were averaged. These mean values were used to compute fractional tissue oxygen extraction for each epoch. Median values for each sleep-wake state were then compared, using a non-parametric one-way ANOVA test. To eliminate artifacts, only data points with SaO2 higher than 60 were included and fractional tissue oxygen extraction calculations that were less than zero were excluded.
Results
Ten infants were included in this pilot study. Demographic and clinical profiles are presented in Table 1. Four infants had hypoxic-ischemic encephalopathy, 3 suspected sepsis/meningitis, 2 neonatal epilepsy, and 1 congenital facial nerve palsy.
Table 1.
Demographic and polysomnographic data for 10 critically ill neonates
| All Subjects N=10a |
|
|---|---|
| Gestational Age (weeks) | 39.0 ± 2.2 |
| Day of Life when Studied (median; range) | 4; 2–21 |
| Birth weight (kg) | 3.24 ± 0.72 |
| Total Recording Time (min) | 703 ± 57b |
| Wakefulness (% of Total Recording Time) | 15.3 ± 9.8 |
| Total Sleep Time (min) | 575 ± 68 |
| Quiet Sleep (% of Total Sleep Time) | 42.8 ± 11.2 |
| Active Sleep (% of Total Sleep Time) | 41.7 ± 8.4 |
| Indeterminate (% of Total Sleep Time) | 15.5 ± 5.9 |
| Sleep Efficiency (%)c | 82.7 ± 9.2 |
| Arousal Index (arousal/hour sleep) | 6.1 ± 3.4 |
| Apnea / Hypopnea index | 10.1 ± 7.0 |
| Quiet sleep Apnea / Hypopnea index | 3.9 ± 2.8 |
| Active sleep Apnea / Hypopnea index | 16.5 ± 10.1 |
| Mean %SpO2 during sleep | 97.2 ± 2.4 |
| Minimum % oxygen saturation during sleep | 80.9 ± 6.3 |
Unless otherwise indicated, data presented are mean ± standard deviation
Total recording time did not always equal sleep time + waking time, as epochs obscured by artifact were eliminated.
Sleep Efficiency = (Total Sleep Time / Total Recording Time) * 100.
All infants had clear sleep staging (Table 1). The apnea/hypopnea index was elevated during active sleep, compared to quiet sleep, in all but one subject (p=0.003). The electroencephalogram background was characterized by one investigator (RAS) according to published criteria5 and was normal in 2 subjects, mildly abnormal in 4, moderately abnormal in 3, and markedly abnormal in 1 (this individual had clear sleep-wake cycling, despite the severe electroencephalogram abnormalities). Only two subjects had electrographic seizures during polysomnography monitoring. All but one of these seizures occurred during indeterminate or transitional sleep stages, and so they were not subjected to further analyses. The remaining seizure occurred during active sleep.
Cerebral rSO2 and fractional tissue oxygen extraction varied with sleep-wake state, with statistically significant within-subject differences between wakefulness and sleep stages for every neonate (Table 2). Using all subjects’ pooled data, we performed a post-hoc analysis using a nonparametric Mann-Whitney U-test to evaluate pair-wise differences between sleep-wake states, and nearly all were significant, even with Bonferroni correction (p<0.0001). However, patterns varied between individual subjects. For example, cerebral fractional tissue oxygen extraction was lower during wakefulness than during active or quiet sleep for 4 subjects (p<0.0001), but was higher during wakefulness in another 3 (p<0.003), with no difference in 2. Systemic near-infrared spectroscopy measures (recorded from the thigh) also varied significantly with behavioral state for every subject, with higher systemic fractional tissue oxygen extraction during wakefulness than active or quiet sleep for 6 subjects, highest fractional tissue oxygen extraction during quiet sleep for 2, and active sleep for another 2. The aggregate values presented in Table 2 show only small absolute differences between sleep-wake states, with small p-values due to the density of the data (collected every 5 seconds over a mean of 703 minutes). However, absolute differences were more clinically significant within individual subjects (Figure 1).
Table 2.
