Abstract
Objective
Adolescents with anorexia nervosa (AN) are amenorrheic and have decreased bone mass accrual and low bone mineral density (BMD). The regulation of mesenchymal stem cell differentiation is an important factor governing bone formation. Preadipocyte factor 1 (Pref-1), an inhibitor of adipocyte and osteoblast differentiation, is elevated in states of estrogen deficiency. In this study, we aim to (i) investigate effects of transdermal estradiol on Pref-1 in adolescent girls with AN, and (ii) examine associations of changes in Pref-1 with changes in lumbar BMD and bone turnover markers.
Design
Adolescent girls with AN and normal-weight controls were studied cross-sectionally. Girls with AN were examined longitudinally in a double-blind study and received transdermal estradiol (plus cyclic medroxyprogesterone) or placebo for twelve months.
Patients
69 girls (44 with AN, 25 normal-weight controls) 13–18 years were studied at baseline; 22 AN girls were followed prospectively.
Measurements
Pref-1 levels, bone formation and resorption markers, and BMD.
Results
Pref-1 levels decreased in girls with AN after treatment with transdermal estradiol compared with placebo (−0.015±0.016 vs. 0.060±0.026 ng/ml, p=0.01), although at baseline, levels did not differ in AN versus controls (0.246±0.015 vs. 0.267±0.022 ng/ml). Changes in Pref-1 over twelve months correlated inversely with changes in lumbar BMD (r=−0.48, p=0.02) and positively with changes in CTX (r=0.73, p=0.006).
Conclusions
For the first time, we show that Pref-1 is negatively regulated by estradiol in adolescent girls with AN. Inhibition of Pref-1 may mediate the beneficial effects of transdermal estradiol replacement on BMD in girls with AN.
Keywords: anorexia nervosa, Pref-1, osteoporosis
Introduction
Mesenchymal stem cells (MSC) are the progenitor cells for osteoblasts and bone marrow adipocytes.1 Differentiation of MSC into an osteoblast or adipocyte lineage may influence bone formation depending on whether there is increased osteoblastogenesis or increased adipogenesis. Recent studies have demonstrated an inverse relationship between bone mineral density (BMD) and bone marrow fat content in adolescents and adults.2–5 Several studies have suggested that age-related bone loss may be linked to higher levels of marrow adiposity.5,6 Pathologic conditions characterized by bone loss, such as prolonged immobilization increase marrow adiposity7 and we have reported higher marrow fat content in women with anorexia nervosa (AN) compared with normal weight controls.8
Multiple hormones regulate MSC differentiation. Whereas estrogen promotes osteoblastogenesis,9 insulin-like growth factor 1 (IGF-1) may dually stimulate adipocyte and osteoblast differentiation.10 Glucocorticoids have also been shown to stimulate adipocyte differentiation.11 Most individuals with AN have low BMD12,13 and are typically estrogen deficient due to hypogonadotropic hypogonadism, relatively IGF-1 deficient due to acquired growth hormone resistance secondary to undernutrition14 and are hypercortisolemic.15
Pref-1 is a member of the epidermal growth factor (EGF)-like protein family that is highly expressed by adipocyte precursor cells and inhibits adipocyte and osteoblast differentiation.16–18 In recent studies in adult women with AN, we showed that Pref-1 levels were elevated compared to normal weight women and normalized in AN after weight recovery. In those studies Pref-1 levels correlated positively with bone marrow fat content and inversely with BMD.19,20 Women with exercised-induced hypothalamic amenorrhea also have elevated levels of Pref-1, and these levels inversely correlate with BMD.21 These studies in women with AN and hypothalamic amenorrhea, pathologic states of hypogonadism, are consistent with data in postmenopausal women regarding the effects of estrogen deficiency on Pref-1 levels. In a large comparison study of pre- and postmenopausal women, Pref-1 levels were elevated in the postmenopausal group and decreased significantly with estrogen replacement therapy.22
Adolescence is a period characterized by marked increases in bone accrual in healthy individuals, and bone marrow fat content is inversely related to bone mineral density in adolescence.2,3 Girls with AN have reduced bone accrual during adolescence compared to healthy controls.23 Bone metabolism and bone turnover in adolescents differs from adults. Adolescence is normally a period of high bone turnover subsequent to bone modeling and remodelling, and bone formation exceeds bone resorption as peak bone mass accrues.24 Although adult women with AN have decreased bone formation and increased bone resorption, adolescents with AN have decreases in both bone formation and resorption. Pref-1 levels and the effect of estrogen administration have not been investigated in adolescents with AN. We hypothesized that Pref-1 levels would be elevated in girls with AN, a state of estrogen deficiency.
