Abstract
Mutations in SCN2A gene cause a variety of epilepsy syndromes. We report a novel SCN2A-associated epilepsy phenotype in monozygotic twins with tonic seizures soon after birth and a suppression-burst EEG pattern. We reviewed the medical records, EEG tracings, MRI, neuropathological findings, and performed whole genome sequencing (WGS) on Twin B’s DNA and Sanger sequencing (SS) on candidate gene mutations. Extensive neurometabolic evaluation and early neuroimaging studies were normal. Twin A died of an iatrogenic cause at 2 weeks of life. His neuropathologic examination was remarkable for dentato-olivary dysplasia and granule cell dispersion of the dentate gyrus. Twin B became seizure-free at 8 months and was off anti-epileptic drugs by 2 years. His brain MRI, normal at 2 months, revealed evolving brainstem and basal ganglia abnormalities at 8 and 15 months that resolved by 20 months. At 2.5 years, Twin B demonstrated significant developmental delay. Twin B’s WGS revealed a heterozygous variant c.788C>T predicted to cause p.Ala263Val change in SCN2A and confirmed to be de novo in both twins by SS. In conclusion, we have identified a de novo SCN2A mutation as the etiology for Ohtahara syndrome in monozygotic twins associated with a unique dentate-olivary dysplasia in the deceased twin.
Keywords: SCN2A, Ohtahara syndrome, Whole genome sequencing, Seizures
Introduction
Mutations in sodium channels alter neuronal excitability, and thereby underlie multiple epilepsy syndromes. Voltage-gated sodium channels in the brain initiate and propagate action potentials, and their subtypes NaV1.1, NaV1.2, NaV1.3, and NaV1.6 are encoded by the SCN1A, SCN2A, SCN3A, and SCN8A respectively. SCN2A mutations have been associated with generalized epilepsy with febrile seizure plus, benign familial neonatal-infantile seizures, intractable epilepsies, infantile spasms and severe myoclonic epilepsy of infancy (Need et al., 2012; Ogiwara et al., 2009). Recently, various SCN2A mutations have been linked to severe intellectual disability and autism without seizures, further expanding the phenotypes due to SCN2A mutations (Rauch et al., 2012; Sanders et al., 2012). We describe a unique neonatal epileptic encephalopathy in monozygotic twins that initially presented with features of Ohtahara syndrome (OS) but evolved into a seizure-free state by late infancy in surviving Twin B due to a de novo missense SCN2A mutation. NaV1.2 channels are known to be widely distributed in the human brain with predominant expression in the cerebellum (Martinez-Hernandez et al., 2012).This is the first neuropathological report of subtle anomalies in the cerebellar architecture, a variant of dentate-olivary dysplasia (DOD) associated with a SCN2A mutation.
Methods
The twins and extended family were enrolled in an IRB-approved study. Blood samples were collected and DNA was extracted. Whole genome sequencing (WGS) was performed on DNA from Twin B using the Complete Genomics (Mountain View, CA) platform as described previously (Veeramah et al., 2012). Non-pathogenic variants were filtered using dbSNP131 and the 1000 Genomes databases and potential disease-causing variants were confirmed using Sanger sequencing (SS).
