Abstract
Phosphorylation of cardiac troponin I serines 43/45 (cTnISer43/45) by protein kinase C (PKC) is associated with cardiac dysfunction and yet there is disagreement about the role this cluster plays in modulating contractile performance. The present study evaluates the impact of phospho-null Ala substitutions at Ser43/45 (cTnISer43/45Ala) on contractile performance in intact myocytes. Viral-based gene transfer of cardiac troponin I (cTnI) or cTnISer43/45Ala resulted in time-dependent increases in expression, with 70-80% of endogenous cTnI replaced within 4 days. Western analysis of intact and permeabilized myocytes along with immunohistochemistry showed each exogenous cTnI was incorporated into the sarcomere of myocytes. In contractile function studies, there were no differences in shortening and re-lengthening for cTnI and cTnISer43/45Ala-expressing myocytes 2 days after gene transfer. However, more extensive replacement with cTnISer43/45Ala after 4 days diminished peak shortening amplitude and accelerated re-lengthening measured as the time to 50% re-lengthening (TTR50%). A decrease in myofilament Ca2+ sensitivity of tension also was observed in permeabilized myocytes expressing cTnISer43/45Ala and is consistent with accelerated re-lengthening observed in intact myocytes under basal conditions. Phosphorylation of cTnI Ser23/24 and the Ca2+ transient were not changed in these myocytes. These results demonstrate extensive sarcomere expression of cTnISer43/45Ala directly modulates myofilament function under basal conditions. In further work, the accelerated re-lengthening observed in control or cTnI-expressing myocytes treated with the PKC agonist, endothelin-1 (ET, 10nM) was slowed in myocytes expressing cTnISer43/45Ala. This outcome may indicate Ser43/45 is targeted for phosphorylation by ET-activated PKC and/or influences transduction of this agonist-activated response.
Keywords: Troponin, Myofilament, Contractile Proteins, Heart, Phosphorylation, Protein Kinase C
Introduction
Thin filament cardiac troponin (cTn) interacts with tropomyosin and actin to regulate actomyosin interactions in response to changes in calcium during cardiac systole and diastole [1, 2]. Cardiac troponin I (cTnI) acts as a molecular rheostat within the Tn complex by strongly interacting with actin in the absence of Ca2+ and then toggling to interact with troponin C (cTnC) as Ca2+ levels increase [2]. Multiple kinases phosphorylate TnI and often modulate myofilament Ca2+ sensitivity. In biochemical studies, protein kinase C (PKC), a serine/threonine kinase, phosphorylated the cardiac isoform of troponin I (cTnI) at three residue clusters, serines 23/24 (Ser23/24), serines 43/45 (Ser43/45), and threonine 144 (Thr144), based on the rat sequence [3]. Phosphorylation of cTnI Ser43/45 is of interest because heart failure is associated with increased phosphorylation of the analogous Ser41 and Ser43 residues in human cTnI [4, 5]. Increased cTnI Ser43 and/or Ser45 phosphorylation in myocardium also develops with cardiac dysfunction caused by pressure overload in rats [6, 7]. In addition, transient increases in cTnI Ser43 phosphorylation are observed in a mouse model of myocardial infarction [8].
Experimental work with PKC phosphorylated cTnI indicate phosphorylation at these residues decreases myofilament Ca2+ sensitivity and peak contractile function. Biochemical studies indicate modrate TnI phosphorylation by PKCδ decreases myofilament Ca2+ sensitivity and peak actomyosin ATPase [9-11]. Further phosphorylation by this isozyme significantly reduces maximum ATPase activity and dampens the Hill coefficient (nH). Extraction and replacement with cTnI containing phospho-mimetic Glu at Ser43/45 produces a similar decrease in Ca2+ sensitivity and peak isometric force as well as peak sliding velocity [12, 13]. In a transgenic (tg) mouse model, replacement of wildtype cTnI with phospho-mimetic Asp residues at all 3 PKC-targeted clusters produced a similar shift in peak actomyosin ATPase activity[14]. These changes are consistent with the slowed rate of contraction measured in vivo.
In contrast to this work, studies with phospho-null cTnI Ser43/45Ala substitutions have generated different ideas about the role played by this cluster in modulating myofilament function. In keeping with phosphorylation and phospho-mimetic studies, replacement of wildtype cTnI with phospho-null Ala substitutions at Ser43/45 blunted the Ca2+ sensitivity shift of actomyosin ATPase activity during moderate cTnI-specific phosphorylation by PKCδ [15]. However, cTnISer43/45Ala also appeared to decrease maximum ATPase and Ca2+ sensitivity compared to cTnI under basal conditions.
The myofilament functional response associated with cTnISer43/45Ala expression could significantly influence organ-level function. However, cardiac performance is not detectably different between wildtype littermates and a tg mouse model expressing about 50% of total cTnI as cTnISer43/45Ala [16]. Further analysis of cellular function in this model indicated there are changes in myofilament function compared to controls [17]. Myofilament Ca2+ sensitivity also appeared to decrease relative to wildtype cTnI in a separate knock-in mouse expressing cTnI with Ala substitutions in all 3 PKC-targeted clusters [18]. Other indices of contractile shortening and the Ca2+ transient remained similar to controls.
Results from these earlier studies in mouse models indicate this construct may significantly impact myofilament function, although it is unclear whether the functional response is caused by a direct or adaptive change to cTnISer43/45Ala expression. While Ala substitutions for Ser are favored as a phospho-null substitution due to similarities between these residues, it remains unclear whether Ser43/45Ala is a functionally conservative substitution in cTnI. In the present study, recombinant adenoviral-mediated gene transfer of cTnISer43/45Ala is used to evaluate the impact of this substitution mutant on contractile function in isolated myocytes. Our experiments also tested whether a PKC agonist targets cTnISer43/45 using this substitution mutant.
Methods
2.1 Mutagenesis Strategy and Adenoviral Constructs
Full-length wild-type rat cTnI cDNA [19] was used to substitute serines 43 and 45 with alanines and produce a phospho-null cTnISer43/45Ala using the QuikChange site-directed mutagenesis kit (Invitrogen) [20]. The mutagenesis primers were 5′-gtctaagatcgccgccgccagaaaacttcag-3′ (sense; mutated residues are shown in bold and underlined) and 5′ctgaagttttctggcggcggcgatcttagac-3′ (anti-sense), which removed a BglII site from cTnI. The cDNA for cTnISer43/45Ala with and without a carboxy-terminal FLAG-tag were then subcloned into the pDC315 shuttle vector [21]. Recombinant adenoviral vectors for each cDNA were created by homologous recombination of the shuttle vector with pBHGloxΔE1,3Cre in HEK293 cells. High titer, recombinant adenovirus was generated and the titer was determined by plaque assay [22].
