Abstract
Food deprivation in mammals results in profound changes in fuel metabolism and substrate regulation. Among these changes are decreased reliance on the counter-regulatory dynamics by insulin-glucagon due to reduced glucose utilization, and increased concentrations of lipid substrates in plasma to meet the energetic demands of peripheral tissues. As the primary storage site of lipid substrates, adipose tissue must then be a primary contributor to the regulation of metabolism in food deprived states. Through its regulation of lipolysis, adipose tissue influences the availability of carbohydrate, lipid, and protein substrates. Additionally, lipid substrates can act as ligands to various nuclear receptors (retinoid x receptor (RXR), liver x receptor (LXR), and peroxisome proliferator-activated receptor (PPAR)) and exhibit prominent regulatory capabilities over the expression of genes involved in substrate metabolism within various tissues. Therefore, through its control of lipolysis, adipose tissue also indirectly regulates the utilization of metabolic substrates within peripheral tissues. In this review, these processes are described in greater detail and the extent to which adipose tissue and lipid substrates regulate metabolism in food deprived mammals is explored with comments on future directions to better assess the contribution of adipose tissue to metabolism.
Keywords: lipid metabolism, fasting, fatty acids, starvation, dyslipidemia
I. Introduction
Regulation of metabolism has been largely associated with the liver because it serves as a critical target for most hormones to mediate their functions. However, in recent years adipose tissue has received greater attention due to increased understanding of its endocrine capabilities and its influence over insulin sensitivity [1–4]. In addition to being the storage site for triacylglycerols (TAG), adipose tissue also secretes various adipokines such as leptin, adiponectin, and apelin, which modulate insulin sensitivity by regulating the utilization of plasma lipids [4–6]. Through the breakdown of stored TAG, adipose tissue also releases free fatty acids (FFA) and glycerol. FFA can be directly oxidized to generate ATP while glycerol can be used as a substrate in gluconeogenesis or lipogenesis. In this manner, adipose tissue can directly and indirectly modulate the availability of other metabolic substrates.
When mammals endure food deprivation, significant changes to metabolism occur to promote the preservation of metabolic substrates [7]. These changes were identified as the three phases of starvation, and characterized by the predominant catabolism of a single class of substrate: 1) carbohydrate, 2) lipid, and 3) protein [8, 9] (Figure 1). Because carbohydrate stores are depleted within a matter of hours, metabolism must transition to reliance on lipids to meet energetic demands [7, 9]. Mammals adapted to prolonged food deprivation, like seals and bears, transition to a metabolism primarily reliant on lipid oxidation as part of their natural life history [10–14]. However, other mammals (e.g., humans and rodents) that are not adapted are incapable of shifting to a completely lipid-dependent metabolism [15]. This can be due to either improper regulation of substrates during fasting (Phase I or II), or simply inadequate lipid stores. Whatever the case, the inability to transition results in protein catabolism and lean tissue degradation (cachexia) for energy. If not prevented, cachexia can eventually lead to death.
Figure 1.
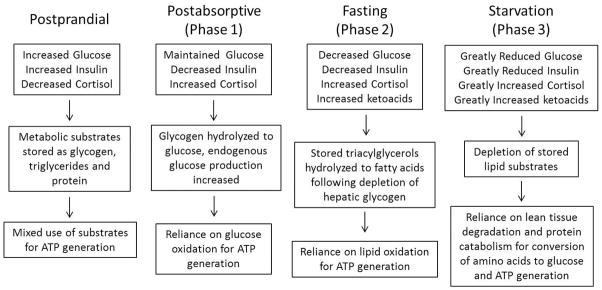
Comparison of the changes to key metabolic parameters in mammals under postprandial, postabsorptive, fasting, and starving conditions.
These distinctions in lipid metabolism during phase II and III of Cahill’s model of starvation provide the basis for differentiating the adapted mammals ability to fast versus the non-adapted mammals endurance of starvation [16].
In the past, research concerning metabolic regulation during food deprivation focused on hormonal control at the systemic level, allowing for the characterization of the typical endocrine response to fasting [8, 9, 17]. However, because most endocrine factors that regulate metabolism postprandially have reduced roles in food-deprived mammals [18], considerable investigation has focused on the contributions of intracellular mechanisms of substrate regulation to metabolism [19–22]. Though the majority of this work has been done in humans and rodents during feeding or short term fasting, data from mammals that endure prolonged bouts of food deprivation, like seals, suggests that lipid substrates may have the same regulatory effects [10, 13, 23].
As the principal storage site of lipids, adipose tissue must contribute to metabolic regulation under food-deprived conditions. Therefore, understanding its contributions, from the systemic to the molecular level, is important to assess metabolic regulation during food deprivation in mammals. Because hepatic regulation of metabolism is prominent, a better understanding of the cross-talk between the liver and peripheral tissues would be useful. Therefore, this review focuses on the regulation of substrate availability by adipose tissue, the influence by the liver on this regulation, and how this may assist in the regulation of fuel metabolism in prolong-fasted mammals.
II. Mechanisms Regulating Substrate Availability
Most mammals suppress sympathetic nervous system activity [24, 25], various endocrine factors that regulate postprandial metabolism [18], and the activity of adipokines [26], in order to reduce energy expenditure under food deprived states. Food deprivation also increases the concentrations of slow-acting hormones like cortisol or biomolecules like retinoic acid that control the expression of genes within the liver, adipose, and other peripheral tissues to generate metabolism-regulating proteins like lipases or fatty acid transporters [27, 28]. These proteins are responsible for shifting metabolism and maintaining the availability and utilization of substrates within a tolerable range. Therefore, the regulation of their activity and expression is of critical importance to the survival of the organism.
A. Lipolysis
Intracellular lipases are responsible for breaking down the stored TAG molecules to release three FA and glycerol. Adipose triglyceride lipase (ATGL), hormone-sensitive lipase L), and monoglyceride lipase (MGL) are the principal lipases. Each removes a single FA in a stepwise fashion, converting the TAG to diacylglycerol (DAG), then monoacylglycerol (MAG), and finally free glycerol. This ensures a steady supply of substrates for ATP generation, but also allows for the utilization of glycerol in either hepatic lipogenesis or gluconeogenesis [29]. Because hepatic synthesis of FFA is reduced during food deprived conditions [30], essentially all of the FFA in circulation result from adipose tissue lipolysis.