Results
Clinical characteristics
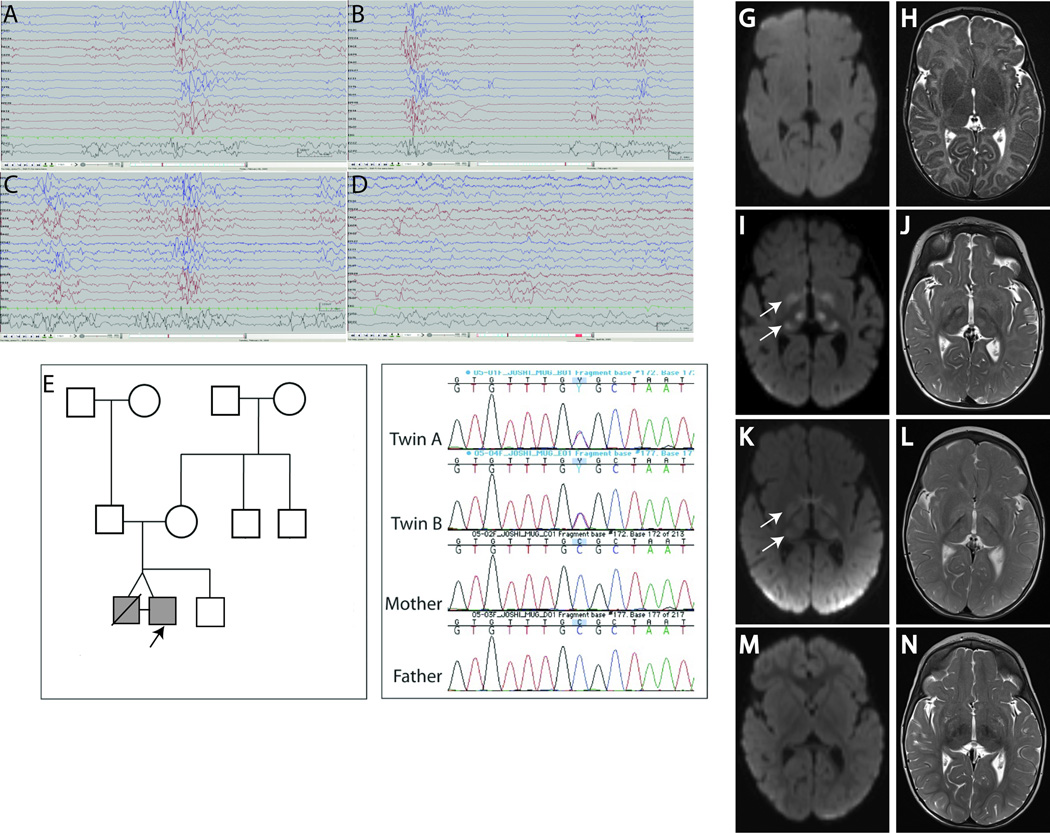
Monozygotic twin boys, born by uncomplicated cesarean delivery at 37 6/7 gestational weeks developed 50–60 seizures/day on day 1, lasting <30 seconds, and presenting with crying followed by tonic stiffening of the extremities and apnea. They received several doses of phenobarbital, phenytoin, pyridoxine, levetiracetam, sodium valproate, clonazepam, topiramate without improvement. On Day 19, Twin A experienced a fatal cardiorespiratory arrest of iatrogenic cause, and an autopsy was performed. Twin B became seizure-free at 8 months on topiramate, levetiracetam, phenobarbital, vigabatrin, and lamotrigine, and seizure-free without medications at 2 years. At 2.5 years, he was able to grab objects with an immature grasp, say short sentences, but did not crawl, and had intermittent esotropia, diffuse axial hypotonia and head lag. An electroencephalogram (EEG) recording from each twin at 2 weeks showed a Suppression-Burst (S-B) pattern with bursts of sharp activity lasting 1–2 seconds, and periods of relative voltage suppression (Fig. 1A–B). Twin B’s EEG evolved to a discontinuous and asynchronous background with frequent multifocal sharp waves by 4 weeks (Fig. 1C), and further improved later (Fig. 1D). At 1.5 and 2 years, EEG revealed occasional multifocal spikes in parietal regions with symmetric background.
Figure 1.