2.2 Myocyte Isolation and Gene Transfer
Myocytes were isolated from adult, female Sprague-Dawley rats (200g) as previously described in detail [22]. All animal procedures were approved and performed following the guidelines outlined by the University Committee on the Use and Care of Animals at the University of Michigan. Briefly, hearts removed from heparinized rats were perfused on a Baker perfusion apparatus and digested with collagenase and hyaluronidase. Isolated, Ca2+-tolerant myocytes were plated onto laminin-coated coverslips and cultured in DMEM supplemented with 5% FBS, penicillin (50 U/ml), and streptomycin (50μg/ml: P/S) at 37°C in a 5% CO2 incubator for 2 hrs. Recombinant adenovirus containing cTnI (±FLAG) or cTnISer43/45Ala (±FLAG) was diluted in serum-free media and added to myocytes. One hr later, additional serum-free M199 media supplemented with P/S was added to each well. Media was changed the next day, and then every other day thereafter for non-paced myocytes. Other coverslips were transferred into a stimulation chamber and paced at 0.2Hz with media changed every 12 hours.
2.3 Western blot Analysis
The levels of expression and myofilament replacement for each cTnI substitution mutant, myofilament stoichiometry, and cTnI phosphorylation of Ser23/24 were determined via Western blot. Sarcomeric proteins were separated by SDS-PAGE on a 12% acrylamide gel then transferred onto PVDF membranes [23]. Membranes were blocked with 5% milk or albumin and then probed with primary antibody (Ab), followed by conjugated secondary antibodies (Abs). Primary Abs included an anti-troponin I monoclonal Ab (mAb; 1:4000, MAB1691, Millipore), anti-phospho-cTnI Ser23/24 polyclonal Ab (pAb; 1:1000, Cell Signaling), and anti-troponin T mAb (1:500, Fitzgerald). Secondary Abs included Alexa-Fluor 680-conjugated goat anti-mouse (GAM; 1:25,000, Invitrogen), horseradish peroxidase (HRP)-conjugated GAM (1:2000, GAM-HRP, Cell Signaling), and HRP-conjugated goat anti-rabbit (GAR) (1:2000, GAR-HRP, Cell Signaling) Abs. Alexa Fluor-conjugated Ab was detected using an Odyssey infrared imaging system, while a BioRad imaging system detected HRP-conjugated Ab using enhanced chemiluminescence (ECL, Pierce). Quantity One software (BioRad) was used for quantitative analysis of expression. Expression of cTnI, Tm or cTnT were normalized to a silver (Ag)-stained band on the gel or Sypro-stained band on the blot (cTnI only) and phosphorylation of Ser23/24 was normalized to cTnI.
2.4 Immunofluorescence
Cellular localization was determined with cTnIFLAG and cTnISer43/45AlaFLAG using indirect immunohistochemistry, as previously described [22]. Localization of each construct was compared to non-treated controls or cTnIFLAG-expressing myocytes. Briefly, myocytes were fixed in paraformaldehyde, treated with NH4Cl4 to minimize excess aldehydes, and then blocked in phosphate buffered saline (PBS) containing 20% normal goat serum (NGS) plus 0.5% Triton X-100. TnI immunostaining was performed with the anti-troponin I mAb described in the Western protocol (1:500), washed and blocked again prior to incubation in secondary GAM Ab conjugated to fluorescein isothiocyanate (FITC; 1:500, Invitrogen). After blocking with GAM IgG overnight and GAM IgG Fab fragments for 1 hr, FLAG-tagged cTnI was detected using anti-FLAG mAb (1:500, Invitrogen) and secondary GAM Ab conjugated to Texas Red (TR) (1:500, Sigma). Cells were imaged with a Fluoview 500 laser scanning confocal microscope (Olympus) and images were de-convoluted with Autoquant X (Media Cybernetics).
2.5 Phosphorylation Detected by Radiolabeling in Intact Myocytes
Myofilament proteins in intact myocytes were labeled with [32P]orthophosphate in M199 media containing calyculin A (10 nM), as described previously [20]. Radiolabeled proteins were separated with 12% SDS-PAGE. Then, gels were exposed to a phosphorimage cassette to detect myofilament protein phosphorylation.
2.6 Sarcomere Shortening and Calcium Transient Measurements
Myocytes were paced at 0.2 Hz starting 24 hrs after isolation, and then paced for up to 4 days after gene transfer. Resting sarcomere length, peak shortening amplitude (% baseline), time to peak (TTP) shortening, and times to 25%, 50%, and 75% re-lengthening (TTR25%, TTR50%, and TTR75%) were measured on signal averaged traces collected with a CCD-camera (Ionoptix). Coverslips with myocytes were perfused in a 37°C chamber. For some experiments, signal-averaged Ca2+-transient and sarcomere shortening measurements were recorded in myocytes loaded with Fura-2AM, as described in detail earlier [23]. Resting and peak Ca2+ ratios, the rate of Ca2+ rise and decay within the myocyte, and the times to 25%, 50% and 75% Ca2+-transient decay (TTD25%, TTD50%, TTD75%) were measured 4 days after gene-transfer for these studies.
