Abstract
The phosphorylation state of several cardiac myofilament proteins changes with the level of stretch in intact, twitch-contracting cardiac muscles. It remains unclear which kinases are involved in the length-dependent phosphorylation of these proteins. We set out to investigate which kinases are involved after a step-wise change in cardiac muscle length. We hypothesize that myofilament protein phosphorylation by PKCβII and PKA alters contractile kinetics during length-dependent activation. Right ventricular intact trabeculae were isolated from New Zealand White rabbit hearts and stimulated to contract at 1 Hz. Twitch force recordings where taken at taut and optimal muscle lengths before and after administration of kinase inhibitors at 37 °C. PKCβII inhibition significantly decreased time from stimulation to peak force (TTP), time from peak force to 50% relaxation (RT50), and 90% relaxation (RT90) at optimal muscle length. This led to a loss in the length-dependent increase of RT50 and RT90 in the presence of the PKCβII inhibitor, whereas the length-dependent increase in RT50 and RT90 was seen in the controls. PKA inhibition using H-89 significantly decreased TTP at both taut and optimal muscle lengths. Detection of Ser/Thr phosphorylation with ProQ-diamond staining indicates a role for PKCβII in the phosphorylation of tropomyosin and myosin light chain-2 (MLC2) and PKA for tropomyosin, troponin-I, MLC2, myosin binding protein-C, troponin-T (TnT) 3 and TnT4. Our data provide evidence for two signaling kinases acting upon myofilament proteins during length-dependent activation, and provide further insight for length-dependent myofilament function.
Keywords: Frank-Starling mechanism, Contraction, Kinetics, Protein kinase C, Protein kinase A, Phosphorylation, Rabbit
Introduction
The heart’s ability to increase cardiac output as a result of an increase in venous return is known as the Frank–Starling mechanism [1]. This increase in left ventricular end-diastolic pressure and volume increases the amount of stretching the chamber has to do to accommodate the increase in blood volume. This stretching is also known as an increase in preload of the ventricle. When cardiac muscle tissue is stretched within its physiological range, the force development per cross-sectional area increases, as does myofilament calcium sensitivity [2]. The prolongation of relaxation seen at longer muscle lengths has been attributed to myofilament properties, since the time for intracellular calcium decline does not significantly change at different muscle lengths [3]. Recently, it was demonstrated that phosphorylation of troponin I (TnI),1 tropomyosin (Tm), and myosin light chain-2 (MLC2) is increased at steady-state twitch contractions at a longer muscle length compared to a shorter muscle length in rabbit ventricular myocardium [4]. The same study also demonstrated that TnI phosphorylation at Ser22–23 is increased when cardiac muscle is stretched. Another study showed that tropomyosin is phosphorylatable near the C-terminus at Ser283, possibly by protein kinase C (PKC) [5,6], while another study demonstrated increased myofilament calcium sensitivity by PKCβII phosphorylation of TnI at Thr144 [7].
Efficient phosphorylation of myofilament proteins requires spatially confined pools of kinases anchored in close proximity to their substrates [8]. Troponin T (TnT) serves as a sarcomeric A-kinase anchoring protein, and tethers protein kinase A (PKA) at the myofilaments [8]. Both PKA and PKC have been shown to co-localize with, and dock at, cardiac Z-discs [9], and mechanical strain on cardiac Z-discs may play a role in the Frank–Starling mechanism [10]. Since these two signaling kinases bind to sarcomeric proteins, they are localized in the cell in close proximity to other sarcomeric proteins, many of which are known targets of these two kinases. This led us to speculate that increasing mechanical strain at the myofilament level during ventricular filling could lead to the release and activation of these kinases. While the origin of these kinases is not within the scope of the current study, it provided a starting point to determine which kinases to study in the regulation of sarcomeric protein phosphorylation during length-dependent activation. We therefore chose to investigate whether these two kinases are involved in cardiac stretch-induced changes in myofilament phosphorylation and contractile function during the slow response. The immediate increase in developed twitch tension seen after stretching a muscle is followed by a slow response, during which developed tension further increases on a beat-to-beat basis. It often takes several minutes for the changes in developed tension observed during the slow response to fully stabilize. We hypothesize that myofilament protein phosphorylation by PKCβII and PKA contributes to the length-dependent prolongation of relaxation and post-translational modifications of sarcomeric proteins seen previously [3,4] in cardiac tissue. The results advance the field by identifying two kinases, namely PKCβII and PKA, and several of their target proteins, namely myosin binding protein-C (MyBP-C), TnT3, TnT4, Tm, TnI, and MLC2, involved in the regulation of the Frank–Starling mechanism.
Materials and methods
All protocols were approved by The Ohio State University’s Animal Care and Use Committee and comply with the laws of The United States of America. Male New Zealand White Rabbits (weighing approximately 2 kg and 3 months old) were intravenously injected with 5000 units/kg Heparin and anesthetized with 50 mg/kg pentobarbital sodium. The chest was opened by bilateral thoracotomy, the heart was rapidly excised, and ultra-thin trabeculae were dissected from the right ventricle. Muscles of this size were chosen so as to avoid core hypoxia that will be present in muscles greater than approximately 150 µm thick [11] under the conditions used. The muscles were dissected in a Krebs–Henseleit solution containing (in mM) 137 NaCl, 5 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 20 NaHCO3, 10 glucose, and 0.25 CaCl2. Twenty millimolar 2,3-butanedione monoxime (BDM) was added to this solution to minimize cutting damage and to arrest the heart [12]. Muscles were mounted into the setup as previously described [13–15] and stimulated for a 3 ms duration at 120% threshold voltage at a frequency of 1 Hz, which is slightly below the physiological resting rate, while perfused with an oxygenated Krebs–Henseleit solution, now without BDM and containing 1.5 mM Ca2+. The muscles were stretched until an increase in passive (diastolic) force was no longer accompanied by a substantial increase in developed force. The muscles were allowed time to equilibrate at 37 °C (approximately 20 min, or until twitches were consistent). Previous studies have shown this length (i.e. optimal length) to correspond to a sarcomere length of about 2.2 µm, which approximates the end-diastolic sarcomere length in the in vivo beating heart [16].
