Abstract
Background
Current nutritional approaches have been partially successful in Cystic Fibrosis (CF). Essential amino acids mixtures with high Leucine levels (EAA) have anabolic properties in catabolic conditions, however data in CF are lacking.
Methods
On two days according a randomized crossover design, 15 pediatric CF patients ingested 6.7g EAA versus mixture of total amino acids as present in whey. Whole body protein and Arginine metabolism (as EAA lack Arginine) were assessed by stable isotope methodology.
Results
Protein synthesis (p<0.05) but not protein breakdown was higher after EAA and 70% higher values for net anabolism (P<0.001) were found both in patients with and without nutritional failure. Arginine turnover was lower (P<0.001) and de novo Arginine synthesis tended lower (P=0.09) after EAA. Nitric oxide synthesis was not different.
Conclusions
CF patients are highly responsive to EAA intake independent of their nutritional status. Addition of Arginine to the EAA mixture may be warranted in CF.
Keywords: Cystic Fibrosis, Essential amino acids, Whole body protein anabolism, Arginineinine production, Nitric oxide synthesis, Nutritional failure
Introduction
Fat-free mass depletion, reflecting muscle mass loss, is present in 25–30% of children with Cystic Fibrosis (1, 2), independent of their body mass index, and is associated with reduced lung function and bone mineral loss (1). Previous studies in CF showed that oral supplementation of large amounts of calories to improve nutritional status is only partially successful and muscle gain is difficult to achieve in these patients (3, 4). Insight into the composition of dietary intake that is able to induce protein anabolism in children with CF is therefore of crucial importance to successfully counteract muscle wasting. Studies examining the effects of dietary proteins and amino acids in children with CF are limited, and their specific daily protein requirements are still unclear. A previous study showed that very high levels of dietary protein (5g·kg−1·d−1) are able to stimulate whole body protein synthesis in stable pediatric CF patients (5). When taking into account the impaired protein digestion capacity of CF patients and the increased amino acid need for building muscle and acute phase proteins due to their inflammatory state (6), supplements with specific amino acids might be of clinical importance to increase the dietary nitrogen load in this patient population. In the past years, essential amino acids (EAA) have been shown to be solely responsible for the amino acid induced stimulation of muscle protein anabolism (7). Furthermore, EAA are able to stimulate muscle protein synthesis to more than twice the extent as the same amount of a high quality (whey) protein (8). When the proportion of leucine is increased to 40% in the EAA mixture, the rate of muscle protein synthesis is stimulated to an even greater extent (9). Furthermore, leucine is known for its insulinotropic effect (10), which is of importance as a defect in the suppressive response of proteolysis to insulin is present in CF (11). We therefore hypothesize that an leucine-enriched EAA mixture will stimulate protein anabolism in children with CF. Furthermore, CF is associated with reduced exhaled nitric oxide (NO) (12) despite the presence of chronic airway inflammation. Arginine is the sole precursor of nitric oxide (NO) and plays a key role in many other metabolic processes (i.e protein synthesis, release of anabolic hormones, and modulation of immune function). It remains unclear to what extent dietary amino acid formulations are able to alter Arginine availability and NO production in CF and whether the EAA composition is optimal for CF patients.
In the present study, the acute anabolic effects of a leucine-enriched EAA mixture was examined in pediatric patients with CF and compared to that of a balanced mixture of essential and non-essential amino acids as present in whey protein. We also examined whether differences in the anabolic properties between the amino acid mixtures were associated with changes in arginine metabolism and NO synthesis rate. The data are required to support development of evidence based programs for nutritional support for pediatric CF patients using free amino acids to prevent or ameliorate muscle wasting and to reduce morbidity and mortality.
Materials and methods
Subjects
The study population consisted of 15 children with CF, age 10 to 21 years, and admitted to Arkansas Children’s Hospital for antibiotic treatment of a pulmonary exacerbation. The subjects were clinically stable with no symptoms of pulmonary exacerbation any more or other problems requiring use of drugs other than their usual medications. Furthermore, lung function of each subject at enrollment was back or was within 10% of baseline value (defined as the highest FEV1 value obtained in the preceding year). All subjects were pancreatic insufficient based on 72 hr fecal fat collection or fecal elastase level measurement. The patients were having one or more of the following pathogens: 8/15 (53%) Methicillin-resistant Staphylococcus aureus (MRSA), 6/15 (40%) Methicillin-sensitive Staphylococcus aureus (MSSA), 2/15 (13%) Pseudomonas aeruginosa, 1/15 (7%) Mycobacterium avium-intracellulare infection, 1/15 (7%) Burkholderia cepacia. Exclusion criteria included established diagnosis of diabetes mellitus and unstable metabolic diseases. Written informed consent/assent was obtained from all CF subjects and their parents in case the CF subject was < 18y, and the study was approved by the University of Arkansas for Medical Sciences Institutional Review Board (IRB#104738).
Composition of the nutritional supplements
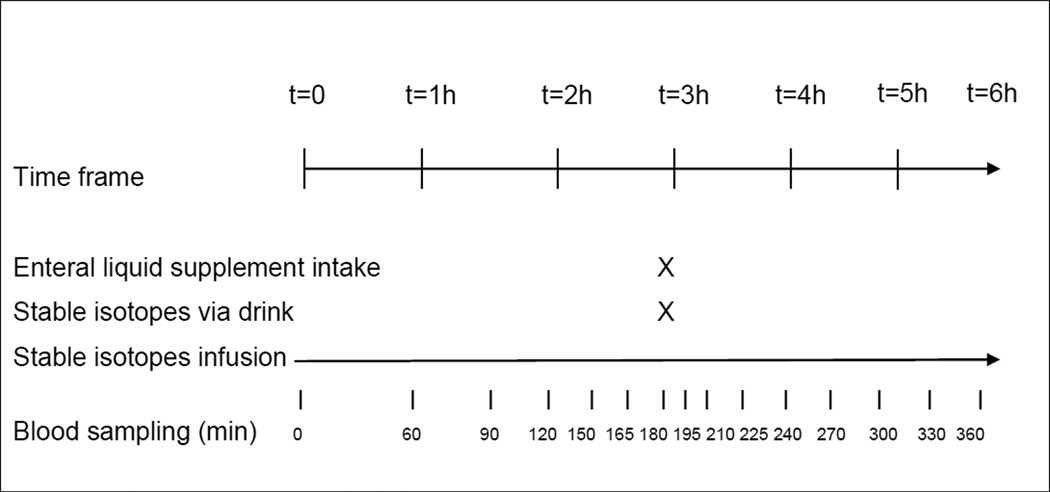
In a double-blind and randomized crossover design, all CF subjects received on 2 subsequent study days one of the two nutritional supplements: 6.7g high-leucine EAA mixture (containing 41% leucine) + 15g carbohydrates or 6.7g balanced mixture of total (EAA and non-essential) amino acids + 15g carbohydrates. The subjects were given orally or enterally (when a feeding tube was present) one serving of each supplement. The content of the nutritional supplements is presented in Table 1. The amino acids and carbohydrate were provided by Ajinomoto Co. For an overview of the study design, see Fig 1.
