Abstract
Diffusion tensor imaging (DTI) studies consistently reported abnormalities in fractional anisotropy (FA) and radial diffusivity (RD), measures of the integrity of white matter (WM), in bipolar disorder (BD), that may reflect underlying pathophysiologic processes. There is, however, a pressing need to identify peripheral measures that are related to these WM measures, to help identify easily-obtainable peripheral biomarkers of BD. Given the high lipid content of axonal membranes and myelin sheaths, and that elevated serum levels of lipid peroxidation are reported in BD, these serum measures may be promising peripheral biomarkers of underlying WM abnormalities in BD. We used DTI and probabilistic tractography to compare FA and RD in ten prefrontal-centered WM tracts, 8 of which are consistently shown to have abnormal FA (and/or RD) in BD, and also examined serum lipid peroxidation (lipid hydroperoxides, LPH and 4-hydroxy-2-nonenal, 4-HNE), in 24 currently euthymic BD adults (BDE)and 19 age- and gender- matched healthy adults (CONT). There was a significant effect of group upon FA in these a priori WM tracts (BDE<CONT:F[1,41]=6.8;p=0.013) and RD (BDE>CONT:F[1,41]=10.3;p=0.003), and a significant between-group difference in LPH (BDE>CONT:t[40]=2.4;p=0.022), but not 4-HNE. Multivariate multiple regression analyses revealed that LPH variance explained, respectively, 59% and 51% of the variance of FA and RD across all study participants. This is the first study to examine relationships between measures of WM integrity and peripheral measures of lipid peroxidation. Our findings suggest that serum LPH may be useful in the development of a clinically-relevant, yet easily obtainable and inexpensive, peripheral biomarkers of BD.
Keywords: global probabilistic tractography, fractional anisotropy, radial diffusivity, lipid peroxidation, oxidative stress, bipolar disorder
Introduction
There is a pressing need to identify biomarkers reflecting underlying pathophysiologic processes for psychiatric disorders1–3 to facilitate more accurate diagnosis of these disorders than reliance on behavioral measures alone, and ultimately provide biological targets to optimize treatment choice and new treatment developments. While neuroimaging may identify biomarkers reflecting central (neural circuitry) pathophysiologic processes, neuroimaging facilities are not available in all clinical settings, and remain relatively expensive to administer routinely in clinical practice. Identifying peripheral biomarkers related to underlying central pathophysiology is one way of obtaining clinically-relevant, yet easily-obtainable and inexpensive biological measures to help with psychiatric diagnosis, and ultimately treatment choice. This is especially important for bipolar disorder (BD), one of the top six most debilitating of all non-communicable illnesses4–5, yet misdiagnosed as recurrent major depressive disorder in 60% of BD individuals seeking treatment for depression4, 6–7.
Diffusion tensor imaging (DTI) is sensitive to the water diffusivity in brain tissue. Given the hydrophobic nature of the lipid components of WM tracts, specifically, axonal membranes and myelin sheaths, water molecules diffuse along the principal (longitudinal) direction of highly-packed fibers in collinear axons, but in two or more directions in non-collinear WM fibers (e.g., crossing tracts). DTI measures include: the diffusivity along the principal axis, λ1, longitudinal/axial diffusivity (L1); the diffusivity along transverse directions (λ2 and λ3) perpendicular to the principal axis of diffusion, radial diffusivity (RD), high in crossing tracts; and fractional anisotropy (FA), the ratio of longitudinal vs. transverse diffusivity in WM tracts. DTI studies consistently reported abnormalities in WM in BD8,9–13 (especially BD type-I), including reduced FA and elevated RD in 8 WM tracts connecting prefrontal with subcortical and other cortical regions14–23. These include: forceps minor (interhemispheric fibers connecting left and right frontal cortices); anterior thalamic radiation (connecting thalamus with prefrontal cortex); and six associative tracts interconnecting functional subdomains of the cerebral cortex: cingulum; inferior longitudinal fasciculus; superior longitudinal fasciculus; and uncinate fasciculus. The cingulum is further divided into the angular bundle and bundle of the cingulate gyrus24; and the superior longitudinal fasciculus, into the arcuate and I-III bundles24.
Animal studies suggest that increased RD may reflect myelin abnormalities25–28, and human postmortem evidence29 suggests that greater RD may reflect myelin abnormalities and axonal damage. Given that WM mostly comprises neuronal axons and glia cell-containing myelinated sheaths, and both axonal membranes (axolemma) and myelin comprise 80% lipids, potential peripheral measures of abnormal WM, suggested by elevated RD, are serum measures of lipid damage (e.g., lipid oxidative stress). Lipid peroxidation is the specific process by which reactive oxygen species (ROS) induces lipid oxidative stress damage, and involves three phases: initiation, propagation and termination of lipid peroxides30. During early stages, lipid hydroperoxides (LPH) are generated by reaction of fatty acids with ROS that can be used as an early stage biomarker of lipid peroxidation. If the lipid peroxidation cascade progresses, two carbon or hydroperoxide radicals react and form non-radical species, that can be detected in serum as measures of late-stage lipid damage. 4-hydroxy-2-nonenal (4-HNE) is one example of a late-stage biomarker of lipid peroxidation31. Myelin is vulnerable to lipid oxidative stress because it contains a large amount of polyunsaturated fatty acid side-chains, specific targets for lipid peroxidation30. Increasing evidence suggests that oxidative stress may be implicated in the pathophysiology of mood disorders, particularly BD type-I32–33, and post mortem findings reported elevated 4HNE in the anterior cingulate cortex in BD34. Assessing early- and late-stage measures of lipid peroxidation in serum samples can provide insight into the level of lipid oxidative stress damage that in turn may relate to abnormal WM (elevated RD and reduced FA) in BD individuals.
As a first stage toward identifying peripheral biomarkers of pathophysiologic processes in BD, the goal of the present study was first to determine whether adults with BD showed abnormalities in both WM and peripheral measures of lipid peroxidation vs. CONT, and then to determine the nature of the relationships between WM and peripheral measures across all participants. We focused on BD type-I adults, given that the majority of DTI findings are reported in BD type-I (see above), and BD adults in the euthymic stage of illness, to avoid any potential confounds of depressed or manic mood episode upon peripheral and WM measures. For DTI analyses, we employed an automated global probabilistic approach in tractography to study 10 major WM tracts, including the above 8 tracts consistently shown to have abnormal FA and RD in BD (Figure1A), and two other “control” tracts that have not been consistently shown to be abnormal in BD: the forceps major, the posterior bundle of the corpus callosum passing through the splenium, and the cortico-spinal tract, a major ascending tract.