In addition, we have previously reported that transdermal estradiol increases BMD in adolescents with AN,25 and this effect is not mediated by sclerostin,26 an osteocyte derived hormone that inhibits osteoblasts and decreases with estrogen use in postmenopausal women and elderly men.27,28 Mediators of estrogen effects on BMD in AN remain to be characterized and may involve a decrease in Pref-1. Utilizing this previously described cohort26 we examined the effects of 12 months of treatment with transdermal estradiol or placebo on Pref-1 levels in adolescents girls with AN. We hypothesized that treatment with transdermal estradiol would reduce Pref-1 levels and that changes in levels of Pref-1 would correlate inversely with changes in BMD and bone formation markers, and directly with changes in bone resorption markers.
Subjects and Methods
The study was performed at the Clinical Research Center of Massachusetts General Hospital (MGH), Boston, MA, USA, and the Clinical Investigation Unit at the Hospital for Sick Children (SickKids), Toronto, ON, Canada. The study was approved by the Institutional Review Board of Partners HealthCare, Boston, and the Research Ethics Board at SickKids, Toronto, Canada. Written informed consent or assent, if the subject was <18 years old, was obtained from all subjects prior to their participation. Parental consent was obtained for subjects under the age of 18 years.
Study subject selection was previously described.26 A subset of adolescent girls with AN and normal-weight controls 13–18 years of age were selected from a prior study25 according to lumbar spine BMD Z-scores [AN: BMD Z-scores of less than −0.5 (n=44); normal-weight controls BMD Z-scores between +1.0 and −1.0 (n=25)]. The BMD cutoffs were chosen in order to investigate the hypothesis that Pref-1 is elevated in adolescents with AN and low BMD compared with normal-weight controls with normal BMD. The diagnosis of AN was confirmed by a study psychiatrist according to DSM-IV criteria. All subjects with AN were actively enrolled in clinical treatment by their health care providers during the course of the study. Study exclusion criteria have been previously described.25
Data from a subset of twenty-two AN subjects with lumbar spine BMD Z-scores of < −0.5 and a bone age of ≥ 15 years were available for longitudinal analysis over 12 months. The treatment protocol has been described previously.25 Briefly, these subjects with AN and a bone age of ≥ 15 years were randomized to transdermal estradiol (100mcg patch applied twice weekly; Novartis Pharmaceuticals, Inc.) or placebo in a double-blind fashion. Subjects with AN also received cyclic medroxyprogesterone (2.5 mg daily for 10 days each month). All subjects received calcium and vitamin D (1200 mg calcium carbonate and 400 IU vitamin D daily). In the subset of patients where data were available for longitudinal analysis, thirteen girls with AN received transdermal estradiol and nine received placebo.
Blood samples were collected at baseline and at 12 months (samples were obtained during the early follicular phase of the menstrual cycle from normal-weight controls). Baseline and 12-month biochemical analysis included serum levels of estradiol, insulin-like growth factor 1 (IGF-1), leptin, a marker of bone resorption [C-terminal cross-linked peptides (CTX)], a marker of bone formation [N-terminal propeptide of type 1 procollagen (P1NP)], parathyroid hormone (PTH), and Pref-1. Samples for serum 25(OH) vitamin D [25(OH)D] and 24-hour urinary free cortisol (24-hr UFC) were also collected at baseline. Serum Pref-1 concentrations were measured by a sandwich ELISA assay (R&D Systems, Inc., Minneapolis, MN; intra-assay coefficient of variation 3.7%, inter-assay coefficient of variation 6.2%; sensitivity 0.012 ng/ml). Additional biochemical assays have been described previously.25
BMD and body composition were determined by dual-energy x-ray absorptiometry (DXA) (Hologic 4500 A, Waltham MA). Bone age was assessed by a single pediatric endocrinologist according to the methods of Greulich and Pyle.29
Statistical analysis was completed using JMP (version 9, SAS Institute). All values are shown as means ± SEM. P values < 0.05 were considered statistically significant. Baseline characteristics and longitudinal comparisons were performed utilizing the Wilcoxon Rank Sum test. Univariate relationships were evaluated using a Pearson’s correlation coefficient (Spearman’s correlation was utilized when normality was not confirmed by the Wilk-Shapiro test). Outliers in Pearson’s correlations were identified by Jackknife distance assessment. One significant outlier was segregated from the analyses of ΔPref-1 and ΔCTX. Multivariate analysis was utilized to examine the effects of potential confounders after log transformation.