2.7 Isometric Force Measurements
Isometric force was measured in myocytes 4 days after gene transfer, as described earlier [24, 25]. Briefly, one end of a myocyte was mounted to a motor (Model 315C, Aurora Scientific) and the other end to a force transducer (model 403A, Aurora Scientific) with a resonance frequency of 1.1MHz on the stage of a Nikon TS-i Eclipse microscope. Myocytes attached to the force transducer and length controller were incubated in ice-cold high relaxing solution (HR; pH 7.0) composed of pCa (-log [Ca2+]) 9.0, 10 mM EGTA, 20 mM Imidazole, 1 mM free Mg2+, 4 mM free ATP, 14.5 mM creatine phosphate and sufficient KCl to bring the ionic strength to 180 mM at 15°C. Myocytes were permeabilized in HR containing 0.1% Triton X-100 and sarcomere length was set to 2.0 μm. Active isometric tension at each pCa was determined using the slack method, with pCa concentrations ranging from 9.0 to 4.5 [24]. Ion concentrations for each pCa were calculated using MATLAB, as described earlier [26]. Tension was measured in low EGTA-containing relaxing (pCa 9.0) and activating (pCa 4.5) solutions after every two measurements in sub-maximal calcium solutions [24]. Each pCa solution was buffered to pH 7.0 at 15°C and contained Ca2+ ranging from 10-9 to 10-4.5 M, 20 mM Imidizole, 7 mM EGTA, 1 mM free Mg2+, 4 mM free ATP, 14.5 mM creatine phosphate along with sufficient KCl to bring the ionic strength to 180 mM. The tension-pCa curve for each group was fitted using the Marquardt-Levenburg nonlinear, least squares algorithm for the Hill equation, where P is the fractional tension, K is the midpoint or −log [Ca2+] producing 50% peak tension (pCa50) and nH is the Hill coefficient for the equation: P = [Ca2+]nH/(KnH + [Ca2+]nH.
2.8 Statistical Analysis
Results are expressed as mean ± SEM. Myocyte contractile function was analyzed using an unpaired Student's t-test for data collected 2 days after gene transfer. Protein expression and contractile function measured 4 days after gene was analyzed using a one-way analysis of variance (ANOVA) and post-hoc Newman-Keuls tests. Statistical significance was set at p< 0.05 (*) for all comparisons.
Results
Our initial experiments evaluated sarcomere expression and endogenous cTnI replacement with FLAG-tagged versions of cTnI and cTnISer43/45Ala 2 and 4 days after gene transfer. Sarcomere incorporation of cTnISer43/45AlaFLAG and cTnIFLAG was comparable over this time interval. A second important component of this study evaluated whether Ala acts as a neutral amino acid substitution for cTnISer43/45, meaning Ala is functionally conservative and has no impact on contractile function in myocytes. Our results show this substitution produces direct, functional changes in contractile performance following extensive cTnISer43/45Ala replacement without generating adaptive responses within the myocyte.
3.1 Expression and Sarcomeric Replacement with cTnI Substitution Mutants
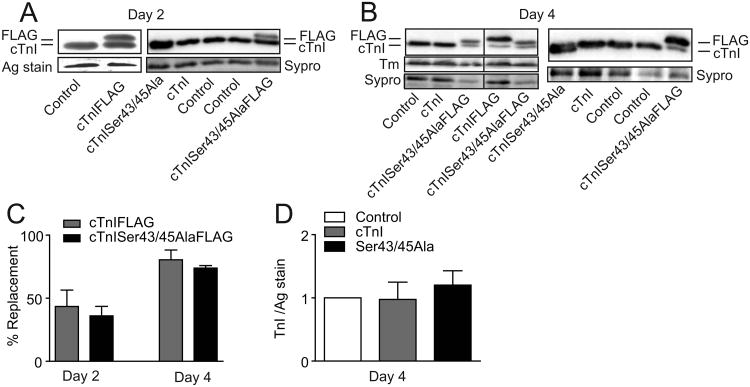
Our initial work focused on analyzing expression and sarcomere replacement of endogenous cTnI with cTnISer43/45Ala. Western analysis showed both cTnIFLAG and cTnISer43/FLAG replaced endogenous cTnI over time, with 35-45% replacement after 2 days and 70-80% by day 4 (Figs. 1A-C). In myocytes expressing cTnI and cTnISer43/45Ala, protein expression was normalized to a band on the silver-stained gel or Sypro-stained blot, which indicated replacement with either cTnI construct did not change total cTnI expression (Fig. 1D). The expression level of other thin filament proteins such as tropomyosin (Tm, Fig. 1B) and troponin T (TnT; results not shown) also showed preservation of stoichiometry in myocytes expressing FLAG-tagged cTnI and cTnISer43/45 Ala. These results are consistent with sarcomeric cTnI replacement and maintenance of thin filament stoichiometry reported in earlier studies [14, 27-29].
Figure 1. Expression of cTnI and cTnISer43/45Ala with and without FLAG 2 and 4 days after gene transfer.
A. Representative Western blot illustrating cTnI expression in myocytes 2 days after adenoviral-mediated gene transfer of cTnI, cTnIFLAG, cTnISer43/45Ala, and cTnISer43/45AlaFLAG into adult rat myocytes. B. Representative Western showing cTnI expression 4 days after gene transfer of the same recombinant viral constructs listed in A. A representative example of tropomyosin (Tm) also is shown in the left panel of B to demonstrate maintenance of thin filament stoichiometry. A silver-stained portion of gel is included in A (left panel) and a Sypro stained-band on the blot in A (right panel) and B to indicate protein loading. Samples shown in the left panel of B were separated on the same gel and the black line between samples indicates separation by additional samples. C. Quantitative comparison of endogenous cTnI replacement by cTnIFLAG and cTnISer43/45AlaFLAG 2 and 4 days after gene transfer. Results are expressed as FLAG expression as a percentage of total TnI expression. D. Quantitative analysis of cTnI stoichiometry in myocytes 4 days after gene transfer of cTnI or cTnISer43/45Ala. TnI expression is normalized to expression in control myocytes. A one-way ANOVA indicated there were no significant differences (p>0.05) in cTnI expression compared to controls in panel D.
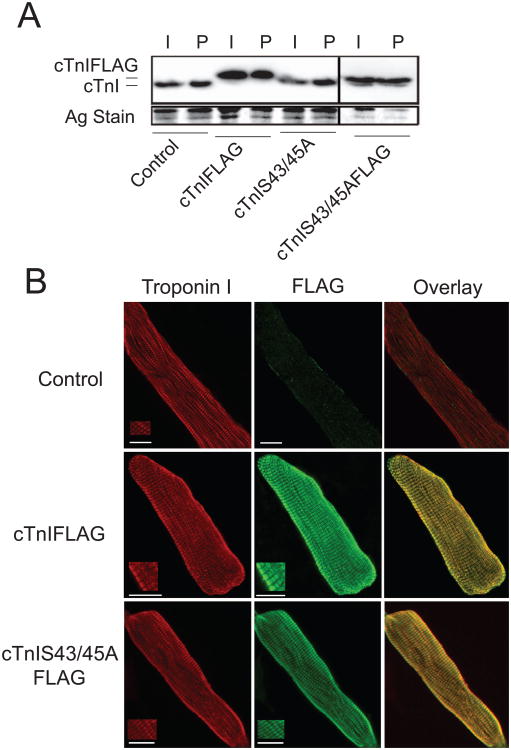
Biochemical studies and indirect immunohistochemistry indicated both constructs are incorporated into the sarcomere. Similar levels of non- and FLAG-tagged cTnI and cTnISer43/45Ala expression were detected by Western analysis in intact and detergent permeabilized myocytes (Fig. 2A). Confocal image analysis of immunostained myocytes provided further proof these exogenous cTnIs were incorporated into sarcomeres (Fig. 2B). The striated staining observed with anti-FLAG Ab overlapped with the sarcomere pattern detected with anti-TnI Ab in myocytes expressing cTnIFLAG or cTnISer43/45AlaFLAG (Fig 2B).