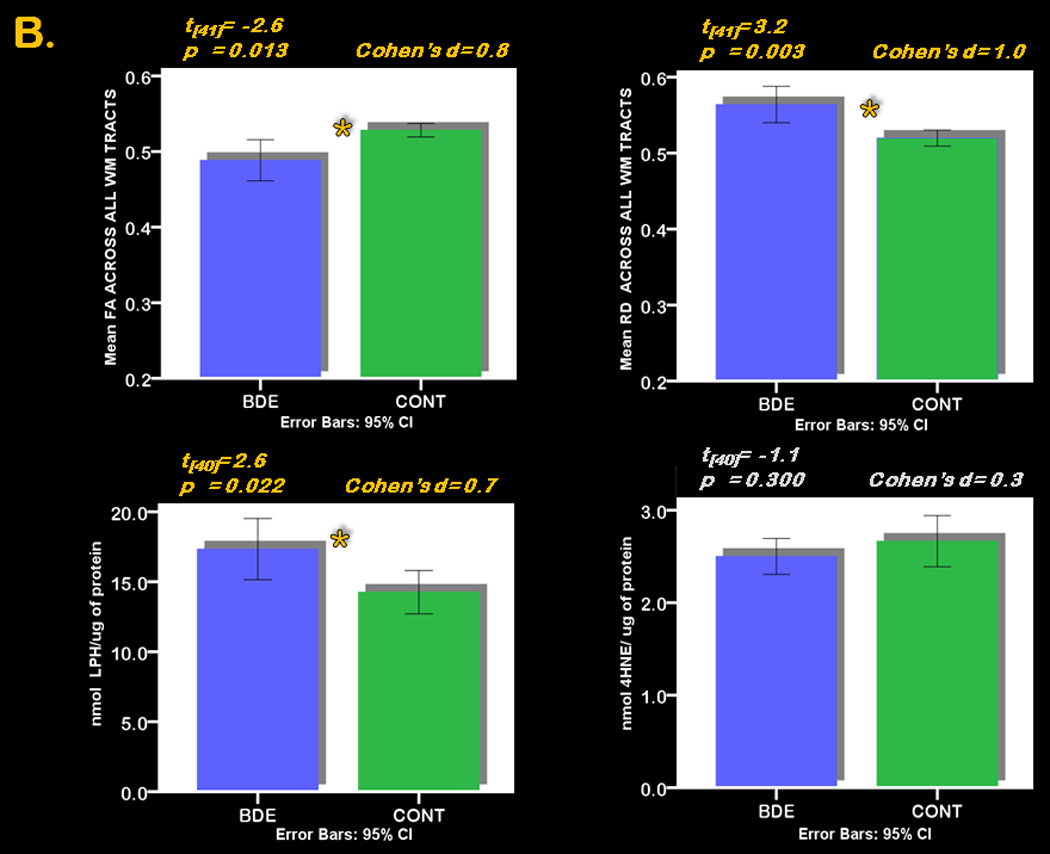
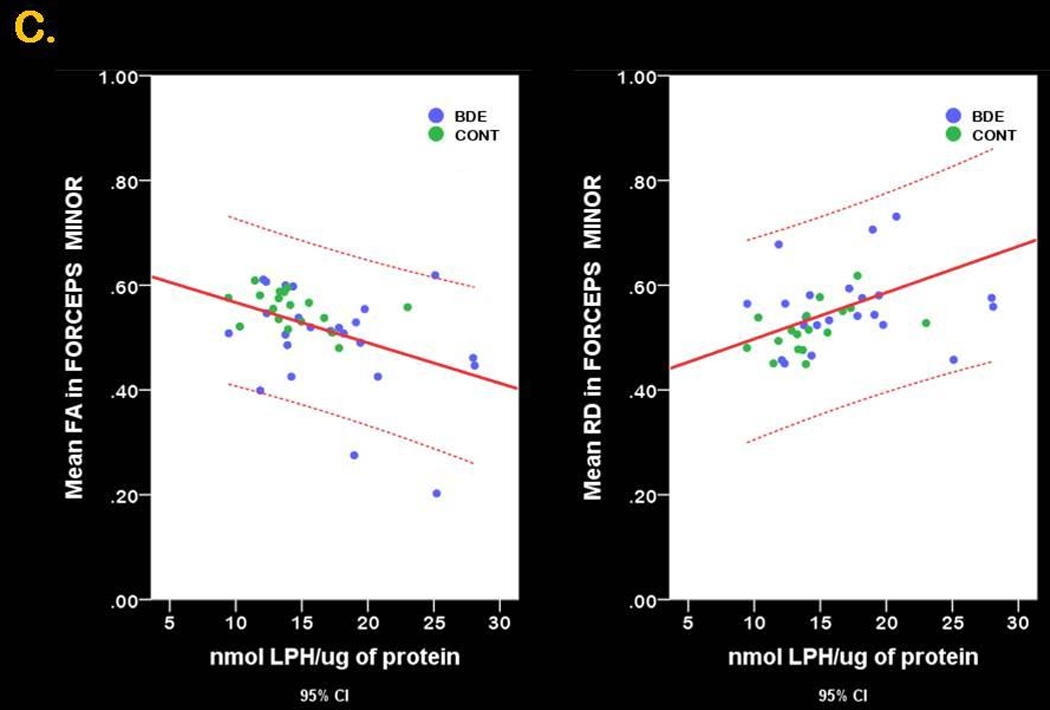
Figure 1. 3-D anatomical representation of reconstructed WM tracts and graphical representation of between group differences in FA, RD and LPH.