Table 1.
Composition of the high-leucine essential amino acid (EAA) mixture and the isonitrogenous balanced mixture of total (essential and non-essential) amino acids
| Amino Acid | EAA (g) | Total AA (g) |
|---|---|---|
| Histidine | 0.239 | 0.33 |
| Isoleucine | 0.614 | 0.31 |
| Leucine | 2.790 | 0.53 |
| Lysine | 1.069 | 0.65 |
| Methionine | 0.284 | 0.17 |
| Phenylalanine | 0.398 | 0.27 |
| Threonine | 0.751 | 0.33 |
| Valine | 0.580 | 0.36 |
| Other amino acids | 0 | 3.67* |
| Total | 6.726 g | 6.726 g |
| Maltodextrin | 15g | 15g |
Other amino acids: alanine (0.46 g), arginine (0.41 g), aspartic acid (0.61 g), cystine (0.08 g), glutamic acid (0.96 g), glycine (0.31 g), proline (0.31 g), serine (0.28 g), and tyrosine (0.25 g)
Fig 1.
Overview of the study design
Anthropometric data and body composition
Body weight and height were measured by a digital beam scale and stadiometer, respectively. Height, weight and body mass index (BMI) percentiles of the CF subjects were calculated in accordance with the CF consensus report (13). Whole body fat mass (FM) and fat-free mass (FFM) were obtained by dual-energy X-ray absorptiometry (DXA) (Hologic QDR 4500/Version 12.7.3.1 Bedford, MA), standardized for height (14) to obtain FFM-index and FM-index, and expressed as percentage of published reference data (15, 16). The DXA procedure was done once during hospital stay or the data were copied from subject’s file when DXA was performed in the preceding month of the study as part of CF care.
Lung function
Forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) was measured by spirometry (nSpire Health, Longmont, CO) and reference equations were used to calculate predicted values (17).
Study protocol
The study days were performed on two subsequent days after overnight fasting in the patient’s hospital room at Arkansas Children’s Hospital during the last days of 2 weeks of antibiotic treatment for a CF exacerbation. On the first study day, a catheter for blood sampling was placed in a superficial vein of the lower armor hand and the line was kept patent until the end of the second study day. For infusion of the stable isotopes, a venous entry point (port or antecubital) was used. The arm with the catheter for blood sampling was placed in a thermostatically controlled hot box to obtain arterialized-venous blood (18). After 3 hours of intravenous infusion, each subject consumed a bolus of 6.7g of leucine enriched EAA or same dose of an essential and non-essential amino acid (TAA) mixture to which 15g of maltodextrin was added. Both mixtures were dissolved in 250 mL of a non-caloric soft-drink and consumed through ingestion or delivering enterally (when feeding tube was present). The tracer of 15N-Phenylalanine (3.98 mg/ml) was mixed with the supplements to study first-pass splanchnic amino acid extraction. Blood samples were taken throughout the study for analysis of concentrations and tracer-tracee ratios of amino acids corrected for their baseline value (cTTR). Blood was treated as discussed before (19). Analysis for enrichment and concentrations was done by LC-ESI-MS (QTrap 5500 MS; AB Sciex, Foster Citrulliney, CA) with Express HT Ultra LC (Eksigent Div.; AB Sciex, Foster Citrulliney, CA) after derivatization with 9-fluorenylmethoxycarbonyl (Fmoc). Fmoc was fragmented to obtain specific and high sensitivity fragments. All arginine and protein metabolic data were determined and calculated as described before (19) and in online supplement.
Statistical analysis
Results are expressed as mean ± standard error (SE). Two-factor repeated-measures analysis of variance (general linear model, SPSS version 12; SPSS Inc, Chicago, IL) with interaction was performed with time and amino acid mixture effect (EAA vs. TAA) to test the effects on the change in time (from postabsorptive until 3 hours after intake of amino acid mixtures) on the amino acid concentrations and TTR of the infused isotopes. If there was a significant time-by-amino acid mixture interaction, the Bonferroni multiple comparisons was used to evaluate the mixture effect on each time point.
The mean value at the time points 150, 165 and 180 min was used as the postabsorptive value as this is the time period a steady state in tracer enrichment of the infused isotopes is present. Steady state enrichment values were also obtained in the postabsorptive state in our CF group (defined as difference among time points < 5% and non-significant from zero, data not shown). The prandial state was expressed as 3 hours integral after intake of the mixtures and expressed per hour (nmol/kg FFM/h) to compare postabsorptive vs. prandial state. Two-factor repeated-measures analysis of variance with interaction was performed with time and amino acid mixture effect to test the effects of the enteral intake of the EAA vs. TAA mixture on metabolic measures. The 3 hours integral was calculated (nmol/kg FFM/3h) as the prandial value and the paired t-test was used to determine the difference in metabolic measures between the mixtures. The level of significance was set at p<0.05. The statistical package within Graphpad Prism (Version 5.04), and SPSS (version 20) was used for data analysis.