EEG and MRI findings in affected twins, pedigree and sequencing results for SCN2A mutation. (A) EEG for twin A at 2 weeks of age showed background suppression with bursts lasting 1–2 seconds of high-voltage (100–150uV) sharp activity. (B) The EEG for Twin B at 2 weeks of age showed a similar pattern. (C) At 4 weeks age, EEG shows a similar pattern as 2 weeks but with more discontinuous background and asynchronous suppression. (D) The EEG pattern improved further at 10 weeks of age. Pedigree (E) with proband Twin B indicated by an arrow, and Sanger sequencing of genomic PCR products illustrating the alteration c.788C>T in the Twins A and B (F). MRI findings in Twin B on axial diffusion weighted (G,I,K,M) and axial T2 Turbo Spin Echo (TSE) (H,J,L,N) images. (G–H) MRI at 2 mo of age showed no diffusion or T2 signal abnormalities, MRI reported as normal. (I–J) MRI at 8 mo revealed symmetric bright signal due to decreased diffusion (arrows) on diffusion weighted imaging (DWI) (I) and abnormal increased T2 signal within the globus pallidus, thalami on axial T2 images (J). (K–L) MRI at 15 months age revealed similar findings as I–J although less bright signal (arrows) on DWI (K). (M–N) MRI at 20 months shows interval resolution of decreased diffusion (M) and T2 signal abnormalities (N).
Clinical Investigations
Plasma lactate, ammonia, biotinidase activity, acylcarnitine profile, very long chain fatty acids, and amino acids, white blood cell lysosomal enzymes, urine organic acids, urine sulfites and acylglycines, and cerebrospinal fluid lactate, amino acids, neurotransmitter metabolites, and 5-tetrahydrofolate were normal. Skin biopsy of Twin B was negative for storage disorders. Brain CT scan, MRI (Fig. 1G–H), and magnetic resonance spectroscopy on both twins in the neonatal period were normal. Subsequently, Twin B’s brain MRI at 8 and 15 months revealed symmetrically abnormal signal within the globi pallidi, thalami, red nuclei, cerebral peduncles, dorsal pons, and ventral medulla with associated restricted diffusion consistent with cytotoxic edema (Fig. 1I–L), with resolution at 20 months (Fig. 1M–N). Genes associated with early-onset epilepsy, STXBP1, KCNQ2, CDKL5, SCN1A, MECP2 and ARX, and several genes associated with neurometabolic disease including POLG1 and NDUFAF1 were sequenced with no significant variations. Chromosomal microarray for copy number variation revealed no pathogenic variants. Histopathological analysis of muscle biopsy at one year age was normal. Oxidative phosphorylation enzymology revealed complex I and III defects and a reduction in complex III subunit on Western blot, possibly related to disuse atrophy.
Neuropathology findings in Twin A
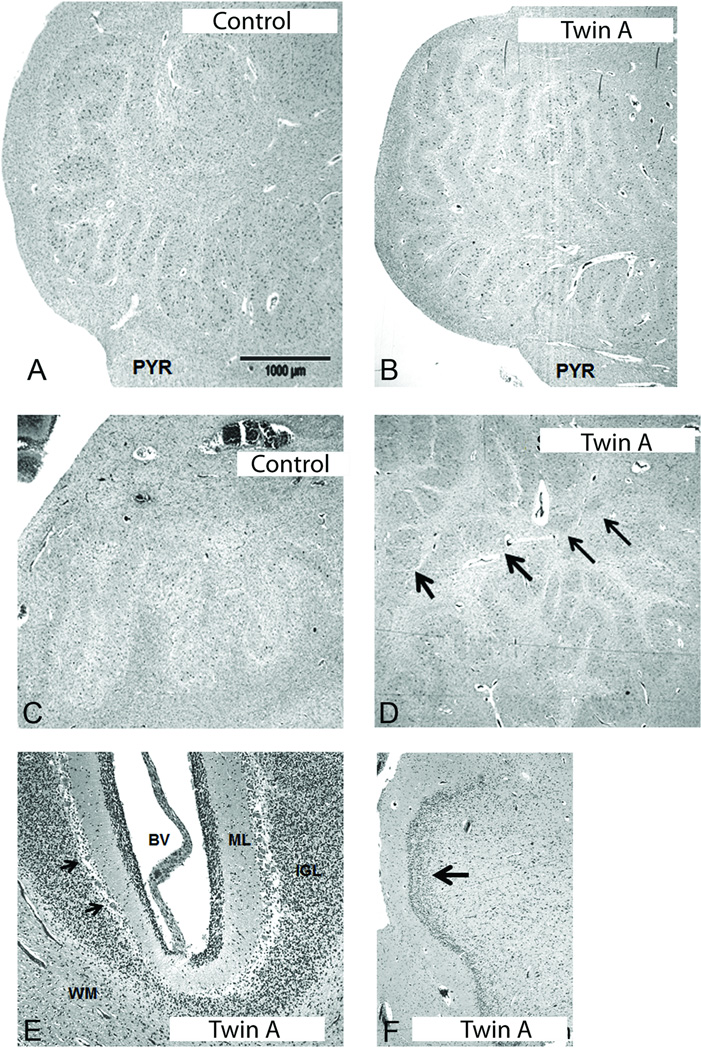