Twitch contractions were continuously recorded throughout the experiment. Force development was normalized to the cross sectional area of the trabeculae to allow for comparison between muscles of different diameters. Twitches were recorded at each experimental condition upon stabilization of developed tension. Data were collected and analyzed using custom-designed software (in LabView, National Instruments).
After muscles were allowed to stabilize in the experimental setup, twitch recordings were taken both when the muscle was taut (that is, not stretched yet not slack) and when the muscle was at optimal length (stretched until an increase in developed force is accompanied by a disproportional increase in diastolic force, which corresponds to a sarcomere length of about 2.2 µm [16]), to serve as baseline measurements. All measurements were taken upon stabilization of force, which was several minutes after the muscle length was changed. Therefore, the data represent twitch dynamics during the slow phase response. The kinase inhibitor was then added, and a micrometer was used to ensure that twitch recordings were taken again at the same muscle lengths. This allowed each muscle to serve as its own control. In the first subset of trabeculae (n = 9), staurosporine (Calbiochem), a broad spectrum serine–threonine kinase inhibitor, was applied for 20 min at a final concentration of 0.1 µM. In another subset of trabeculae (n = 8), bisindolylmaleimide VIII acetate salt (Sigma), a non-specific PKC inhibitor, was applied for 10 min at a final concentration of 1 µM. Likewise, in another subset of trabeculae (n = 24), PKCβII peptide inhibitor I trifluoroacetate salt (Sigma), a specific PKCβII inhibitor, was applied for 15 min at a final concentration of 7.5 nM. Finally, H-89 (Sigma), a widely utilized PKA inhibitor, was applied at a final concentration of 20 µM for 2 min. All experiments were performed at 1 Hz to avoid frequency-dependent phosphorylation of the myofilament proteins, since phosphorylation of TnI and MLC2 increases at 4 Hz compared to 1 Hz in rabbit myocardium [17]. Trabeculae twitching at 1 Hz at either no preload or optimal preload were flash frozen with liquid nitrogen by dousing the twitch-contracting muscle beating in the set-up with ~20 ml of liquid N2. The muscle was quickly removed from the setup while still frozen and stored at −80 °C for protein analysis. Additional muscles were frozen without drug administration as controls for ProQ analysis.
Phosphoprotein levels were determined by ProQ analysis. Proteins were separated by 1D-PAGE on 12% polyacrylamide gels. Gels were loaded semiquantitatively (equal volume of protein/lane). Phosphorylated proteins were detected by PRO Q Diamond stain following the protocol of the supplier (Invitrogen). Briefly, the gels were fixed in 10% trichloroacetic acid/50% methanol and stained with Pro Q Diamond (1.5 h). The gel was destained and scanned using a Typhoon 9400 (GE Healthcare). Subsequently, the gel was stained with 80 ml of Coomassie Brilliant Blue for 3 h to visualize total protein. The gel was destained with 10% methanol, 10% acetic acid for at least 30 min, and scanned on the Gel Doc XR System (Bio-Rad) using a Coomassie filter set. Phosphobands were normalized to total lane individually, and then averaged. N = 3–5 per group.