RESULTS
The study group consisted of 9 boys and 6 girls with CF (TABLE 3). The patients had mild to moderate airflow obstruction and were characterized by reduced values for FFM and FM. Eight CF patients had nutritional failure (FFM-index <5th (6) and/or BMI <10th percentile (age ≤20 years) (13). Body weight and FEV1 increased between hospital admission and study days by 1.9 ± 0.5 kg and 15 ± 5%, respectively but the changes were not different between patients with and without nutritional failure.
Table 3.
General characteristics of the CF group
| Total CF group | ||||
|---|---|---|---|---|
| Gender | m/f | 9 | / | 6 |
| Age | y | 15.8 | ± | 4.2 |
| Tanner stage: pre-pub/pub/post-pub | 0/11/4 | |||
| Height | Percentile | 39.1 | ± | 10.5 |
| BMI | Percentile | 33.9 | ± | 9.1 |
| FFM-index | %norm | 91.9 | ± | 25.5 |
| FM-index | %norm | 87.5 | ± | 23.4 |
| Nutritional failure | y/n | 8/7 | ||
| FEV1 | %pred | 83.1 | ± | 22.2 |
| FVC | %pred | 98.6 | ± | 26.3 |
Values are means ± SEM. CF: Cystic Fibrosis, Pre-pubertal: Tanner stage 1, Pubertal: Tanner stage 2–4, Post-pubertal: Tanner stage 5, BMI: Body mass index, FFM-index: Fat-free mass index, FM-index: Fat mass index, FEV1: forced expiratory volume in one second, FVC: forced vital capacity. Nutritional failure was defined as BMI <10 percentile and/or FFM-index < 5th percentile
Response in TTR of Phenylalanine, Tyrosine, Leucine, and Arginine corrected for their baseline value
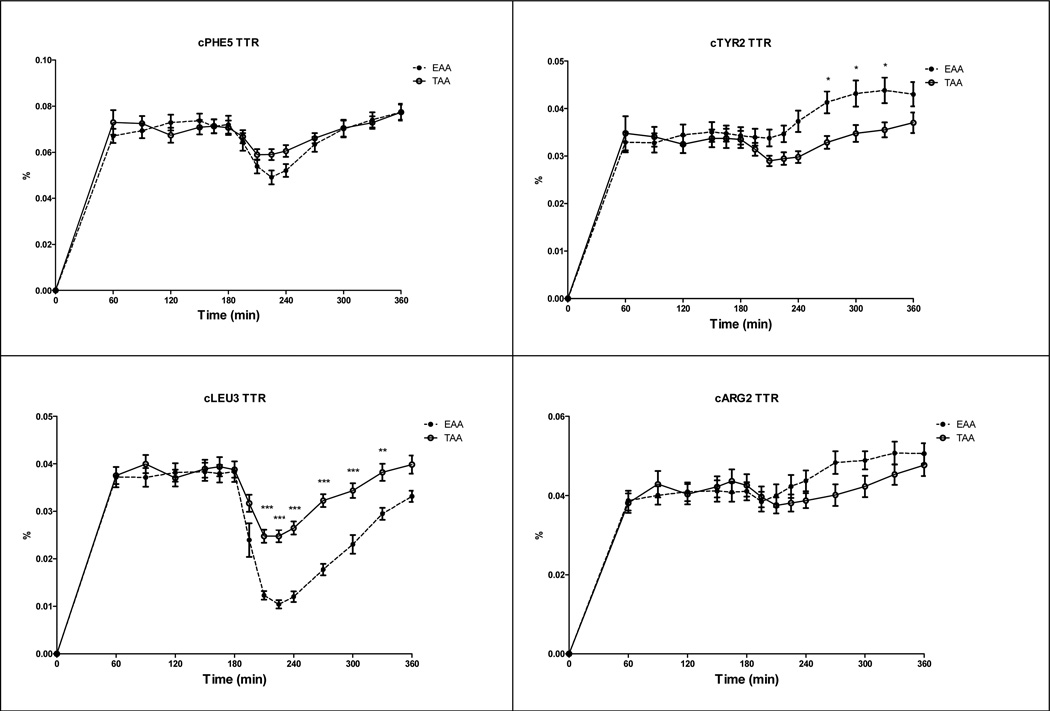
There were time effects for cTTR of Phenylalanine, Tyrosine, Leucine, and Arginine (Fig 2) (P<0.001) indicating reductions for Phenylalanine and Leucine cTTR, and increases in Tyrosine and Arginine cTTR after intake of the amino acid mixtures. There were amino acid mixture effects for cTTR Tyrosine and Leucine, indicating higher values for cTTR Tyrosine and lower values for Leucine after intake of EAA mixture (P<0.001). There was a time-by-amino acid mixture interaction for cTTR Leucine (P<0.001).
Fig 2.