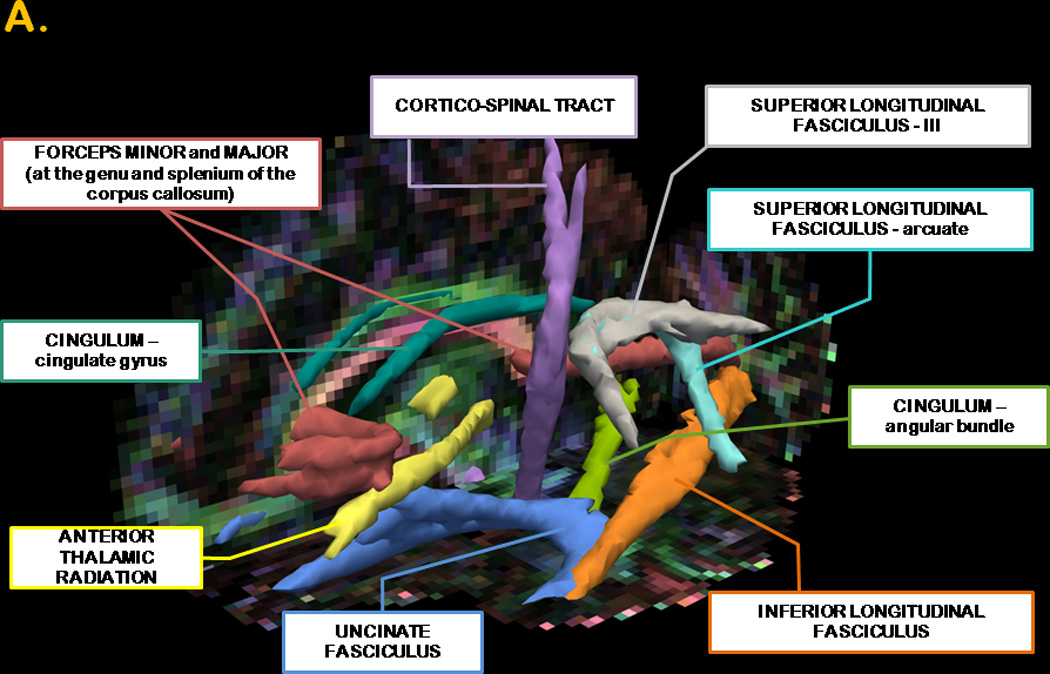
Panel A. The posterior distribution of each WM tract is displayed in isosurface mode. The forceps minor and major are represented in red; the anterior thalamic radiation in yellow, the angular bundle of the cingulum in light green; the cingulate gyrus of the cingulum in emerald green; the cortico-spinal tract in purple; the inferior longitudinal fasciculus in orange; the arcuate bundle of the superior longitudinal fasciculus in aquamarine; the III bundle of the superior longitudinal fasciculus in gray and uncinate fasciculus in blue. All fibers were thresholded at 20% of their maximum. The background image depicts the FA image in color-code convention in one of our participants: voxels with a red color define left-to-rightly oriented fibers; voxels with a blue color define inferior-to- superiorly oriented fibers and voxels with a green color define anterior-to-posteriorly oriented fibers. The isosurface superimposed on the sagittal view of the colored FA image shows the characteristic anterior-posterior alignment of the cingulum (green voxels) and the origin of the forceps minor and major (red voxels in the genu and splenium of the corpus callosum).
Panel B. Error-Bars graphs depict the between group differences in central measures (top; FA in the left corner and RD in the right corner) across all WM tracts, and in peripheral measures (bottom; LPH in the left corner and 4-HNE in the right corner) in 24 BDE and 19 CONT.
Panel C. Scatter plot graphs represent the linear relationship between mean FA (left) and mean RD (right) and LPH across all study participants in the forceps minor
We had the following specific hypotheses:
BD (type-I) vs. healthy adults would show significantly reduced FA and elevated RD in the above a priori eight WM tracts.
BD (type-I) vs. healthy adults would show significantly elevated LPH and 4-HNE.
There would be a negative relationship across all adults between FA, and a positive relationship between RD, in these WM tracts and peripheral measures of lipid peroxidation.
Methods
Participants
Forty-three right-handed participants were recruited, including 24 euthymic BD individuals, type-I (BDE), diagnosed according to DSM-IV criteria and the Structured Clinical Interview for DSM-IV (SCID-I/P)35 (male/female=8/16; mean age[SD]=33.1[8.3]), who fulfilled criteria for remission (in remission for at least 2 months at the time of scanning, having Hamilton Depression Rating Scale (HDRS-25 item)36 score≤18 and Young Mania Rating Scale (YMRS)37 score ≤10. One BDE had a HDRS-25 score of 23, but only on the scanning day). We also recruited 19 age- and gender-matched healthy adults (CONT; male/female=9/10; mean age[SD]=31.7[6.7]; no previous history of psychiatric illness). All participants were right-handed (Annett criteria38). All BDE had experienced at least two episodes of illness in the past 4 years. All BDE were taking one or more psychotropic medications: 50% were taking antidepressants(n=12/24), 58.3% antipsychotics(n=14/24), 75% mood stabilizers(n=18/24), and 12.5% anxiolytics(n=3/24; Table1). Only participants for whom there were ≤30 days between the day of scan and blood withdrawal were included in analyses, in order to obtain peripheral measures that were as close to the scanning day as possible for each participant.
Table 1.
Demographic and clinical variables in 24 BDE and 19 CONT
| GROUP | N | MEAN | [SD] | Statistics | df | P value | |
|---|---|---|---|---|---|---|---|
| AGE AT MRI | BDE | 24 | 33.2 | [7.7] | t= −0.4 | 41 | 0.658 |
| CONT | 19 | 34.0 | [4.4] | ||||
| GENDER RATIO [M/F] | BDE | 8/16 | c2= 0.874 | 1 | 0.35 | ||
| CONT | 9/10 | ||||||
| NART | BDE | 24 | 112.7 | [9.6] | t= 0.2 | 41 | 0.84 |
| CONT | 19 | 112.1 | [8.5] | ||||
| LEVEL OF EDUCATION | BDE | 24 | 6.4 | [1.1] | t= 0.0 | 41 | 0.991 |
| CONT | 19 | 6.4 | [1.3] | ||||
| HDRS-25 item | BDE | 24 | 8.2 | [5.9] | t= 5.0 | 29# | <.001 |
| CONT | 19 | 1.8 | [1.9] | ||||
| YMRS | BDE | 24 | 2.3 | [2.3] | t= 4.1 | 28# | <.001 |
| CONT | 19 | 0.3 | [0.7] | ||||
| TRAIT ANXIETY [STAI] | BDE | 17 | 39.9 | [9.0] | t= 5.0 | 33# | <.001 |
| CONT | 18 | 26.6 | [6.6] | ||||
| AGE OF ILLNESS ONSET | BDE | 24 | 19.4 | [6.2] | |||
| ILLNESS DURATION | BDE | 24 | 13.8 | [7.4] | |||
| LIFETIME NUMBER OF MOOD EPISODES | BDE | 24 | 4.0 | [1.4] | |||
| MONTHS OF REMISSION TO MRI | BDE | 24 | 26.8 | [29.9] | |||
| OFF/ON | |||||||
| ANTIDEPRESSANTS | BDE | 12/ 12 | |||||
| ANTIPSYCHOTICS | BDE | 10/ 14 | |||||
| MOOD STABILIZERS | BDE | 6/ 18 | |||||
| ANXIOLYTICS | BDE | 21/ 3 | |||||
missing information in 1 CONT and 7 BD euthymic.
The study was approved by the University of Pittsburgh Institutional Review Board. Participants were recruited through the WPIC, Mood Disorder Treatment and Research Program (MDTRP) and local advertising. Participants reflected the demographics of Pittsburgh and surrounding area. All participants were made aware of the purpose of, and signed informed consent to participate in, the study. (Details on exclusion criteria and data acquisition are in Supplemental Materials; lifetime comorbidities in BDE are in Supplemental Table1).