Pooled near-infrared spectroscopy data across sleep-wake states for ten critically-ill neonates.
| Awake | Quiet Sleep |
Active Sleep |
Kruskal -Wallisa |
Pair-Wise Comparisons (Mann-Whitney p-valuea) |
|||
|---|---|---|---|---|---|---|---|
| Median(IQ R) |
Median(IQ R) |
Median(IQ R) |
p-value | Awake- Quiet |
Quiet- Active |
Awake- Active |
|
| Left rSO2 | 76.6 (5.8) | 78.2 (13.7) | 77.4 (8.0) | <0.0001 | <.0001 | 0.0014 | <.0001 |
| Right rSO2 | 80.0 (7.8) | 79.0 (11.4) | 77.5 (8.2) | <0.0001 | 0.7515 | <.0001 | <.0001 |
| Systemic rSO2 | 72.5 (17.6) | 84.8 (11.8) | 78.8 (14.0) | <0.0001 | <.0001 | <.0001 | <.0001 |
| SaO2 | 96.0 (5.6) | 97.4 (3.4) | 96.8 (3.5) | <0.0001 | <.0001 | <.0001 | <.0001 |
| Left FTOE | 0.19 (0.09) | 0.18 (0.15) | 0.19 (0.11) | <0.0001 | 0.0353 | <.0001 | 0.4640 |
| Right FTOE | 0.17 (0.09) | 0.19 (0.13) | 0.20 (0.10) | <0.0001 | 0.0066 | <.0001 | <.0001 |
| Systemic FTOE | 0.24 (0.18) | 0.13 (0.12) | 0.19 (0.15) | <0.0001 | <.0001 | <.0001 | <.0001 |
| Heart Rateb | 137.0 (27.0) | 114.2 (25.8) | 117.3 (23.0) | <0.0001 | <.0001 | <.0001 | <.0001 |
| Mean BPb | 56.0 (20.0) | 49.0 (15.0) | 49.0 (20.0) | <0.0001 | <.0001 | 0.10 | <.0001 |
IQR = Interquartile range
SaO2=systemic oxygen saturation
FTOE = Fractional Tissue Oxygen Saturation
Mean BP = Mean arterial blood pressure
Applying a Bonferroni correction, with 9 comparisons across 3 behavioral states, a p-value less than 0.002 is considered significant.
Data for Heart Rate and Mean Blood Pressure were available for 8 of the 10 subjects.
Figure 1.
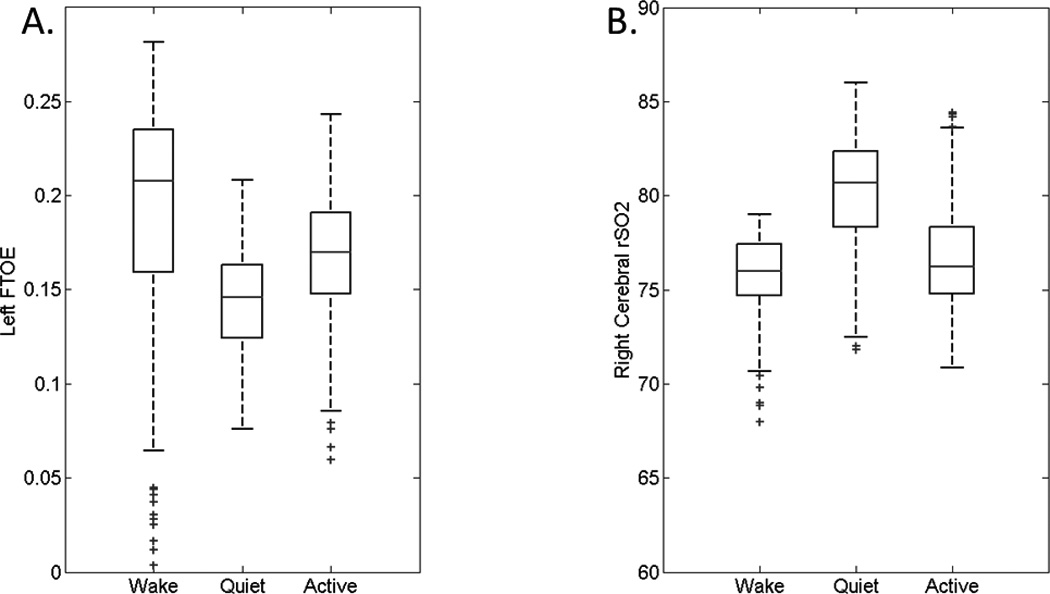
Sample plots of fractional tissue oxygen extraction (FTOE) across sleep-wake states from an infant with seizures of uncertain etiology (panel A) and cerebral rSO2 values from an infant with hypoxic ischemic encephalopathy (panel B). Boxplots demonstrate the median, 25th, 75th, 10th, and 90th percentiles, with outliers. For each subject, there were statistically significant differences among sleep-wake states (p<0.0001).