ATGL is the rate-limiting enzyme in TAG hydrolysis [31]. ATGL expression increases during food deprivation in humans, rats, and seals [21, 23, 32] and so is likely responsible for the increased circulating FFA concentration during fasting. AMP-activated protein kinase (AMPk) activity has also been reported to increase with fasting in rats and elephant seals [19, 33, 34], and because its upregulation increases ATGL expression in vitro [35, 36], it is likely responsible for the increased ATGL expression in vivo during prolonged fasting. Though its affinity for TAG molecules is much less than that of ATGL, HSL is capable of hydrolyzing TAG, DAG, and MAG molecules [37]. As its name implies, HSL is responsive to various hormones, such as catecholamines and insulin and so can also increase or decrease lipolysis depending on the needs of the organism. Insulin decreases substantially during fasting in mammals [18, 38] while the concentration of catecholamines (e.g. epinephrine, norepinephrine) increases [39], thus promoting increased lipolysis through HSL. However, HSL lipolytic activity decreases when AMPk is chronically activated [40], likely to limit the pro-lipolytic effects of catecholamines.
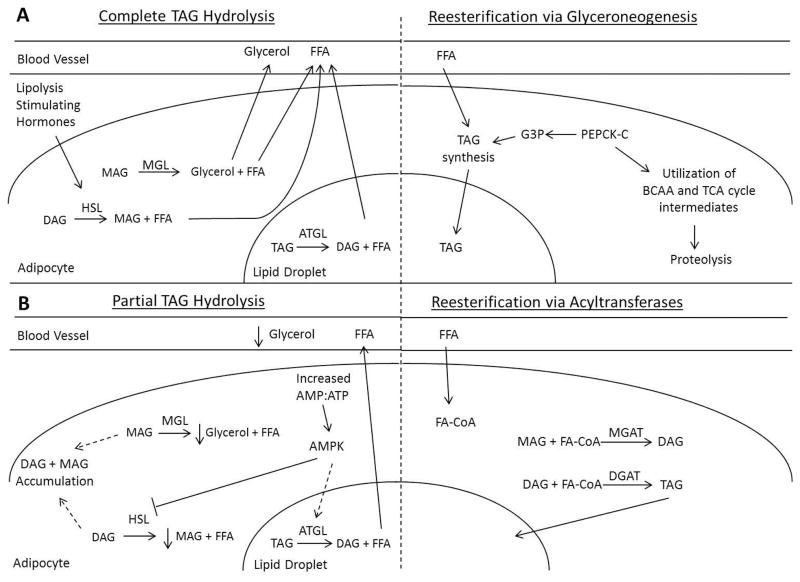
The inhibition of HSL and increased expression of ATGL by AMPk has been proposed as an adaptive response to fasting lifestyles [23]. Increased ATGL activity would maintain rates of lipolysis, while decreased HSL activity would reduce DAG hydrolysis, preventing premature depletion of lipid stores [41] (Figure 2). As seen in fasting elephant seals [23], this would increase the FFA:glycerol turnover ratio, potentially reducing the amount of free glycerol available in plasma for subsequent conversion to carbohydrate via gluconeogenesis. Interestingly, decreased HSL and increased ATGL activities are also associated with the dysregulation of fat metabolism seen in high fat diet-induced obesity in mice [40]. The only thing separating the two conditions is the impairment of AMPk activity due to obesity.
Figure 2.
Schematic of the simplified A) complete hydrolysis of triacylglycerols and subsequent re-esterification, and B) the proposed partial hydrolysis of triglycerides and subsequent re-esterification of monoacylglycerols and diacylglycerols. Solid lines denote direct effects, dashed lines denote indirect effects. Short downward pointing arrows denote a decrease. Abbreviations: AMPK, AMP kinase; ATGL, adipose triglyceride lipase; BCAA, branched chain amino acids; DAG, diacylglycerol; DGAT, diglyceride acyltransferase; FA-CoA, fatty acyl-CoA; FFA, free fatty acid; G3P, glycerol-3-phosphate; HSL, hormone-sensitive lipase; MAG, monoacylglycerol; MGAT, monoglyceride acyltransferase; MGL, monoglyceride lipase; PEPCK-c, phosphoenolpyruvate carboxykinase cytosolic; TAG, triacylglycerol; TCA, tricarboxylic acid
The effects of fasting on MGL have not been thoroughly investigated so these data are scarce. However, in the postabsorptive state, MGL is responsible for catalyzing the final step in the separation of glycerol and fatty acids by hydrolyzing MAG [42]. Several MAG species have been shown to promote lipid storage and reduce energy expenditure by binding to and activating the cannabinoid receptors in rats and humans [43]. Decreased MGL activity and maintained endocannabinoid signaling may be beneficial to hibernators, since they decrease their metabolic rate and do not maintain normothermic rates of energy expenditure [43]. However, mammals maintaining their body temperature and metabolic rate during food deprivation may increase MGL activity, since increased lipid storage would not benefit their survival under these circumstances [44]. Alternatively, if increased AMPk activity results in the accumulation of DAG molecules, then MGL activity would not be as crucial because a limited amount of MAG would be produced and available for further metabolism.
B. Fatty Acid Uptake
Fatty acid transporters are the primary mediators of long chain fatty acid (LCFA) uptake into cells and therefore have substantial control over the availability of FA in circulation. Fatty acid translocase (CD36), fatty acid binding protein (FABP), and fatty acid transport protein 1 (FATP1) are the three principal transporters regulating FA uptake by cells [45]. Retinoic acid has been reported to regulate the expression of CD36 and FATP1 through the retinoic acid receptor (RAR) and peroxisome proliferator-activated receptor gamma (PPARγ) in cells and diabetic rats [46, 47]. Regulation of the transporters appears to be tissue specific as fasting increases their expression in muscle [48], but decreases their expression in the liver and adipose tissue [23, 49, 50]. This differential expression suggests that regulation of the transporters during fasting involves the activation of different subtypes of RAR and PPAR within the different tissues. Additionally, the differential expression of fatty acid transporters is likely associated with the availability of energy stores within the tissues, as muscle stores are limited compared to liver and adipose tissue. This may also result from the need to maintain elevated concentrations of FFA in circulation during fasting to support a lipid-based metabolism [23]. The liver and adipose tissue actively participate in the futile cycling of FA (re-esterification of FFA into TAG) even when attempting to maintain elevated plasma FFA [51]. Because both tissues account for approximately 70% of fatty acid uptake in the postabsorptive state [52], decreased transporter content may be the principal mechanism contributing to a decrease in FA uptake under prolonged food deprived conditions.
