Abstract
Inflammatory demyelinating diseases like multiple sclerosis are characterized by mononuclear cell infiltration into the central nervous system. The glycosaminoglycan hyaluronan and its receptor, CD44, are implicated in the initiation and progression of a mouse model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE). Digestion of hyaluronan tethered to brain vascular endothelial cells by a hyaluronidase blocks the slow rolling of lymphocytes along activated brain vascular endothelial cells and delays the onset of EAE. These effects could be due to the elimination of hyaluronan or the generation of hyaluronan digestion products that influence lymphocytes or endothelial cells. Here, we found that hyaluronan dodecasaccharides impaired activated lymphocyte slow rolling on brain vascular endothelial cells when applied to lymphocytes but not to the endothelial cells. The effects of hyaluronan dodecasaccharides on lymphocyte rolling were independent of CD44 and a receptor for degraded hyaluronan, toll-like receptor-4. Subcutaneous injection of hyaluronan dodecasaccharides or tetrasaccharides delayed the onset of EAE in a manner similar to subcutaneous injection of hyaluronidase. Hyaluronan oligosaccharides can therefore act directly on lymphocytes to modulate the onset of inflammatory demyelinating disease.
Keywords: CD44, Toll-like receptor, endothelial cell, hyaluronan, myelin
1. Introduction
Multiple Sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE) are autoimmune inflammatory diseases characterized by the infiltration of myelin-reactive lymphocytes into the central nervous system (CNS) (Kuerten and Lehmann, 2011; Nylander and Hafler, 2009). These encephalitogenic cells, particularly CD4+ T-cells in the case of EAE, cause demyelination, axonal damage and both glial cell and neuronal death (Goverman, 2009). Impairing lymphocyte extravasation across CNS vascular endothelium delays disease progression and reduces disease severity (Yednock et al., 1992; Baron et al., 1993; Polman et al., 2006). However, our understanding of the molecular mechanisms underlying this process is incomplete and the molecules involved have been disputed (Kerfoot et al., 2006; Doring et al., 2007).
One molecule implicated in inflammatory demyelinating lesions is the glycosaminoglycan hyaluronan (HA). HA is composed of repeating units of D-glucuronic acid and N-acetyl-D-glucosamine. In mammals, it is synthesized at the inner leaflet of the cell membrane by one of three synthases (HAS1–3) that extrude HA into the extracellular matrix as a linear, non-sulfated molecule reaching sizes in excess of 107 Da. During inflammation, HAS expression increases, resulting in local accumulation of high molecular weight (HMW) HA (Kennedy et al., 2000; Tammi et al., 2005). This HMW HA is typically digested by hyaluronidases and degraded by reactive oxygen species, resulting in the accumulation of HA fragments within inflammatory microenvironments (Sampson et al., 1992; Girish and Kemparaju, 2007; Noble, 2002).
Both HMW HA and HA fragments (including HA oligosaccharides) generated in inflammatory microenvironments signal through transmembrane HA receptors. CD44 is an HA receptor that is expressed by many cell types, including CNS vascular endothelial cells (ECs) and lymphocytes (Pure and Cuff, 2001; Lokeshwar et al., 1996; Ponta et al., 2003). CD44 promotes EAE pathogenesis by facilitating lymphocyte extravasation (Brennan et al., 1999; Brocke et al., 1999; Laman et al., 1998). This role depends at least in part on lymphocyte interactions with HMW HA tethered to endothelial cells by CD44 to promote lymphocyte capture, rolling and adhesion (DeGrendele et al., 1996; DeGrendele et al., 1997; Nandi et al., 2004; Winkler et al., 2012). In the CNS, these interactions occur through a mechanism that is independent of CD44 on lymphocytes (Winkler et al., 2012). Disrupting these interactions by digestion of vascular HA with a hyaluronidase leads to delayed disease onset and reduced demyelination in mice with EAE.
Because HA synthases and hyaluronidases are simultaneously active in inflammatory lesions, it is possible that both HMW HA and fragments of HA generated in the vasculature around areas of CNS inflammation could contribute to the onset and progression of inflammatory demyelinating disease. HA oligosaccharides (including molecules of sizes predicted to be generated by hyaluronidases) are capable of interfering with CD44-HA interactions via several mechanisms. These include CD44 receptor cleavage (Sugahara et al., 2003), displacement of bound HMW HA (Tammi et al., 1998) or competitive binding to CD44 (Teriete et al., 2004). Conceivably, loss of CD44 or displacement of HMW HA by either endogenous or added hyaluronidases would result in decreased receptor aggregation, diminished cell-cell adhesion interactions, and altered intracellular signaling. These effects, singly or in combination, may interrupt activated lymphocyte rolling and adhesion in the CNS.
Another way that digestion of HMW HA by hyaluronidases could influence inflammatory demyelination is through the activation of Toll-like receptors (Taylor et al., 2004; Jiang et al., 2005; Scheibner et al., 2006). Toll-like receptors (TLRs) are a family of pattern-recognition receptors involved in microbial recognition by the immune system (Barton and Medzhitov, 2003). HA oligosaccharide-mediated signaling through TLRs is generally considered to be pro-inflammatory (Stern et al., 2006; Jiang et al., 2007), however their roles in HA-mediated CNS inflammation and lymphocyte extravasation have not been addressed. Naïve lymphatic-derived mouse CD4+ T cells express transcripts for TLR4 but not TLR2 (Gelman et al., 2004). Furthermore, TLR4 signaling inhibits T cell activation (Gonzalez-Navajas et al., 2010) and HA oligosacchardies can induce elevated CD44 and TLR4 expression (Campo et al., 2010; Campo et al., 2012). HA oligosaccharide-induced TLR4 signaling may therefore influence T-cell mediated inflammation during EAE progression leading to reduced extravasation of encephalitogenic cells.