Functional data were statistically analyzed using two-way ANOVA followed by student’s t-tests with repeated measures by JMP statistical software. ProQ data were statistically analyzed using two-way ANOVA followed by student’s t-test without repeated measures, also using JMP statistical software. A two-tailed value of P < 0.05 was considered significant. Data are represented as mean ± SEM.
Results
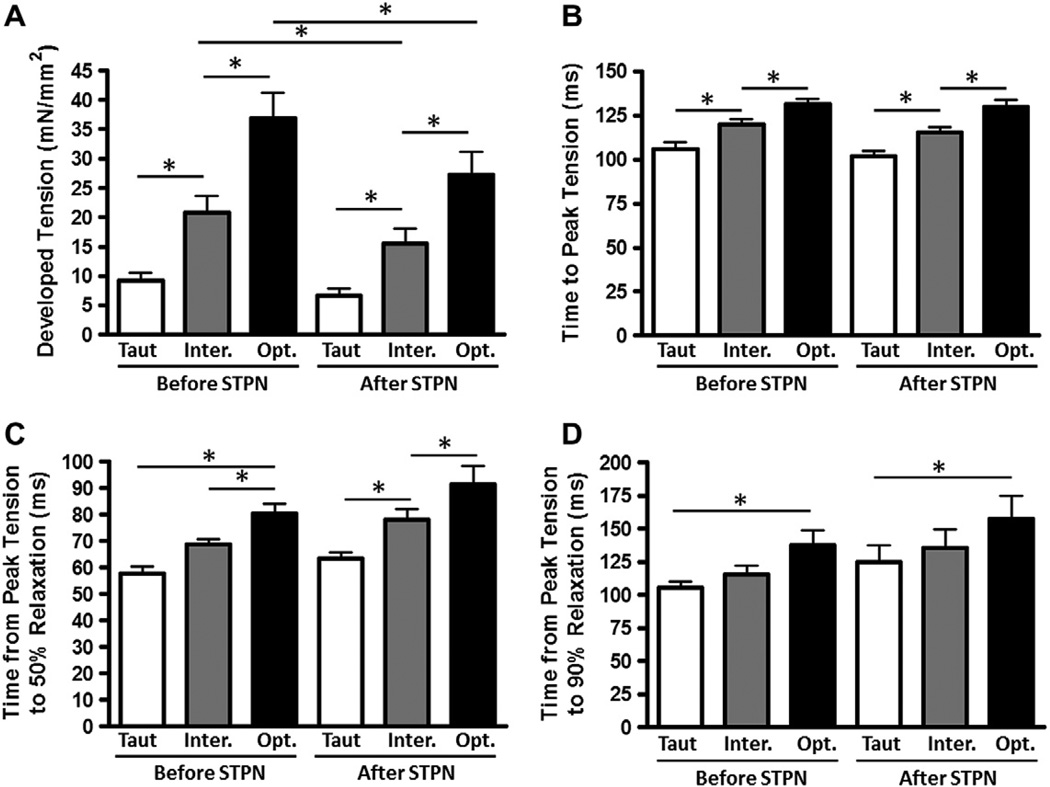
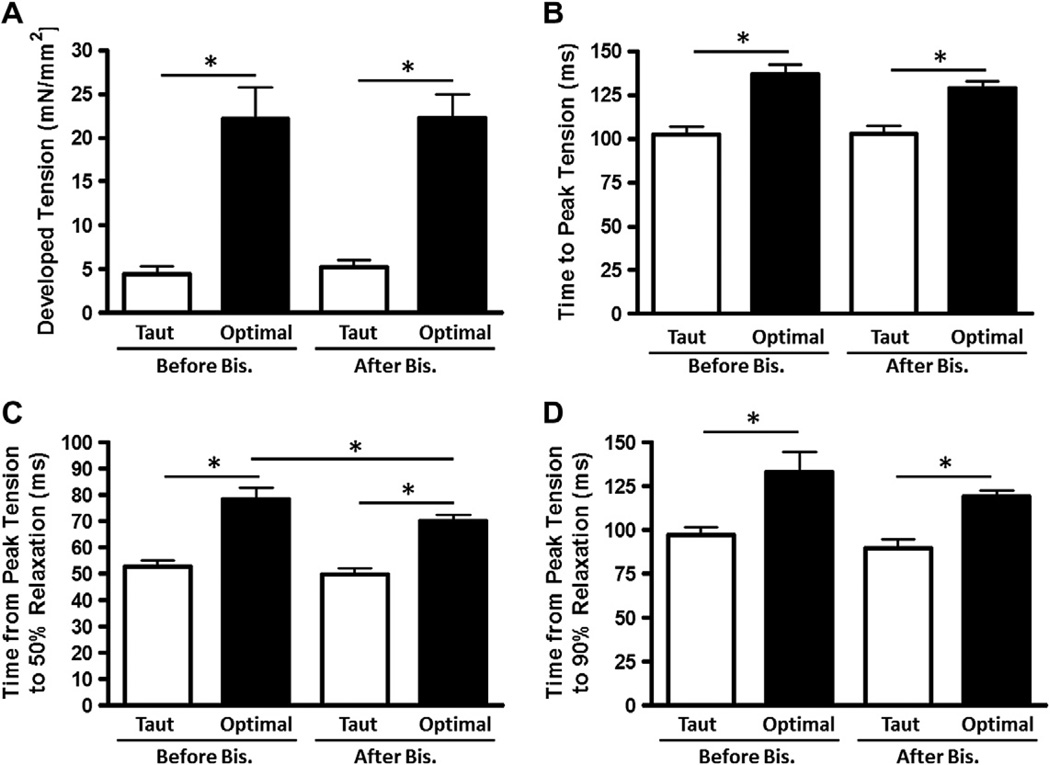
First, we set out to determine whether there may be any myocardial functional changes at different muscle lengths due to serine or threonine phosphorylation, and to quantify any mechanical response in intact, field-stimulated myocardium. To test this, we used administration of staurosporine, a broad spectrum serine–threonine kinase inhibitor, and measured development of tension, time to peak tension (TTP), and time from peak tension to 50% and 90% relaxation (RT50 and RT90, respectively). These mechanical myocardial responses to administration of staurosporine are shown in Fig. 1. Administration of staurosporine resulted in a significant decrease in developed tension at the intermediate and optimal muscle lengths, and a decreasing trend for developed tension at the taut muscle length. TTP, RT50, and RT90 were not significantly affected by staurosporine administration. However, the effect on developed tension prompted further investigation using more specific kinase inhibitors. Thus, in a separate set of experiments, we investigated whether there could be a role for any PKC isoforms by application of the non-specific PKC inhibitor bisindolylmaleimide VIII acetate salt (Fig. 2). Non-specific PKC inhibition resulted in a significant decrease in RT50 and a decrease (although not statistically significant) in RT90 at optimal muscle length.
Fig. 1.
Staurosporine decreases developed tension. Developed tension (A), time to peak tension (B), time from peak tension to 50% relaxation (C), and time from peak tension to 90% relaxation (D) before and after application of Staurosporine, a non-specific serine/threonine kinase inhibitor, at taut, intermediate, and optimal muscle lengths. Data collected at 1 Hz, 37 °C, n = 9.
Fig. 2.
Non-specific PKC inhibition decreases RT50 and, to an extent, RT90. Developed tension (A), time to peak tension (B), time from peak tension to 50% relaxation (C), and time from peak tension to 90% relaxation (D) before and after application of bisindolylmaleimide VIII acetate salt (Bis), a non-specific PKC inhibitor, at taut and optimal muscle lengths. Data collected at 1 Hz, 37 °C, n = 8.