Response in Phenylalanine TTR (cTTR Phenylalanine 5, panel a), Tyrosine TTR (cTTR Tyrosine 2, panel b), Leucine TTR (cTTR Leucine 3, panel c), and Arginine (cTTR Arginine 2, panel d) corrected for baseline in the CF group before and after intake of the TAA and EAA mixture (t=180 min). cTTR Phenylalanine 5: Time effect (P<0.001), cTTR Tyrosine 2: Time and Amino acid mixture effect (P<0.001), cTTR Leucine 3: Time and Amino acid mixture effect (P<0.001), and Time by Amino acid mixture interaction (P<0.001), cTTR Arginine 2: Time effect (P<0.001). Significance of difference between the EAA and TAA mixture: *: P<0.05; **: P<0.01; ***: P<0.001
Metabolic response to intake of the EAA vs. TAA mixture
Phenylalanine, Leucine, Arginine, EAA, and TAA concentration
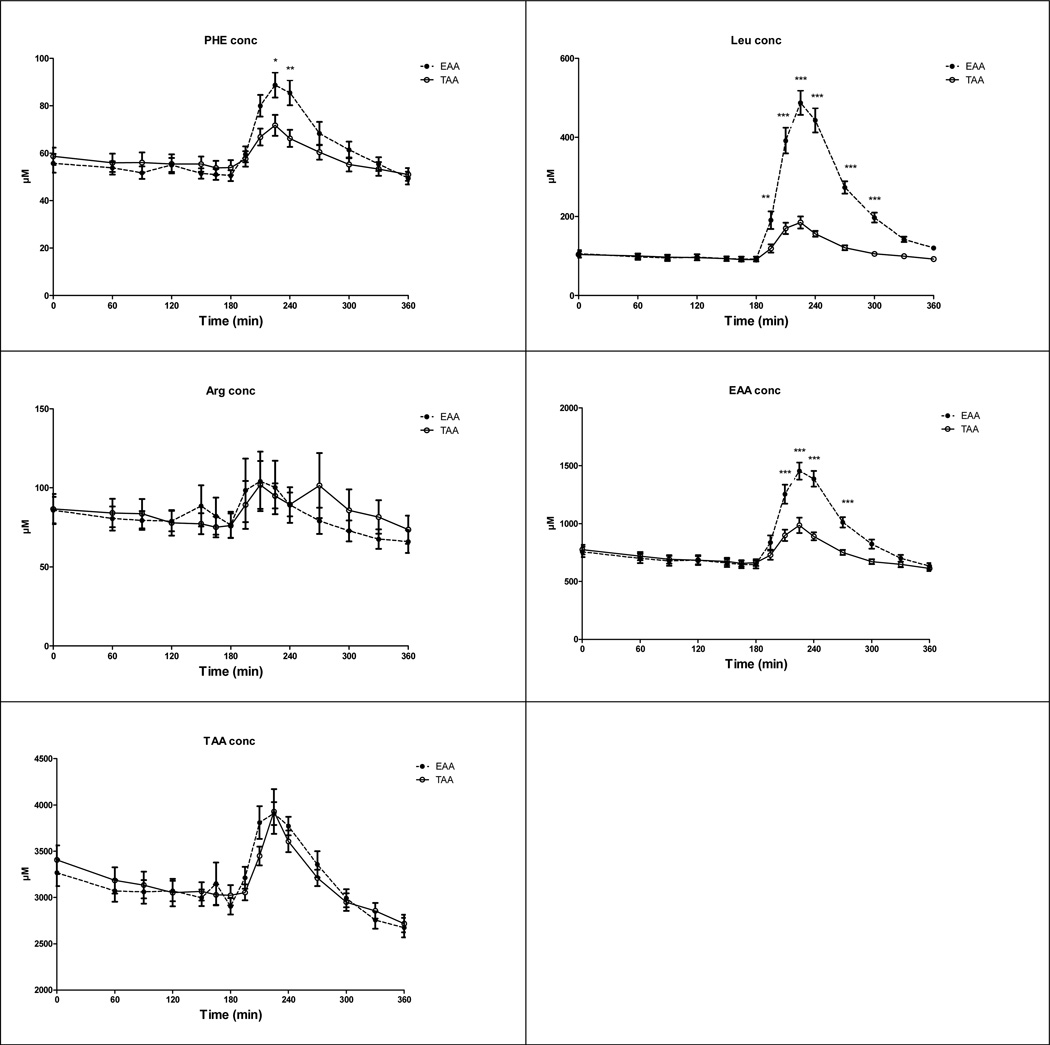
There were time effects for Phenylalanine, Leucine, EAA and sumAA (Fig 3) (P<0.001), indicating increased plasma concentrations after intake of both mixtures. The amino acid mixture effects for the concentration of Phenylalanine, Leucine, and EAA (P<0.001) indicated higher concentrations after intake of EAA mixture. There were time-by-amino acid mixture interaction for the concentration of Phenylalanine (P<0.01), Leucine, and EAA (P<0.001) but no time or amino acid mixture effect for Arginine.
Fig 3.
Response in Phenylalanine (panel a), Leucine (panel b), Arginine (panel c), EAA (panel d) and TAA (panel e) concentration in the CF group before and after intake of the TAA and EAA mixture (t=180 min). Phenylalanine conc: Time (P<0.001) and Amino acid mixture effect (P<0.05), Time by Amino acid mixture interaction (P<0.01); Leucine conc: Time and Amino acid mixture effect (P<0.001), and Time by Amino acid mixture interaction (P<0.001); EAA conc: Time and Amino acid mixture effect (P<0.001), and Time by Amino acid mixture interaction (P<0.001); TAA conc: Time effect (P<0.001). Significance of difference between the EAA and TAA mixture: *: P<0.05; **: P<0.01; ***: P<0.001
Postabsorptive vs. prandial WbPS and WbPB (expressed in nmol/kg FFM/h)
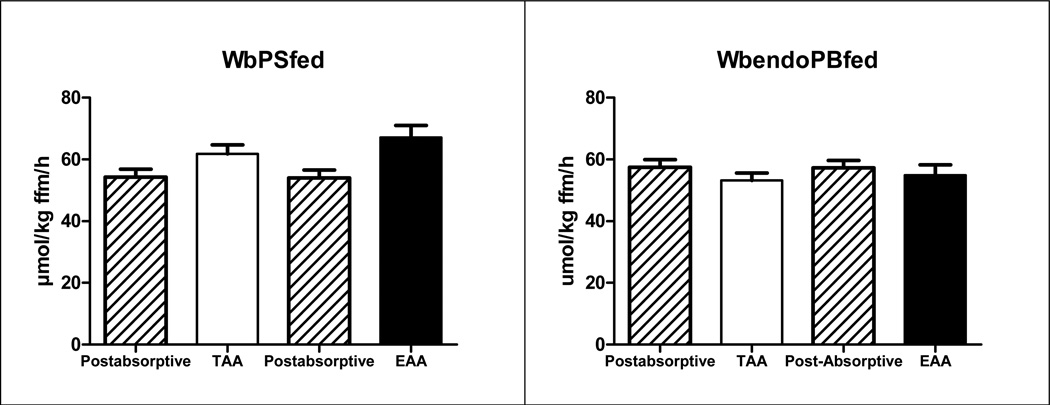
The time effects for both WbPS and WbPB (Fig 4) (P<0.05) indicated an increase in WbPS and a reduction in WbPB after intake of both mixtures. There was a time-by-amino acid mixture interaction for WbPS (P<0.05).
Fig 4.