In this study, we tested the ability of HA oligosaccharides to influence lymphocyte-EC interactions and the onset and progression of EAE. We found that dodecasaccharides of HA (HA12) impaired lymphocyte slow-rolling interactions with CNS ECs, however CD44 and TLR4 did not mediate this effect. HA12 also delayed the onset of EAE and transiently reduced disease burden. All together, our findings suggest that alternative HA receptors on lymphocytes signal in response to HA12, and that HA12 can modulate the onset and course of inflammatory demyelinating disease by interfering with lymphocyte-EC slow rolling.
2. Results
2.1 HA oligosaccharides impair activated lymphocyte rolling on CNS ECs
We previously determined that HA tethered to CD44 on the surface of CNS ECs facilitates lymphocyte rolling that contributes to the onset of EAE (Winkler et al., 2012). In this study, degradation of HA by a hyaluronidase delayed EAE onset. These effects may have resulted from the removal of HMW HA from the lumen of CNS blood vessels or the generation of low molecular weight digestion products of HA that have distinct biological activities on ECs or lymphocytes. In particular, HA dodecasaccharides (HA12) have a number of biological activities including influencing endothelial cell differentiation (Takahashi et al., 2005) and wound healing (David-Raoudi et al., 2008). We therefore tested if HA12 influences T cell rolling on ECs. We utilized an in vitro static adhesion assay where calcein-AM-loaded WT lymphocytes or CNS WT ECs grown as a monolayer were pre-treated with HA12 prior to interaction in a co-culture for one hour. Following two washes with culture medium, fluorescence intensity emitted by lymphocytes was significantly decreased in wells where lymphocytes had been treated with 50 μg/mL or 10 μg/mL HA12 (Fig. 1A). In contrast, no change in fluorescence intensity was observed in co-cultures where ECs had been pre-treated with HA12 (Fig. 1B). These findings demonstrate that HA12 impairs activated lymphocyte adherence through a lymphocyte-dependent mechanism.
Figure 1.
Pre-treatment of activated lymphocytes, not CNS ECs with HA12 disrupts lymphocyte adhesion. CD3/CD28 stimulated lymphocytes were loaded with calcein-AM dye for 15 min. Activated lymphocytes (A) or CNS ECs (B) were pre-incubated with various concentration of HA12 (50, 10 and 1 ug/mL) or PBS 30 minutes prior to a one hour co-culture. Fluorescence was measured at 538 nm after 2 washes with culture medium. * p<0.05, t- test.
Because CD44-HA interactions are proposed to be critical for lymphocyte rolling on ECs (DeGrendele et al., 1996), we utilized an in vitro parallel-plate physiologic flow assay to assess how HA oligosaccharides influence lymphocyte-EC interactions under flow conditions. Confirming our static adhesion assay results, HA12 treatment of lymphocytes prior to use in the flow assay significantly reduced the number of interacting cells compared to PBS controls (Fig. 2A vs. 2B). Quantification of the parallel plate assays revealed a 27.9% decrease in the number of interacting cells (Fig. 2C, *p<0.05 t-test). Using particle-tracking software, the average rolling speed of all cells was determined. Bins for slowest (<0.5 μm/sec), slow (0.5–1 μm/sec), medium (1–5 μm/sec) and fast (5+ μm/sec) rolling cells were generated and plotted against the mean number of interacting cells for both treatment groups (Fig. 2D). Within the slowest and slow groups, HA12 treatment significantly decreased the number of rolling lymphocytes (Fig. 2D, *p<0.05, t-test). HA12 treatment also trended toward increasing the number of fast rolling interacting cells although not significantly. Collectively, these data indicate that HA12 treatment interferes with the capture and slow rolling of lymphocytes on CNS ECs.
Figure 2.

Treating WT lymphocytes with HA12 impairs activated lymphocyte rolling on CNS ECs. Representative bright-field images of psuedo colored (black) of PBS treated WT lymphocytes (A) and HA12 treated WT lymphocytes bound to WT ECs at the end of a 7 minute experiment. (C) HA12 treated (30min, 10 ug/mL) WT lymphocytes had significantly fewer interactions with CNS endothelial cell than PBS treated controls. D) Treatment of WT lymphocytes with HA12 significantly decreased the number of slowest (<0.5 μm/sec) and slow (0.5–1 μm/sec) rolling cells compared to PBS control. Data from all experimental groups represent two independent experiments performed in triplicate. *p<0.05, t- test compared to WT lymphocytes+Vehicle.
2.2 The effect of HA12 on activated lymphocyte rolling is independent of CD44
CD44 on lymphocytes is dispensable for the adhesive interactions with high molecular weight HA tethered to CNS endothelial cells (Winkler et al., 2012). However, CD44 expressed by lymphocytes can mediate other aspects of lymphocyte interactions with ECs that could be altered by HA12 (Ponta et al., 2003). To address this possibility, CD44−/− lymphocytes were pretreated with HA12 or PBS prior to use in the parallel plate assay. Similar to WT lymphocytes, HA12 treatment of CD44−/− lymphocytes significantly reduced the number of interacting cells compared to controls (28.1%, Fig. 3A, *p<0.05 t-test). Furthermore, like WT cells, HA12 treatment significantly decreased the number of CD44−/− lymphocytes in the slowest rolling bin and trended toward an increase in the number of fast rolling cells (Fig. 3B, *p,0.05 t-test). Interestingly, the number of slow rolling cells was not significantly different between the groups; however, this may be accounted for by mild deficits in lymphocyte extravasation previously observed in the CD44−− phenotype (Protin et al., 1999). Overall, these data imply that the impaired slowest/slow rolling phenotype generated by HA12 treatment is not mediated by CD44 expressed by activated lymphocytes.