Data analysis
Neuroimaging
Data analysis was performed using three freely available software packages: ExploreDTI(www.exploredti.com), FreeSurfer(www.surfer.nmr.mgh.harvard.edu) and TRACULA, diffusion toolbox of Functional MRI tool of Brain Software Library(FSL; www.fmrib.ox.ac.uk/fsl). ExploreDTI is an advanced quality assurance tool for diffusion images, and can examine individual images to check for gross artifacts, such as signal dropouts and interleave artifacts. Details on preprocessing are in Supplemental material. After tensor estimation, the probability distributions of multiple fiber directions of our a priori WM tracts was based on the Bayesian framework for global tractography proposed in TRACULA39, that uses reproducible tracking protocols24, validated on a set of training subjects, and is therefore suitable for the study of well-characterized WM tracts40. Measures of interest (FA, RD and L1) were then extracted for each reconstructed pathway in each participant. Structural images were processed in FreeSurfer41 to automatically parcellate the cortex and segment subcortical regions for each subject, in order for TRACUA to derive the end regions for the automated tractography.
Peripheral measures of lipid peroxidation
These analyses were performed blind to subject diagnosis. (1). LPH and (2). 4-HNE were measured. Details are in Supplemental Materials.
Statistical analysis
Demographic data, clinical data, peripheral measures, FA RD and L1 were all imported into well-established statistical software (Predictive Analytics SoftWare Statistics 17.0, for Windows) to test main hypotheses and exploratory analyses. Rather than considering the 10 WM tracts separately, our approach was to examine them simultaneously. This allowed us to balance type-I and type-II errors and avoid problems associated with multiple comparisons. Furthermore, given the absence of a group by laterality (left, right hemispheres) interaction for the overall mean FA (and RD/L1) for the 8 bilateral WM tracts in BDE and CONT (FA:F[1,41]=0.1;p=0.732; RD:F[1,41]=0.2;p=0.669), we computed mean FA (or RD/L1) across left and right hemispheres for each of the 8 bilateral tracts, and entered these mean values, together with the values of the 2 interhemispheric fibers, forceps major and minor, into the same model (total of 10 WM tracts). 2(group)×10(tract) repeated measures analyses of variance (ANOVAs; one each for FA, RD, and L1) were used. Main effects of group (BDE, CONT),tract (10 tracts) and between-effect interaction were examined for FA, RD, and L1. We then performed post-hoc analyses of individual measures for testing our a priori approach (8 tracts of interest; 2 control tracts), for hypothesis generating and future meta-analytic work. We examined the main effect of group upon peripheral measures (LPH, 4-HNE), using a 2×2 repeated measures ANOVA.
To examine possible effects of age and gender on these a priori dependent measures, Pearson or Spearman coefficients were used, as appropriate, for each group, and across groups. Given the no significant effects of age and gender upon WM and peripheral measures (for statistical values, see footnotes of Tables 2A,2C and 3A), these variables were not entered as covariates in the ANOVA models.
Table 2.
| A. 2-way Repeated Measure ANOVA of effects of group (2 levels: BDE and CONT) and WM tract (10 levels: forceps major and minor, anterior thalamic radiation, cingulum –angular bundle and cingulate gyrus, cortico-spinal tract, inferior longitudinal fasciculus, superior longitudinal fasciculus –arcuate and SLF I-III and uncinate fasciculus) on FA. | ||
|---|---|---|
| FACTORS$ | F [1,41] | Sig. |
| GROUP | 6.8 | 0.013 |
| GROUP * WM TRACT# | 1.4 | 0.237 |
| B. Post hoc analysis of between group (BDE and CONT) differences in FA in WM tracts. | ||||||
|---|---|---|---|---|---|---|
| WM TRACT | GROUP | N | MEAN | SD | t [41] | P (2-tailed) |
| FORCEPS MAJOR | BDE | 24 | 0.601 | 0.148 | ||
| CONT | 19 | 0.686 | 0.102 | −2.2 | 0.032 | |
| FORCEPS MINOR | BDE | 24 | 0.495 | 0.100 | ||
| CONT | 19 | 0.556 | 0.034 | −2.8# | 0.01 | |
| ANTERIOR THALAMIC RADIATION | BDE | 24 | 0.418 | 0.050 | ||
| CONT | 19 | 0.436 | 0.021 | −1.5 | 0.152 | |
| CINGULUM [ANGULARBUNDLE] | BDE | 24 | 0.407 | 0.074 | ||
| CONT | 19 | 0.452 | 0.059 | −2.2 | 0.035 | |
| CINGULUM [CINGULATEGYRUS] | BDE | 24 | 0.578 | 0.080 | ||
| CONT | 19 | 0.617 | 0.040 | −2 | 0.057 | |
| CORTICO-SPINAL TRACT | BDE | 24 | 0.535 | 0.079 | ||
| CONT | 19 | 0.548 | 0.028 | −0.7 | 0.498 | |
| INFERIOR LONGITUDINAL FASCICULUS | BDE | 24 | 0.494 | 0.085 | ||
| CONT | 19 | 0.526 | 0.043 | −1.5 | 0.142 | |
| SUPERIOR LONGITUDINAL FASCICULUS [SLF I-III] | BDE | 24 | 0.465 | 0.068 | ||
| CONT | 19 | 0.495 | 0.028 | −2 | 0.073 | |
| SUPERIOR LONGITUDINAL FASCICULUS [ARCUATE] | BDE | 24 | 0.472 | 0.086 | ||
| CONT | 19 | 0.511 | 0.025 | −2.1# | 0.046 | |
| UNCINATE FASCICULUS | BDE | 24 | 0.420 | 0.075 | ||
| CONT | 19 | 0.456 | 0.028 | −2.2# | 0.039 | |
| C. 2-way Repeated Measure ANOVA of effects of group (2 levels: BDE and CONT) and WM tract (10 levels: forceps major and minor, anterior thalamic radiation, cingulum –angular bundle andcingulate gyrus, cortico-spinal tract, inferior longitudinal fasciculus, superior longitudinal fasciculus –arcuate and SLF I-III and uncinate fasciculus) on RD. | ||
|---|---|---|
| FACTORS$ | F [1,41] | Sig. |
| GROUP | 10.3 | 0.003 |
| GROUP * WM TRACT # | 1 | 0.375 |
| D. Post hoc analysis of between group (BDE and CONT) differences in RD in WM tracts. | ||||||
|---|---|---|---|---|---|---|
| WM TRACT | GROUP | N | MEAN | SD | t [41] | P (2-tailed) |
| FORCEPS MAJOR | BDE | 24 | 0.531 | 0.248 | ||
| CONT | 19 | 0.425 | 0.152 | 1.6 | 0.11 | |
| FORCEPS MINOR | BDE | 24 | 0.576 | 0.119 | ||
| CONT | 19 | 0.514 | 0.043 | 2.1 | 0.04 | |
| ANTERIOR THALAMIC RADIATION | BDE | 24 | 0.592 | 0.061 | ||
| CONT | 19 | 0.570 | 0.022 | 1.5 | 0.143 | |
| CINGULUM [ANGULARBUNDLE] | BDE | 24 | 0.650 | 0.081 | ||
| CONT | 19 | 0.599 | 0.057 | 2.3 | 0.026 | |
| CINGULUM [CINGULATEGYRUS] | BDE | 24 | 0.472 | 0.064 | ||
| CONT | 19 | 0.432 | 0.033 | 2.6# | 0.012 | |
| CORTICO-SPINAL TRACT | BDE | 24 | 0.489 | 0.063 | ||
| CONT | 19 | 0.477 | 0.021 | 0.9# | 0.384 | |
| INFERIOR LONGITUDINAL FASCICULUS | BDE | 24 | 0.585 | 0.080 | ||
| CONT | 19 | 0.555 | 0.048 | 1.4 | 0.158 | |
| SUPERIOR LONGITUDINAL FASCICULUS [SLF I-III] | BDE | 24 | 0.556 | 0.073 | ||
| CONT | 19 | 0.518 | 0.025 | 2.4# | 0.023 | |
| SUPERIOR LONGITUDINAL FASCICULUS [ARCUATE] | BDE | 24 | 0.545 | 0.087 | ||
| CONT | 19 | 0.507 | 0.026 | 2.1# | 0.048 | |
| UNCINATE FASCICULUS | BDE | 24 | 0.643 | 0.094 | ||
| CONT | 19 | 0.600 | 0.032 | 2.1# | 0.044 | |
There was no effect of age (F[39]=1.7; p=0.203) or gender (F[39]=0.001; p=0.977) in the comparison of central FA between BDE and CONT, therefore these factors were not entered in the model.