C. Hepatic Re-esterification
While most tissues will oxidize FA to generate ATP, a very small fraction of the plasma FFA are re-esterified into TAG by the liver, packaged into very low density lipoproteins (VLDL), and returned into circulation [53]. In the postabsorptive state, this process could also entail the de novo synthesis of FA by fatty acid synthase (FAS) [30]. However, FAS expression and activity decrease with fasting as a result of decreased insulin [54], so FFA released by adipose tissue should account for the majority of the FA re-esterified into TAG by the liver. Synthesis of glycerol-3-phosphate (G3P) is also necessary for hepatic re-esterification because G3P serves as the backbone of the TAG molecule [55]. This involves the phosphorylation of free glycerol by glycerol kinase (GK), or de novo synthesis of G3P by phosphoenolpyruvate carboxykinase-cytosolic (PEPCK-C)[56]. The fasting induced increase in plasma cortisol promotes hepatic PEPCK-C expression [28], which may predominantly contribute to G3P synthesis via glyceroneogenesis. However, because PEPCK-C is also involved in gluconeogenesis [57], its increased expression may facilitate both processes. Though endogenous glucose production (EGP) has been reported to decrease with fasting duration in seals, rats, and dogs [16, 58, 59], a basal level must be maintained to ensure that glucose is available for tissues that do not rely on lipid oxidation (e.g., CNS & RBC). Therefore, because TAG synthesis must be balanced against gluconeogenesis, the liver contributes to a limited amount of FFA re-esterification.
D. Adipose Tissue Re-esterification
Fatty acid re-esterification is predominantly mediated by adipose tissue, which internalizes FFA as well as the FA released from VLDL-TAG by lipoprotein lipase (LPL) [51]. However, like the FA transporters, LPL decreases as a result of fasting [60], likely in an attempt to maintain plasma FFA concentration elevated. Similar to re-esterification in the liver, a pool of fatty acid acceptors is necessary to synthesize the TAG molecule [55]. Because GK activity in adipose tissue of humans and rodents is relatively very low, even in the fed state [61, 62], G3P must be derived from either conversion of glucose to dihydroxyacetone (DHA) and then G3P [63], or by glyceroneogenesis using branched chain amino acids (BCAA) or TCA cycle intermediates [64]. However, in rats fasted for 48 hours, adipose tissue glucose uptake decreases by 68% while PEPCK-C activity increases by 400% [65], so glyceroneogenesis may be the preferred method of G3P synthesis during fasting.
Contrary to what is seen in the liver, cortisol decreases the expression of PEPCK-C in adipose tissue [64, 66]. This differential regulation of PEPCK-C expression may be indicative of the decreased need for TAG storage in adipocytes and increased need for FA mobilization during prolonged food deprivation. Alternatively, because up to 40% of the FFA released by lipolysis are re-esterified by adipocytes [67], a shift to partial hydrolysis during fasting could allow for the accumulation of DAG and MAG within adipose tissue that could serve as fatty acid acceptors (Figure 2). This would allow for reduced reliance on glyceroneogenesis because remodeling of MAG and DAG by monoglyceride acyltransferase (MGAT) and diglyceride acyltransferase (DGAT) [68] could achieve TAG synthesis. Both MGAT and DGAT activities increase in adipose tissue of marmots prior to hibernation [69] demonstrating that these enzymes are involved in the preservation of lipid substrates in food-deprived mammals. Additionally, short-term fasting in rodents increases DGAT expression in adipose tissue due to relatively low levels of carbohydrate [70] suggesting that the same increase may be seen in adipose tissue of prolong-fasting mammals. This process could potentially reduce the impact of futile cycling on energy stores by: 1) reducing the amount of FFA released through lipolysis, 2) keeping a pool of acylglycerols in adipocytes to serve as FA acceptors, and 3) reducing the need for glyceroneogenesis.
III. Substrate Availability & Fuel Metabolism
The effects of substrate availability on fuel metabolism at the systemic level have been described as the three phases of starvation [8, 17]. Each phase is characterized by the predominant catabolism of a different class of substrate and so has a different duration depending on the stores of the specific mammal: 1) carbohydrate (24–48 hours), 2) lipid (2–12 weeks), and 3) protein (1–3 days) [17, 71]. As carbohydrate stores are depleted, defined mechanisms shift metabolism to reliance on lipid oxidation, with a small degree of protein catabolism, until either a food source is found or the organism enters irreversible terminal cachexia and succumbs. Though the characterization of the three phases was a substantial addition to our understanding of starvation metabolism, there has been substantial work demonstrating that the regulation of substrate utilization goes beyond the hormone-mediated hydrolysis of available substrate [36, 46, 72–74]. Lipids exhibit substantial control over metabolism through transcription regulation and through β-oxidation [72, 73, 75, 76], and increase in circulation as a result of food deprivation [9]. Therefore, in addition to serving as sources of energy, lipids could potentially be key regulators of metabolism by serving as cellular signals during food deprivation in mammals.
A. Transcriptional Regulation
Lipid substrates can bind to and activate nuclear receptors like the liver X receptor (LXR), retinoid X receptor (RXR), or PPAR to influence the expression of genes involved in the regulation of metabolism in peripheral tissues [20, 46, 47, 77, 78]. Investigation into the effects of different lipids on nuclear receptors has demonstrated consistency in both affinity for receptor subtypes and effect across multiple species [20, 47, 77–79]. Polyunsaturated fatty acids (PUFA) have been reported tobind to PPARα and promote the expression of genes involved in lipid oxidation in muscle and liver, while downregulating hepatic fatty acid synthase in rodents [20, 73, 80]. PUFA inhibit sterol regulatory element-binding protein (SREBP) activity via deactivation of hepatic LXR, which impedes lipogenesis [55]. Monounsaturated fatty acids (MUFA) promote lipolysis through inhibition of PPARγ, but do not significantly affect oxidation in rats and hamsters [72, 74]. Saturated fatty acids (SFA) increase low-density lipoprotein (LDL) production, but have also been suggested to contribute to FA chain elongation and to increase the expression of genes involved in hepatic de novo lipogenesis in mice [81–83]. Furthermore, derivatives of LCFA oxidation maintain the expression of glucose transporter type 4 (GLUT4) by activating LXR in cultured adipocytes [79] suggesting that lipid metabolism may contribute to cellular glucose availability via effects on LXR.
Furthermore, glucose may be necessary for the binding of lipid ligands to these nuclear receptors [77]. This suggests that in mammals that do not regularly endure prolonged food deprivation, such as humans, reduced glucose availability could reduce lipid ligand-induced activation of these nuclear receptors. If this is the case, then the elevated rates of EGP seen in certain adapted mammals undergoing prolonged fasting [84] may retain or augment the regulatory function of the lipid ligands on nuclear receptor activation. Additionally, maintenance of GLUT4 expression in adipose by LCFA derivatives could preserve the regulation of nuclear receptors in the absence of elevated rates of EGP, thereby preventing impairments to lipid metabolism. For adapted mammals dependent on a lipid-derived metabolism, alleviation of such impairments in lipid metabolism would be critically important.