Figure 3.
HA12 impaired lymphocyte rolling phenotype is not altered in CD44−/− lymphocytes. (A) HA12 treated (30min, 10 ug/mL) CD44−/− lymphocytes had significantly fewer interactions with activated CNS ECs than PBS treated CD44−/− lymphocyte controls. D) Treatment of CD44−/− lymphocytes with HA12 significantly decreased the number of slowest (<0.5 μm/sec) rolling cells compared to PBS control. Data from all experimental groups represent two independent experiments performed in triplicate. *p<0.05, t- test compared to CD44−/− lymphocytes+Vehicle.
2.3 The effect of HA12 on activated lymphocyte rolling is independent of TLR4
HA oligosaccharides signal through TLR4, typically inducing expression of pro-inflammatory cytokines (Taylor et al., 2004; Shimada et al., 2008) although TLR4 signaling can also inhibit T-cell mediated inflammation (Gonzalez-Navajas et al., 2010). TLR4 mRNA transcripts in T-cells decrease with CD3/CD28 activation out to 24 hrs (Gelman et al., 2004; Gonzalez-Navajas et al., 2010). However, at the 72 hrs post-activation time-point utilized for our experiments, we found a modest induction of TLR4 transcripts in activated lymphocytes (Fig. 4). We therefore tested if the effects of HA12 on lymphocyte rolling are regulated by TLR4. We assayed activated lymphocyte recruitment and rolling in WT lymphocytes pre-treated with HA12 and a TLR4 neutralizing antibody (anti-TLR4) or an isotype control (anti-Iso) antibody. Additionally, we assayed TLR4−/− lymphocytes pretreated with HA12. HA12 treatment alone decreased the number of interacting lymphocytes as expected (45%, Fig. 5A). This effect was not altered by the TLR4 blocking antibody or an isotype control antibody (49% and 52% respectively Fig. 5A). Furthermore, activated TLR4−/− lymphocytes treated with HA12 had a 49% decrease in interacting cells relative to PBS controls (Fig. 5A). No significant difference in the number of interacting lymphocytes was observed in any of the HA12- treated groups (Fig. 5A). Similarly, no significant difference was observed in the number of interacting cells within speed bins between the HA12 only, HA12 + TLR4 blocking antibody, HA12 + isotype control antibody or HA12 + TLR4−/− lymphocyte treatment groups (Fig. 5B). These data indicate that the effects of HA12 on lymphocyte-EC interactions are not dependent on TLR4.
Figure 4.

TLR4 mRNA transcript is modestly increased at 72hr of activation in lymphocytes. qRT analysis of Tlr4 mRNA in WT lymphocytes stimulated with anti-CD3/CD28 for 0 and 72hr. There is a modest, but statistically insignificant increase in transcript at this time point.
Figure 5.
Blockage or knockout of TLR4 signaling does not alter HA12 mediated lymphocyte rolling impairment. A) WT lymphocytes treated with PBS (vehicle) interacted more frequently with activated CNS ECs than did WT lymphocytes treated with HA12 (10 ug/mL, 30 min) only, HA12+TLR4 blocking antibody (anti-TLR4, clone MTS510, 100 ng/mL, 10min prior to HA12), HA12+Isotype antibody (anti-Iso, Rat IgG2a,κ, 100ng/mL, 10min prior to HA12) or HA12 treated TLR4−/− lymphocytes. B) PBS treated lymphocytes has significantly more rolling cells in the slowest (<0.5 mm/sec) speed bin and the medium (1–5 mm/sec). However, no significance (NS) was observed in the number of rolling cells between any of the HA12 treated lymphocyte + treatment conditions in any speed bin. Data from all experimental groups represent one experiment performed in triplicate. *p<0.05, t- test compared to WT lymphocytes+HA12.
2.4 HA oligosaccharides delay the onset of EAE
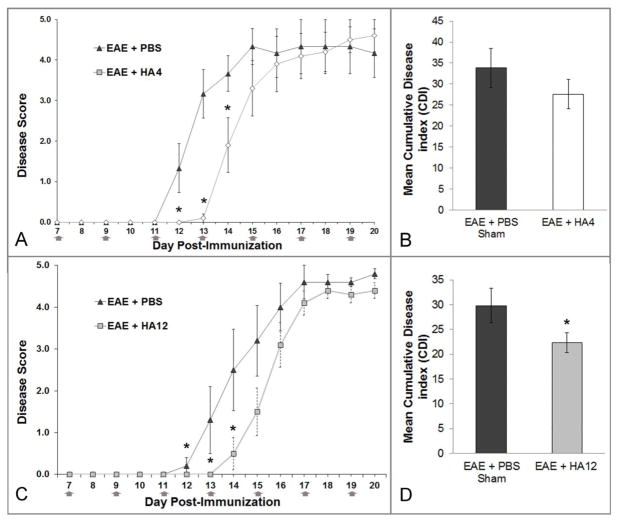
Given the effects of HA12 on lymphocyte-endothelial cell interactions in vitro, we tested if HA12 and another HA oligosaccharide, HA4, influenced EAE onset and progression. HA4, HA12 or PBS alone (vehicle control) were subcutaneously administered to mice every two days starting seven days after the induction of chronic EAE. Clinical scores were rated over a 20-day period in eight-week-old C57/Bl6 mice immunized with MOG35–55 to induce active EAE. Symptoms were evident in PBS-treated animals beginning 12 days post-inoculation with a mean onset on day 13 (Fig. 6A, 6C). In contrast animals treated with HA4 (Fig. 6A, 6B) or HA12 (Fig. 6C, 6D) on average had a two-day delay in disease onset, with a mean onset on day 15 (*p<0.05 repeated measures ANOVA). Although not statistically significant, mean daily EAE scores of HA12 treated animals after day 15 remained lower than PBS treated controls through the end of the experiment while animals treated with HA4 had disease scores that, on average, slightly exceeded controls by day 20 post-inoculation. Reinforcing this observation, the mean cumulative disease index (CDI) score of HA12 treated animals was significantly less than PBS controls (*p<0.05 t-test, Fig. 6B) but there was no significant difference in the CDIs of animals treated with HA4. These experiments were performed 3 times with similar results. Thus, both HA12 and HA4 delay the onset of EAE, but only HA12 results in an improved CDI.