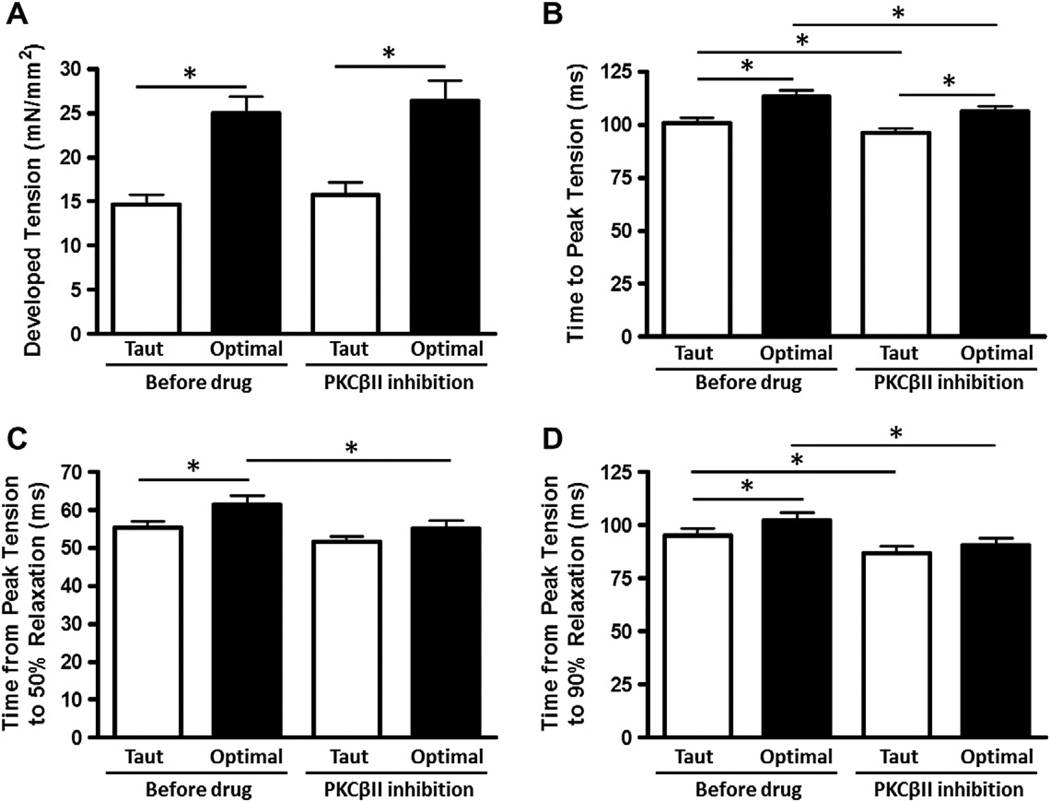
Given the significant effect of a non-specific PKC inhibitor on RT50, we expanded our investigation with yet a more specific kinase inhibitor. The mechanical myocardial responses to administration of a specific PKCβII inhibitor are shown in Fig. 3. Specific PKCβII inhibition resulted in a statistically significant decrease in TTP, RT50, and RT90 at optimal muscle length. Importantly, length-dependent increases in RT50 and RT90 seen in control were abolished after PKCβII inhibition. Developed tension was not significantly different with PKCβII inhibition.
Fig. 3.
PKCβII inhibition decreases contractile and relaxation kinetics. Developed tension (A), time to peak tension (B), time from peak tension to 50% relaxation (C), and time from peak tension to 90% relaxation (D) before and after application of PKCβII peptide inhibitor I trifluoroacetate salt, at taut and optimal muscle lengths. Data collected at 1 Hz, 37 °C, n = 24.
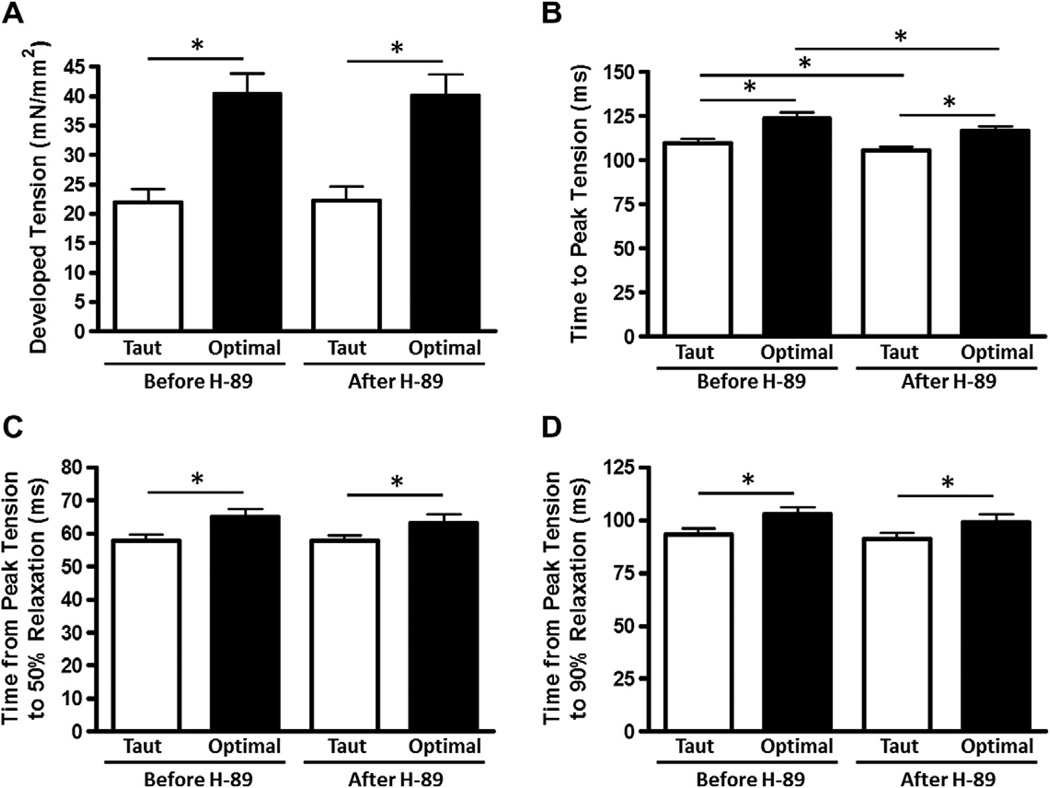
Next, we similarly set out to determine the mechanical response in intact, field-stimulated myocardium to inhibition of PKA. Since PKA is one of the most powerful kinases known to impact on cardiac contractile performance, it is interesting to note that the net mechanical function as assessed by developed tension, RT50, and RT90 were not significantly affected by PKA inhibition by H89. However, TTP was decreased at both taut and optimal muscle lengths with PKA inhibition, although the myocardium maintained a significant length-dependent increase in TTP after PKA inhibition (Fig. 4).
Fig. 4.
H89, a non-specific PKA inhibitor, decreases contractile but not relaxation kinetics. Developed tension (A), time to peak tension (B), time from peak tension to 50% relaxation (C), and time from peak tension to 90% relaxation (D) before and after application of H-89, a PKA inhibitor, at taut and optimal muscle lengths. Data collected at 1 Hz, 37 °C, n = 23.