Figure 2. Analysis of sarcomere incorporation of cTnI and cTnISer43/45Ala with and without FLAG tags.
A. A representative Western comparing cTnIFLAG, cTnISer43/45Ala, and cTnISer43/45AlaFLAG expression in intact (I) and detergent permeabilized (P) myocytes 4 days after gene transfer. Samples shown were separated on the same gel and the black line between samples indicates separation by additional samples. A silver-stained portion of the gel is shown to indicate protein loading in each lane. There were no detectable differences in the expression detected in intact and permeabilized myocytes, which provides evidence of sarcomere incorporation for each construct. B. Projection confocal images showing cTnI (left panel; TR) and FLAG (middle panel; FITC) immunostaining along with the overlay (right panel) for control myocytes (top panels) and myocytes expressing cTnIFLAG (middle panels) and cTnISer43/45AlaFLAG (lower panel). Dual immunostaining for cTnI and FLAG were detected with TR and FITC, respectively as described in the Methods section. Insets for each myocyte are shown to demonstrate a higher resolution striated pattern of immunostaining. These results show sarcomere localization of cTnIFLAG and cTnISer43/45AlaFLAG in the myofilament.
3.2 Influence of cTnISer43/45Ala on Basal Contractile Performance
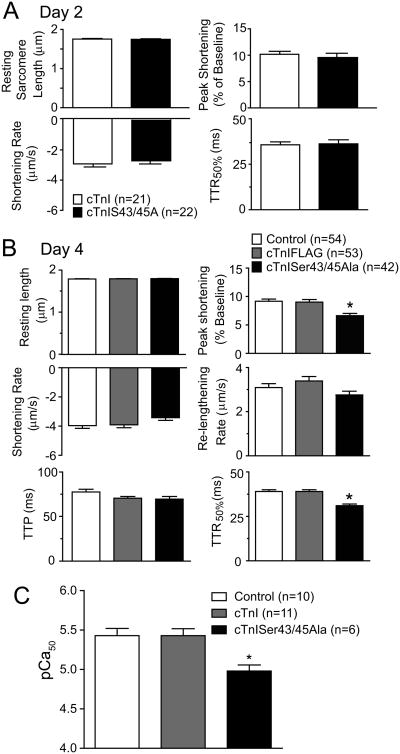
In the next set of studies, sarcomere shortening and re-lengthening in intact myocytes was measured to evaluate the impact of cTnISer43/45Ala expression on contractile function. There were no significant differences in the mechanics and kinetics of shortening and re-lengthening between myocytes expressing cTnI versus cTnISer43/45Ala 2 days after gene transfer (Fig 3A). Resting sarcomere length, shortening and re-lengthening rate, and time to peak shortening (TTP) also were similar in myocytes expressing cTnI or cTnISer43/45Ala compared to non-treated controls 4 days after gene transfer (Fig. 3B). However, peak shortening amplitude was significantly decreased in myocytes expressing cTnISer43/45Ala compared to controls (Fig. 3B). In addition, the time to 50% re-lengthening (TTR50%) was significantly accelerated in cTnISer43/45Ala-expressing myocytes compared to myocytes expressing cTnI. These results indicate more extensive replacement of endogenous cTnI with cTnISer43/45Ala diminished shortening amplitude and accelerated re-lengthening 4 days after gene transfer.
Figure 3. Cardiac myocyte contractile function 2 and 4 days after gene transfer of cTnI constructs.
A. Comparison of sarcomere shortening and re-lengthening measurements in control myocytes and myocytes expressing cTnISer43/45Ala 2 days after gene transfer. An unpaired Student's t-test indicated there were no significant differences between myocyte groups. In addition to these measurements, the time to peak (TTP), re-lengthening rate, and times to 25% and 75% re-lengthening also were not different between these 2 groups. B. Comparison of cardiac myocyte shortening and re-lengthening 4 days after gene transfer of cTnI and cTnISer43/45Ala. Results were compared using a one-way ANOVA and Newman-Keuls post-hoc test with p<0.05 (*) considered statistically significant from control values. Resting sarcomere length, shortening and re-lengthening rates and TTP were not different in control, and myocytes expressing cTnIFLAG, and cTnISer43/45Ala. The shortening amplitude decreased and there was an acceleration midway through re-lengthening (TTR50%) detected in myocytes expressing cTnISer43/456Ala. C. Quantification of pCa50 in control myocytes 4 days after gene transfer. Myofilament Ca2+ sensitivity was significantly reduced in Ser43/45Ala myocytes versus controls (Control 5.43±0.091, n=10, cTnI 5.43±0.088, n=11 and cTnISer43/45Ala-expressing myocytes 4.98±0.077, n=6). Statistical differences between the pCa50 values were identified using one-way ANOVA and Newman-Keuls post-hoc test with (*p<0.05) considered statistically significant.
Isometric tension measurements were performed to determine whether cTnISer43/45Ala directly influence myocyte contractile function. Analysis of the pCa50 derived from the tension/pCa relationship indicated cTnISer43/45Ala produced a significant decrease in myofilament Ca2+ sensitivity of tension (Fig. 3C). However, similar peak tension values were observed in control, cTnI- and cTnISer43/45Ala-expressing myocytes (controls: 3.48 mN±0.51, n=10; cTnI: 5.00±0.54, n =11; cTnISer43/45Ala: 3.20 mN±0.67, n=6). The accelerated re-lengthening in intact myocytes and rightward shift in Ca2+-activated tension indicate cTnISer43/45Ala decreases myofilament Ca2+ sensitivity. However, the reduction in shortening amplitude without peak tension changes may result from adaptive changes within the myocyte.