Whole body protein synthesis (=PS, panel a) and breakdown (PB, panel b) in the CF group before and after intake of the TAA and EAA mixture. Mean values ± SE and are expressed in nmol/kg FFM/h. WbPS: Time effect (P<0.05), and Time by Amino acid mixture interaction (P<0.05), WbPB: Time effect (P<0.05)
Whole body protein and arginine kinetics (expressed in nmol/kg FFM/3h)
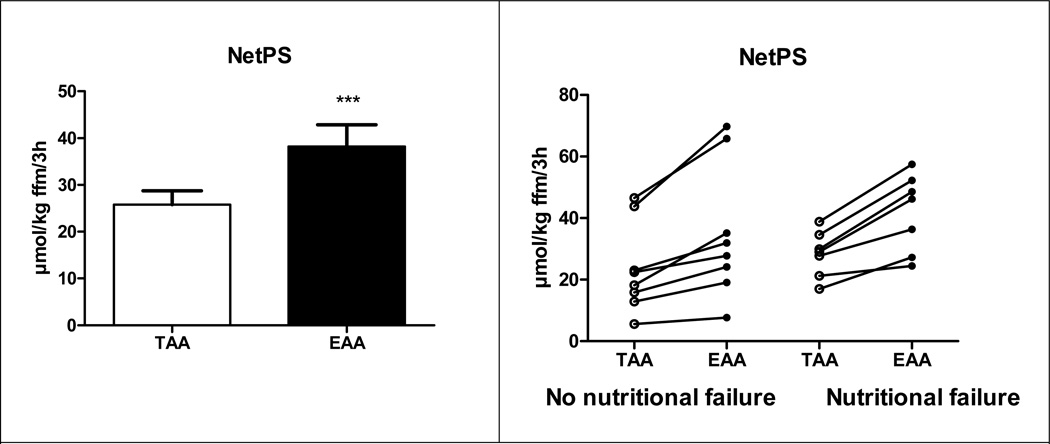
WbPS (P<0.01) and Leucine Ra (P<0.001) were higher, and Arginine Ra (P<0.001) was lower after intake of the EAA mixture (Table 4, Fig 5). Splanchnic extraction of Phenylalanine tended to be lower after EAA intake (P=0.06). No differences were found in WbPB, Citrulline Ra and Q Arginine to Citrulline (=NO synthesis rate). Q Citrulline to Arginine (= de novo Arginine production, P=0.09) tended to be lower. NetPS (Fig 5) was higher after intake of the EAA mixture (P<0.001)in all individual CF subjects, and comparable values were found in patients with and without nutritional failure (increase of 47.6 vs. 49.3%, respectively).
Table 4.
Protein and Arginine kinetics in the CF group after intake of the EAA and TAA mixture
| TAA | EAA | |||||
|---|---|---|---|---|---|---|
| Protein synthesis | 185.4 | ± | 8.7 | 197.1 | ± | 11.6** |
| Protein breakdown | 159.6 | ± | 7.0 | 171.1 | ± | 10.7 |
| Net Protein synthesis | 25.8 | ± | 3.0 | 38.2 | ± | 4.6*** |
| Splanchnic extraction | 0.50 | ± | 0.04 | 0.47 | ± | 0.05p=0.06 |
| WbRa Leucine | 372.8 | ± | 17.7 | 645.5 | ± | 41.6*** |
| WbRa Arginine | 439.7 | ± | 33.3 | 394.5 | ± | 31.4*** |
| WbRa Citrulline | 47.8 | ± | 3.8 | 44.8 | ± | 3.0 |
| Q Citrulline to Arginine | 52.2 | ± | 5.0 | 47.1 | ± | 4.1p=0.09 |
| Q Arginine to Citrulline | 1.3 | ± | 0.3 | 0.9 | ± | 0.2 |
Values are means ± SEM and expressed in nmol/kg ffm/3h. CF: Cystic Fibrosis, WbRa: Whole body rate of appearance, Q Citrulline to Arginine: de novo arginine synthesis rate, Q Arginine to Citrulline: Nitric oxide production rate. Significance of difference after intake of the total amino acid mixture (TAA) and the essential amino acid mixture (EAA):
: P < 0.01,
: P<0.001.
Fig 5.
Net Protein synthesis (=PS-PB, panel a) in total group of patients with CF, and the individual values for Net Protein synthesis in CF patients with and without nutritional failure (panel b) after intake of the TAA and EAA mixture. Mean values ± SE (expressed in nmol/kg FFM/3h) are shown for panel a. Significance of difference as compared to the TAA mixture (***: P<0.001).
Relationship between NetPS and total amino acid and phenylalanine intake
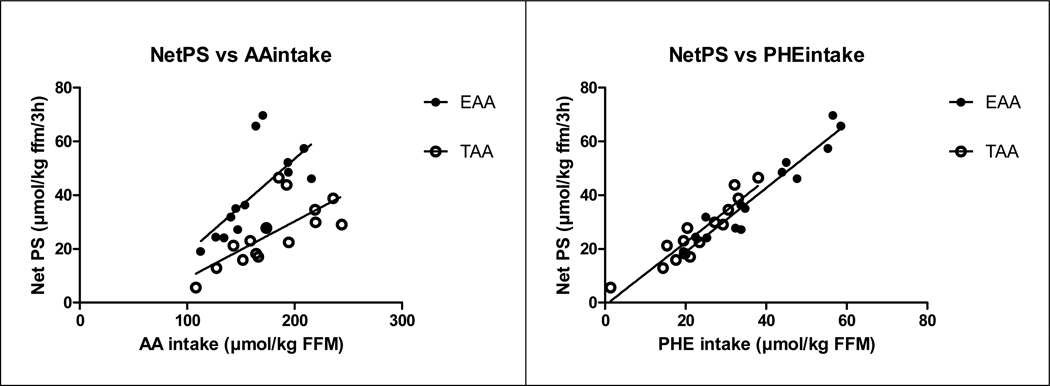
Significant relationships between NetPS (in nmol/kg FFM/3h) and total amino acid intake for the EAA mixture (r:0.68, P<0.01) and for the TAA mixture (Fig 6) (r:0.73, P<0.01) were found. There was a significant correlation between NetPS and Phenylalanine intake after correction for splanchnic extraction for the EAA (r:0.96, P<0.001) and TAA mixture (r:0.93, P<0.001).