Mauchly's test of non-sphericity was significant, as such Greenhouse-Geisser corrections were used, corrected degrees of freedom=(2, 68).
Equal variances not assumed (Levene's test)
There was no effect of age (F[39]=0.2; p=0.658) or gender (F[39]=0.4; p=0.555) in the comparison of peripheral markers between BDE and CONT, therefore these factors were not entered in the model
Mauchly's test of non-sphericity was significant, as such Greenhouse-Geisser corrections were used, corrected degrees of freedom=(2, 81).
Equal variances not assumed (Levene's test)
Table 3.
| A. 2-way Repeated Measure ANOVA of effects of group (2 levels: BDE and CONT) and peripheral measures of lipid peroxidation (2 levels:
b asal LPH and 4-HNE) | ||
|---|---|---|
| FACTORS$ | F[1,39] | Sig. |
| GROUP | 4.6 | 0.037 |
| PERIPHERAL MEASURE of lipid oxidative stress# | 0.2 | 0.652 |
| GROUP* PERIPHERAL MEASURE of lipid oxidative stress # | 6 | 0.018 |
| B. Post hoc analysis of between group (BDE and CONT) differences in peripheral measures of lipid peroxidation (LPH and 4-HNE) | ||||||
|---|---|---|---|---|---|---|
| PERIPHERAL MEASURE of lipid oxidative stress | GROUP | N | MEAN $ | SD$ | t [40] | Sig |
| LPH | BDE | 24 | 17.3 | 5.2 | 2.4# | 0.022 |
| CONT | 18 | 14.3 | 3.1 | |||
| 4HNE | BDE | 24 | 2.5 | 0.5 | −1.1 | 0.300 |
| CONT | 18 | 2.7 | 0.6 | |||
There was no effect of age (F[38]=1.8; p=0.192) or gender (F[38]=1.4; p=0.250) in the comparison of peripheral markers between BDE and CONT, therefore these factors were not entered in the model
Given the different unit between LPH and 4-HNE, the three peripheral markers were centered on the mean (data range between −1 and +1) before being entered in the model.
Mauchly's test of non-sphericity was significant, as such Greenhouse-Geisser corrections were used, corrected degrees of freedom=(2, 68).
Equal variances not assumed (Levene's test)
Original (not centered on the mean) descriptives of peripheral markers are reported.
To estimate the variance in the WM measures explained by the peripheral measures of lipid peroxidation across all study participants, multivariate multiple regression analyses were used, with the two peripheral measures as independent variables, and FA(or RD/L1) of the 10 WM tracts as 10 dependent variables. For any significant relationships between WM and peripheral measures, post hoc Spearman rank order correlational analyses determined the direction (positive or negative correlation) between individual tract FA (and RD/L1) and peripheral measures across all participants, and in each group separately, using a statistical threshold of p<0.005 (p<0.05/10), to control for the 10 parallel tests for individual tracts.
In BDE, to assess potential effects of medication on peripheral and WM measures, independent-sample t-tests were performed in BDE not taking vs. BDE taking each medication class (antidepressants, antipsychotics, mood stabilizers and anxiolytics). To assess the potential contribution of lifetime comorbid anxiety disorders and substance use/dependence on peripheral and WM measures, independent sample t-tests were performed with absence vs. presence of history of comorbidity as between-subjects factor.
Results
Demographic and Clinical Characteristics
There were no significant between-group differences in age, gender ratio, years of education, premorbid IQ, handedness. As expected, BDE had significantly more anxious (STAI), depressive(HDRS) and manic(YMRD) symptoms than CONT(Table1).
Between group-differences in FA and RD in the ten a priori WM tracts
FA
There was a significant main effect of group (BDE<CONT:F[1,41]=6.8;p=0.013) but no significant group by tract interaction (Table2A). Post hoc analyses revealed that BDE vs. CONT showed significantly lower mean FA across tracts (t[41]=−2.6;p=0.013;Cohen’s d=0.8). Given our a priori approach (8 tracts of interest and 2 control tracts) we further explored the between-group difference in each WM tract separately. These analyses revealed that BDE vs. CONT showed significantly reduced FA specifically in the forceps major and minor of the corpus callosum, in the angular bundle of the cingulum, in the arcuate fasciculus of the superior longitudinal fasciculus, and in the uncinate fasciculus (all p≤0.05;Table2B;Figure1B).
RD
There was a significant main effect of group (BDE>CONT:F[1,41]=10.3;p=0.003), although no significant group by tract interaction (Table2C). Post hoc analyses revealed that BDE vs. CONT showed significantly greater mean RD across tracts (t[41]=3.2;p=0.003;Cohen’s d=1.0). BDE vs. CONT showed significantly greater RD specifically in the forceps minor of corpus callosum, the cingulum (cingulate gyrus and angular bundle), the superior longitudinal fasciculus (both bundles), and the uncinate fasciculus (all p≤0.05;Table2D;Figure1B).
L1
There were no significant between-group differences in L1 in any tracts (Supplemental Table2).
Between group-differences in peripheral measures of lipid peroxidation
There was a significant main effect of group (BDE>CONT:F[1,39]=4.6;p=0.037) and a group by peripheral measure interaction (F[1.7,68]=6.0;p=0.018;Table3A;Figure1B). Post hoc analyses revealed that BDE showed significantly greater LPH (t[40]=2.4;p=0.022;Cohen’s d=0.7), but did not differ significantly from CONT in 4-HNE (Table3B; Figure1B).