3.5 Compensatory Adaptations in Cardiac Myocytes Expressing cTnISer43/45Ala
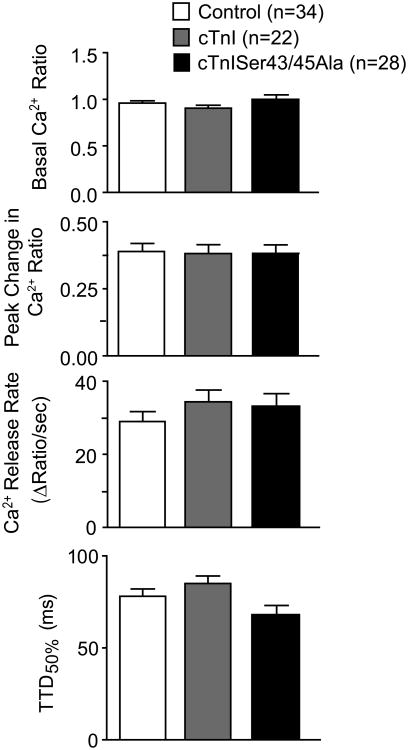
The cellular Ca2+ transient was measured in loaded myocytes to determine whether direct or adaptive changes contributed to the diminished shortening amplitude in myocytes. In agreement with our earlier results (Fig. 3), shortening amplitude in Fura-2AM-loaded myocytes remained significantly lower and TTR50% was accelerated in cTnISer43/45 Alapressing myocytes relative to cTnI-expressing myocytes (results not shown). Basal and peak Ca2+ ratios were not significantly different between myocytes expressing cTnI versus cTnISer43/45Ala (Fig. 4). There was a trend toward accelerated TTD values in Fura-loaded myocytes expressing cTnISer43/45Ala, although this change was not statistically different from controls (Fig. 4).
Figure 4. Comparison of Ca2+-transients in Fura-2AM loaded myocytes 4 days after gene transfer.
Basal and peak Ca2+ levels were similar in control, cTnI-, and cTnISer43/45Ala-expressing myocytes. The rates of Ca2+ release and decay also were similar among the 3 groups. There was a trend toward acceleration of Ca2+ decay, as indicated in the TTD50%, although a 1-way ANOVA indicated there was no statistically significant difference from control values (p>0.05).
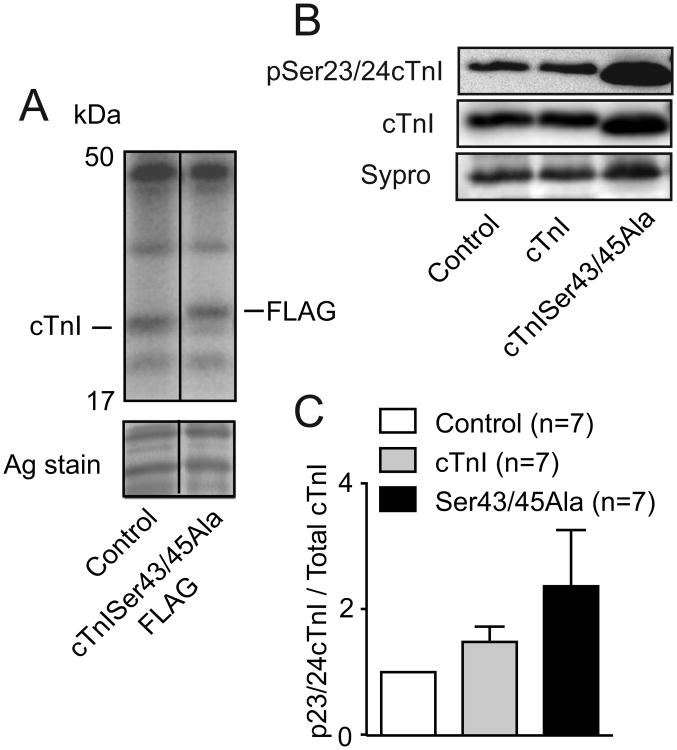
The potential for myofilament adaptations to differentially impact peak shortening versus tension was evaluated in our next group of studies. There were no detected differences in 32P incorporation into proteins when comparing radiolabeled control and cTnISer43/45Ala-expressing myocytes (Fig. 5A). Additional work with a Ser23/24 specific phospho-Ab showed pSer23/24 cTnI/total cTnI ratios tended to increase in cTnISer43/45Ala-expressing myocytes, but were not significantly different from controls (Fig. 5B-C). The absence of change in the Ca2+ transient and myofilament phosphorylation suggest this cTnI substitution mutant directly influences crossbridge cycling rate, but not peak tension.
Figure 5. Myofilament phosphorylation 4 days after gene transfer.
A. Radiolabeling of proteins observed after 32P incorporation into control and cTnISer43/45Ala expressing myocytes. B. Representative Western blot showing Ser23/24 phosphorylation (top panel), cTnI expression (middle panel) and a band on the Sypro-stained blot (lower panel). C. Quantitative comparison of Ser23/24 phosphorylation in myocytes expressing cTnISer43/45Ala compared to controls and myocytes expressing cTnI. Total cTnI was normalized to a quantified band on Sypro-stained blots. A 1-way ANOVA and Newman-Keuls post-hoc tests showed there was no significant elevation in Ser23/24 phosphorylation in cTnISer43/45Ala- or cTnI-expressing myocytes.