Results
Baseline Characteristics
Baseline clinical and biochemical characteristics of the study subjects were as previously described.26 Girls with AN and normal-weight controls did not differ in bone age, height, Tanner stage, or exercise activity. Girls with AN had had lower body mass index (BMI), lean mass, and percent body fat. Subjects with AN also had significantly lower BMD at the spine and hip versus controls and significantly higher calcium and vitamin D intake including supplements (Table 1).
Table 1.
Baseline Characteristics: Subjects with Anorexia Nervosa (AN) versus Normal-weight Controls, and Anorexia Nervosa Treated with Estradiol (AN E+) versus Anorexia Nervosa Treated with Placebo (AN E−)
| Cross-Sectional Study | Prospective Interventional Study | |||||
|---|---|---|---|---|---|---|
| AN (n=44) |
Controls (n=25) |
p (AN vs. Controls) |
AN E+ (n=13) |
AN E− (n=9) |
p (AN E+ vs AN E−) |
|
| Clinical Characteristics | ||||||
| Age (y) | 16.7±0.22 | 15.7±0.23 | 0.006 | 17.2±0.3 | 16.8±0.4 | NS |
| Bone age (y) | 16.2±0.17 | 15.7±0.25 | NS | 16.7±0.2 | 16.0±0.3 | NS |
| Weight (kg) | 46.7±0.8 | 57.2±1.4 | <0.0001 | 47.5±1.3 | 45.2±2.3 | NS |
| BMI (kg/m2) | 17.2±0.21 | 21.11±0.55 | <0.0001 | 17.4±0.4 | 16.7±0.4 | NS |
| Percent body fat (%) | 17.9±0.8 | 25.4 ±1.0 | <0.0001 | 17.8±1.8 | 15.0±1.8 | NS |
| Lean mass (kg) | 37.3±0.7 | 41.8±0.8 | 0.0003 | 37.7±1.0 | 37.3±2.1 | NS |
| Tanner stage (breasts) | 4.6±0.1 | 4.76±0.1 | NS | 4.7±0.1 | 4.7±0.2 | NS |
| Amenorrhea duration (y) | 0.94±0.12 | - | - | 0.90±0.19 | 0.84±0.13 | NS |
| Exercise Activity (h) | 17.0±1.9 | 16.2±2.2 | NS | 15.4±3.1 | 16.5±3.3 | NS |
| Calcium intake (mg) | 2055±139 | 1096±102 | <0.0001 | 1756±231 | 2033±312 | NS |
| Vitamin D intake (IU) | 570±42 | 174±31 | <0.0001 | 460±78 | 622±93 | NS |
| Hormonal Parameters | ||||||
| Pref-1 (ng/ml) | 0.246±0.015 | 0.267±0.022 | 0.51 | 0.235±0.030 | 0.261±0.036 | NS |
| CTX (ng/ml) | 0.73±0.06 | 1.04±0.07 | 0.002 | 0.62±0.08 | 0.99±0.09 | 0.02 |
| P1NP (ng/ml) | 97.2±11.2 | 194.8±23.6 | <0.0001 | 90.8±13.5 | 107.7±34.6 | NS |
| 25(OH) vitamin D (nmol/l) | 75.4±3.5 | 55.4±2.7 | <0.0001 | 69.1±5.5 | 88.9±10.2 | NS |
| PTH (pg/ml) | 21.3±2.0 | 9.6±2.1 | <0.0001 | 30.4±4.4 | 17.8±3.5 | NS |
| Estradiol (pmol/l) | 107.6±8.8 | 158.2±7.3 | <0.0001 | 125.9±18.7 | 89.2±13.2 | NS |
| 24-hr UFC (nmol) | 179.6±8.2 | 106.8±6.4 | 0.003 | 150.4±28.4 | 139.1±41.9 | NS |
| IGF-1 (ng/ml) | 227±13 | 366±20 | <0.0001 | 219±21 | 259±37 | NS |
| Leptin (ng/ml) | 5.0±0.6 | 11.2±1.1 | <0.0001 | 4.9±0.7 | 3.0±1.1 | NS |
| Bone Mineral Density | ||||||
| Lumbar BMD (g/cm2) | 0.833±0.010 | 0.944±0.014 | <0.0001 | 0.847±0.020 | 0.821±0.028 | NS |
| Lumbar BMD Z-score | −1.37±0.09 | −0.14±0.11 | <0.0001 | −1.33±0.20 | −1.53±0.27 | NS |
NS: not significant
Compared to the control group, girls with AN had significantly lower levels of IGF-1, estradiol, leptin, CTX, and P1NP and significantly higher levels of 25(OH) vitamin D and PTH (although PTH levels remained within the normal range) (Table 1).