Discussion
This series of critically ill neonates, perhaps the first to be studied with measures of brain metabolism and sleep simultaneously, shows that cerebral oxygen metabolism varies significantly with sleep-wake state. Although the direction and degree of these changes are variable, our observation could reflect periods of relative vulnerability. During behavioral states with increased fractional tissue oxygen extraction, accelerated metabolic demand or declines in oxygen delivery could in theory result in injury, or exacerbate it. Additional study of this phenomenon is warranted. If predictable periods of potential vulnerability are discovered, then focused neuromonitoring could be employed to integrate behavioral state assessment along with brain oxygen metabolism measurement as critical guides for neonatal intensive care. Avoidance of potential perturbations during states with higher baseline cerebral oxygen metabolism might mitigate susceptibility to ongoing or developing injury.
Using near-infrared spectroscopy, decreased spontaneous hemodynamic cerebral activity during slow wave sleep, compared to wakefulness, rapid eye movement and stage N2 sleep, has been demonstrated among healthy adult volunteers6. These authors speculated that the decreased spontaneous oscillations in oxygen metabolism (recorded by near-infrared spectroscopy over the right frontal region) during slow wave sleep could be related to the increasingly synchronized electroencephalogram delta activity during this state.
In healthy newborn lambs, cerebral oxygen metabolism was found to vary according to behavioral state7. Invasive blood samples from the superior sagittal sinus and carotid artery of neonatal lambs demonstrated that oxygen consumption was similar in wakefulness and active sleep, but significantly lower in quiet sleep, a pattern seen in some of our study subjects.
Cyclic patterns in cerebral blood volume (derived from near-infrared spectroscopy) have been associated with apnea of prematurity and periodic breathing in healthy infants 8,9. These studies evaluated infants during quiet sleep and demonstrated moment-to-moment variability in cerebral blood volume associated with respiratory patterns, at a cycling frequency of 3–6/minute. Our subjects demonstrated no consistent change in near-infrared spectroscopy measurements surrounding apnea events (data not shown), but our sampling rate was much slower than that described by these authors. Given our subjects’ particularly high mean apnea-hypopnea index during active sleep, any changes in cerebral oxygen metabolism associated with both a behavioral state with high fractional tissue oxygen extraction and simultaneous frequent apnea could be clinically meaningful.
Systemic oxygen metabolism might be expected to vary with behavioral state, as infants have more limb movement during wakefulness than during active sleep or quiet sleep. This hypothesis held true for our pooled study data, although systemic oxygen extraction was paradoxically highest during quiet sleep for two subjects. We did not account for medication or blood pressure effects in the present analyses and hypothesize that these or other unstudied variables could have played a role in these two subjects’ surprising systemic metabolism data. Our findings highlight the critical need to control for systemic metabolism in future studies, an approach that has been lacking in prior work with near-infrared spectroscopy.
Our study has some limitations. Although we demonstrate statistically significant differences in near-infrared spectroscopy measures across behavioral states, the patterns vary among individual subjects. Our small sample includes infants with varying underlying brain pathology. Larger samples focusing on more homogenous populations might demonstrate consistent near-infrared spectroscopy changes between individuals and are required prior to the wide-spread clinical application of coregistered rSO2 and polysomnography in neonatal intensive care. Normative data derived from healthy infants are also currently lacking and should be a focus of future work.
Additionally, statistically significant results do not always translate to clinically relevant findings. Our pooled data indicated small, albeit statistically significant, absolute differences between rSO2 and fractional tissue oxygen extraction across sleep-wake states. The results for individual subjects revealed larger absolute differences, yet the magnitude of the variability that would result in exacerbation of brain injury remains to be determined.