3.3 The Effects of cTnISer43/45Ala on the Contractile Response to Endothelin-1
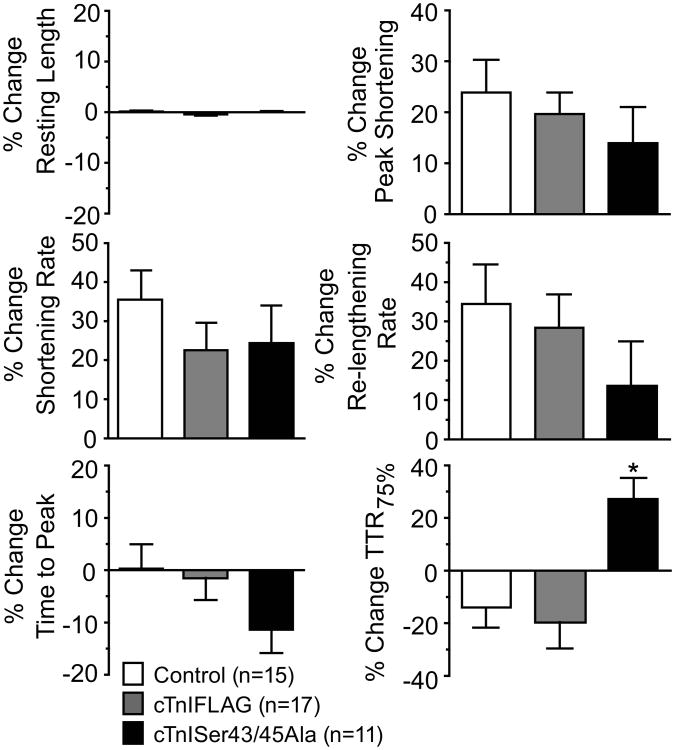
Introduction of a phospho-null cTnI into the myofilament, such as cTnISer43/45Ala, is often used to determine whether specific residue(s) are targeted by kinases such as PKC. Thus, the ability of the endogenous PKC activating neurohormone, endothelin-1 (ET) to modulate contractile function was compared in cTnI- and cTnISer43/45Ala- expressing myocytes. In earlier work, cTnI phosphorylation by ET preserved re-lengthening rate [20]. Studies with a cTnISer23/24Ala phospho-null indicated this cluster contributed to re-lengthening rate after 60 min of ET (10 nM) treatment, but had little influence during an acute 15 min treatment with ET. In the present studies, ET increased shortening amplitude and accelerated the shortening rate in all 3 groups of myocytes, in keeping with the earlier work (Fig. 6). There was a trend for re-lengthening rate to be slower in cTnISer43/45Ala-expressing myocytes compared to myocytes expressing cTnI or cTnIFLAG, although this difference was not statistically significant (Fig. 6). However, myocytes expressing cTnISer43/45Ala developed a significant increase in the time to 75% re-lengthening (TTR75%) compared to control or cTnIFLAG-expressing myocytes. This slowed re-lengthening also was matched by a trend toward an accelerated time to peak shortening (TTP) with cTnISer43/45Ala, although the ET-induced change in TTP was not statistically different from controls. Taken together, these results suggest the Ser43/45 cluster helps to preserve re-lengthening rate during the ET response. ET activation of PKC presumably phosphorylates this cluster to maintain re-lengthening rate, which is absent from myocytes expressing cTnISer43/45Ala.
Figure 6. Sarcomere shortening and re-lengthening in response to the PKC agonist, endothelin-1 (ET, 10 nM).
Experiments were performed using control myocytes and myocytes expressing cTnIFLAG or cTnISer43/45Ala 4 days after gene transfer. Results are expressed as a % change from basal during 15 minutes of ET. A 1-way ANOVA analysis and post-hoc Neuman-Keuls tests (*p<0.05) showed significant increases in TTR75% for cTnISer43/45Ala-expressing myocytes compared to controls. This increase indicates a slowing of relaxation in myocytes expressing cTnISer43/45Ala, and suggests Ser43/45 phosphorylation may preserve relaxation rate during the ET response. Measurement of times to 25% and 50% re-lengthening were not significantly different between the 3 groups.
Discussion
The goals of the present study were to determine whether cTnISer43/45Ala is a functionally conservative substitution and to identify whether this residue cluster contributes to functional responses to the PKC agonist, ET. Ala substitution for Ser at 43/45 in cTnI is expected to be functionally conservative and not diminish the myocyte contractile function response to PKC. This substitution mutant has no detectable influence on intact myocyte shortening when it replaced less than 50% of endogenous cTnI 2 days after gene transfer (Figs 1,3). However, more extensive sarcomere incorporation of cTnISer43/45Ala by day 4 decreased myofilament Ca2+ sensitivity and accelerated re-lengthening, in addition to reducing myocyte shortening amplitude under basal conditions (Figs. 1-3). Collectively, this work demonstrates Ser residues at cTnI43/45 contribute to basal contractile function in the myofilament. In addition, high cTnISer43/45Ala levels slowed re-lengthening in response to the PKC agonist ET compared to controls (Fig. 6). These later results are consistent with Ala substitutions at Ser43/45 acting as phospho-null residues during the agonist response, and support the idea this cluster contributes to PKC agonist-induced modulation of contractile function.
The functional changes caused by extensive replacement with cTnISer43/45Ala may be due to the polar attributes of Ser compared to Ala. In the x-ray crystal structures for the Tn core domain, both Ser43 and Ser45 reside in an α-helical region at the start of the IT arm and are in close proximity to the N-lobe of cardiac troponin C (cTnC) [30]. Kobayashi and colleagues suggested cTnISer45 interacts with Glu10 in cTnC and Ser45 phosphorylation would disrupt this interaction [31]. Introduction of a less polar Ala at this site and at Ser43 also could modify interactions between cTnI and cTnC and lead to the decreased Ca2+ sensitivity predicted to result from phosphorylation. The shift in myofilament Ca2+ sensitivity of tension and the accelerated TTR50% in intact myocytes (Fig. 3) are consistent with this interpretation. Our findings also indicate cTnISer43/45Ala may be a useful tool to investigate cTnI residues leading into the amino-terminus of the I-T arm (aa 33-45). This region has the potential to interact with multiple domains within cTnI and cTnC, as it traverses the region between the amino- and carboxyl-globular TnC domains.
The results showing a reduction in myofilament Ca2+ sensitivity with cTnISer43/45Ala expression are predicted to accelerate relaxation in intact myocytes. Indeed re-engthening was accelerated in myocytes, although this acceleration was detected midway through re-lengthening (Fig 3). Diminished myofilament Ca2+ sensitivity also was evident in a cTnIAla5nb mouse model expressing cTnI with Ala substitutions at Ser23/24, Ser43/45 and Thr144 [18, 32]. In addition, there was a trend toward reduced myofilament Ca2+ sensitivity in a transgenic mouse expressing cTnISer43/45Ala, although the change was not statistically significant [33]. While cTnISer43/45Ala replaced about 50% of endogenous cTnI in the transgenic model, expression of cTnI-Ala5 on a null background left no endogenous cTnI in these hearts. Changes in re-lengthening and Ca2+ sensitivity were detected with more extensive cTnISer43/45Ala replacement at day 4 in our study (Fig. 3). These results, together with findings in mouse models suggest extensive cTnISer43/45Ala replacement is required shift myofilament Ca2+ sensitivity.