Pref-1 Levels in Girls with AN and Healthy Controls
Pref-1 levels did not differ between the two groups at baseline even after controlling for levels of IGF-1 (p = 0.43), levels of estradiol and PTH (p = 0.34), or after matching the groups for chronological age (p = 0.58).
We found no significant associations between baseline levels of Pref-1 and baseline levels of IGF-1, leptin, estradiol, PTH, 24-hr urine free cortisol, CTX, or P1NP in girls with AN or controls (although the correlation between Pref-1 and leptin did approach statistical significance in controls). Levels of Pref-1 positively correlated with 25(OH) vitamin D in controls (r = 0.55, p = 0.003), but this relationship was not observed in girls with AN or when the two groups were analyzed together. Pref-1 levels did not correlate with fat mass or percent body fat in girls with AN or controls.
Impact of Estradiol Replacement on Pref-1 Levels
Twenty-two adolescent girls with AN were randomized to receive transdermal estradiol or placebo for twelve months. Baseline levels of Pref-1 did not differ between the two groups (p = 0.59). As previously described,26 the baseline characteristics of the two groups did not significantly differ except for levels of CTX (Table 1).
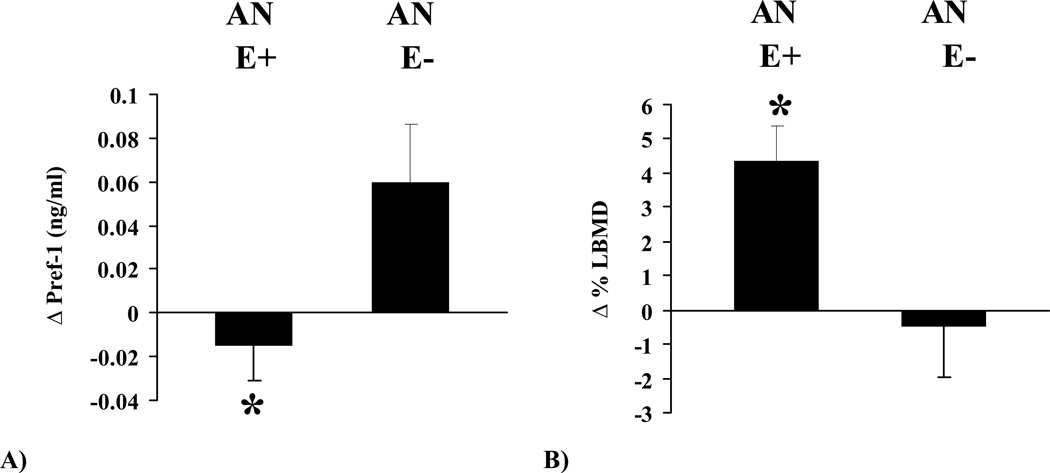
Compared to placebo, treatment with transdermal estradiol significantly decreased Pref-1 levels over 12 months (p = 0.01) in girls with AN. The group receiving transdermal estradiol had significant improvement in lumbar BMD versus the placebo group at twelve months (p = 0.02) (Figure 1). These differences in Pref-1 and lumbar BMD remained significant after controlling for baseline age and weight changes during the period of treatment (p = 0.02 for both parameters). Changes in body composition parameters (total body weight, fat mass, and lean mass) and biochemical parameters (IGF-1, PTH, and leptin) did not differ between the two groups (data not shown).
Figure 1.
Changes in Pref-1 (Panel A) and lumbar BMD (Panel B) after 12 months of treatment with transdermal estradiol (AN E+) or placebo (AN E−). Asterisk denotes statistical significance (p < 0.05).