Because the only lipid substrates available to fasting and hibernating mammals are those released from TAG stores through lipolysis, FA composition of TAG stores should be the first consideration in evaluating the potential regulatory capacity of lipid substrates on metabolism under food deprived conditions. Analysis of the FA composition in adipose tissue of mammals adapted to prolonged bouts of food deprivation (e.g., bears, seals) demonstrates a consistently greater abundance of MUFA than other FA, similar to that observed in humans [10, 11, 13, 14, 85–93]. However, the relative amounts of PUFA and saturated fatty acids (SFA) vary among the different species (Table 1, [10, 11, 14, 85–93]) in a manner that appears to be related to their energy requirements. Fasting animals remain normothermic and only slightly decrease their metabolic rate, while hibernators decrease both body temperature and metabolic rate substantially [14]. The lipid composition of fasting mammals [10, 13, 91] is consistent with what would be expected given the regulatory effects described because PUFA would inhibit lipid synthesis and increase lipid oxidation, while MUFA would maintain lipolysis. Similarly, the relatively high levels of MUFA along with a lower PUFA:SFA ratio seen in hibernators [14, 86, 90, 92] would maintain lipolysis without necessarily increasing lipid oxidation, allowing for the preservation of energy stores to support the energetic burdens associated with prolonged food deprivation.
Table 1.
Fatty acid composition (presented as % of total) of fat depots of various feeding, fasting, or hibernating mammals
| Feeding | Food Deprived | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Species | Manipulation | SFA (%) | MUFA (%) | PUFA (%) | SFA (%) | MUFA (%) | PUFA (%) | References |
|
| ||||||||
| Erignathus barbatus | 15 | 54 | 30 | 10 | ||||
|
| ||||||||
| Eutamias amoenus | SFA diet | 20 | 65 | 15 | ||||
| MUFA diet | 10 | 77 | 13 | 86 | ||||
| PUFA diet | 10 | 44 | 46 | |||||
|
| ||||||||
| Homo sapiens | Healthy | 29 | 55 | 14 | 87 | |||
| Obese | 27 | 55 | 18 | 89 | ||||
| 38 | 45 | 17 | 88 | |||||
|
| ||||||||
| Marmota marmota | 16 | 65 | 17 | 92 | ||||
|
| ||||||||
| Martes americana | 37 | 42 | 21 | 34 | 43 | 23 | 93 | |
|
| ||||||||
| Meles meles | 34 | 42 | 20 | 85 | ||||
|
| ||||||||
| Mirounga angustirostris | Nursing | 21 | 64 | 14 | 91 | |||
|
| ||||||||
| Phoca groenlandica | 15 | 55 | 30 | 10 | ||||
|
| ||||||||
| Phoca hispida | 14 | 56 | 30 | 10 | ||||
|
| ||||||||
| Rhinopoma microphyllum | 50 | 43 | 7 | 90 | ||||
|
| ||||||||
| Spermophilus parryii | 19 | 50 | 31 | 14 | ||||
|
| ||||||||
| Ursus americanus | 33 | 36 | 31 | 39 | 36 | 25 | 11 | |
|
| ||||||||
| Ursus maritimus | 11 | 71 | 18 | 10 | ||||
Abbreviations: SFA-saturated fatty acids, MUFA-monounsaturated fatty acids, PUFA-polyunsaturated fatty acids
Interestingly, humans maintain adipose tissue PUFA:SFA ratios lower than that of fasting and hibernating mammals [87–89]. The respiratory quotient (RQ) of food deprived humans at Phase II of starvation is approximately 0.82 [15] compared to an RQ of 0.71 throughout the 10–12 weeks of fasting in elephant seals [94]. An adipose tissue FA composition that promotes the preservation of lipids may be responsible for the inability of humans to rely solely on β-oxidation under food deprived conditions. Because humans are not able to adjust their metabolic rate to the same extent as fasting adapted mammals, this could explain why humans, and other nonadapted mammals, starve (enter phase III) rather than fast during prolonged periods of food deprivation.
B. β-oxidation
Besides the potential transcriptional regulation of metabolism during food deprivation, lipids can also affect the availability and utilization of metabolic substrates through the mitochondria [76, 95, 96]. For example, the acetyl-CoA remaining from oxidation of FA can be used as a substrate in hepatic ketogenesis, decreasing glucose utilization by the CNS [7, 97] and not just by peripheral tissues, ultimately decreasing the need for EGP. Furthermore, medium chain fatty-acyl carnitines derived from oxidation of LCFA are capable of increasing lipid oxidation in murine muscle cells [76]. Though this suggests that greater availability of lipid substrates, as seen in food deprived mammals [20, 23], promotes proper lipid utilization, increased flux of SFA through the mitochondria results in the downregulation of oxidative capacity and leads to the production of lipid derivatives that interfere with insulin signaling [96, 98–100].
SFA and MUFA are preferentially mobilized from TAG stores in food deprived humans and some adapted mammals [87, 93]. SFA- and MUFA-carnitines also make up 51% and 41%, respectively, of the plasma acylcarnitines in fasting elephant seal pups [23] suggesting that the SFA and MUFA mobilized are being directed towards mitochondrial oxidation. This also suggests that: 1) PUFA are spared from oxidation, regardless of whether a mammal is adapted to food deprivation or not, likely to conserve PUFA for other purposes (e.g., maintenance of membrane fluidity, activation of nuclear receptors), and 2) downregulation of oxidative capacity and interference of insulin signaling may be purposeful because more SFA are oxidized even though MUFA content is greater. Inhibition of cellular insulin signaling reduces glucose uptake, which could further decrease the need for EGP and utilization of BCAA. Therefore insulin resistance associated with increased lipid utilization may be an evolved strategy used by adapted mammals that frequently experience food deprivation to prevent the protein oxidation and cachexia associated with starvation in humans.
IV. Summary and Future Directions
The ultimate goal of any organism undergoing food deprivation is to find food before endogenous energy stores are depleted. In the interim, metabolism is drastically altered to facilitate the availability and utilization of substrates via tightly regulated mechanisms. The mechanisms described here demonstrate the important cross talk that exists between liver and adipose tissue during fasting, emphasizing the increased contribution of adipose tissue as the storage site of the primary metabolic substrate in fasting-adapted mammals. Additionally, the ability of lipid metabolites to activate nuclear receptors and regulate gene expression of proteins associated with lipogenesis, lipolysis, and lipid oxidation provides an indication of the contribution of lipids to the regulation of fasting metabolism beyond that as merely a metabolic fuel. Future studies will benefit from profound examinations of the contributions of nuclear receptors (i.e., LXR, RXR, and PPAR) and FA on the mechanisms regulating lipid metabolism during prolonged fasting.