Conclusions
In this series of neonates with varying risk factors for brain pathology, sleep-wake cycling was documented by polysomnography and measures of cerebral and systemic oxygen metabolism were found to vary between sleep-wake states. These novel findings suggest a potential concern for increased vulnerability to evolving or new brain injury among critically ill neonates during certain behavioral states. If confirmed, this observation conceivably could lead to individualized neuromonitoring and novel interventions to moderate the risk for injury and adverse outcomes in this vulnerable patient population.
Acknowledgements
The authors thank Judy Fetterolf and Lora Merley for their assistance in scoring the polysomnograms.
Financial Disclosure / Funding: This research was supported by grants from NICHD (K23 HD068402), the Child Neurology Foundation, and the University of Michigan’s Janette Ferrantino Award.
Footnotes
Author Contributions: Dr. Shellhaas designed the study, interpreted the data, and drafted the manuscript. Dr. Burns analyzed the data, assisted in writing the manuscript and revised the manuscript. Ms. Wiggins assisted in study design and revised the manuscript. Ms. Christensen assisted in study design and revised the manuscript. Dr. Barks assisted in the study design and data interpretation and revised the manuscript. Dr. Chervin assisted in the study design and data interpretation and revised the manuscript.
Conflicts of Interest: Somanetics, Inc (Troy, MI) donated NIRS equipment for research conducted in our neonatal intensive care unit, but had no input into this study’s design, data analysis, or writing of the manuscript.
Ethical Approval: The University of Michigan Institutional Review Board approved this study. The parent of each study subject provided written informed consent.
References
- 1.Madsen PL, Schmidt JF, Wildschiodtz G, Friberg L, Holm S, Vorstrup S, et al. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. Journal of Applied Physiology. 1991;70(6):2597–2601. doi: 10.1152/jappl.1991.70.6.2597. [DOI] [PubMed] [Google Scholar]
- 2.Oskredar D, Toet MC, van Rooij LGM, van Huffelen AC, Groenendaal F, De Vries LS. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hpoxic-ischemic encephalopathy. Pediatrics. 2005;115:327–332. doi: 10.1542/peds.2004-0863. [DOI] [PubMed] [Google Scholar]
- 3.Wahr JA, Tremper KK, Samra S, Delpy DT. Near-infrared spectroscopy: theory and applications. Journal of Cardiothoracic and Vascular Anesthesia. 1996;10(3):406–418. doi: 10.1016/s1053-0770(96)80107-8. [DOI] [PubMed] [Google Scholar]
- 4.Anders T, Emde R, Parmalee A. A Manual of Standardized Terminology, Techniques and Criteria for the Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles, CA: UCLA Brain Information Services; 1971. [Google Scholar]
- 5.Shellhaas RA, Gallagher PR, Clancy RR. Assessment of neonatal electroencephalography (EEG) background by conventional and two amplitude-integrated EEG classification systems. Journal of Pediatrics. 2008;153:369–374. doi: 10.1016/j.jpeds.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Nasi T, Virtanene J, Noponen T, Toppila J, Salmi T, Ilmoniemi RJ. Spontenous hemodynamic oscillations during human sleep and sleep stage transitions characterized with nearinfrared spectroscopy. PLoSOne. 2011;6(10):e25415. doi: 10.1371/journal.pone.0025415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvani A, Asti V, Berteotti C, Ferrari V, Franzini C, Lenzi P, et al. Sleep-dependent changes in cerebral oxygen consumption in newborn lambs. Journal of Sleep Research. 2006;15:206–211. doi: 10.1111/j.1365-2869.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 8.Urlesberger B, Kaspirek A, Pichler G, Muller W. Apnoea of prematurity and changes in cerebral oxygenation and cerebral blood volume. Neuropediatrics. 1999;30:29–33. doi: 10.1055/s-2007-973453. [DOI] [PubMed] [Google Scholar]
- 9.Urlesberger B, Trip K, Ruchti JJI, Kerbl R. Quantification of cyclical fluctuations in cerebral blood volume in healthy infants. Neuropediatrics. 1998;29:208–211. doi: 10.1055/s-2007-973562. [DOI] [PubMed] [Google Scholar]