Relationships between WM and peripheral measures of lipid peroxidation showing significant between-group differences across all CONT and BDE
Multivariate multiple regression analysis with LPH and FA
LPH explained 59% of the variance in FA (Wilks’lambda=0.40;F[10,31]=4.50;p=0.001;partial eta2=0.59) across tracts. There was a significant negative linear relationship between LPH and mean FA in the forceps minor (rho= −0.40;p=0.005 across all participants; Figure1C), and negative relationships, at the trend level (given our conservative threshold of p<0.005), between LPH and mean FA in the cingulum (angular bundle: rho=−0.36;p=0.016 across all participants; and rho=−0.52;p=0.026 in CONT). CONT also showed trend-level negative linear relationships between LPH and mean FA in inferior longitudinal and uncinate fasciculi (both rho< −0.51; p≤0.030).(Table4).
Table 4.
Relationships between WM measures and peripheral measures of lipid peroxidation across all study participants.
| Effect of LPH upon FA | Partial Eta Squared | ||||||||
| Independent variable | Wilks' Lambda | F[10,31] | Sig. | ||||||
| LPH | 0.4 | 4.5 | 0.001 | 0.590 | |||||
| Effect of LPH upon RD | Partial Eta Squared | ||||||||
| Independent variable | Wilks' Lambda | F[10,31] | Sig. | ||||||
| LPH | 0.5 | 3.3 | 0.006 | 0.512 | |||||
| TRACT | Spearman's correlation between FA and LPH | Spearman's correlation between RD and LPH | |||||||
| GROUP | rho | Sig. | N | rho | Sig. | N | |||
| FORCEPS MAJOR | TOT | .033 | .837 | 42 | −.028 | .859 | 42 | ||
| BDE | .137 | .525 | 24 | −.160 | .454 | 24 | |||
| CONT | .216 | .390 | 18 | −.139 | .581 | 18 | |||
| FORCEPS MINOR | TOT | −.428** | .005 | 42 | .446** | .003 | 42 | ||
| BDE | −.295 | .161 | 24 | .284 | .179 | 24 | |||
| CONT | −.445 | .064 | 18 | .544* | .020 | 18 | |||
| ANTERIOR THALAMIC RADIATION | TOT | −.005 | .973 | 42 | −.008 | .961 | 42 | ||
| BDE | .115 | .593 | 24 | −.162 | .450 | 24 | |||
| CONT | .006 | .981 | 18 | −.056 | .826 | 18 | |||
| CINGULUM [ANGULAR BUNDLE] | TOT | −.362* | .018 | 42 | .350* | .023 | 42 | ||
| BDE | −.121 | .574 | 24 | .109 | .612 | 24 | |||
| CONT | −.523* | .026 | 18 | .481* | .043 | 18 | |||
| CINGULUM [CINGULATE GYRUS] | TOT | −.265 | .090 | 42 | .285 | .067 | 42 | ||
| BDE | −.129 | .548 | 24 | .089 | .679 | 24 | |||
| CONT | −.310 | .211 | 18 | .375 | .125 | 18 | |||
| CORTICO-SPINAL TRACT | TOT | −.185 | .242 | 42 | .166 | .293 | 42 | ||
| BDE | −.305 | .147 | 24 | .266 | .210 | 24 | |||
| CONT | .130 | .607 | 18 | −.019 | .942 | 18 | |||
| INFERIOR LONGITUDINAL FASCICULUS | TOT | −.150 | .342 | 42 | .175 | .268 | 42 | ||
| BDE | .167 | .435 | 24 | −.114 | .595 | 24 | |||
| CONT | −.512* | .030 | 18 | .507* | .032 | 18 | |||
| SUPERIOR LONGITUDINAL FASCICULUS [SLF I-III] | TOT | −.089 | .573 | 42 | .062 | .698 | 42 | ||
| BDE | .167 | .434 | 24 | −.174 | .415 | 24 | |||
| CONT | −.098 | .699 | 18 | .131 | .604 | 18 | |||
| SUPERIOR LONGITUDINAL FASCICULUS [ARCUATE] | TOT | −.086 | .588 | 42 | .087 | .585 | 42 | ||
| BDE | .144 | .502 | 24 | −.171 | .425 | 24 | |||
| CONT | −.289 | .245 | 18 | .433 | .073 | 18 | |||
| UNCINATE FASCICULUS | TOT | −.266 | .089 | 42 | .273 | .080 | 42 | ||
| BDE | −.048 | .824 | 24 | .055 | .799 | 24 | |||
| CONT | −.570* | .014 | 18 | .595* | .009 | 18 | |||
Significance level was set at p<0.005 (p<0.05/10), to control for the ten parallel tests for individual tracts. Trend-level significance was set between 0.05 and 0.005.
Given the overall non-normality of the data (Levene's test < 200), Spearman's coefficients were reported between LPH and FA (and RD) in BDE, CONT and across the whole sample.
Multivariate multiple regression analysis with LPH and RD
LPH explained 51% of the variance in RD (Wilks’lambda=0.50;F[10,31]=3.30;p=0.006;partial eta2=0.51) across tracts. There was a significant positive linear relationship between LPH and mean RD in the forceps minor across all participants (rho=0.45;p=0.003;Figure1C) and a trend-level positive linear relationship between LPH and mean RD in this tract in CONT (rho=0.54;p=0.020). There were trend-level positive linear relationships between LPH and mean RD in the cingulum (angular bundle: rho=0.35;p=0.023 across all participants and rho=0.48;p=0.043 in CONT). CONT also showed trend-level positive relationships between LPH and mean RD in the inferior longitudinal and uncinate fasciculi (both rho>0.51; p≤0.032;Table 4).
Exploratory analyses
There was a positive relationship between HDRS-25 score and RD in the forceps minor and uncinate fasciculus (all p≤0.034;Supplemental Table3). There was a significant relationship between taking antidepressants and RD in the cingulum (cingulate gyrus), superior longitudinal fasciculus (SLF-III) and uncinate fasciculus (all p≤0.044): RD was significantly greater in BDE taking vs. BDE not-taking antidepressants (Supplemental Table4). BDE were subdivided into 4 categories (1.not-taking 2.low 3.average 4.high therapeutic dosage of antidepressants). Further analysis revealed that BDE taking higher dosage of antidepressants had higher HDRS-25 scores (F[3,20]=4.6;p=0.013;Footnote Supplemental Table4).
Discussion
The major goal of the present study was to identify whether peripheral biomarkers of lipid peroxidation reflected alterations in WM in individuals with BD, as a primary step toward identification of clinically-relevant peripheral biomarkers. We demonstrated that BDE vs. CONT showed significantly greater RD and reduced FA overall, and specifically in the forceps minor, cingulum, superior longitudinal fasciculus and uncinate fasciculus, tracts connecting prefrontal cortex with other cortical and subcortical limbic regions that have previously been shown to be abnormal in BD. We also showed significantly greater LPH, but not 4-HNE, in BDE vs. CONT. Furthermore, across BDE and CONT, LPH explained 59% of the variance in FA, and 51% of the variance in RD in a priori WM tracts.