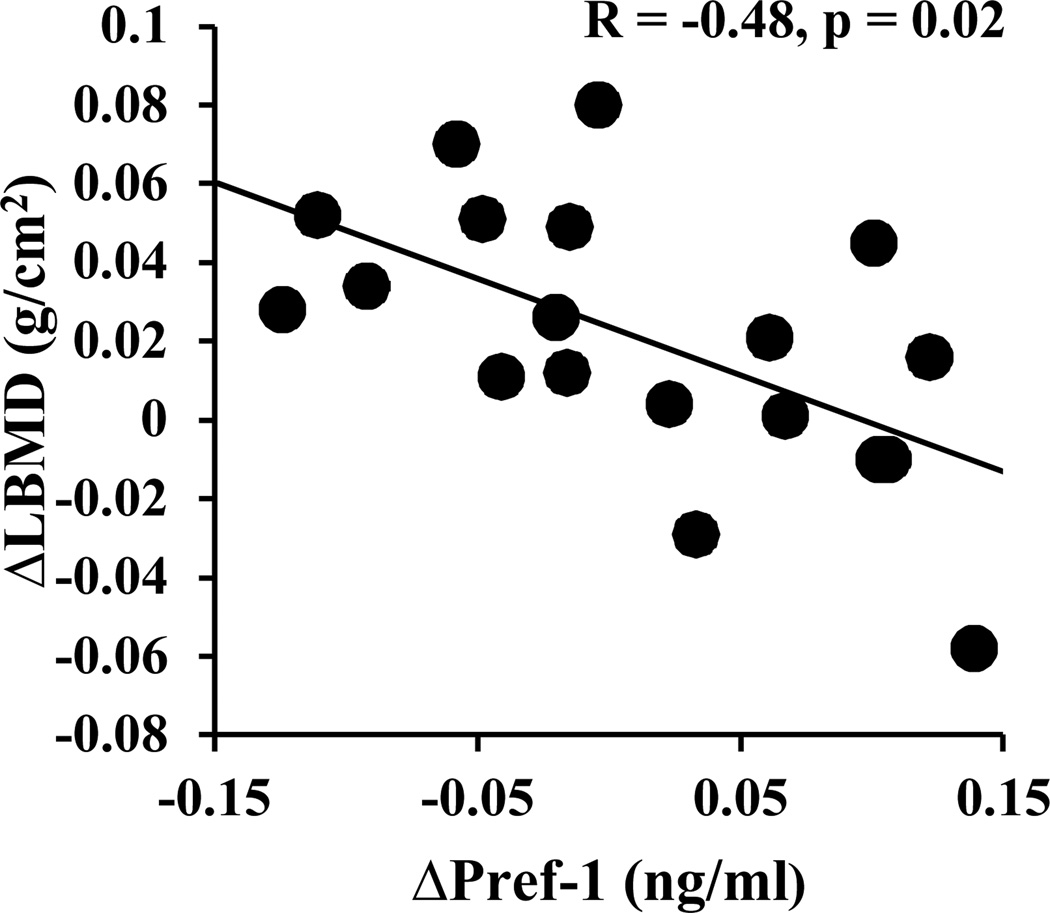
Changes in Pref-1 levels after 12 months of treatment with transdermal estradiol or placebo correlated inversely with changes in lumbar BMD (Figure 2). In a multiple regression model, changes in Pref-1 contributed to 22% of the variability in changes in lumbar BMD (p = 0.05), even after controlling for longitudinal changes in estradiol. There was also a strong positive association between changes in levels of Pref-1 and levels of CTX (r = 0.73, p = 0.006). After 12 months of treatment with transdermal estradiol or placebo, there were inverse associations between the absolute levels of Pref-1 and estradiol (r = −0.64, p = 0.006). Longitudinal changes in Pref-1 and IGF-1 did not correlate (r = 0.12, p = 0.69).
Figure 2.
Correlations of changes in Pref-1 with changes in lumbar BMD after 12 months of treatment with transdermal estradiol or placebo. R and p values were derived from Spearman’s correlation.
Discussion
We demonstrate for the first time that treatment with transdermal estradiol significantly decreases levels of Pref-1 in adolescent girls with AN compared to placebo. As previously reported in the larger cohort, this subset of girls with AN who received transdermal estradiol had significant improvement in lumbar BMD over the treatment period. Changes in lumbar BMD after 12 months of treatment with transdermal estradiol or placebo were associated inversely with changes in levels of Pref-1. This association persisted after controlling for longitudinal changes in estradiol levels, thereby suggesting that the relationship between the relative reduction of Pref-1 and improvement in lumbar BMD might be causal rather than a representation of two independent effects of treatment with transdermal estradiol. Additionally, changes in levels of CTX over 12 months were positively associated with changes in Pref-1.
Although treatment with transdermal estradiol significantly reduced Pref-1 in girls with AN compared to placebo, baseline levels did not differ in girls with AN versus controls. In addition, baseline levels of Pref-1 and estradiol did not correlate in the AN or control groups. These results emphasize the importance of not extrapolating data derived in an adult population with AN to adolescents. In adults with AN, Pref-1 levels are higher than controls.19 However, an important consideration is that serum samples in this study in adolescents were obtained from controls during the early follicular phase of the menstrual cycle; consequently, differences in estradiol levels between girls with AN and controls were only marginal (107.56 ± 8.81 pmol/l and 158.22 ± 7.34 pmol/l, respectively). In contrast, in the study of adult women with AN, estradiol levels were markedly lower in women with AN than controls (126.65 ± 214.75 pmol/l and 558 ± 899.4 pmol/l, respectively).19 It is possible that in the absence of marked differences in estradiol levels between groups in the adolescent study, differences in Pref-1 levels between the groups were masked. However, we did observe a relative decrease in Pref-1 in girls with AN following 12 months of treatment with estradiol compared to placebo. Additionally, estradiol levels at 12 months differed significantly in the treatment versus placebo groups (392.8 ± 62.77 and 123.35 ± 16.89 pmol/l, respectively), and consistent with these differences, we observed a strong inverse association between levels of Pref-1 and estradiol in girls with AN at 12 months. Based on these data, we speculate that Pref-1 is regulated by estrogen status, and may fluctuate during the menstrual cycle due to variations in estradiol concentrations.