Figure 6.
Subcutaneously injected HA 4 and HA12 delays the clinical onset of active EAE and ameliorates chronic disease burden. A–D) EAE was induced in 10 C57BL/6 female mice in two different experiments. For each experiment, half (n=5) of the animals were randomly selected to receive subcutaneous PBS (vehicle) injections (50 ul) and the other half, HA4 (A, B) or HA12 (C, D) (both at 50 mg/kg, in 50 ul PBS). Injections were given in the hind flank every other day beginning 7 days post inoculation (gray arrows). Mice were injected and scored by a blinded experimenter. HA4 (A) and HA12 (C) treated animals had delayed onset of disease by two days (*). Animals were perfused for histology 20 days post inoculation. *p<0.05, repeated measures ANOVA. B and D) Mean cumulative disease score was determined by summing individual daily scores for each animal and averaging across the group. HA4 (B) CDI scores were not significantly different than PBS control. However HA12 (D) treated animals had significantly lower CDI than control (*). *p<0.05 t-test.
2.5 HA oligosaccharides limit demyelination during EAE progression
Our findings that HA12-treated animals have delayed disease onset and less chronic disease burden are consistent with our previous observations using subcutaneous injections of a hyaluronidase (Winkler et al., 2012). Treatment with hyaluronidase resulted in reduced infiltration of CD3+ T cells at early times post-inoculation, but not at later times (e.g. by 20 days post-inoculation) and significantly reduced demyelination. Therefore, EAE lumbar spinal cord sections from HA12-treated animals with EAE were histologically assayed for myelin and infiltrating CD3+ T-cells. PBS-treated controls exhibited diffuse and robust demyelination as visualized by FluoroMyelin stain (Fig. 7A, 7B red). In contrast, HA12-treated animals had significantly less demyelination as determined by stereologic quantification (Fig. 7D, E and 7G). CD3+ cells were distributed fairly evenly across demyelinated areas in PBS controls (Fig. 7B, 7C). However, CD3+ T-cells were concentrated toward a presumably perivascular focal point within the lesions of HA12-treated animals (Fig. 7E, 7F). Interestingly, there was no significant difference in the mean total number of CD3+ T-cells between groups as assessed by stereology (Fig. 7H). These data are consistent with the hypothesis that subcutaneous treatment with HA12, like hyaluronidase treatment, transiently inhibits the extravasation of myelin-reactive T cells into the CNS therefore preventing demyelination.
Figure 7.
HA12 treated EAE animals have less diffuse demyelination and more concentrated sites of infiltration than PBS sham controls 20 days post inoculation. Representative ventral funicular lesions of lumbar spinal cord from PBS (vehicle, A–C) and HA12 treated (D–F) EAE animals taken on EAE day 20. Myelin FluoroMyelin) is shown as red, CD3+ T-cells are green and cell nuclei are stained blue (Hoechst). PBS-treated animals have more demyelination than PEG-PH20 treated animals as quantified by stereology in (G). No significant difference in CD3+ cell number is was quantified (H), however infiltrates are concentrated at presumably perivascular sites consistent with recent infiltration. Scale bar=100μm.* p<0.05, t- test.
3. Discussion
Digestion of HA tethered to CNS ECs in mice with EAE by a hyaluronidase is sufficient to delay the onset of disease and impair lymphocyte rolling (Winkler et al., 2012). HA digestion by hyaluronidases produces HA fragments, including oligosaccharides, that accumulate in areas of inflammation. We found that HA12 acts on lymphocytes to impair lymphocyte-EC interactions, and that subcutaneously injected HA12 delays EAE onset and reduces disease burden. Additionally, histological analysis revealed less demyelination in discrete spinal cord EAE lesions in HA12-treated animals 20 days post-immunization, consistent with the idea that HA12 treatment delays demyelinating disease blocking lymphocyte interactions with ECs.
Although our myelin staining and findings are consistent with the idea that HA12 delays lymphocyte extravasation, we did not find a significant difference in the number of infiltrating CD3+ T-cells at the day 20 time point. Our previous study indicated that digestion of HA leads to impaired lymphocyte extravasation with quantifiable differences in infiltrating T-cells at disease onset but not at later times of disease (Winkler et al., 2012). Therefore, the observation of similar numbers of infiltrating cells in the treatment group compared to controls could be accounted for by perivascular proliferation of T-cells consistent with newly active lesions (Serafini et al., 2006; Liu et al., 2007).