Based on earlier work, it was unclear whether peak isometric tension would change in myocytes expressing cTnISer43/45Ala compared to controls. No changes in peak tension are observed in our work, although decreased peak tension is observed in fiber bundles from transgenic hearts expressing cTnISer43/45Ala compared to non-transgenic controls [16]. This difference is not likely explained by the level of cTnISer43/45Ala expression, as 70-80% replacement is achieved in the present study (Fig. 1), which exceeds the ∼50% replacement in tg myocytes [16]. Instead, the dissimilar outcomes may be explained by elevated cTnI and troponin T (TnT) phosphorylation in the transgenic fiber bundles [33, 34]. There are no detectable changes in myofilament phosphorylation compared to control levels in the present study (Fig. 5). The reductions in peak tension and sliding velocity observed with a phospho-mimetic cTnISer43/45Glu during in vitro studies [12] also are consistent with diminished peak tension caused by adaptive Tn phosphorylation in the transgenic mouse model [16]. In addition, the lack of change in peak tension and myofilament phosphorylation detected in myocytes from the cTnIAla5nb mouse [18, 32] are similar to results in the present study (Figs. 3, 5).
In contrast to peak tension, extensive replacement with cTnISer43/45Ala is associated with diminished shortening amplitude in myocytes in the present study (Fig. 3). Changes in the amplitude and re-lengthening rate are not observed in myocytes from the cTnIAla5nb mouse [18, 32]. The divergent outcomes between our work and the cTnIAla5nb may be due to different experimental conditions, such as pacing frequency, temperature and/or animal model. Alternatively, there may be other adaptive differences between the 2 studies, such as changes in the Ca2+ transient. Similar Ca2+ transients are observed in cTnISer43/45Ala and cTnI-expressing myocytes for the current study (Fig. 4), and it is unclear whether myocytes expressing cTnIAla5nb develop differences in the basal Ca2+ transient[18, 32]. However, cTnISer43/45Ala also reduced unloaded peak actomyosin ATPase activity by 50% in biochemical studies [10]. The later results suggest the Ala substitution may significantly impact unloaded or lightly loaded myofilament crossbridge cycling.
A final component of our study compared the contractile response to the PKC agonist, ET in cTnISer43/45Ala and controls. As anticipated, ET enhanced peak shortening in myocytes expressing cTnI or cTnISer43/45Ala (Fig. 6). Earlier work established cellular alkalosis produces this enhanced peak shortening response to ET [23, 35]. In contrast, ET accelerated re-lengthening in controls, as measured by TTR75% [23] while cTnISer43/45Ala slowed this re-lengthening response (Fig 6). This result is consistent with cTnISer43/45 Ala acting as a phospho-null for Ser43/45 during the ET response, although there are alternative explanations for these results. For example, cTnISer43/45Ala-induced decrease in myofilament Ca2+ sensitivity under basal conditions may prevent a further shift in Ca2+ sensitivity during the ET response and/or modify the troponin conformation such that it is unable to respond to phosphorylation of other target residues. Our work, along with results obtained in several earlier studies [17, 18, 33] are not able to distinguish between these possibilities. However, more extensive myofilament phosphorylation appeared to be required to decrease the actomyosin ATPase Ca2+ sensitivity in response to PKC in at least one study [15], which provides some support for these alternative possibilities.
In conclusion, results from the present study show sarcomeric incorporation of cTnISer43/45Ala in myocytes decreases myofilament Ca2+ sensitivity of tension and peak shortening amplitude without triggering adaptive changes in the Ca2+ transient or Ser23/24 phosphorylation within cTnI. The slowed re-lengthening produced by cTnISer43/45Ala during the ET response is consistent with this construct acting as a phospho-null substitution during the response to this PKC agonist.
Highlights.
Gene transfer of cTnISer43/45Ala is expressed and incorporated into myocyte sarcomeres
Myofilament contractile function is altered by cTnISer43/45Ala
Functional changes are not due to alterations in Ca2+ transients or phosphorylation
The response to the PKC agonist ET is changed by cTnISer43/45Ala
Acknowledgments
Immunohistochemical studies utilized the University of Michigan Morphology and Imaging core of the Michigan Diabetes Research and Training Center funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases. The technical assistance of Gail Romanchuk is gratefully acknowledged. This work was supported by the National Institutes of Health HL067254 (MVW) and an American Heart Association pre-doctoral award (SEL) 12PRE8830022.