In a recent study, normal weight women with exercise-induced hypothalamic amenorrhea had significantly higher levels of Pref-1 than controls.21 Although the distribution of estradiol levels in that study were similar to the AN and control groups in our study, we did not observe a difference in Pref-1 levels. It is possible that additional factors besides estradiol affect Pref-1 in exercise-induced hypothalamic amenorrhea.
Experiments in mice suggest that growth hormone negatively regulates Pref-1,30 and we have shown in a study in rat pituitary cells that Pref-1 (delta-like protein 1) in turn inhibits growth hormone expression.31 Further studies in 3T3 predipocytes indicate that Pref-1 may impair IGF-1 signaling.32 Data from humans are less clear. Levels of Pref-1 do not differ between healthy individuals and patients with growth hormone deficiency or acromegaly. However, growth hormone replacement in individuals with growth hormone deficiency increases Pref-1 levels.33 In our study, girls with AN had significantly lower levels of IGF-1 than controls. Levels of Pref-1 and IGF-1 did not correlate in subjects with AN or controls. Longitudinal changes in Pref-1 and IGF-1 were not significantly correlated in girls with AN.
A study with 3T3 preadipocytes also showed that administration of exogenous dexamethasone is capable of repressing Pref-1 transcription and enhancing adipocyte differentiation.34 Although girls with AN had significantly higher 24-hour urinary free cortisol levels than controls, there was no correlation with Pref-1 in girls with AN or controls.
Leptin administration in healthy women or women with hypothalamic amenorrhea does not affect Pref-1 levels.21 In our study, girls with AN had significantly lower levels of leptin than controls. Leptin levels did not significantly correlate with Pref-1 in the girls with AN. Unlike a prior analysis in adult women with AN,19 Pref-1 levels were not inversely associated with percent body fat in our study. We hypothesize that this correlation may have been evident if samples from controls were obtained at mid-cycle when estradiol levels are elevated (and Pref-1 levels are possibly lower), rather than in the early follicular phase when estradiol levels are low.
Assessment of serum Pref-1 levels may also be complicated by specific assay considerations. Pref-1 exists as a transmembrane protein with four isoforms that may be cleaved to create two alternate soluble products.35,36 The large soluble product appears to be the biologically active form.37 Current antibody-based assays are unable to distinguish between the two soluble forms. Pref-1 production has also been demonstrated (in mice and rats) in cell types other than preadipocytes including thymic stromal cells, adrenal glands, and pancreatic islet Beta cells.38–40 The relative contribution from these sources to the overall serum level of Pref-1 is unknown. Whether peripheral serum Pref-1 levels correlate with local levels in the bone marrow is also unknown.
It is interesting that serum levels of Pref-1 increased longitudinally in the group receiving placebo. To our knowledge there is no published normative data set which describes the impact of age on Pref-1 levels in adolescents. Like other factors which regulate bone formation, such as sclerostin, it may be possible that serum levels of Pref-1 change with advancing chronological age in adolescents and that this increase is augmented by estrogen deficiency.
The lack of serum vitamin A levels in study subjects represents a potential limitation. A recent study in mice demonstrated that retinoic acid administration stimulates Pref-1 expression in vitro and in vivo.41 Our group has previously reported higher vitamin A intake in women with anorexia nervosa compared to healthy controls, and hypercarotenemia has been described in women with anorexia nervosa.42–44 It is theoretically possible that alterations in vitamin A levels may also influence Pref-1 in subjects with anorexia nervosa.
Pref-1 has pleiotropic effects. Recent studies have advanced the link between estrogen, inflammation, and bone loss. DNA microarray analysis has shown that Pref-1 overexpression (or the addition of exogenous Pref-1) upregulates the expression of a variety of pro-inflammatory factors, including IL-6 and NF-κB.45 A prior study by our group demonstrated that IL-6 levels are elevated in adolescent girls with AN and decrease after weight gain.46 Therefore Pref-1 may mediate elevations of IL-6 in AN.