Our findings also demonstrated that HA12 treatment of activated lymphocytes but not CNS ECs is sufficient to impair lymphocyte interactions with CNS endothelia. This implies that an HA receptor competent to interact with HA oligosaccharides is mediating this process. CD44 and TLR4 are known HA oligosaccharide receptors expressed by lymphocytes; however our results indicated they are not involved in lymphocyte rolling and capture. Therefore the question remains: how is HA12 treatment of activated lymphocytes mediating this effect? It is possible that another HA receptor is assuming this function. The receptor for hyaluronan-mediated motility (RHAMM) is known to mediate cell adhesion and migration in both normal and pathological states (Sherman et al., 1994) and has been implicated in T-cell development and migration (Pilarski et al., 1993). RHAMM is expressed at the cell surface of lymphocytes and facilitates cytoskeletal re-arrangements that could influence T-cell extravasation (Turley et al., 2002). However, HA and HA oligosaccharides interact with RHAMM to promote leukocyte infiltration in an arthritis model (Nedvetzki et al., 2004), and macrophage recruitment following lung injury (Zaman et al., 2005). While not directly studied in an inflammatory CNS model, these data imply that RHAMM-HA interactions in immune cells are generally pro-inflammatory and would likely promote lymphocyte extravasation at the CNS endothelium. Nevertheless, examining the contribution of RHAMM to lymphocyte rolling on CNS ECs may provide useful insight into MS pathogenesis
Our finding that TLR4 signaling is not required for the effects of HA12 on lymphocyte rolling was surprising in light of several studies implicating TLR4 in the activities of HA oligosaccharides. TLR4 has been implicated in inhibiting activation of naïve T-cells and may be functionally expressed on circulating T-cells (Gonzalez-Navajas et al., 2010; Komai-Koma et al., 2004). HA oligosaccharides also signal through TLR2 which may have some over-lapping functions with TLR4 (Jiang et al., 2005; Shimada et al., 2008), although HA oligosaccharides in some studies functioned through TLR4 and not TLR2 (e.g. Termeer et al., 2002). Impairing signaling of both receptors could also be required to completely abolish HA oligosaccharide-induced activity (e.g. Jiang et al., 2005; Shimada et al., 2008). Therefore, if TLR signaling were involved in lymphocyte rolling at the CNS endothelium we would expect a modest phenotype when TLR4 signaling is impaired that is magnified when TLR2 signaling is abolished. Nonetheless, experiments impairing signaling through TLR2 either alone or in combination with TLR4 could further establish if TLR signaling is involved in the effects of HA12 on lymphocyte rolling.
Extracellular HA-binding proteins could also influence the effects of HA oligosaccharides on lymphocyte rolling. Tumor necrosis factor stimulated gene-6 (TSG-6), for example, is a secreted protein whose expression is up-regulated in many cell types including peripheral blood mononuclear cells in response to cytokines and growth factors (Milner et al., 2003). Its expression by lymphocytes is unknown; however a gene with significant sequence homology to TSG-6 was identified in lymphocytes in lampreys (Mayer et al., 2002). TSG-6 is principally known to rearrange components of the ECM through catalytic transfer with serine proteases (Jiang et al., 2011), but can also facilitate lymphocyte capture and rolling on ECs by promoting HA adhesion to the cell surface in vitro (Lesley et al., 2004). Furthermore, HA oligosaccharides (8–10mers) can competitively bind to TSG-6 displacing HMW species (Heng et al., 2008). This suggests that TSG-6 could function to support HA mediated adhesion with lymphocytes at the CNS vascular endothelium. Conversely, TSG-6 has been shown to prevent neutrophil infiltration into sites of inflammation during arthritis presumably by interfering with HA-CD44 interactions at the synovial endothelium (Szanto et al., 2004). Therefore HA oligosaccharide-induced expression of TSG-6 at sites of inflammation may also prevent immune cell extravasation.
Collectively, our data support the notion that digestion products of HWM HA species can delay the onset of CNS inflammatory disease and impair the rolling and subsequent extravasation of activated lymphocytes. These products likely mediate this effect through HA receptor interactions on lymphocytes, although the molecular mechanism remains unknown. We propose that HA degradation products produced during CNS inflammation by hyaluronidases or other mechanisms, may function as a molecular brake on lymphocyte extravasation in a concentration-dependent manner. As such, HA oligosaccharides represent a potential therapeutic tool aimed at slowing inflammatory demyelinating disease progression and reducing severity.
4. Experimental Procedures
4.1 Induction of EAE
EAE in 4–5-month-old female C56BL/6 mice was induced using mouse myelin oligodendrocyte glycoprotein, peptides 35–55 (MOG35–55), synthesized artificially by Peptides International (Louisville, KY, USA). MOG35–55 was emulsified with complete Freund’s adjuvant containing heat-inactivated mycobacterium tuberculosis as previously described (Tuohy et al., 2004). Female mice were used because they are more susceptible to EAE onset compared to male mice largely as a result of the protective effect of testosterone in male mice (Voskuhl and Palaszynski, 2001).
4.2 EAE scoring
Beginning one day after EAE induction, a blind experimenter assigned a clinical disease score daily as previously described (Winkler et al., 2012) until the end of the experiment (day 20). The following clinical disease scoring scale was used: 0, no symptoms; 1, tail-weakness (completely flaccid); 2, hindlimb weakness (animal can be easily flipped radially onto its back when grasped at base of tail); 3, animal walks with hind limbs splayed outwards; 4, one hindlimb partially or substantially paralyzed; 5, both hindlimbs completely paralyzed, no spastic movement; 6, moribund (animal is euthanized immediately). Increments of 0.5 were used for disease severity between indicated scores.