The brain was macroscopically normal. Histologically, the cerebral cortex, thalami, basal ganglia and spinal cord were unremarkable. The white matter was intensely gliotic. The hippocampus was gliotic in the hilum with focal duplication of the dentate gyrus (Fig. 2F). The dentate nucleus in the cerebellum was hyper-convoluted, and subdivided into small and disconnected islands of neurons, rather than forming a continuous ribbon (Fig. 2D). The overlying cerebellar cortex in the vermis and lateral hemisphere was well formed (Fig. 2E). In the medulla, there was hyper-convolution of the principal inferior olives (Fig. 2B); the ribbons of the lateral lamella were folded multiple times in a back-to-back configuration (Fig. 2B).
Figure 2.
Histopathological findings of the inferior olive, dentate nucleus of the cerebellum, cerebellar cortex and dentate gyrus of the hippocampus in Twin A. Comparison of the principal inferior olive in an age-related control (A) to Twin A (B) with SCN2A mutation; there is increased anomalous folding of the laminae in Twin A (B). Abbreviation: PYR, pyramidal tract. Scale bar=1000 microns. Comparison of the dentate nucleus of the cerebellum in an age-related control (C) to Twin A (D), indicating the segmentation of the nucleus into disconnected islands (arrows) in the latter (D), ×20. There are no histological abnormalities of the cerebellar cortex in Twin A (E) x10. The Purkinje cells are intact (arrows). Abbreviations: BV, blood vessel; ML, molecular layer; IGL, internal granular layer; PC, Purkinje cell (arrows); WM, white matter. There is focal duplication of the dentate gyrus (arrow) in Twin A (F), ×20.
Whole Genome Sequencing
The pedigree is shown in Fig. 1E. WGS was performed on Twin B’s DNA. We identified 19,766 variants within exons or splice-sites, of which 702 variants were novel. We evaluated these novel variants for epilepsy-associated genes (Table S1). A de novo variant c.788C>T predicted to cause p.Ala263Val change in SCN2A (Fig. 1F) was identified and confirmed by SS in both twins. The same SCN2A mutation has been previously reported to cause neonatal seizures where the resulting ion channel was predicted to cause gain-of-function neuronal hyperexcitability with a 3-fold increase in persistent Na+ current on cell culture experiments (Liao et al., 2010).
Discussion
We describe neonatal encephalopathy with OS in monozygotic twins carrying a de novo p.Ala263Val-SCN2A mutation. Their clinical presentation was quite similar until the sudden death of twin A. The same mutation has been previously identified in a patient with neonatal tonic-clonic seizures controlled with phenobarbital and phenytoin, with normal intelligence and episodes (1–3/month) of ataxia, slurred speech, myoclonic jerks, headache, back pain and hyperventilation (Liao et al., 2010). This is unlike tonic seizures resistant to multiple AEDs, and EEG consistent with OS along with significant developmental delay in our patient. This suggests marked phenotypic heterogeneity and possible role of modifiers. Further, SCN2A mutations are linked to a wide range of epilepsies, autism-spectrum disorders and intellectual disabilities without seizures. While there are no obvious mutation hotspots, de novo SCN2A mutations are generally associated with more severe phenotypes than the autosomal dominant ones (Ogiwara et al., 2009).