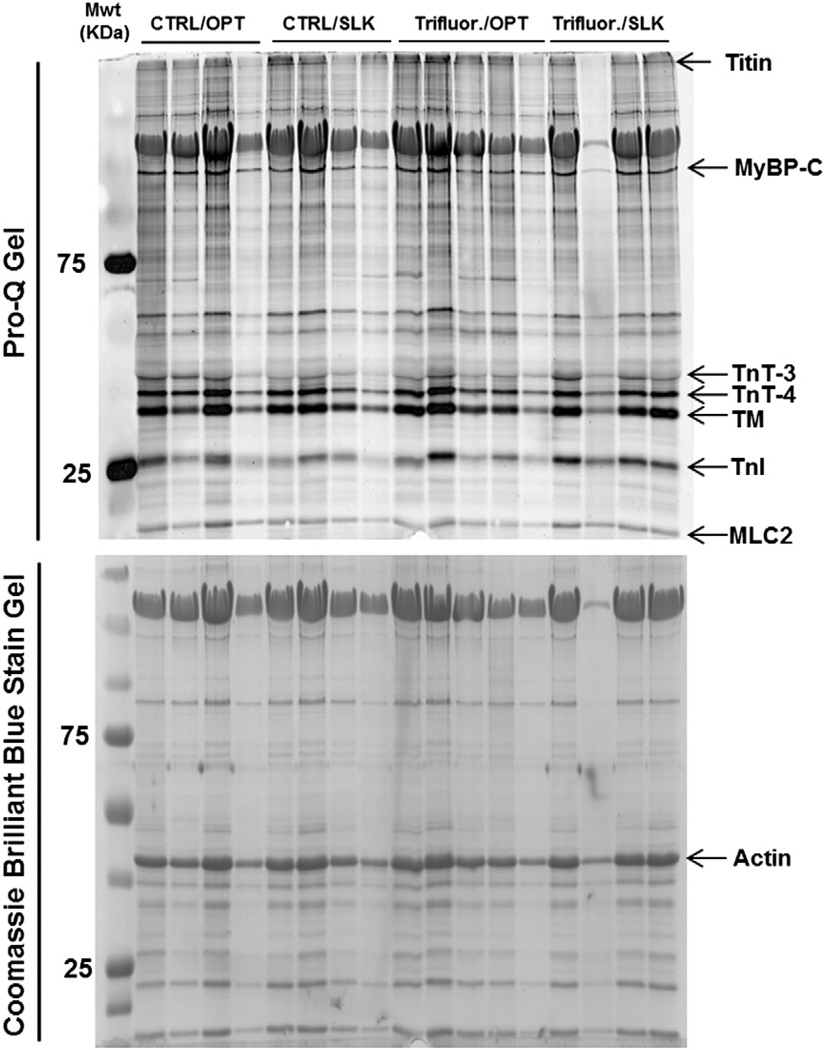
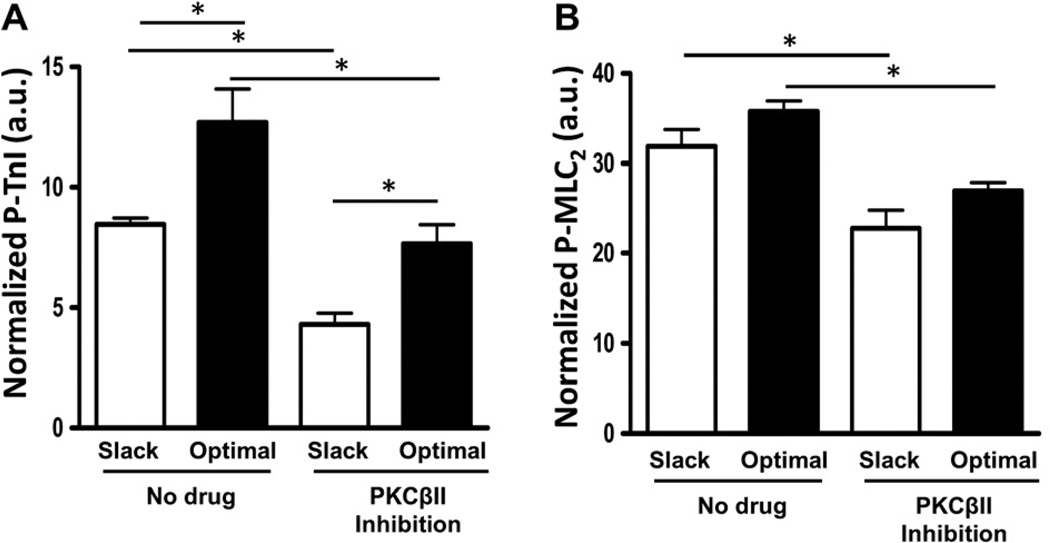
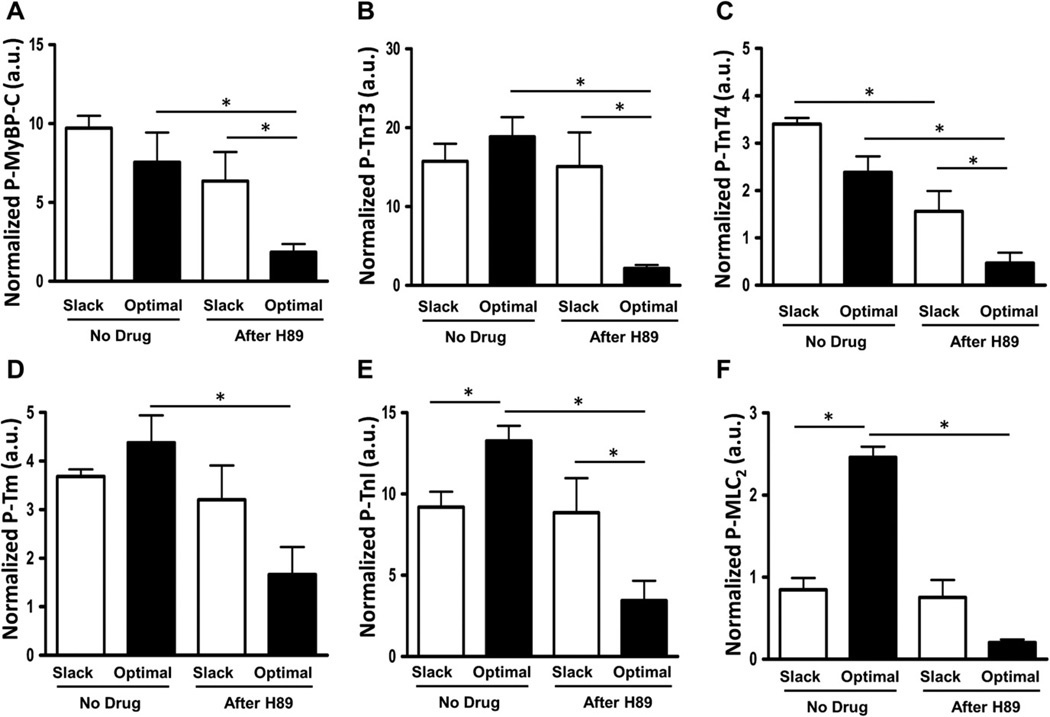
To detect whether myofilament protein post-translational modifications may play a role in the differential mechanical responses observed, we employed ProQ analysis to assess the phosphorylation level of several major sarcomeric proteins. A representative gel with ProQ and Coomassie Brilliant Blue staining is shown in Fig. 5. PKCβII inhibition by PKCβII peptide inhibitor I trifluoroacetate salt administration results in significantly reduced TnI and MLC2 phosphorylation at both taut and optimal muscle lengths (Fig. 6). Phosphorylation of tropomyosin, MyBP-C, TnT3, and TnT4 (Figure S1) was not significantly different after PKCβII inhibition between any of the groups. However, PKA inhibition by H89 administration results in significantly reduced MyBP-C, TnT3, TnT4, Tm, TnI, and MLC2 phosphorylation at optimal muscle length compared to non-treated muscle at the same length (Fig. 7). In addition, phosphorylation of MyBP-C, TnT3, TnT4, and TnI was significantly reduced at optimal length with H89 treatment compared to slacked muscles also with H89 treatment (Fig. 7). This indicates that the net increased phosphorylation of TnI normally seen at longer muscle lengths compared to shorter muscle lengths is actually reversed by PKA inhibition; phosphorylation of TnI is decreased at optimal length compared to slack with PKA inhibition. These findings indicate an important role for PKA signaling to sarcomeric proteins in the regulation of length-dependent activation.
Fig. 5.
Representative assessment of myofilament phosphorylation status. Pro-Q and Coomassie Gels demonstrating changes in phosphorylation of various myofilament proteins in intact cardiac trabeculae after or without administration of PKCβII peptide inhibitor I trifluoroacetate salt (Trifluor.) when the muscle is held slack or at optimal muscle length.
Fig. 6.
PKCβII inhibition reduces TnI and MLC2 phosphorylation. ProQ results showing phosphorylation status of myofilament proteins troponin-I (TnI) and myosin light chain-2 (MLC2) with or without PKCβII peptide inhibitor I trifluoroacetate salt administration to induce inhibition of PKCβII at a short (muscle slacked) and long (optimal) muscle length.
Fig. 7.
PKA inhibition reduces phosphorylation of several myofilament proteins. ProQ results showing phosphorylation status of myofilament proteins myosin binding protein-C (MyBP-C), troponin T-3 (TnT3), troponin T-4 (TnT4), tropomyosin (Tm), troponin-I (TnI), and myosin light chain-2 (MLC2), with or without H89 administration to induce inhibition of PKA at a short (muscle slacked) and long (optimal) muscle length.