4.3 HA oligosaccharide administration in mice with EAE
Endotoxin-free HA 4 and HA12 are proprietary reagents generously provided by Glycoscience Laboratories, Inc. (Tokyo, Japan) and Halyozyme Therapeutics Inc. (San Diego, CA, USA) respectively. Aliquots of HA oligosaccharide stock solutions were prepared the same day that they were used. HA 4 and HA 12 oligosaccharide aliquots were diluted in phosphate-buffered saline (PBS) to a concentration of 20 mg/mL and sterilized by filtration. Mice were randomly assigned to two groups to receive injections every other day of either 50 μl subcutaneous sterile PBS (vehicle control) or 50 μl subcutaneous HA12 into the lumbar spine area. Injections began seven days after immunization and continued every-other until 20 days post-immunization. Eight animals were in each treatment group, and each experiment was performed a total of three times. Effective in vivo HA4 and HA12 treatment concentrations were determined by titration in a small number of EAE animals (data not shown).
4.4 Immunohistochemistry and Stereology
Mice were euthanized and perfused transcardially with heparin saline followed by 4% paraformaldehyde as previously described (Winkler et al., 2012). Lumbar spinal cords were removed, freeze-embedded and serially-sectioned at a thickness of 10 μm on a cryostat (Leica, Wetzler, Germany) and placed on glass slides. Sections were washed, blocked and permeabilized according as described (Winkler et al., 2012). A primary antibody against CD3 (1:150, AbD Serotec, Raleigh, NC, USA) and a secondary goat anti-rat Alexa 488 antibody (1:1000, Invitrogen, Grand Island, NY, USA) were used to visualize infiltrating T-cells. FluoroMyelin (1:300, Invitrogen) was used to visualize myelin and Hoechst 33342 (1:5000, Invitrogen) was used to label cell nuclei. Sections were mounted with Prolong Gold mounting media (Invitrogen) then imaged using a Zeiss Axiovert 200M (Zeiss, Oberkochen, Germany) fluorescence microscope interfaced with a Marianas Digital Microscopy Workstation (Intelligent Imaging Innovation Inc., Denver, CO, USA ). Twelve sections of the lumbar spinal cord montages were analyzed from each animal (n=5 per group). Stereologic analysis of demyelination and CD3+ cell counts was performed using SlideBook™ software as previously described (Winkler et al., 2012).
4.5 Splenocyte culture and lymphocyte isolation
Splenocytes from wild type (WT; C57BL/6), CD44−/− (Jackson Labs, Bar Harbor, ME, USA) and TLR4−/− mice (Jackson Labs) were cultured in T75 flasks coated with anti-CD3 and anti-CD28 (eBioscience, San Diego, CA, USA) antibodies for 72 hr to induce T-cell-specific activation and clonal expansion. Lymphocytes were harvested using a Lympholyte® (Sigma, St. Louis, MO, USA) gradient according to the manufacturer’s protocol, washed and prepared for flow assay experiments as previously described (Winkler et al., 2012). Lymphocytes were maintained in RPMI medium supplemented with 1% FBS, 2mM L-Glutamine, 50μM 2-mercaptoethanol and 1mM sodium pyruvate in a humidified 5% CO2-95% air atmosphere at 37°C prior to use.
4.6 Murine cortical EC culture
Primary cortical microvessels from C57BL/6 mice were isolated as previously described (Deli et al., 2000). Microvessel fragments were separated on a 33% continuous Percoll gradient (1000× g, 10 min), collected, and washed twice in DMEM before plating in 24 well plates or 35 mm plastic dishes coated with rat tail collagen and human fibronectin (Sigma) for static adhesion or parallel plate assays respectively. Cultures were maintained in DMEM supplemented with 20% plasma-derived bovine serum (Atlas Biologicals, Atlanta, GA, USA), 1 ng/ml fibroblast growth factor-2 (R&D Systems) and 4 μg/ml puromycin (Sigma) in a humidified 5% CO2-95% air atmosphere at 37°C for one week prior to application in either assay.
4.7 Static adhesion assay
CNS EC cell monolayers in 24 well plates were stimulated with tumor necrosis factor-alpha (TNFα; 10 ng/mL in 1 mL in culture media) 4 hr prior to co-culture. For individual well co-cultures, 1×104 WT CD3/CD28 stimulated lymphocytes were loaded with calcein-AM dye (10μM, Molecular Probes) for 15 min, followed by a spin, wash with fresh medium and a 30 min incubation according to the manufacturer’s protocol. During lymphocyte incubation either activated CNS ECs or activated lymphocytes were treated with varying concentration of HA12 (50, 10 and 1 ug/mL in 1 mL of culture media) or PBS. Following HA12 treatment, 1×104 activated lymphocytes in 1 mL of media were added to ECs in the 24 well plates and allowed to co-culture for 1 hr. Cultures were washed twice with co-culture medium and fluorescence was measured at 538 nm by a FlexStation plate reader (Molecular Devices, Sunnyvale, CA, USA). Relative fluorescence of HA12 treated wells was compared as percent of PBS treated controls. All treatments were performed in triplicate in a minimum of two experiments.