An early diagnosis of OS in our twins was based on neonatal tonic seizures and a S-B pattern on EEG, but the clinical course in Twin B with seizure resolution and marked EEG improvement in late infancy was distinct. In addition, his brain MRI at 8 and 15 months revealed diffuse basal ganglia and brain stem abnormalities, initially raising the possibility of a progressive neurodegenerative disorder. A similar course has been reported with KCNQ2 mutations, with early tonic seizures, a S-B pattern, and transient MRI abnormalities (Weckhuysen et al., 2012). Interestingly, genetic interaction between Scn2a and Kcnq2 has been hypothesized in mouse models of epilepsy (Kearney et al., 2006). Further, a similar pattern of abnormal signal intensity in deep gray nuclei and dorsal brainstem has been reported in patients treated with vigabatrin (Simao et al., 2011). We also highlight that patients with apparently progressive neurologic disease and encephalopathic epilepsy should be evaluated for epilepsy-associated channelopathies, whose features may overlap with those of a neurodegenerative process in infancy.
The combined anomaly of the cerebellar dentate nucleus and inferior olive in Twin A may represent a variant of DOD. Intractable epilepsy of infancy has been associated with DOD, notably with S-B patterns where the inferior olive is hypo-convoluted with a hook shape and the dentate nucleus forms a compact mass of grey matter islands (Harding & Boyd, 1991). While similar dentate nucleus findings were identified in Twin A, the inferior olive was hyper- as opposed to hypo-convoluted, thereby suggesting a variant of abnormal olivary folding. The role of the NaV1.2 channel in olivary and dentate folding is unknown, as the developmental expression of this channel relative to these structures has not been highlighted in neuroanatomical mapping studies, which focus rather upon the granule cells and molecular layer. He also had a variant of granule cell dispersion of the hippocampal dentate gyrus i.e., focal duplication that may reflect a primary developmental abnormality predisposing to seizures; they may result, however, from epilepsy-induced secondary changes (Blumcke et al., 2009).
In summary, this report describes a novel SCN2A-associated phenotype with unique neuropathological findings. We suggest adding SCN2A sequencing to the diagnostic evaluation of neonatal seizures. Further, next generation sequencing may be preferable to the single gene/s sequencing in the determination of genetic basis for neonatal epilepsies.
Supplementary Material
Acknowledgments
This work was made possible by generous support from the Gene Discovery Core of The Manton Center for Orphan Disease Research including funding for the whole genome sequencing (M.T.). Sanger sequencing was performed by the Intellectual and Developmental Disabilities Research Center (IDDRC) Molecular Genetics Core, supported by NIH-P30-HD18655. A.P. was supported by the NINDS/NIH (K23 NS069784) and P.B.A. by a Boston Children’s Hospital Career Development Award and NIAMS/NIH (K08 AR055072). We wish to thank Dr. Alan H. Beggs for his critical advice and support. We would like to acknowledge the work of Dr. Debbie Schoefield in interpreting the histopathological slides and Dr. Larry White in reading the EEGs. We would also like to thank the parents for their enrollment and support for the study.
Footnotes
DISCLOSURE:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Dr. Joshi, Dr. Touma, Ms. Connolly, Dr. Grant, Dr. Hansen, Dr. Berry, Dr. Kinney, Dr. Poduri and Dr. Agrawal report no disclosures. Dr Khwaja holds a Corporate Appointment and stock options with F.Hoffmann-La Roche. He has received funding from Autism Speaks and the International Rett Syndrome Foundation.
Contributor Information
Marlin Touma, Email: MTouma@mednet.ucla.edu.
Mugdha Joshi, Email: mugdha.joshi@childrens.harvard.edu.
Meghan C. Connolly, Email: meghan.connolly@childrens.harvard.edu.
P. Ellen Grant, Email: p.ellen.grant@gmail.com.
Anne R. Hansen, Email: anne.hansen@childrens.harvard.edu.
Omar Khwaja, Email: omarkhwaja@me.com.
Gerard T. Berry, Email: Gerard.berry@childrens.harvard.edu.
Hannah C. Kinney, Email: Hannah.kinney@childrens.harvard.edu.
Annapurna Poduri, Email: Annapurna.poduri@childrens.harvard.edu.