Discussion
The current study has advanced our understanding of the mechanism of length-dependent activation by identifying two important signaling proteins, PKCβII and PKA, which alter myofilament protein phosphorylation states during length-dependent activation. Length-dependent prolongation of relaxation kinetics was abolished after treatment with a PKCβII inhibitor. The data are in agreement with our previous study, which found posttranslational modifications of myofilament proteins are involved in length-dependent activation and modulation of myofilament calcium sensitivity in intact, field-stimulated muscle at physiological temperature and frequencies [4]. However, the current study provides an initial insight into which kinases are involved in this process, and provides further insight into which sarcomeric proteins are involved. In addition, phospho-proteomic analysis in twitch-contracting single preparations, often providing no more than 0.05 mg total tissue, allows us to investigate signal pathways at physiological temperature, where signaling pathways are present. In addition, these assessments can be made in an animal model (rabbit) that closely resembles human EC-coupling.
Intact cardiac trabeculae were first subjected to a non-specific serine/threonine kinase inhibitor, staurosporine, to generally assess whether contractile parameters can be prominently influenced by phosphorylation at serine or threonine residues. Our results indicate that the main effect is a reduced developed tension after the application of staurosporine, whereas contraction and relaxation parameters are not significantly affected. This could be for a number of reasons, including but not limited to the fact that phosphorylation may also occur at tyrosine residues, that staurosporine may be poorly selective for PKC [18], which was found to play a role in relaxation in the present study, and that staurosporine inhibits a large number of protein kinases [18]. Moreover, the specificity and effectiveness of “general” inhibitors may only have been tested against the few readily available kinases (usually PKA, PKC, PKG, MLCK, and CaMKII) [18] and may not be effective for less studied kinases, though many kinases may actually play a role in regulation of myofilament function. Therefore, we chose to investigate further using more specific inhibitors.