V. Translational Potential
Because of the nature of studies conducted on these types of animals, identifying potential mechanisms using the available data is more challenging, as the original methods performed were not necessarily designed to investigate cellular metabolism. However, because there is substantial data that agrees in key changes to either biochemistry or cellular protein expression, mechanisms can be inferred and compared to those seen in humans and rodents. Mammals adapted to fasting lifestyles can depend primarily on lipid metabolism and still maintain tight control of both substrate availability and utilization. Because they do so despite experiencing decreased nervous system activity as well as decreased endocrine regulation, they offer the unique opportunity to investigate the cellular contributions to systemic metabolic regulation. As stated earlier, fasting seals experience cellular and biochemical changes similar to that seen in obese humans, and appear to develop fasting-induced insulin resistance. Unlike humans, the seal maintains control of its metabolism, and appears to benefit from the reduced insulin action. Therefore, delineating the mechanisms that allow seals, and other fasting-adapted mammals, to maintain control of metabolism has the potential to improve our understanding of the cellular perturbations that lead to dyslipidemia and insulin resistance in humans.
Acknowledgments
JAV was supported by NIH NHLBI HL091767-S4 and RMO was partially supported by NIH NHLBI K02HL103787 during the writing of this review.
Abbreviations
- AMPk
AMP kinase
- ATGL
Adipose Triglyceride Lipase
- BCAA
Branched chain amino acid
- CD36
Fatty acid Translocase
- DAG
Diacylglycerol
- DHA
Dihydroxyacetone
- DGAT
Diglyceride acyltransferase
- EGP
Endogenous glucose production
- FAS
Fatty acid Synthase
- FATP
Fatty acid Transport Protein
- G3P
Glycerol-3-phosphate
- GK
Glycerol Kinase
- GLUT4
Glucose transporter type 4
- HSL
Hormone-sensitive Lipase
- LCFA
Long chain fatty acid
- LPL
Lipoprotein Lipase
- LXR
Liver X Receptor
- MAG
Monoacylglycerol
- MGAT
Monoglyceride acyltransferase
- MGL
Monoglyceride Lipase
- MUFA
Monounsaturated fatty acid
- PEPCK-c
Phosphoenolpyruvate Carboxykinase-cytosolic
- PPAR
Peroxisome Proliferator –activated Receptor
- RAR
Retinoic Acid Receptor
- RQ
Respiratory Quotient
- RXR
Retinoid X Receptor
- SFA
Saturated fatty acid
- SNS
Sympathetic nervous system
- SREBP
Sterol regulatory element-binding protein
- TAG
Triacylglycerol
Footnotes
Disclosure: Authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotamisligil G, Shargill N, Spiegelman B. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 2.Tschritter O, Fritsche A, Thamer C, et al. Plasma Adiponectin Concentrations Predict Insulin Sensitivity of Both Glucose and Lipid Metabolism. Diabetes. 2003;52(2):239–243. doi: 10.2337/diabetes.52.2.239. [DOI] [PubMed] [Google Scholar]
- 3.Sabio G, Das M, Mora A, et al. A Stress Signaling Pathway in Adipose Tissue Regulates Hepatic Insulin Resistance. Science. 2008;322(5907):1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y-h, Magkos F, Mantzoros CS, et al. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60(12):1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 6.Higuchi K, Masaki T, Gotoh K, et al. Apelin, an APJ Receptor Ligand, Regulates Body Adiposity and Favors the Messenger Ribonucleic Acid Expression of Uncoupling Proteins in Mice. Endocrinology. 2007;148(6):2690–2697. doi: 10.1210/en.2006-1270. [DOI] [PubMed] [Google Scholar]
- 7.Cahill GF, Owen OE, Morgan AP. The consumption of fuels during prolonged starvation. Adv Enzyme Regul. 1968;6:143–150. doi: 10.1016/0065-2571(68)90011-3. [DOI] [PubMed] [Google Scholar]
- 8.Goodman MN, Larsen PR, Kaplan MM, et al. Starvation in the rat. II. Effect of age and obesity on protein sparing and fuel metabolism. Am J Physiol Endocrinol Metab. 1980;239(4):E277–286. doi: 10.1152/ajpendo.1980.239.4.E277. [DOI] [PubMed] [Google Scholar]
- 9.Cahill GF, Herrera MG, Morgan AP, et al. Hormone-Fuel Interrelationships during Fasting. J Clin Invest. 1966;45(11):1751–69. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grahl-Nielsen O, Andersen M, Derocher AE, et al. Fatty acid composition of the adipose tissue of polar bears and of their prey: ringed seals, bearded seals and harp seals. Mar Ecol Prog Ser. 2003;265:275–282. [Google Scholar]
- 11.LeBlanc PJ, Obbard M, Battersby BJ, et al. Correlations of plasma lipid metabolites with hibernation and lactation in wild black bears Ursus americanus. J Comp Physiol [B] 2001;171(4):327–334. doi: 10.1007/s003600100180. [DOI] [PubMed] [Google Scholar]
- 12.Lohuis TD, Beck TDI, Harlow HJ. Hibernating black bears have blood chemistry and plasma amino acid profiles that are indicative of long-term adaptive fasting. Can J Zool. 2005;83(9):1257–1263. [Google Scholar]
- 13.Thiemann GW, Iverson SJ, Stirling I. Seasonal, sexual and anatomical variability in the adipose tissue of polar bears (Ursus maritimus) J Zool. 2006;269:65–76. [Google Scholar]
- 14.Frank CL, Karpovich S, Barnes BM. Dietary Fatty Acid Composition and the Hibernation Patterns in Free Ranging Arctic Ground Squirrels. Physiol Biochem Zool. 2008;81(4):486–495. doi: 10.1086/589107. [DOI] [PubMed] [Google Scholar]
- 15.Stull AJ, Galgani JE, Johnson WD, et al. The contribution of race and diabetes status to metabolic flexibility in humans. Metabolism. 2010;59(9):1358–1364. doi: 10.1016/j.metabol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellini MA, Rea LD. The biochemistry of natural fasting at its limits. Cell Mol Life Sci. 1992;48(6):575–582. doi: 10.1007/BF01920242. [DOI] [PubMed] [Google Scholar]
- 17.Cahill GF. Starvation in Man. N Engl J Med. 1970;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 18.Penicaud L, Kande J, Le Magnen J, et al. Insulin action during fasting and refeeding in rat determined by euglycemic clamp. Am J Physiol Endocrinol Metab. 1985;249(5):E514–E518. doi: 10.1152/ajpendo.1985.249.5.E514. [DOI] [PubMed] [Google Scholar]