Abbreviations
- ANOVA
Analysis of variance
- Ab
antibody
- Abs
antibodies
- cTn
cardiac troponin
- cTnC
cardiac troponin C
- cTnI
cardiac troponin I
- cTnT
cardiac troponin T
- DMEM
Dulbecco's Modified Eagle Medium
- ET
endothelin-1
- ECL
enhanced chemiluminescence
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GAM
goat anti-mouse
- GAR
goat anti-rabbit
- HR
high relaxing
- nH
Hill coefficient
- HRP
horseradish peroxidase
- pCa
-log[Ca2+]
- pCa50
-log[Ca2+]50
- M199
medium 199
- mAb
monoclonal antibody
- NGS
normal goat serum
- P/S
penicillin/streptomycin
- PBS
phosphate buffered saline
- pAb
polyclonal antibody
- PKC
protein kinase C
- Ag stain
silver stain
- tg
transgenic
- TR
Texas Red
- TTP
time to peak
- TTR
time to re-lengthening
- TTD
time to decay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon AM, Regnier M, Homsher E. Skeletal and cardiac muscle contractile activation: tropomyosin “rocks and rolls”. News Physiol Sci. 2001;16:49–55. [PubMed] [Google Scholar]
- 2.Davis J, et al. Designing heart performance by gene transfer. Physiol Rev. 2008;88(4):1567–651. doi: 10.1152/physrev.00039.2007. [DOI] [PubMed] [Google Scholar]
- 3.Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989;264(34):20778–85. [PubMed] [Google Scholar]
- 4.Noguchi T, et al. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004;110(8):982–7. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, et al. Multiple Reaction Monitoring to Identify Site-Specific Troponin I Phosphorylated Residues in the Failing Human Heart. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.112.096388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopher B, et al. Reduced force production during low blood flow to the heart correlates with altered troponin I phosphorylation. J Muscle Res Cell Motil. 2009;30(3-4):111–23. doi: 10.1007/s10974-009-9180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X, et al. Augmented phosphorylation of cardiac troponin I in hypertensive heart failure. J Biol Chem. 2012;287(2):848–57. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker LA, et al. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2010;48(6):1180–6. doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jideama NM, et al. Phosphorylation specificities of protein kinase C isozymes for bovine cardiac troponin I and troponin T and sites within these proteins and regulation of myofilament properties. J Biol Chem. 1996;271(38):23277–83. doi: 10.1074/jbc.271.38.23277. [DOI] [PubMed] [Google Scholar]
- 10.Noland TA, Jr, et al. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca(2+)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995;270(43):25445–54. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 11.Noland TA, Jr, Kuo JF. Protein kinase C phosphorylation of cardiac troponin I and troponin T inhibits Ca(2+)-stimulated MgATPase activity in reconstituted actomyosin and isolated myofibrils, and decreases actin-myosin interactions. J Mol Cell Cardiol. 1993;25(1):53–65. doi: 10.1006/jmcc.1993.1007. [DOI] [PubMed] [Google Scholar]
- 12.Burkart EM, et al. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003;278(13):11265–72. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 13.Sumandea MP, et al. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- 14.Sakthivel S, et al. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280(1):703–14. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 15.Noland TA, Jr, et al. Differential regulation of cardiac actomyosin S-1 MgATPase by protein kinase C isozyme-specific phosphorylation of specific sites in cardiac troponin I and its phosphorylation site mutants. Biochemistry. 1996;35(47):14923–31. doi: 10.1021/bi9616357. [DOI] [PubMed] [Google Scholar]
- 16.MacGowan GA, et al. Ischemic dysfunction in transgenic mice expressing troponin I lacking protein kinase C phosphorylation sites. Am J Physiol Heart Circ Physiol. 2001;280(2):H835–43. doi: 10.1152/ajpheart.2001.280.2.H835. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery DE, et al. alpha-Adrenergic response and myofilament activity in mouse hearts lacking PKC phosphorylation sites on cardiac TnI. Am J Physiol Heart Circ Physiol. 2002;282(6):H2397–405. doi: 10.1152/ajpheart.00714.2001. [DOI] [PubMed] [Google Scholar]
- 18.Pi Y, et al. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circ Res. 2002;90(6):649–56. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 19.Murphy AM, et al. Molecular cloning of rat cardiac troponin I and analysis of troponin I isoform expression in developing rat heart. Biochemistry. 1991;30(3):707–12. doi: 10.1021/bi00217a018. [DOI] [PubMed] [Google Scholar]
- 20.Westfall MV, Borton AR. Role of troponin I phosphorylation in protein kinase C-mediated enhanced contractile performance of rat myocytes. J Biol Chem. 2003;278(36):33694–700. doi: 10.1074/jbc.M305404200. [DOI] [PubMed] [Google Scholar]
- 21.Michele DE, Albayya FP, Metzger JM. Thin filament protein dynamics in fully differentiated adult cardiac myocytes: toward a model of sarcomere maintenance. J Cell Biol. 1999;145(7):1483–95. doi: 10.1083/jcb.145.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westfall MV, et al. Adenovirus-mediated myofilament gene transfer into adult cardiac myocytes. Methods Cell Biol. 1997;52:307–22. [PubMed] [Google Scholar]
- 23.Westfall MV, Lee AM, Robinson DA. Differential contribution of troponin I phosphorylation sites to the endothelin-modulated contractile response. J Biol Chem. 2005;280(50):41324–31. doi: 10.1074/jbc.M506043200. [DOI] [PubMed] [Google Scholar]
- 24.Sweitzer NK, Moss RL. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990;96(6):1221–45. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger JM, et al. Effects of myosin heavy chain isoform switching on Ca2+-activated tension development in single adult cardiac myocytes. Circ Res. 1999;84(11):1310–7. doi: 10.1161/01.res.84.11.1310. [DOI] [PubMed] [Google Scholar]
- 26.Reitz FB, Pollack GH. Labview virtual instruments for calcium buffer calculations. Comput Methods Programs Biomed. 2003;70(1):61–9. doi: 10.1016/s0169-2607(01)00196-1. [DOI] [PubMed] [Google Scholar]
- 27.Westfall MV, et al. Myofilament calcium sensitivity and cardiac disease: insights from troponin I isoforms and mutants. Circ Res. 2002;91(6):525–31. doi: 10.1161/01.res.0000034710.46739.c0. [DOI] [PubMed] [Google Scholar]
- 28.Westfall MV, Metzger JM. Single amino acid substitutions define isoform-specific effects of troponin I on myofilament Ca2+ and pH sensitivity. J Mol Cell Cardiol. 2007;43(2):107–18. doi: 10.1016/j.yjmcc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda S, et al. Cardiac transgenic and gene transfer strategies converge to support an important role for troponin I in regulating relaxation in cardiac myocytes. Circ Res. 2007;101(4):377–86. doi: 10.1161/CIRCRESAHA.106.145557. [DOI] [PubMed] [Google Scholar]
- 30.Takeda S, et al. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003;424(6944):35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, et al. Effects of protein kinase C dependent phosphorylation and a familial hypertrophic cardiomyopathy-related mutation of cardiac troponin I on structural transition of troponin C and myofilament activation. Biochemistry. 2004;43(20):5996–6004. doi: 10.1021/bi036073n. [DOI] [PubMed] [Google Scholar]
- 32.Pi Y, et al. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552(Pt 3):845–57. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyle WG, et al. Troponin I serines 43/45 and regulation of cardiac myofilament function. Am J Physiol Heart Circ Physiol. 2002;283(3):H1215–24. doi: 10.1152/ajpheart.00128.2002. [DOI] [PubMed] [Google Scholar]
- 34.MacGowan GA, et al. Troponin I protein kinase C phosphorylation sites and ventricular function. Cardiovasc Res. 2004;63(2):245–55. doi: 10.1016/j.cardiores.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Kramer BK, Smith TW, Kelly RA. Endothelin and increased contractility in adult rat ventricular myocytes. Role of intracellular alkalosis induced by activation of the protein kinase C-dependent Na(+)-H+ exchanger. Circ Res. 1991;68(1):269–79. doi: 10.1161/01.res.68.1.269. [DOI] [PubMed] [Google Scholar]