Previous studies demonstrated myofilament calcium sensitivity is increased by phosphorylation of TnI by PKCβII [7], and phosphorylation of TnI occurs concomitant with an increase in myofilament calcium sensitivity during length-dependent activation [4]. This is in agreement with the current study, in which PKCβII inhibition results in decreased TTP, RT50, and RT90, suggestive of decreased calcium sensitivity, and loss of length-dependent prolongation of relaxation. Additionally, these functional responses occur concomitant with decreased TnI phosphorylation. This is in agreement with another study which found increased myofilament calcium sensitivity is associated with increased phosphorylation of TnI by PKCβII [7]. Furthermore, MLC2 phosphorylation was decreased with PKCβII inhibition in the present study. Increases in MLC2 phosphorylation have been implicated in length dependent activation, occurring together with increases in TnI and Tm phosphorylation [4]. However, Tm phosphorylation was not significantly altered with PKCβII inhibition. This suggests a role for multiple kinases in length-dependent regulation of myofilament function. Finally, this is in agreement with another study which found a decrease in TnI and MLC2 phosphorylation is associated with increased contractile kinetics during frequency-dependent activation [17]. These results indicate a role for PKCβII in the phosphorylation of TnI and MLC2 during length-dependent activation.
PKA is a well-known signaling protein which post-translationally modifies several myofilament proteins. However, it remains unclear whether stretch in intact twitching muscles activates PKA, and thus we aimed to determine whether this protein plays a role in stretch-induced activation and how those effects may contribute to the prolonged contractile and relaxation kinetics and increased tension development induced by stretch. PKA inhibition resulted in a significantly reduced TTP at both muscle lengths studied. The decreased TTP at optimal length seen with PKA inhibition compared to untreated control occurred concomitant with decreased phosphorylation of several important sarcomeric proteins, including MyBP-C, TnT3, TnT4, Tm, TnI, and MLC2. The decrease in TTP with PKA inhibition suggests a decrease in calcium sensitivity. This is in agreement with the phospho-protein analysis of the present study in that simultaneous decreases in Tm, TnI, and MLC2 phosphorylation have been previously shown to occur concomitant with decreased myofilament calcium sensitivity [4]. However, developed tension, RT50, and RT90 were not significantly altered with PKA inhibition, suggesting a role for multiple kinases in length-dependent activation.
The compounding effect of multiple post-translational modifications of myofilament proteins is still poorly understood, despite the vast number of studies. PKC and PKA may phosphorylate myofilament proteins, including TnI [19], at different, distinct sites [19], and this may cause opposing functional effects of the myofilament response [19]. Additionally, the functional response of the myocardium is a result of the sum of all of the post-translational modifications and other factors (lattice spacing) which occur during length-dependent activation. Therefore, it would be rather ambiguous to attribute an in vivo functional response to a single posttranslational modification, and it is best to remember that different combinations of protein modifications may result in a different response phenotype. These concepts should be considered during interpretation of the findings. Thus, future studies will address further the effects of site-specific post-translational modifications.
Previous studies investigating length-dependent activation have considered an important role for calcium during the slow force response [20,21]. However, regarding changes in kinetics during this slow response, a more recent study which simultaneously measured force development and calcium transients demonstrated that the time for calcium decline does not change at different muscle lengths, and is therefore not mainly responsible for prolonged force relaxation at longer muscle lengths [3]. Although the level of developed force may be largely dictated by the amount of calcium [20,21], the rate of relaxation appears mainly determined by the myofilaments [3], and this is in agreement with several other studies [22,23]. Therefore, we would speculate that any post-translational changes in calcium handling proteins would have only a minor or negligible effect on the kinetics of length-dependent activation, since major modifications in calcium handling proteins would be thought to modify calcium transient kinetics.
Myofilament proteins may contribute to prolonged relaxation rates at longer muscle lengths by several mechanisms. One mechanism is through cooperativity, whereby crossbridges recruit the formation of additional crossbridges, which can prolong relaxation times [23] in the absence of calcium [23,24]. Another mechanism is through post-translational modifications to specific proteins [4]. PKC has been shown to phosphorylate MLC2 [25], TnT [6], MyBP-C [26], TnI [27], and titin [28]. PKA has also been shown to phosphorylate TnI [19] and MyBP-C [29], and is tethered to the myofilaments by TnT [8]. Our laboratory has shown that MLC2 phosphorylation may also modify other sarcomeric proteins (e.g., cardiac troponins) as substrates for kinases and phosphatases [30–32]. We previously demonstrated that TnI phosphorylation increases at Ser22–23 during length-dependent activation at optimal length compared to slack [4]. This is in agreement with the current study in which TnI phosphorylation was increased with length in controls. An interesting finding is that TnI phosphorylation not only decreased but the length-dependency was actually reversed, in that phosphorylation was less at optimal length compared to slack. This indicates that phosphorylation of TnI may play an important role in the regulation of length-dependent activation.
A limitation of the study is that the small preparation size of the trabeculae (~0.02–0.05 mg total) typically requires that the entire preparation be loaded onto the gel for any ProQ or Western blot analysis. Because of this limitation, we focused on the myofilament proteins rather than on proteins involved in calcium handling in this study. A complete differential analysis to further elucidate the relevance of the quantitative involvement of each individual phosphorylation site will likewise require an experimental design of a significantly large scope. Additionally, sometimes preparations are so small that functional data may be obtained, but not enough protein is present for use in molecular analysis. Therefore, for each gel that is run, several muscles are needed, while only 1–2 muscles per heart lend themselves for inclusion in the contractile physiology part of such study. Currently, the present study has advanced the field by identifying two highly investigated signaling kinases and linking them to length-dependent activation. Future studies will have to be undertaken to address quantitative functional relevance for each potential target amino acid involved. A second limitation is that not all pharmacological inhibitors are 100% specific, and thus may (partially) block additional kinases, and thus further investigations are warranted into fully quantifying the impact of each individual kinase more precisely.