- 19.Kajita K, Mune T, Ikeda T, et al. Effect of fasting on PPARγ and AMPK activity in adipocytes. Diabetes Res Clin Pract. 2008;81(2):144–149. doi: 10.1016/j.diabres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: The PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96(13):7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen TS, Vendelbo MH, Jessen N, et al. Fasting, But Not Exercise, Increases Adipose Triglyceride Lipase (ATGL) Protein and Reduces G(0)/G(1) Switch Gene 2 (G0S2) Protein and mRNA Content in Human Adipose Tissue. J Clin Endocrinol Metab. 2011;96(8):E1293–E1297. doi: 10.1210/jc.2011-0149. [DOI] [PubMed] [Google Scholar]
- 22.Pilegaard H, Osada T, Andersen LT, et al. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism. 2005;54(8):1048–1055. doi: 10.1016/j.metabol.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Viscarra JA, Vázquez-Medina JP, Rodriguez R, et al. Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris) J Exp Biol. 2012;215(14):2455–2464. doi: 10.1242/jeb.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young JB. Developmental plasticity in sympathetic nervous system response to fasting in adipose tissues of male rats. Metabolism. 2003;52(12):1621–1626. doi: 10.1016/s0026-0495(03)00331-7. [DOI] [PubMed] [Google Scholar]
- 25.Young JB, Landsberg L. Suppression of sympathetic nervous system during fasting. Science. 1977;196(4297):1473–1475. doi: 10.1126/science.867049. [DOI] [PubMed] [Google Scholar]
- 26.Ahima RS. Adipose Tissue as an Endocrine Organ. Obesity. 2006;14(S8):242S–249S. doi: 10.1038/oby.2006.317. [DOI] [PubMed] [Google Scholar]
- 27.Kiefer FW, Orasanu G, Nallamshetty S, et al. Retinaldehyde Dehydrogenase 1 Coordinates Hepatic Gluconeogenesis and Lipid Metabolism. Endocrinology. 2012 doi: 10.1210/en.2011-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J-C, Gray N, Kuo T, et al. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell & Bioscience. 2012;2(1):19. doi: 10.1186/2045-3701-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bortz WM, Paul P, Haff AC, et al. Glycerol turnover and oxidation in man. J Clin Invest. 1972;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res. 1998;39(6):1280–1286. [PubMed] [Google Scholar]
- 31.Haemmerle G, Lass A, Zimmermann R, et al. Defective Lipolysis and Altered Energy Metabolism in Mice Lacking Adipose Triglyceride Lipase. Science. 2006;312(5774):734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 32.Caimari A, Oliver P, Palou A. Adipose triglyceride lipase expression and fasting regulation are differently affected by cold exposure in adipose tissues of lean and obese Zucker rats. The Journal of Nutritional Biochemistry. 2012;23(9):1041–1050. doi: 10.1016/j.jnutbio.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Viscarra JA, Champagne CD, Crocker DE, et al. 5′ AMP-activated protein kinase activity is increased in adipose of northern elephant seal pups during prolonged fasting-induced insulin resistance. J Endocrinol. 2011;209:317–325. doi: 10.1530/JOE-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viscarra JA, Vazquez-Medina JP, Crocker DE, et al. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol. 2011;300:R150–R154. doi: 10.1152/ajpregu.00478.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaidhu MP, Fediuc S, Anthony NM, et al. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J Lipid Res. 2009;50(4):704–715. doi: 10.1194/jlr.M800480-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo Y-T, Lin T-H, Chen W-L, et al. Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells. Eur J Pharmacol. doi: 10.1016/j.ejphar.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Fredrikson G, Strålfors P, Nilsson NO, et al. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem. 1981;256(12):6311–6320. [PubMed] [Google Scholar]
- 38.Merl V, Peters A, Oltmanns KM, et al. Preserved circadian rhythm of serum insulin concentration at low plasma glucose during fasting in lean and overweight humans. Metabolism. 2004;53(11):1449–1453. doi: 10.1016/j.metabol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Jensen MD, Haymond MW, Gerich JE, et al. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. The Journal of Clinical Investigation. 1987;79(1):207–213. doi: 10.1172/JCI112785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaidhu MP, Anthony NM, Patel P, et al. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am J Physiol Cell Physiol. 2010;298(4):C961–C971. doi: 10.1152/ajpcell.00547.2009. [DOI] [PubMed] [Google Scholar]
- 41.Gauthier M-S, Miyoshi H, Souza SC, et al. AMP-activated Protein Kinase Is Activated as a Consequence of Lipolysis in the Adipocyte. J Biol Chem. 2008;283(24):16514–16524. doi: 10.1074/jbc.M708177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taschler U, Radner FPW, Heier C, et al. Monoglyceride Lipase Deficiency in Mice Impairs Lipolysis and Attenuates Diet-induced Insulin Resistance. J Biol Chem. 2011;286(20):17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaughn LK, Denning G, Stuhr KL, et al. Endocannabinoid signalling: has it got rhythm? Br J Pharmacol. 2010;160(3):530–543. doi: 10.1111/j.1476-5381.2010.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherel Y, Robin J-P, Heitz A, et al. Relationships between lipid availability and protein utilization during prolonged fasting. J Comp Physiol [B] 1992;162(4):305–313. doi: 10.1007/BF00260757. [DOI] [PubMed] [Google Scholar]
- 45.Nickerson JG, Momken I, Benton CR, et al. Protein-mediated fatty acid uptake: regulation by contraction, AMP-activated protein kinase, and endocrine signals. Appl Physiol Nutr Metab. 2007;32(5):865–873. doi: 10.1139/H07-084. [DOI] [PubMed] [Google Scholar]
- 46.Wuttge DM, Romert A, Eriksson U, et al. Induction of CD36 by all-trans retinoic acid: retinoic acid receptor signaling in the pathogenesis of atherosclerosis. FASEB J. 2001 doi: 10.1096/fj.00-0488fje. [DOI] [PubMed] [Google Scholar]
- 47.Martin G, Poirier H, Hennuyer N, et al. Induction of the Fatty Acid Transport Protein 1 and Acyl-CoA Synthase Genes by Dimer-selective Rexinoids Suggests That the Peroxisome Proliferator-activated Receptor-Retinoid X Receptor Heterodimer Is Their Molecular Target. J Biol Chem. 2000;275(17):12612–12618. doi: 10.1074/jbc.275.17.12612. [DOI] [PubMed] [Google Scholar]