Pankaj B. Agrawal, Email: pagrawal@enders.tch.harvard.edu.
REFERENCES
- 1.Blumcke I, Kistner I, Clusmann H, Schramm J, Becker AJ, Elger CE, Bien CG, Merschhemke M, Meencke HJ, Lehmann T, Buchfelder M, Weigel D, Buslei R, Stefan H, Pauli E, Hildebrandt M. Towards a clinico-pathological classification of granule cell dispersion in human mesial temporal lobe epilepsies. Acta Neuropathol. 2009;117:535–544. doi: 10.1007/s00401-009-0512-5. [DOI] [PubMed] [Google Scholar]
- 2.Harding BN, Boyd SG. Intractable seizures from infancy can be associated with dentato-olivary dysplasia. J Neurol Sci. 1991;104:157–165. doi: 10.1016/0022-510x(91)90305-q. [DOI] [PubMed] [Google Scholar]
- 3.Kearney JA, Yang Y, Beyer B, Bergren SK, Claes L, Dejonghe P, Frankel WN. Severe epilepsy resulting from genetic interaction between Scn2a and Kcnq2. Hum Mol Genet. 2006;15:1043–1048. doi: 10.1093/hmg/ddl019. [DOI] [PubMed] [Google Scholar]
- 4.Liao Y, Anttonen AK, Liukkonen E, Gaily E, Maljevic S, Schubert S, Bellan-Koch A, Petrou S, Ahonen VE, Lerche H, Lehesjoki AE. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010;75:1454–1458. doi: 10.1212/WNL.0b013e3181f8812e. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Hernandez J, Ballesteros-Merino C, Fernandez-Alacid L, Nicolau JC, Aguado C, Lujan R. Polarised Localisation of the Voltage-Gated Sodium Channel Na(v)1.2 in Cerebellar Granule Cells. Cerebellum. 2012 doi: 10.1007/s12311-012-0387-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Need AC, Shashi V, Hitomi Y, Schoch K, Shianna KV, McDonald MT, Meisler MH, Goldstein DB. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet. 2012;49:353–361. doi: 10.1136/jmedgenet-2012-100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogiwara I, Ito K, Sawaishi Y, Osaka H, Mazaki E, Inoue I, Montal M, Hashikawa T, Shike T, Fujiwara T, Inoue Y, Kaneda M, Yamakawa K. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology. 2009;73:1046–1053. doi: 10.1212/WNL.0b013e3181b9cebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, Albrecht B, Bartholdi D, Beygo J, Di Donato N, Dufke A, Cremer K, Hempel M, Horn D, Hoyer J, Joset P, Ropke A, Moog U, Riess A, Thiel CT, Tzschach A, Wiesener A, Wohlleber E, Zweier C, Ekici AB, Zink AM, Rump A, Meisinger C, Grallert H, Sticht H, Schenck A, Engels H, Rappold G, Schrock E, Wieacker P, Riess O, Meitinger T, Reis A, Strom TM. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 9.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, Ercan-Sencicek AG, DiLullo NM, Parikshak NN, Stein JL, Walker MF, Ober GT, Teran NA, Song Y, El-Fishawy P, Murtha RC, Choi M, Overton JD, Bjornson RD, Carriero NJ, Meyer KA, Bilguvar K, Mane SM, Sestan N, Lifton RP, Gunel M, Roeder K, Geschwind DH, Devlin B, State MW. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simao GN, Zarei Mahmoodabadi S, Snead OC, Go C, Widjaja E. Abnormal axial diffusivity in the deep gray nuclei and dorsal brain stem in infantile spasm treated with vigabatrin. AJNR Am J Neuroradiol. 2011;32:199–203. doi: 10.3174/ajnr.A2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veeramah KR, O'Brien JE, Meisler MH, Cheng X, Dib-Hajj SD, Waxman SG, Talwar D, Girirajan S, Eichler EE, Restifo LL, Erickson RP, Hammer MF. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012;90:502–510. doi: 10.1016/j.ajhg.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, Peter de J. KCNQ2 encephalopathy: Emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.