In summary, although length-dependent processes alter myofilament properties virtually instantaneously by altered geometry of the myofilaments [2], slower processes also contribute to the final steady-state contractile output of the myocardium after a length-change. These myofilament-based targets of kinases fine-tune the myocardium both in contraction amplitude, as well as in the kinetics of the contraction and relaxation [33]. These myofilament proteins need to be thought of as part of a complex system, rather than what effect phosphorylation of one particular protein may have in isolation. The use of new technologies now allows us to better understand these mechanisms by attempting to “trap” the posttranslational modifications, as described by Marston and Walker [34], during more physiologically relevant conditions. It is important to investigate the role of individual kinases and their target proteins with an emphasis on their role as part of a complex system, rather than focusing on a single protein acting as the proverbial “silver bullet”. The emphasis of the current study is to highlight the fact that at least two signaling kinases play a role in length-dependent activation and that these kinases signal to multiple sarcomeric proteins. Future studies are required to fully elucidate the exact molecular/biophysical mechanisms upstream of the activation of the kinases and post-translational modifications of sarcomeric proteins involved in late-modulating effects of length-dependent activation as described in this study.
Supplementary Material
Acknowledgments
Grants
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute Grants R01 HL746387 and R01 HL113084 (to P.M.L.J.), an American Heart Association National Center Established Investigator award 0740040N (to P.M.L.J.), and P01 HL 062426 (R.J.S.). An American Heart Association Great Rivers Affiliate Pre-doctoral Fellowship 0815457D, and NIH/NHLBI Grants T32 HL 07692-16-20 and F32 HL 116094 supported M.M.M. A grant from the UIC Center for Clinical and Translational Science supported D.M.T.
The authors gratefully thank Chad M. Warren and Dr. Brandon J. Biesiadecki for useful discussions.
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.abb.2012.10.005.
Footnotes
Abbreviations used: BDM, 2,3-butanedione monoxime; MLC2, myosin light chain-2; MyBP-C, myosin binding protein-C; Tm, tropomyosin; TnI, troponin I; TnT, Troponin T; PKC, protein kinase C.
Disclosures
None.
References
- 1.Solaro RJ. Biophys. J. 2007;93:4095–4096. doi: 10.1529/biophysj.107.117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. J. Mol. Cell. Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monasky MM, Varian KD, Davis JP, Janssen PM. Pflugers Arch. 2008;456:267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 4.Monasky MM, Biesiadecki BJ, Janssen PM. J. Mol. Cell. Cardiol. 2010;48:1023–1028. doi: 10.1016/j.yjmcc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao VS, Marongelli EN, Guilford WH. Cell Motil. Cytoskeleton. 2009;66:10–23. doi: 10.1002/cm.20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SC, Solaro RJ. J. Biol. Chem. 2007;282:30691–30698. doi: 10.1074/jbc.M703670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. J. Mol. Cell. Cardiol. 2006;41:823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Sievert GA, Balke CW, Sumandea MP. J. Biol. Chem. 2011;286:530–541. doi: 10.1074/jbc.M110.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyle WG, Solaro RJ. Circ. Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- 10.Hoshijima M. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman S, Kelley MA, Janssen PM. Pflugers Arch. 2006;451:625–630. doi: 10.1007/s00424-005-1500-9. [DOI] [PubMed] [Google Scholar]
- 12.Mulieri LA, Hasenfuss G, Ittleman F, Blanchard EM, Alpert NR. Circ. Res. 1989;65:1441–1449. doi: 10.1161/01.res.65.5.1441. [DOI] [PubMed] [Google Scholar]
- 13.Varian KD, Raman S, Janssen PM. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2092–H2097. doi: 10.1152/ajpheart.01241.2005. [DOI] [PubMed] [Google Scholar]
- 14.Monasky MM, Janssen PM. J. Comp. Physiol. B. 2009;179:469–479. doi: 10.1007/s00360-008-0331-3. [DOI] [PubMed] [Google Scholar]
- 15.Monasky MM, Varian KD, Janssen PM. J. Comp. Physiol. B. 2008;178:307–313. doi: 10.1007/s00360-007-0223-y. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. Am. J. Physiol. Heart Circ. Physiol. 1992;263:H293–H306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- 17.Varian KD, Janssen PM. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2212–H2219. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- 18.MacKintosh C, MacKintosh RW. Trends Biochem. Sci. 1994;19:444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 19.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. J. Physiol. 2003;552:845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White E, Boyett MR, Orchard CH. J. Physiol. 1995;482(Pt 1):93–107. doi: 10.1113/jphysiol.1995.sp020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calaghan SC, Colyer J, White E. Pflugers Arch. 1999;437:780–782. doi: 10.1007/s004240050846. [DOI] [PubMed] [Google Scholar]
- 22.Allen DG, Kurihara S. J. Physiol. (Lond) 1982;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon AM, Homsher E, Regnier M. Physiol. Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 24.Poggesi C, Tesi C, Stehle R. Pflugers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- 25.Venema RC, Raynor RL, Noland TA, Jr, Kuo JF. Biochem. J. 1993;294(Pt 2):401–406. doi: 10.1042/bj2940401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barefield D, Sadayappan S. J. Mol. Cell. Cardiol. 2010;48:866–875. doi: 10.1016/j.yjmcc.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu QW, Hinken AC, Patrick SE, Solaro RJ, Kobayashi T. J. Biol. Chem. 2010;285(1817):11810–11811. doi: 10.1074/jbc.M109.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solaro RJ. J. Biol. Chem. 2008;283:26829–26833. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel JR, Fitzsimons DP, Buck SH, Muthuchamy M, Wieczorek DF, Moss RL. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2732–H2739. doi: 10.1152/ajpheart.2001.280.6.H2732. [DOI] [PubMed] [Google Scholar]
- 30.Scruggs SB, Hinken AC, Thawornkaiwong A, Robbins J, Walker LA, de Tombe PP, Geenen DL, Buttrick PM, Solaro RJ. J. Biol. Chem. 2009;284:5097–5106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM. Mol. Cell. Proteomics. 2010;9:1804–1818. doi: 10.1074/mcp.M110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scruggs SB, Solaro RJ. Arch. Biochem. Biophys. 2011 doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen PM. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H1741–H1749. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marston SB, Walker JW. J. Muscle Res. Cell Motil. 2009;30:93–95. doi: 10.1007/s10974-009-9184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.