Myelin is a specific target for lipid peroxidation30. Lipid peroxidation of myelin has been shown in brain injury42, and spinal cord dysfunction43, and myelin isolated in vitro studies is subject to oxidative stress in a time and dose-dependent manner44. The forceps minor, an inter-hemispheric tract connecting left and right prefrontal cortices, showed the most consistent pattern of significantly reduced FA and elevated RD in BDE vs. CONT, and was the WM tract for which there were significant relationships among peripheral (LPH) and WM (FA, RD) measures, across all study participants. To a lesser extent, BDE showed significantly altered FA and RD in the cingulum and uncinate fasciculus, and there were trend-level relationships among LPH and FA and/or RD in these tracts across all study participants. Our significant findings in the forceps minor may reflect the proximity of this WM tract to the dopamine-rich ventral prefrontal cortical-striatal reward system, known to be dysfunctional in BD45, given that dopamine can be auto-oxidized in the brain to form quinones, that increase oxidative stress by inhibiting mitochondrial functioning46. There were no consistent patterns of abnormal WM integrity in our two control tracts in BDE, namely, the forceps major and the cortico-spinal tract, and no significant relationships among LPH and either FA or RD in these tracts across all participants. Together, these findings support our three main hypotheses, and suggest that LPH may be a peripheral measure of WM integrity, particularly of key tracts connecting prefrontal cortical with subcortical and other prefrontal cortical regions supporting reward and mood regulation47.
Our findings of significantly greater RD (and reduced FA) in the forceps minor, cingulum and uncinate fasciculus in BDE vs. CONT support previous findings of abnormalities in WM integrity in the genu of corpus callosum and in the (anterior) cingulum in region-of-interest14, voxel-based15, tract-based spatial statistics16, and tractography-based17–18 studies, and in studies of the uncinate fasciculus18–23 in BD. Our findings of reduced FA in these tracts in BDE vs. CONT likely resulted from elevated RD, given that there were no between-group differences in L1 in these tracts. Given that elevated RD may be associated with myelin and axolemma abnormalities25–28, our findings may reflect myelin and axonal membrane abnormalities in these WM tracts in BDE.
BDE vs. CONT also showed significantly greater RD and reduced FA in the arcuate fasciculus of the superior longitudinal fasciculus, supporting previous findings in BD18–19. Unlike the forceps minor, cingulum, and uncinate fasciculus, there were no significant relationships between FA or RD in this tract and peripheral measures of lipid peroxidation in study participants. This may suggest that WM abnormalities in this fasciculus may reflect a more complex architecture, rather than abnormalities in myelin or axolemma components, in BD vs. CONT. The specific role of this tract in the pathophysiology of BD remains unclear.
In a recent meta-analysis, we48 found that thiobarbituric acidic reactive substances (TBARS), a measure of late-stage lipid peroxidation, was significantly elevated in all phases of BD type-I. In the present study, we measured both LPH and 4-HNE, and found between-group differences only in LPH. Elevated early-stage measures of lipid peroxidation (i.e., LPH) can be removed from cells by antioxidant enzymes31, 49, and increases in these enzymes, e.g., glutathione-S-transferase, are reported in individuals with BD50. Such increases in antioxidant systems are thought to be activated in BD in response to oxidative stress to brain lipids, given that reduced levels of total glutathione are observed in postmortem prefrontal cortex of BD subjects51. The activation of this antioxidant system may help prevent a rise in measures of late-stage lipid peroxidation, and may explain in part the absence of elevated 4-HNE in BDE in the present study. Future studies assessing other late-stage products of lipid peroxidation (e.g., 8-isoprostane and acrolein) and antioxidant enzyme levels can clarify this. Further focus on the mechanism through which oxidative damage to lipids may affect specific WM tracts in BD is clearly needed.
There were limitations to the present study. BDE were medicated, as is frequently the case to allow BDE to remain in remission52. Exploratory analyses revealed that BDE taking antidepressants had greater RD in a priori WM tracts than BDE not-taking antidepressants, although BDE with higher subsyndromal depression severity also had greater RD in the forceps minor and uncinate fasciculus. Furthermore, BDE taking higher dosage antidepressants had higher subsyndromal depression severity, suggesting that greater RD may be associated with greater depression severity, rather than with antidepressants, and parallel previous findings of greater mean diffusivity (an indirect index of RD) in depressed BD individuals vs. BDE53.
While significant relationships between LPH and FA/RD in the forceps minor, and trend-level relationships between LPH and FA/RD in the cingulum(angular bundle), were observed in all participants, only CONT showed linear relationships between these measures in these tracts (and also in uncinate and inferior longitudinal fasciculi). Interindividual variability (e.g., in subsyndromal symptoms, treatments and response to treatment) in BDE may in part explain the loss of linear relationships between LPH and FA/RD in these tracts. Additional studies should examine relationships between WM and peripheral measures in young BDE in early stages of illness, and also between symptom severity, medication, and WM and peripheral measures in BDE, and whether other factors (e.g., diet54) impact these measures. Future studies should also examine relationships between WM and peripheral measures in BD individuals across different mood states, and in individuals with other mood disorders, to determine whether these relationships represent persistent or mood state-dependent features of BD, or BD-specific pathology versus dimensions of pathology across the mood disorders spectrum.
This is the first study to use a combination of DTI, to examine measures of WM integrity, and peripheral (serum) measures of lipid peroxidation in BDE and CONT. Our findings suggest that peripheral measures of early-stage lipid peroxidation are associated with underlying pathophysiologic processes involving WM abnormalities in key prefrontal-subcortical and prefrontal-prefrontal cortical tracts in BD that, in turn, may underlie the mood dysregulation that is characteristic of the illness. These findings offer insight into one of the first steps for biomarker development, the identification of a robust biological candidate. There are critical next steps to understand whether serum LPH is a potential biomarker, including replication and determination of the sensitivity, specificity and predictive value for the biomarker. Our findings suggest that examination of these peripheral measures is a promising way forward to help identify pathophysiologically-relevant, yet easily obtainable and inexpensive, peripheral biomarkers of BD.
Supplementary Material
Acknowledgments
This study was supported in part by R01 MH076971-01 (Dr Phillips) and MH63480 (Dr Nimgaonkar) from National Institutes of Health, by a NARSAD (National Alliance for Research on Schizophrenia and Depression) Young Investigator Award (Dr Versace) and by Canadian Institute of Health Research (CIHR; Dr. Young, Dr. Andreazza).
Footnotes
Conflict of interest
None of the authors have competing financial interest to report.