4.8 Parallel plate assay
Lymphocyte adhesion and rolling along CNS ECs was quantified under flow conditions using a parallel-plate flow chamber as previously described (Winkler et al., 2012). Briefly, EC monolayers in 35 mm dishes were stimulated with TNFα (10 ng/mL in 2 mL of culture media) for 4 hr prior to assembly with the flow chamber (150-μm channel depth, 1.26-mm channel width). The chamber was mounted on the stage of a Zeiss Axiovert 200M microscope (Zeiss) and maintained at 37°C. An infuse and withdraw syringe pump (Harvard Apparatus, Holliston, MA, USA) controlled the flow rate of 1×106 CD3/CD28 stimulated WT, CD44−/− or TLR4−/− lymphocytes in 0.6 mL of Hanks buffered salt solution through the chamber. WT lymphocytes were pre-treated in the buffered salt solution with either PBS, TLR4 blocking antibody (Clone MTS510, BD Pharmigen, San Jose, CA, USA, 100 ng/mL) or isotype control antibody (Clone R35–95, BD Pharmigen, 100 ng/mL) for 10 min prior to treatment with PBS or HA12 (10 ug/mL) for 30min. CD44−/− and TLR4−/− lymphocytes in 0.6 mL Hanks buffered salt solution were treated with HA12 (10 ug/mL) for 30 min prior to use in the parallel plate. Treated lymphocytes were superfused through the chamber for 7 minutes at 0.5 dyn/cm2. The microscope was equipped with a CCD camera (Axiocam MRm, Zeiss) and imaging software (Stallion SlideBook v5.0.0.10, Intelligent Imaging Innovations, Inc.) to digitally record cell movements. A single field of view (10x; 0.55 mm2) was monitored during each trial. The number of total interacting cells and the average rolling velocity of each interacting cell were analyzed for each experiment using particle tracking software.
4.9 Real-time PCR
Total RNA from Lympholyte® isolated splenocyte cultures stimulated for 0 hr or 72 hr by CD3/CD28 was obtained and single stranded cDNAs were synthesized using the ImProm-II Reverse Transcriptase synthesis kit (Promega Corporation, Fitchburg, WI, USA) according to the manufacturer’s protocol. TLR4-specific TaqMan primers and probes (Mm00445274_m1) were obtained from Applied Biosystems (Carlsbad, CA, USA). 18S ribosomal RNA was used as a normalizing unit for each reaction. Primer sets were purchased as a kit (TaqMan Ribosomal RNA Control Reagents Kit; Applied Biosystems). cDNA was amplified in 1× Universal Master Mix (Applied Biosystems) with the following thermal cycler protocol: 50°C for two minutes and 95°C for 10 minutes, followed by 45 cycles of 95°C for 15 seconds and 60°C for one minute. Assays were performed in triplicate in two separate experiments. The normalized expression of the target gene with respect to 18S was computed for all samples using the ΔΔCT method in Microsoft Excel.
4.10 Statistical Analysis
Differences between treatment groups in the static adhesion and parallel plate assays were analyzed by a Student’s t-test. Differences in mean EAE disease score between groups were analyzed by a repeated Measures ANOVA. Statistical significance was defined as p<0.05 for all analyses.
Acknowledgments
This work was supported by a grant from Halozyme Therapeutics Inc.; from the Laura Fund for Multiple Sclerosis Research; from a collaborative grant from the National Multiple Sclerosis Society and by grants P51 RR000163 and P30 NS061800 from the National Institutes of Health. CWW and IA are supported by Vertex Pharmaceutical scholar awards. CWW is an ARCS scholar. We thank Dr. Fatima Banine, Dr. Weping Su and Dr. Anda Cornea for technical assistance and intellectual contributions to these studies.
Abbreviations
- CDI
cumulative disease index
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- EC
endothelial cell
- HA
hyaluronan
- HA4
HA tetrasaccharide
- HA12
HA dodecasaccharide
- HMW
high molecular weight
- MOG
myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- PBS
phosphate-buffered saline
- TLRs
Toll-like receptors
- TNFα
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- Brennan FR, O’Neill JK, Allen SJ, Butter C, Nuki G, Baker D. CD44 is involved in selective leucocyte extravasation during inflammatory central nervous system disease. Immunol. 1999;98:427–435. doi: 10.1046/j.1365-2567.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci (USA) 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharm. 2010;80:480–490. doi: 10.1016/j.bcp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, D’Ascola A, Scuruchi M, Prestipino V, Nastasi G, Calatroni A, Campo S. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem. 2012;113:1852–1867. doi: 10.1002/jcb.24054. [DOI] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Deli MA, Sakaguchi S, Nakaoke R, Abraham CS, Takahata H, Kopacek J, Shigematsu K, Katamine S, Niwa M. PrP fragment 106–126 is toxic to cerebral endothelial cells expressing PrP(C) Neuroreport. 2000;11:3931–3936. doi: 10.1097/00001756-200011270-00064. [DOI] [PubMed] [Google Scholar]
- Doring A, Wild M, Vestweber D, Deutsch U, Engelhardt B. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol. 2007;179:8470–8479. doi: 10.4049/jimmunol.179.12.8470. [DOI] [PubMed] [Google Scholar]
- Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genasetti A, Vigetti D, Viola M, Karousou E, Moretto P, Rizzi M, Bartolini B, Clerici M, Pallotti F, De Luca G, Passi A. Hyaluronan and human endothelial cell behavior. Connect Tiss Res. 2008;49:120–123. doi: 10.1080/03008200802148462. [DOI] [PubMed] [Google Scholar]