- 48.Turcotte LP, Srivastava AK, Chiasson J-L. Fasting increases plasma membrane fatty acid-binding protein (FABPPM) in red skeletal muscle. Mol Cell Biochem. 1997;166(1–2):153–158. doi: 10.1023/a:1006846907394. [DOI] [PubMed] [Google Scholar]
- 49.Qiao L, Zou C, Shao P, et al. Transcriptional Regulation of Fatty Acid Translocase/CD36 Expression by CCAAT/Enhancer-binding Protein α. J Biol Chem. 2008;283(14):8788–8795. doi: 10.1074/jbc.M800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung L, Andersen M, Gustavsson C, et al. Hormonal and nutritional regulation of alternative CD36 transcripts in rat liver - a role for growth hormone in alternative exon usage. BMC Mol Biol. 2007;8(1):60. doi: 10.1186/1471-2199-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalderon B, Mayorek N, Berry E, et al. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279(1):E221–E227. doi: 10.1152/ajpendo.2000.279.1.E221. [DOI] [PubMed] [Google Scholar]
- 52.Luiken JJFP, Arumugam Y, Dyck DJ, et al. Increased Rates of Fatty Acid Uptake and Plasmalemmal Fatty Acid Transporters in Obese Zucker Rats. J Biol Chem. 2001;276(44):40567–40573. doi: 10.1074/jbc.M100052200. [DOI] [PubMed] [Google Scholar]
- 53.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284(2):457–0. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989;264(1):574–577. [PubMed] [Google Scholar]
- 55.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43(2):134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 56.Lin ECC. Glycerol Utilization and its Regulation in Mammals. Annu Rev Biochem. 1977;46(1):765–795. doi: 10.1146/annurev.bi.46.070177.004001. [DOI] [PubMed] [Google Scholar]
- 57.Burgess SC, He T, Yan Z, et al. Cytosolic Phosphoenolpyruvate Carboxykinase Does Not Solely Control the Rate of Hepatic Gluconeogenesis in the Intact Mouse Liver. Cell Metab. 2007;5(4):313–320. doi: 10.1016/j.cmet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cherel Y, Burnol A-F, Leturque A, et al. In vivo glucose utilization in rat tissues during the three phases of starvation. Metabolism. 1988;37(11):1033–1039. doi: 10.1016/0026-0495(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 59.Cowan JS, Vranic M, Wrenshall GA. Effects of preceding diet and fasting on glucose turnover in normal dogs. Metabolism. 1969;18(4):319–330. doi: 10.1016/0026-0495(69)90053-5. [DOI] [PubMed] [Google Scholar]
- 60.Wu G, Brouckaert P, Olivecrona T. Rapid downregulation of adipose tissue lipoprotein lipase activity on food deprivation: evidence that TNF-α is involved. Am J Physiol Endocrinol Metab. 2004;286(5):E711–E717. doi: 10.1152/ajpendo.00257.2003. [DOI] [PubMed] [Google Scholar]
- 61.Ryall R, Goldrick R. Glycerokinase in human adipose tissue. Lipids. 1977;12(3):272–277. doi: 10.1007/BF02533346. [DOI] [PubMed] [Google Scholar]
- 62.Koschinsky T, Gries F, Herberg L. Regulation of glycerol kinase by insulin in isolated fat cells and liver of Bar Harbor obese mice. Diabetologia. 1971;7(5):316–322. doi: 10.1007/BF01219464. [DOI] [PubMed] [Google Scholar]
- 63.Botion LM, Brito MN, Brito NA, et al. Glucose contribution to in vivo synthesis of glyceride-glycerol and fatty acids in rats adapted to a high-protein, carbohydrate-free diet. Metabolism. 1998;47(10):1217–1221. doi: 10.1016/s0026-0495(98)90326-2. [DOI] [PubMed] [Google Scholar]
- 64.Reshef L, Hanson RW, Ballard FJ. A Possible Physiological Role for Glyceroneogenesis in Rat Adipose Tissue. J Biol Chem. 1970;245(22):5979–5984. [PubMed] [Google Scholar]
- 65.Frasson D, Boschini RP, Chaves VE, et al. The sympathetic nervous system regulates the three glycerol-3P generation pathways in white adipose tissue of fasted, diabetic and high-protein diet-fed rats. Metabolism. 2012;61(10):1473–1485. doi: 10.1016/j.metabol.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Ballard FJ, Hanson RW, Leveille GA. Phosphoenolpyruvate Carboxykinase and the Synthesis of Glyceride-Glycerol from Pyruvate in Adipose Tissue. J Biol Chem. 1967;242(11):2746–2750. [PubMed] [Google Scholar]
- 67.Hammond VA, Johnston DG. Substrate cycling between triglyceride and fatty acid in human adipocytes. Metabolism. 1987;36(4):308–313. doi: 10.1016/0026-0495(87)90199-5. [DOI] [PubMed] [Google Scholar]
- 68.Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297(1):E10–E18. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mostafa N, Everett DC, Chou SC, et al. Seasonal changes in critical enzymes of lipogenesis and triacylglycerol synthesis in the marmot (Marmota flaviventris) J Comp Physiol [B] 1993;163(6):463–469. doi: 10.1007/BF00346930. [DOI] [PubMed] [Google Scholar]
- 70.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem Biophys Res Commun. 2002;298(3):317–323. doi: 10.1016/s0006-291x(02)02466-x. [DOI] [PubMed] [Google Scholar]
- 71.Bertile F, Oudart H, Criscuolo F, et al. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem Biophys Res Commun. 2003;303(4):1106–1113. doi: 10.1016/s0006-291x(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 72.Liao FH, Liou TH, Chiu WC, et al. Differential effects of high MUFA with high or low P/S ratio (polyunsaturated to saturated fatty acids) on improving hepatic lipolytic enzymes and mediating PPAR[gamma] related with lipoprotein lipase and hormone-sensitive lipase of white adipose tissue in diet-induced obese hamster. Int J Obes. 2010;34(11):1608–1617. doi: 10.1038/ijo.2010.88. [DOI] [PubMed] [Google Scholar]
- 73.Ren B, Thelen AP, Peters JM, et al. Polyunsaturated Fatty Acid Suppression of Hepatic Fatty Acid Synthase and S14 Gene Expression Does Not Require Peroxisome Proliferator-activated Receptor α. J Biol Chem. 1997;272(43):26827–26832. doi: 10.1074/jbc.272.43.26827. [DOI] [PubMed] [Google Scholar]
- 74.Soriguer F, Moreno F, Rojo-Martínez G, et al. Monounsaturated n9 fatty acids and adipocyte lipolysis in rats. Br J Nutr. 2003;90:1015–1022. doi: 10.1079/bjn2003993. [DOI] [PubMed] [Google Scholar]