Fig 6.
Relationship between NetPS (expressed in nmol/kg FFM/3h) and AA intake (panel a) and between NetPS and Phenylalanine AA intake after correction for splanchnic extraction(cPhenylalanine intake, panel b) in the CF group after intake of the TAA (open circles) and EAA (closed circles) mixture.
NetPS vs. AA intake: EAA: R2: 0.46, P<0.01, NetPS = (0.35 * AA intake) − 16.5; TAA: R2: 0.53, P<0.01, NetPS = (0.21 * AA intake) − 12.0.
NetPS vs. cPhenylalanine intake: EAA: R2: 0.92, P<0.001, NetPS = (1.19 * cPhenylalanine intake) − 5.0; TAA: R2: 0.87, P<0.001, NetPS = (1.17 * cPhenylalanine intake) − 1.0
DISCUSSION
In the present study, intake of an EAA mixture stimulated protein anabolism to a greater extent than a standard balanced mixture of all essential and non-essential amino acids in pediatric subjects with CF, which supports the concept of EAA as an anabolic stimulus in CF.
Multiple factors like reduced anorexia, inflammation, and hormonal changes are present in CF patients and contribute to loss of lean tissue (6, 20, 21). Conventional nutritional supplementation is often ineffective in stimulating protein anabolism in CF as it did not lead to sufficient muscle gain (4). We have previously demonstrated a strong theoretical rationale for the use of amino acid supplements in clinical populations suffering from muscle wasting. Amino acids mixtures are capable of acutely stimulating protein synthesis while not increasing urea production (22) and they do not require digestion by the gut.
Stimulation of protein synthesis by EAA but no further reduction in protein breakdown
In the present study, 6.7g of total amino acids was able to induce protein anabolism in CF children, indicating that balanced amino acid mixtures as present in high quality milk proteins are able to stimulate WbPS in CF pediatric subjects. A previous CF study illustrated that 1,000–1,500 kcal more than ad libitum intake increases WbPS only in the supplemented group during a pulmonary exacerbation (23). Furthermore, feeding very high protein diets (to 5g/kg/d) for 4 days was able to enhance WbPS to 30% in stunted CF children (24). However, high protein intake in combination with reduced protein digestion capacity in CF will lead to a high load of undigested proteins in the colon which results in diarrhea and the production of harmful toxins by gut bacteria (25).The EAA mixture was able to induce higher levels of WbPS in CF patients than a similar dose of a TAA mixture. Previously, it has been suggested that the high leucine content of a meal can effectively compensate for the blunted muscle protein synthetic response to food intake in the elderly (9). However, the relation between the amount of phenylalanine and the measured net protein synthesis inCF shows that the lines of the TAA and the EAA groups overlap. One would have expected that when leucine would add to the anabolic stimulation of the amino acid mixture, the line after EAA intake would be higher. Our data indicate that adding leucine to a maltodextrin-EAA mixture did not have additional anabolic properties in CF, which confirms previous studies in young adults showing that additional leucine did not improve the anabolic capabilities of whey protein (26). Our data therefore suggest that it is even possible to develop higher anabolic supplements in CF by reducing the leucine content of the 6.7g EAA mixture to the level as present in whey protein and to add more of the other essential amino acids.
Splanchnic extraction (SPE) of phenylalanine was around 48% in CF which is slightly higher than the SPE recently observed by us in healthy young subjects (39%) (27). Interestingly, SPE was lower after EAA than after TAA intake in the CF subjects. Previously we found in COPD that SPE decreased when branched-chain amino acids were added to soy protein (28) indicating that leucine, valine and isoleucine have a lowering effect on SPE resulting in enhanced amino acid availability in the circulation and/or reduced protein synthesis in the gut itself.
Both amino acid mixtures were able to reduce WbPB in CF which is remarkable as previous studies showed no or only a marginal suppression of protein breakdown with protein feeding in CF patients as compared to healthy controls (5). Feeding of high protein diets for 4 days (5g/kg/d) was not able to reduce WbPB in stunted CF children (24). It has been suggested that the attenuated suppression of WbPB by feeding in CF is related to defective insulin secretion due to pancreatic insufficiency. However in all published studies in CF, endogenous PB was not measured like in our study, as it requires the addition of oral isotopes to be able to measure SPE. We observed that endogenous WbPB suppression in CF is independent of the type of free amino acid mixture ingested.
Relationship net protein synthesis and AA intake
We observed a direct relation between net protein synthesis and the total amount of amino acid supplement/kg fat-free mass delivered to the peripheral compartment which is in line with our previous study showing a strong relationship between amino acid delivery to muscle and muscle protein synthesis (29). As the oral amino acid dose was identical in all subjects and SPE not different between CF patients with and without nutritional failure (data not shown), the highest amino acid delivery in CF was to those with low fat-free mass. The higher the line indicated the more anabolic/gram amino acid mixture, and there was no maximal threshold in net anabolic response in CF. Furthermore, as phenylalanine is one of the EAA, the amount of EAA intake is also directly related to net protein synthesis in children with CF. Therefore, these data suggest that the amount of EAA available to the peripheral compartment determines the anabolic effect of an amino acid supplement in CF, and that the high leucine component of the EAA mixture may not be needed in this circumstance.
Effects EAA mixture on arginine / NO metabolism
We examined whether an EAA mixture that was able to induce a positive protein balance was associated with a reduced stimulation of Arginine appearance in plasma and NO synthesis. This response might be expected since, in contrast to the TAA, the EAA mixture contained no arginine. We found that Arginine rate of appearance was significantly lower after intake of the EAA mixture, however no differences were found in NO synthesis rate. Recently we have shown that the response of Arginine appearance and NO synthesis can be significantly altered during meal intake in critically ill children by changing the composition of the meal (De Betue unpublished data). In these patients, increased values for Arginine production and increased NO synthesis were found using a protein-energy enriched as compared to a standard formula, indicating that a well-balanced protein-energy enriched formula may be an effective way to improve Arginine production and NO synthesis. Incorporation of arginine or citrulline (as precursor of Arginine) into the EAA mixture might therefore be a way to increase arginine production in CF.