In conclusion, we demonstrate for the first time that estradiol administration downregulates Pref-1 in AN. In our study, the effect of transdermal estradiol on Pref-1 levels was inversely related to changes in BMD and correlated positively with changes in CTX, a marker of bone resorption. Our data in adolescents are consistent with previous adult studies in postmenopausal women and women with AN or hypothalamic amenorrhea.19–22 Pref-1 may potentially mediate the harmful effects of estrogen deficiency on BMD in adolescent girls with AN, and a decrease in Pref-1 with estradiol administration may mediate the beneficial effects of transdermal estradiol on bone accrual. Further studies are warranted to examine the impact of oral versus transdermal estrogen on Pref-1 levels and the effects of estradiol replacement on MSC differentiation, bone marrow fat content, and markers of inflammation in AN.
Acknowledgements
We thank the staff at the Clinical Research Center of Massachusetts General and the Clinical Investigation Unit at the Hospital for Sick Children for their nursing and bionutrition support.
This study was supported by NIH grants R01 DK062249 and 1 UL1 RR025758-01
Footnotes
Financial Disclosure: Nothing to declare
References
- 1.Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Di Iorgi N, Rosol M, Mittelman SD, et al. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adult. Journal of Clinical Endocrinology and Metabolism. 2008;93:2281–2286. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Iorgi N, Mo AO, Grimm K, et al. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. Journal of Clinical Endocrinology and Metabolism. 2010;95:2977–2982. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wren TA, Chung SA, Dorey FJ, et al. Bone marrow fat is inversely related to cortical bone in young and old subjects. Journal of Clinical Endocrinology and Metabolism. 2011;96:782–786. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Rajaratnam JH, Denton J, et al. Adipocyte proportion of bone marrow is inversely related to bone formation in osteoporosis. Journal of Clinical Pathology. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeung DK, Griffith JF, Antonio GE, et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. Journal of Magnetic Resonance Imaging. 2005;22:279–285. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 7.Trudel G, Payne M, Madler B, et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. Journal of Applied Physiology. 2009;107:540–548. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 8.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2009;94:2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao JW, Gao ZL, Mei H, et al. Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. American Journal of Medical Sciences. 2011;341:460–468. doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]
- 10.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone-marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cellular and Molecular Life Sciences. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazziotti G, Angeli A, Bilezikian JP, et al. Glucocorticoid-induced osteoporosis: an update. Trends in Endocrinology and Metabolism. 2006;17:144–149. doi: 10.1016/j.tem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Salisbury JJ, Mitchell JE. Bone mineral density and anorexia nervosa in women. American Journal of Psychiatry. 1991;148:768–774. doi: 10.1176/ajp.148.6.768. [DOI] [PubMed] [Google Scholar]
- 13.Bachrach LK, Guido D, Katzman D, et al. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440–447. [PubMed] [Google Scholar]
- 14.Fazeli PK, Lawson EA, Prabhakaran R, et al. Effects of recombinant human growth hormone in anorexia: a randomized, placebo-controlled study. Journal of Clinical Endocrinology and Metabolism. 2010;95:4889–4897. doi: 10.1210/jc.2010-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. Journal of Clinical Endocrinology and Metabolism. 2004;89:4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 16.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 17.Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histology and Histopathology. 2000;15:119–129. doi: 10.14670/HH-15.119. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Sul HS. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metabolism. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazeli PK, Bredella MA, Misra M, et al. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2010;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fazeli PK, Bredella MA, Freedman L, et al. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. Journal of Bone and Mineral Research. 2012;27:1864–1871. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronis KN, Klim H, Chamberland JP, et al. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. Journal of Clinical Endocrinology and Metabolism. 2011;96:E1634–E1639. doi: 10.1210/jc.2011-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdallah BM, Bay-Jensen A, Srinivasan B, et al. Estrogen inhibits Dlk1/FA1 production: a potential mechanism for estrogen effects on bone turnover. Journal of Bone and Mineral Research. 2011;26:2548–2551. doi: 10.1002/jbmr.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soyka LA, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. Journal of Clinical Endocrinology and Metabolism. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 24.Van Coeverden SCCM, Netelenbos JC, De Ridder CM, et al. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clinical Endocrinology. 2002;57:107–116. doi: 10.1046/j.1365-2265.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 25.Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. Journal of Bone and Mineral Research. 2011;26:2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faje AT, Fazeli PK, Katzman DK, et al. Sclerostin levels and bone turnover markers in adolescents with anorexia nervosa and healthy adolescent girls. Bone. 2012;51:474–479. doi: 10.1016/j.bone.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modder UI, Roforth MM, Hoey K, et al. Effects of estrogen on osteoprogenitor cells and cytokines/bone-regulatory factors in postmenopausal women. Bone. 2011;49:202–207. doi: 10.1016/j.bone.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modder UI, Clowes JA, Hoey K, et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. Journal of Bone and Mineral Research. 2011;26:27–34. doi: 10.1002/jbmr.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: Stanford University Press; 1959. [Google Scholar]
- 30.Abdallah BM, Ding M, Jensen CH, et al. DLK1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007;148:3111–3121. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 31.Ansell PJ, Zhou Y, Schjeide BM, et al. Regulation of growth hormone expression by delta-like protein 1 (Dlk1) Molecular and Cellular Endocrinology. 2007;271:55–63. doi: 10.1016/j.mce.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Nohr J, Jensen CH, et al. Insulin-like growth factor-1/insulin bypases Pref-1/FA-1-mediated inhibition of adipocyte differentiation. Journal of Biological Chemistry. 2003;278:20906–20914. doi: 10.1074/jbc.M300022200. [DOI] [PubMed] [Google Scholar]
- 33.Andersen M, Jensen CH, Stoving RK, et al. Fetal antigen 1 in healthy adults and patients with pituitary disease: relation to physiological, pathological, and pharmacological GH levels. Journal of Clinical Endocrinology and Metabolism. 2001;86:5465–5470. doi: 10.1210/jcem.86.11.7990. [DOI] [PubMed] [Google Scholar]
- 34.Smas CM, Chen L, Zhao L, et al. Transcriptional repression of pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. Journal of Biological Chemistry. 1999;274:12632–12641. doi: 10.1074/jbc.274.18.12632. [DOI] [PubMed] [Google Scholar]
- 35.Smas CM, Green D, Sul HS. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein pref-1. Biochemistry. 1994;33:9257–9265. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- 36.Smas CM, Chen L, Sul HS. Cleavage of membrane-associated pref-1 generates a soluble inhibitor of adipocyte differentiation. Molecular and Cellular Biology. 1997;17:977–988. doi: 10.1128/mcb.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mei B, Zhao L, Chen L, et al. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochemical Journal. 2002;364:137–144. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneta M, Osawa M, Sudo K, et al. A role for pref-1 and HES-1 in thymocyte development. Journal of Immunology. 2000;164:256–264. doi: 10.4049/jimmunol.164.1.256. [DOI] [PubMed] [Google Scholar]
- 39.Halder SK, Takemori H, Hatano O, et al. Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells. Endocrinology. 1998;139:3316–3328. doi: 10.1210/endo.139.7.6081. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson C, Tornehave D, Lindberg K, et al. Growth hormone and prolactin stimulate the expression of rat preadipocyte factor-I/Delta-like protein in pancreatic islets: molecular cloning and expression pattern during development and growth of the endocrine pancreas. Endocrinology. 1997;138:3940–3948. doi: 10.1210/endo.138.9.5408. [DOI] [PubMed] [Google Scholar]
- 41.Berry DC, DeSantis D, Soltanian H, et al. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61:1112–1121. doi: 10.2337/db11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadigan CM, Anderson EJ, Miller KK, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. International Journal of Eating Disorders. 2000;28:284–292. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Robboy MS, Sato AS, Schwabe AD. The hypercarotenemia in anorexia nervosa: a comparison of vitamin A and carotene levels in various forms of menstrual dysfunction and cachexia. American Journal of Clinical Nutrition. 1974;27:362–367. doi: 10.1093/ajcn/27.4.362. [DOI] [PubMed] [Google Scholar]
- 44.Casper RC, Kirschner B, Sandstead HH, et al. An evaluation of trace metals, vitamins, and taste function in anorexia nervosa. American Journal of Clinical Nutrition. 1980;33:1801–1808. doi: 10.1093/ajcn/33.8.1801. [DOI] [PubMed] [Google Scholar]
- 45.Abdallah BM, Boissy P, Qihua T, et al. dlk1/FA1 regulates the function of human bone marrow mesenchymal stem cells by modulating gene expression of pro-inflammatory cytokines and immune response-related factors. Journal of Biological Chemistry. 2007;282:7339–7351. doi: 10.1074/jbc.M607530200. [DOI] [PubMed] [Google Scholar]
- 46.Misra M, Miller KK, Tsai P, et al. Uncoupling of cardiovascular risk markers in adolescents girls with anorexia nervosa. Journal of Pediatrics. 2006;149:763–769. doi: 10.1016/j.jpeds.2006.08.043. [DOI] [PubMed] [Google Scholar]