References
- 1.Phillips ML, Frank E. Redefining bipolar disorder: toward DSM-V. Am J Psychiatry. 2006;163(7):1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 2.Kupfer DJ, Angst J, Berk M, Dickerson F, Frangou S, Frank E, et al. Advances in bipolar disorder: selected sessions from the 2011 International Conference on Bipolar Disorder. Ann Ny Acad Sci. 2011;1242:1–25. doi: 10.1111/j.1749-6632.2011.06336.x. [DOI] [PubMed] [Google Scholar]
- 3.Frey BN, Andreazza AC, Houenou J, Jamain S, Goldstein B, Frye MA, et al. Biomarkers in Bipolar Disorder: A Positional Paper from the International Society for Bipolar Disorders Biomarkers Committee. ANZJP. doi: 10.1177/0004867413478217. submitted July, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Hirschfeld RM, Vornik LA. Bipolar disorder--costs and comorbidity. Am J Manag Care. 2005;11(3 Suppl):S85–S90. [PubMed] [Google Scholar]
- 5.WHO. The global burden of disease: 2004 update. Geneva, Switzerland: 2008. [Google Scholar]
- 6.Goodwin FK, Jamison KR. Manic-depressive illness : bipolar disorders and recurrent depression. 2 edn. New York, N.Y.: Oxford University Press; 2007. v.<2>pp. [Google Scholar]
- 7.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64(2):161–174. [PubMed] [Google Scholar]
- 8.Vederine F-E, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1820–1826. doi: 10.1016/j.pnpbp.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla P, Bellani M, Yeh P-H, Soares JC, Tansella M. White matter connectivity in bipolar disorder. International Review of Psychiatry. 2009;21(4):380–386. doi: 10.1080/09540260902962172. [DOI] [PubMed] [Google Scholar]
- 10.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review of Diffusion Tensor Imaging Studies in Affective Disorders. Biol Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Womer FY, Kalmar JH, Wang F, Blumberg HP. A ventral prefrontal-amygdala neural system in bipolar disorder: a view from neuroimaging research. Acta Neuropsychiatrica. 2009;21(5):228–238. doi: 10.1111/j.1601-5215.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34(4):533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J Neural Transm. 2010;117(5):639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- 14.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 15.Chaddock CA, Barker GJ, Marshall N, Schulze K, Hall MH, Fern A, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194(6):527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- 16.Benedetti F, Yeh P-H, Bellani M, Radaelli D, Nicoletti MA, Poletti S, et al. Disruption of White Matter Integrity in Bipolar Depression as a Possible Structural Marker of Illness. Biol Psychiatry. 2011;69(4):309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti F, Absinta M, Rocca MA, Radaelli D, Poletti S, Bernasconi A, et al. Tract-specific white matter structural disruption in patients with bipolar disorder. Bipolar Disord. 2011;13(4):414–424. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: a DTI tractography study. Journal of Affective Disorders. 2011;131(1–3):299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED, et al. White Matter Tractography in Bipolar Disorder and Schizophrenia. Biol Psychiatry. 2008;64(12):1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 22.Versace A, Almeida JRC, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65(9):1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and Structural Connectivity Between the Perigenual Anterior Cingulate and Amygdala in Bipolar Disorder. Biol Psychiatry. 2009;66(5):516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 27.Sun S-W, Liang H-F, Cross AH, Song S-K. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40(1):1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie M, Wang Q, Wu TH, Song SK, Sun SW. Delayed axonal degeneration in slow Wallerian degeneration mutant mice detected using diffusion tensor imaging. Neuroscience. 2011;197(0):339–347. doi: 10.1016/j.neuroscience.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klawiter EC, Schmidt RE, Trinkaus K, Liang H-F, Budde MD, Naismith RT, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55(4):1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 1984;1(8391):1396–1397. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th edn. Oxford ; New York: Oxford University Press; 2007. p. 851. xxxvi, 858 p. of platespp. [Google Scholar]
- 32.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 33.Andreazza AC. Combining redox-proteomics and epigenomics to explain the involvement of oxidative stress in psychiatric disorders. Mol Biosyst. 2012 doi: 10.1039/c2mb25118c. [DOI] [PubMed] [Google Scholar]
- 34.Young LT. Is bipolar disorder a mitochondrial disease? Journal of Psychiatry & Neuroscience. 2007;32(3):160–161. [PMC free article] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon ML, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. vol. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 36.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 38.Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 39.Yendiki A, Panneck P, Srinivasan P, Stevens A, Z?llei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics. 2011;5 doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jbabdi S, Woolrich MW, Andersson JLR, Behrens TEJ. A Bayesian framework for global tractography. Neuroimage. 2007;37(1):116–129. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 41.Fischl B. FreeSurfer. Neuroimage. ((0)) doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi R, Rickett T, Sun W. Acrolein-mediated injury in nervous system trauma and diseases. Mol Nutr Food Res. 2011;55(9):1320–1331. doi: 10.1002/mnfr.201100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Y, Sun W, McBride JJ, Cheng JX, Shi R. Acrolein induces myelin damage in mammalian spinal cord. J Neurochem. 2011;117(3):554–564. doi: 10.1111/j.1471-4159.2011.07226.x. [DOI] [PubMed] [Google Scholar]
- 44.Konat GW, Wiggins RC. Effect of reactive oxygen species on myelin membrane proteins. J Neurochem. 1985;45(4):1113–1118. doi: 10.1111/j.1471-4159.1985.tb05530.x. [DOI] [PubMed] [Google Scholar]
- 45.Almeida JRC, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical neuroimaging perspectives. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J Neurochem. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 47.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreazza AC, Kauer-Sant'Anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: A meta-analysis. Journal of Affective Disorders. 2008;111(2–3):135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 50.Andreazza AC, Kapczinski F, Kauer-Sant'Anna M, Walz JC, Bond DJ, Goncalves CA, et al. 3-Nitrotyrosine and glutathione antioxidant system in patients in the early and late stages of bipolar disorder. J Psychiatry Neurosci. 2009;34(4):263–271. [PMC free article] [PubMed] [Google Scholar]
- 51.Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Yatham LN, Young LT. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. The International Journal of Neuropsychopharmacology. 2011;14(08):1069–1074. doi: 10.1017/S1461145711000617. [DOI] [PubMed] [Google Scholar]
- 52.Altshuler L, Suppes T, Black D, Nolen WA, Keck PE, Jr, Frye MA, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry. 2003;160(7):1252–1262. doi: 10.1176/appi.ajp.160.7.1252. [DOI] [PubMed] [Google Scholar]
- 53.Zanetti MV, Jackowski MP, Versace A, Almeida JR, Hassel S, Duran FL, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259(6):316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smit LA, Katan MB, Wanders AJ, Basu S, Brouwer IA. A High Intake of trans Fatty Acids Has Little Effect on Markers of Inflammation and Oxidative Stress in Humans. J Nutr. 2011;141(9):1673–1678. doi: 10.3945/jn.110.134668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