In conclusion, formulation of a high anabolic free amino acid supplement is clinically relevant for CF patients, as protein maldigestion is present in CF patients with pancreatic insufficiency (30). Furthermore free EAA are preferred above protein intake as only the EAA and not the non-essential amino acids in proteins are needed to induce a high anabolic response which is of importance particularly when patients with CF have a reduced appetite (20). The present study shows that EAA modulation appears to be a very effective dietary strategy to augment protein anabolism to food intake in stable children with CF. The high leucine component of the EAA mixture may not be needed in CF and could be replaced by arginine (or it’s precursor citrulline) which might be advantageous in terms of arginine metabolism. Future studies are warranted to investigate whether incorporation of chronic EAA intervention in the treatment program prevents or ameliorates muscle loss of CF patients, and benefits quality of life and outcome.
Supplementary Material
Table 2.
Infusion rates of stable isotopes
| Isotope | Priming (µmol/kg BW) |
Infusion rate (µmol/kg BW/h) |
|---|---|---|
| L-[guanidine-15N2]arginine | 3.75 | 3.75 |
| L-[ureido-15N-2H2]citrulline | 0.88 | 0.30 |
| L-[ring-2H5]-phenylalanine | 3.60 | 3.60 |
| L-[ring-2H2]-tyrosine | 1.14 | 1.14 |
| L-[ring-2H4]-tyrosine | 0.31 | - |
| L-[ring-2H3]-leucine | 3.60 | 3.60 |
Primed, constant, and continuous infusion of the stable was done to measure whole body rate of appearance of Arginine, Citrulline, Phenylalanine, Tyrosine and Leucine as well as the conversion of Phenylalanine to Tyrosine, Arginine to Citrulline (Nitric Oxide production) and Citrulline to Arginine (de novo Arginine synthesis). The stable isotopes were purchased from Cambridge Isotopic Laboratories (Woburn, MA).
Acknowledgement
Support was provided by the Arkansas Children’s Hospital Research Institute and the Arkansas Biosciences Institute, a partnership of scientists from Arkansas Children’s Hospital, Arkansas State University, the University of Arkansas for Medical Sciences, the University of Arkansas Division of Agriculture, and the University of Arkansas, Fayetteville. The Arkansas Biosciences Institute is the major research component of the Tobacco Settlement Proceeds Act of 2000. The project was also supported by NIH S10RR027047. Ajinomoto Co provided the amino acids and carbohydrate as an unrestricted gift. The funding agencies had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Abbreviations
- BMI
body mass index
- CF
Cystic Fibrosis
- cTTR
tracer-tracee ratios of amino acids corrected for their baseline value
- DXA
dual-energy X-ray absorptiometry
- EAA
essential amino acids
- FEV1
forced expiratory volume in one second
- FFM
fat-free mass
- FM
fat mass
- FVC
forced vital capacity
- NetPS
net protein synthesis
- NO
nitric oxide
- Q Arginine to Citrulline
nitric oxide production
- Q Citrulline to Arginine
de novo Arginine production
- Ra
rate of appearance
- SE
standard error
- TAA
balanced mixture of essential and non-essential amino acids
- TTR
tracer tracee ratio
- WbPB
whole body protein breakdown
- WbPS
whole body protein synthesis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors have no financial or other forms of conflicts of interest.
References
- 1.Engelen MP, Schroder R, Van der Hoorn K, Deutz NE, Com G. Use of body mass index percentile to identify fat-free mass depletion in children with cystic fibrosis. Clinical nutrition (Edinburgh, Scotland) 2012 May 17; doi: 10.1016/j.clnu.2012.04.012. PubMed PMID: 22607714. Epub 2012/05/23. Eng. [DOI] [PubMed] [Google Scholar]
- 2.McNaughton SA, Shepherd RW, Greer RG, Cleghorn GJ, Thomas BJ. Nutritional status of children with cystic fibrosis measured by total body potassium as a marker of body cell mass: lack of sensitivity of anthropometric measures. The Journal of pediatrics. 2000 Feb;136(2):188–194. doi: 10.1016/s0022-3476(00)70100-4. PubMed PMID: 10657824. Epub 2000/02/05. eng. [DOI] [PubMed] [Google Scholar]
- 3.Kalnins D, Corey M, Ellis L, Pencharz PB, Tullis E, Durie PR. Failure of conventional strategies to improve nutritional status in malnourished adolescents and adults with cystic fibrosis. The Journal of pediatrics. 2005 Sep;147(3):399–401. doi: 10.1016/j.jpeds.2005.06.038. PubMed PMID: 16182685. [DOI] [PubMed] [Google Scholar]
- 4.Poustie VJ, Russell JE, Watling RM, Ashby D, Smyth RL Group CTC. Oral protein energy supplements for children with cystic fibrosis: CALICO multicentre randomised controlled trial. BMJ. 2006 Mar 18;332(7542):632–636. doi: 10.1136/bmj.38737.600880.AE. PubMed PMID: 16467348. Pubmed Central PMCID: 1403226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kien CL, Zipf WB, Horswill CA, Denne SC, McCoy KS, O'Dorisio TM. Effects of feeding on protein turnover in healthy children and in children with cystic fibrosis. Am J Clin Nutr. 1996 Oct;64(4):608–614. doi: 10.1093/ajcn/64.4.608. PubMed PMID: 8839507. eng. [DOI] [PubMed] [Google Scholar]
- 6.Ionescu AA, Nixon LS, Evans WD, Stone MD, Lewis-Jenkins V, Chatham K, et al. Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med. 2000 Sep;162(3 Pt 1):789–794. doi: 10.1164/ajrccm.162.3.9910118. PubMed PMID: 10988084. eng. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002 Oct;132(10):3219S–3224S. doi: 10.1093/jn/131.10.3219S. PubMed PMID: 12368421. eng. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Experimental gerontology. 2006 Feb;41(2):215–219. doi: 10.1016/j.exger.2005.10.006. PubMed PMID: 16310330. eng. [DOI] [PubMed] [Google Scholar]