- 75.Bandyopadhyay GK, Yu JG, Ofrecio J, et al. Increased Malonyl-CoA Levels in Muscle From Obese and Type 2 Diabetic Subjects Lead to Decreased Fatty Acid Oxidation and Increased Lipogenesis; Thiazolidinedione Treatment Reverses These Defects. Diabetes. 2006;55(8):2277–2285. doi: 10.2337/db06-0062. [DOI] [PubMed] [Google Scholar]
- 76.Lehmann R, Zhao X, Weigert C, et al. Medium Chain Acylcarnitines Dominate the Metabolite Pattern in Humans under Moderate Intensity Exercise and Support Lipid Oxidation. PLoS ONE. 2010;5(7):e11519. doi: 10.1371/journal.pone.0011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hostetler HA, Balanarasimha M, Huang H, et al. Glucose regulates fatty acid binding protein interaction with lipids and peroxisome proliferator-activated receptor α. J Lipid Res. 2010;51(11):3103–3116. doi: 10.1194/jlr.M005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kersten S, Seydoux J, Peters JM, et al. Peroxisome proliferator–activated receptor α mediates the adaptive response to fasting. J Clin Invest. 1999;103(11):1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griesel BA, Weems J, Russell RA, et al. Acute Inhibition of Fatty Acid Import Inhibits GLUT4 Transcription in Adipose Tissue, but Not Skeletal or Cardiac Muscle Tissue, Partly Through Liver X Receptor (LXR) Signaling. Diabetes. 2010;59(4):800–807. doi: 10.2337/db09-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke SD. Polyunsaturated Fatty Acid Regulation of Gene Transcription: A Molecular Mechanism to Improve the Metabolic Syndrome. J Nutr. 2001;131(4):1129–1132. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]
- 81.Oosterveer MH, van Dijk TH, Tietge UJF, et al. High Fat Feeding Induces Hepatic Fatty Acid Elongation in Mice. PLoS ONE. 2009;4(6):e6066. doi: 10.1371/journal.pone.0006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin J, Yang R, Tarr PT, et al. Hyperlipidemic Effects of Dietary Saturated Fats Mediated through PGC-1β Coactivation of SREBP. Cell. 2005;120(2):261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 83.Sampath H, Miyazaki M, Dobrzyn A, et al. Stearoyl-CoA Desaturase-1 Mediates the Pro-lipogenic Effects of Dietary Saturated Fat. J Biol Chem. 2007;282(4):2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 84.Champagne CD, Houser DS, Crocker DE. Glucose production and substrate cycle activity in a fasting adapted animal, the northern elephant seal. J Exp Biol. 2005;208:859–868. doi: 10.1242/jeb.01476. [DOI] [PubMed] [Google Scholar]
- 85.Zalewski K, Martysiak-Żurowska D, Iwaniuk M, et al. Characterization of Fatty Acid Composition in Eurasian Badger (Meles meles) Pol J Env Stu. 2007;16(4):645–50. [Google Scholar]
- 86.Geiser F, McAllan BM, Kenagy GJ. The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J Comp Physiol [B] 1994;164(4):299–305. doi: 10.1007/BF00346446. [DOI] [PubMed] [Google Scholar]
- 87.Yli-Jama P, Haugen TS, Rebnord HM, et al. Selective mobilisation of fatty acids from human adipose tissue. Eur J Int Med. 2001;12(2):107–115. doi: 10.1016/s0953-6205(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 88.Halliwell KJ, Fielding BA, Samra JS, et al. Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J Lipid Res. 1996;37(9):1842–8. [PubMed] [Google Scholar]
- 89.Garaulet M, Pérez-Llamas F, Pérez-Ayala M, et al. Site-specific differences in the fatty acid composition of abdominal adipose tissue in an obese population from a Mediterranean area: relation with dietary fatty acids, plasma lipid profile, serum insulin, and central obesity. Am J Clin Nutr. 2001;74(5):585–591. doi: 10.1093/ajcn/74.5.585. [DOI] [PubMed] [Google Scholar]
- 90.Levin E, Yom-Tov Y, Hefetz A, et al. Changes in diet, body mass and fatty acid composition during pre-hibernation in a subtropical bat in relation to NPY and AgRP expression. J Comp Physiol B. 2012:1–10. doi: 10.1007/s00360-012-0689-0. [DOI] [PubMed] [Google Scholar]
- 91.Puppione DL, Kuehlthau CM, Jandacek RJ, et al. Chylomicron triacylglycerol fatty acids in suckling northern elephant seals (Mirounga angustirostris) resemble the composition and the distribution of fatty acids in milk fat. Comp Biochem Physiol Biochem Mol Biol. 1996;114(1):53–57. doi: 10.1016/0305-0491(95)02120-5. [DOI] [PubMed] [Google Scholar]
- 92.Cochet N, Georges B, Meister R, et al. White adipose tissue fatty acids of alpine marmots during their yearly cycle. Lipids. 1999;34(3):275–281. doi: 10.1007/s11745-999-0364-x. [DOI] [PubMed] [Google Scholar]
- 93.Nieminen P, Rouvinen-Watt K, Collins D, et al. Fatty acid profiles and relative mobilization during fasting in adipose tissue depots of the American marten (Martes americana) Lipids. 2006;41(3):231–240. doi: 10.1007/s11745-006-5092-8. [DOI] [PubMed] [Google Scholar]
- 94.Houser DH, Costa DC. Protein catabolism in suckling and fasting northern elephant seal pups (Mirounga angustirostris) J Comp Physiol [B] 2001;171(8):635–642. doi: 10.1007/s003600100214. [DOI] [PubMed] [Google Scholar]
- 95.Abu-Elheiga L, Matzuk MM, Abo-Hashema KAH, et al. Continuous Fatty Acid Oxidation and Reduced Fat Storage in Mice Lacking Acetyl-CoA Carboxylase 2. Science. 2001;291(5513):2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 96.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 97.Hasselbalch SG, Knudsen GM, Jakobsen J, et al. Brain Metabolism During Short-Term Starvation in Humans. J Cereb Blood Flow Metab. 1994;14(1):125–131. doi: 10.1038/jcbfm.1994.17. [DOI] [PubMed] [Google Scholar]
- 98.Sparks LM, Xie H, Koza RA, et al. A High-Fat Diet Coordinately Downregulates Genes Required for Mitochondrial Oxidative Phosphorylation in Skeletal Muscle. Diabetes. 2005;54(7):1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 99.Koves TR, Li P, An J, et al. Peroxisome Proliferator-activated Receptor-γ Co-activator 1α-mediated Metabolic Remodeling of Skeletal Myocytes Mimics Exercise Training and Reverses Lipid-induced Mitochondrial Inefficiency. J Biol Chem. 2005;280(39):33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 100.Hirabara S, Curi R, Maechler P. Saturated Fatty Acid-Induced Insulin Resistance Is Associated With Mitochondrial Dysfunction in Skeletal Muscle Cells. J Cell Physiol. 2010;222:187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]