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Navajas JM, Fine S, Law J, Datta SK, Nguyen KP, Yu M, Corr M, Katakura K, Eckman L, Lee J, Raz E. TLR4 signaling in effector CD4+ T cells regulates TCR activation and experimental colitis in mice. J Clin Invest. 2010;120:570–581. doi: 10.1172/JCI40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nature Rev Immuno. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng BC, Gribbon PM, Day AJ, Hardingham TE. Hyaluronan binding to link module of TSG-6 and to G1 domain of aggrecan is differently regulated by pH. J Biol Chem. 2008;283:32294–32301. doi: 10.1074/jbc.M804155200. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Ann Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CI, Diegelmann RF, Haynes JH, Yager DR. Proinflammatory cytokines differentially regulate hyaluronan synthase isoforms in fetal and adult fibroblasts. J Pediatr Surg. 2000;35:874–879. doi: 10.1053/jpsu.2000.6869. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Norman MU, Lapointe BM, Bonder CS, Zbytnuik L, Kubes P. Reevaluation of P-selectin and alpha 4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immuno. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci (USA) 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerten S, Lehmann PV. The immune pathogenesis of experimental autoimmune encephalomyelitis: lessons learned for multiple sclerosis? J Interferon Cytokine Res. 2011;31:907–916. doi: 10.1089/jir.2011.0072. [DOI] [PubMed] [Google Scholar]
- Laman JD, Maassen CB, Schellekens MM, Visser L, Kap M, de Jong E, van Puijenbroek M, van Stipdonk MJ, van Meurs M, Schwarzler C, Gunthert U. Therapy with antibodies against CD40L (CD154) and CD44-variant isoforms reduces experimental autoimmune encephalomyelitis induced by a proteolipid protein peptide. Mult Scler. 1998;4:147–153. doi: 10.1177/135245859800400312. [DOI] [PubMed] [Google Scholar]
- Lesley J, Gal I, Mahoney DJ, Cordell MR, Rugg MS, Hyman R, Day AJ, Mikecz K. TSG-6 modulates the interaction between hyaluronan and cell surface CD44. J Biol Chem. 2004;279:25745–25754. doi: 10.1074/jbc.M313319200. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Carl JW, Jr, Joshi PS, RayChaudhury A, Pu XA, Shi FD, Bai XF. CD24 on the resident cells of the central nervous system enhances experimental autoimmune encephalomyelitis. J Immunol. 2007;178:6227–6235. doi: 10.4049/jimmunol.178.10.6227. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Iida N, Bourguignon LY. The cell adhesion molecule, GP116, is a new CD44 variant (ex14/v10) involved in hyaluronic acid binding and endothelial cell proliferation. J Biol Chem. 1996;271:23853–23864. doi: 10.1074/jbc.271.39.23853. [DOI] [PubMed] [Google Scholar]
- Mayer WE, Uinuk-Ool T, Tichy H, Gartland LA, Klein J, Cooper MD. Isolation and characterization of lymphocyte-like cells from a lamprey. Proc Natl Acad Sci (USA) 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest: CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- Nedvetzki S, Gonen E, Assayag N, Reich R, Williams RO, Thurmond RL, Huang JF, Neudecker BA, Wang FS, Turley EA, Naor D. RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: a different interpretation of redundancy. Proc Natl Acad Sci (USA) 2004;101:18081–18086. doi: 10.1073/pnas.0407378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- Nylander A, Hafler DA. Multiple sclerosis. J Clin Invest. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski LM, Miszta H, Turley EA. Regulated expression of a receptor for hyaluronan-mediated motility on human thymocytes and T cells. J Immunol. 1993;150:4292–4302. [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nature Rev Molec Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Molec Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- Sampson PM, Rochester CL, Freundlich B, Elias JA. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production. Evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. J Clin Invest. 1992;90:1492–1503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, Aloisi F. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropath Exp Neurol. 2006;65:124–141. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, Richards JS. Hyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilization. Development. 2008;135:2001–2011. doi: 10.1242/dev.020461. [DOI] [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Sugahara KN, Murai T, Nishinakamura H, Kawashima H, Saya H, Miyasaka M. Hyaluronan oligosaccharides induce CD44 cleavage and promote cell migration in CD44-expressing tumor cells. J Biol Chem. 2003;278:32259–32265. doi: 10.1074/jbc.M300347200. [DOI] [PubMed] [Google Scholar]
- Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheumat. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- Tammi R, MacCallum D, Hascall VC, Pienimaki JP, Hyttinen M, Tammi M. Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J Biol Chem. 1998;273:28878–28888. doi: 10.1074/jbc.273.44.28878. [DOI] [PubMed] [Google Scholar]
- Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Derm. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, Lowe E, Mahoney DJ, Tammi MI, Kahmann JD, Campbell ID, Day AJ, Jackson DG. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Molec Cell. 2004;13:483–496. doi: 10.1016/s1097-2765(04)00080-2. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy TM, Wallingford N, Liu Y, Chan FH, Rizvi T, Xing R, Bebo B, Rao MS, Sherman LS. CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia. 2004;47:335–345. doi: 10.1002/glia.20042. [DOI] [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Foster SC, Matsumoto SG, Preston M, Xing R, Bebo B, Banine F, Berny-Lang MA, Itakura A, McCarty OJ, Sherman LS. Hyaluronan Anchored to Activated CD44 on CNS Vascular Endothelial Cells Promotes Lymphocyte Extravasation in Experimental Autoimmune Encephalomyelitis. J Biol Chem. 2012;287:33237–33251. doi: 10.1074/jbc.M112.356287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Resp Cell Molec Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]