- 9.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American journal of physiology. 2006 Aug;291(2):E381–E387. doi: 10.1152/ajpendo.00488.2005. PubMed PMID: 16507602. eng. [DOI] [PubMed] [Google Scholar]
- 10.Newsholme P, Brennan L, Rubi B, Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin Sci (Lond) 2005 Mar;108(3):185–194. doi: 10.1042/CS20040290. PubMed PMID: 15544573. eng. [DOI] [PubMed] [Google Scholar]
- 11.Moran A, Milla C, Ducret R, Nair KS. Protein metabolism in clinically stable adult cystic fibrosis patients with abnormal glucose tolerance. Diabetes. 2001 Jun;50(6):1336–1343. doi: 10.2337/diabetes.50.6.1336. PubMed PMID: 11375334. eng. [DOI] [PubMed] [Google Scholar]
- 12.Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatric pulmonology. 1997 Sep;24(3):173–177. doi: 10.1002/(sici)1099-0496(199709)24:3<173::aid-ppul2>3.0.co;2-o. PubMed PMID: 9330413. Epub 1997/10/23 22:20. eng. [DOI] [PubMed] [Google Scholar]
- 13.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002 Sep;35(3):246–259. doi: 10.1097/00005176-200209000-00004. PubMed PMID: 12352509. Epub 2002/09/28. eng. [DOI] [PubMed] [Google Scholar]
- 14.Van Itallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990 Dec;52(6):953–959. doi: 10.1093/ajcn/52.6.953. PubMed PMID: 2239792. Epub 1990/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 15.Demerath EW, Schubert CM, Maynard LM, Sun SS, Chumlea WC, Pickoff A, et al. Do changes in body mass index percentile reflect changes in body composition in children? Data from the Fels Longitudinal Study. Pediatrics. 2006 Mar;117(3):e487–e495. doi: 10.1542/peds.2005-0572. PubMed PMID: 16510627. Epub 2006/03/03. eng. [DOI] [PubMed] [Google Scholar]
- 16.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. The Journal of clinical endocrinology and metabolism. 2007 Jun;92(6):2087–2099. doi: 10.1210/jc.2006-2553. PubMed PMID: 17311856. Epub 2007/02/22. eng. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatric pulmonology. 1993 Feb;15(2):75–88. doi: 10.1002/ppul.1950150204. PubMed PMID: 8474788. Epub 1993/02/01. eng. [DOI] [PubMed] [Google Scholar]
- 18.Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism: clinical and experimental. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. PubMed PMID: 7022111. eng. [DOI] [PubMed] [Google Scholar]
- 19.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009 Jan;89(1):142–152. doi: 10.3945/ajcn.2007.25765. PubMed PMID: 19056593. [DOI] [PubMed] [Google Scholar]
- 20.Ahme ML, Ong KK, Thomson AH, Dunger DB. Reduced gains in fat and fat-free mass, and elevated leptin levels in children and adolescents with cystic fibrosis. Acta Paediatr. 2004 Sep;93(9):1185–1191. PubMed PMID: 15384881. Epub 2004/09/24. eng. [PubMed] [Google Scholar]
- 21.Sermet-Gaudelus I, Souberbielle JC, Azhar I, Ruiz JC, Magnine P, Colomb V, et al. Insulin-like growth factor I correlates with lean body mass in cystic fibrosis patients. Archives of disease in childhood. 2003 Nov;88(11):956–961. doi: 10.1136/adc.88.11.956. PubMed PMID: 14612353. Pubmed Central PMCID: 1719365. Epub 2003/11/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. American journal of physiology. 2005 Apr;288(4):E761–E767. doi: 10.1152/ajpendo.00291.2004. PubMed PMID: 15572657. eng. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd RW, Holt TL, Cleghorn G, Ward LC, Isles A, Francis P. Short-term nutritional supplementation during management of pulmonary exacerbations in cystic fibrosis: a controlled study, including effects of protein turnover. Am J Clin Nutr. 1988 Aug;48(2):235–239. doi: 10.1093/ajcn/48.2.235. PubMed PMID: 3136639. eng. [DOI] [PubMed] [Google Scholar]
- 24.Geukers VG, Oudshoorn JH, Taminiau JA, van der Ent CK, Schilte P, Ruiter AF, et al. Short-term protein intake and stimulation of protein synthesis in stunted children with cystic fibrosis. Am J Clin Nutr. 2005 Mar;81(3):605–610. doi: 10.1093/ajcn/81.3.605. PubMed PMID: 15755829. eng. [DOI] [PubMed] [Google Scholar]
- 25.Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy. 2007 Jun;27(6):910–920. doi: 10.1592/phco.27.6.910. PubMed PMID: 17542772. [DOI] [PubMed] [Google Scholar]
- 26.Tipton KD, Elliott TA, Ferrando AA, Aarsland AA, Wolfe RR. Stimulation of muscle anabolism by resistance exercise and ingestion of leucine plus protein. Appl Physiol Nutr Metab. 2009 Apr;34(2):151–161. doi: 10.1139/H09-006. PubMed PMID: 19370045. [DOI] [PubMed] [Google Scholar]
- 27.Engelen MP, De Castro CL, Rutten EP, Wouters EF, Schols AM, Deutz NE. Enhanced anabolic response to milk protein sip feeding in elderly subjects with COPD is associated with a reduced splanchnic extraction of multiple amino acids. Clinical nutrition (Edinburgh, Scotland) 2012 Jun 6; doi: 10.1016/j.clnu.2012.04.006. PubMed PMID: 22682082. Epub 2012/06/12. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007 Feb;85(2):431–439. doi: 10.1093/ajcn/85.2.431. PubMed PMID: 17284740. eng. [DOI] [PubMed] [Google Scholar]
- 29.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. The American journal of physiology. 1996 Apr;270(4 Pt 1):E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. PubMed PMID: 8928769. Epub 1996/04/01. eng. [DOI] [PubMed] [Google Scholar]
- 30.Engelen MP, Com G, Anderson PJ, Thaden JJ, Aarts N, Deutz NEP. Novel method to measure fat and protein digestion rate and the acute response to pancreatic enzyme intake in pediatric and adult patients with cystic fibrosis. Pediatric pulmonology. 2012;47(S35):410–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.