Abstract
Prolonged neonatal opioid exposure has been associated with: antinociceptive tolerance, long-term neurodevelopmental delay, cognitive, and motor impairment. Morphine has also been shown to induce apoptotic cell death in vitro studies, but its in-vivo effect in developing rat brain is unknown. Thus, we hypothesized that prolongued morphine administration in neonatal rats in a model of antinociceptive tolerance and dependence is associated with increased neuroapoptosis. We analyzed neonatal rats from the following groups (1) naïve group (n=6); (2) control group (normal saline (NS), n=5), and (3) morphine group (n=8). Morphine sulfate or equal volume of NS was injected subcutaneously twice daily for 6.5 days starting on postnatal day (PD) 1. Development of antinociceptive tolerance was confirmed by Hot Plate test on the 7th day. Evidence of neuronal and glial apoptosis was determined by cleaved caspase-3immunofluorescence combined with specific markers. At PD7, morphine administration after 6 ½ days significantly increased the density of apoptotic cells in the cortex and amygdala, but not in the hippocampus, hypothalamus, or periaqueductal gray. Apoptotic cells exhibited morphology analogous to neurons. Irrespective of the treatment, only a very few individual microglia but not astrocytes were caspase-3 positive. In summary, repeated morphine administration in neonatal rats (PD1-7) is associated with increased supraspinal apoptosis in distinct anatomical regions known to be important for sensory (cortex) and emotional memory processing (amygdala). Brain regions important for learning (hippocampus), and autonomic and nociceptive processing (hypothalamus and periaqueductal gray) were not affected. Lack of widespread glial apoptosis or robust glial activation following repeated morphine administration suggests that glia might not be affected by chronic morphine at this early age. Future studies should investigate long-term behavioral sequelae of demonstrated enhanced apoptosis associated with prolonged morphine administration in a neonatal rat model.
Keywords: Amygdala, caspase-3, cortex, development, glia, neurotoxicity
1. Introduction*
Although the impact of chronic maternal opioid exposure on in utero brain development has been widely studied both in humans (Besunder and Blumer, 1990; Hunt et al., 2008; Konijnenberg and Melinder, 2011; McGlone et al., 2009; Walhovd et al., 2009) and animals (Che et al., 2005; He et al., 2010; Nasiraei-Moghadam et al., 2010; Nasiraei-Moghadam et al., 2012; Sadraie et al., 2008; Seatriz and Hammer, 1993; Slamberova et al., 2005), less in known about the long-term effects of morphine when administered postnatally. Human infants are exposed to opioids every day worldwide in the context of perioperative and procedural pain management. Even in the absence of pain, critically ill neonates and children receive prolonged opioids for sedation to reduce anxiety, agitation, stress responses, and to facilitate ventilation (Anand, 2001; Berde and Sethna, 2002; Chambliss and Anand, 1997). Such treatment is associated with markedly high incidence (35-57%) of analgesic tolerance and opioid dependence (Anand et al., 2010; Fonsmark et al., 1999; Katz et al., 1994), as well as long-term neurodevelopmental delay, neurocognitive and motor impairments (de Graaf et al., 2011; McGlone et al., 2009). A recent pilot study at 5-year follow up (Ferguson et al., 2012) reported differences in head circumference, abnormal choice response latencies, and diminished social interactions between morphine and placebo treated preterm neonates. All together, these reports are suggestive of significant alterations in neural pathways resulting from early exposure to opioids. Thus, this report addresses possible central nervous system plasticity that might result from chronic morphine exposure during newborn period in a rodent model.
It is known that neurons undergo programmed cell death (apoptosis) during the brain maturation period that can be triggered by both physiological and pathological stimuli (Blaschke et al., 1996; Oppenheim, 1991; Rabinowicz et al., 1996; Raff et al., 1993; Rakic and Zecevic, 2000). Disruption of physiological apoptotic cell death during development leads to brain malformations and premature death in rodent models (Kuida et al., 1996). Morphine has been shown to induce apoptotic cell death in vitro studies (Goswami et al., 1998; Singhal et al., 2002; Singhal et al., 2000; Singhal et al., 1999; Singhal et al., 1998; Tegeder et al., 2003; Yin et al., 2000). In contrast to the abundance of in vitro studies, only a smal number of studies reported potential neurotoxic effect of opioids in vivo (Emeterio et al., 2006; Mao et al., 2002). Furthermore, the in vivo effect of prolonged morphine administration on the apoptosis in developing rat brain is unknown. We hypothesized that prolonged administration of morphine in a newborn rat is associated with increased apoptotic cell death. Specifically, the primary objective of the study was to quantify density of apoptotic cells using cleaved caspase-3 immunofluorescence in distinct supraspinal regions. These included regions known to be important for sensory (cortex) and emotional memory processing (amygdala), learning (hippocampus), as well as autonomic and nociceptive processing (hypothalamus and periaqueductal gray). Furthermore, oxidative stress and upregulated nitric oxide were implicated not only in mechanisms of neuronal apoptosis, but in sequelae of chronic opioid exposure (see reviews (Babey et al., 1994; Salvemini, 2009). Finally, previous studies reported that, in contrast to neurons and microglia, astrocytes are resistant to apoptosis in culture cell preparation (Hu et al., 2002), but not in vivo chronic morphine-treatment model of adult mice (Emeterio et al., 2006). However, it is not known if (1) any of the apoptotic neurons are immunoreactive to neuronal nitric oxide synthase (nNOS), or (2) how is glia affected by chronic morphine administration in developing rat brain. Thus, our second objective was to determine the cellular type of identified cleaved caspase-3 cells (neurons vs. glia, such as microglia and astrocytes). To test our hypotheses, we utilized previously published model of prolonged morphine administration in newborn rats (post-natal day (PD) 1-7), associated with antinociceptive tolerance and dependence (Jones and Barr, 1995; Zhu and Barr, 2003), which we have also confirmed in our lab (Bajic et al., 2012).
2. Methods
2.1. Animal Care and Use
The Institutional Animal Care and Use Committee at Boston Children’s Hospital approved the experimental protocols for the use of vertebrate animals in this study. Experiments were conducted according to the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), prepared by the National Academy of Sciences’ Institute for Laboratory Animal Research. All efforts were made to minimize the number of animals used and their discomfort. Sprague Dawley rat pups of both sexes were utilized for this study. Pregnant rat dams (Sasco; Charles River Laboratories International, Inc., Wilmington, MA) were received on day 18 of gestation and were handled for several days before delivery. Cages with pregnant dams were checked at 9 AM and 5 PM daily and pups found at either time were termed 0 days of age. Animals were maintained on a 12:12 h light/dark cycle, food and water were given ad libitum, and animals were housed with its litter.
2.2. Pharmacological Treatment
We utilized an established model of morphine dependence(Jones and Barr, 1995) and analgesic tolerance in newborn rats (Zhu and Barr, 2003), which we have also confirmed in our lab (Bajic et al., 2012). The progeny from 3 litters were used. Each litter had betweeen 8 and 11 pups that were randomly assigned to each of the experimental groups. In other words, split-litter design was used (representing all treatment groups within a single litter) (Booze and Mactutus, 1985). Pharmacological groups included: (1) naïve group that received no treatment (n=6); (2) control group that received normal saline (NS) injections for 6 ½ days (n=5); and (3) morphine group (tolerant group) that received morphine injections for 6 ½ days (n=8). We used subcutaneous (sc) injections to minimize nociceptive experience from intraperitoneal (ip) drug administration. Specifically, morphine sulfate (10 mg/kg; Baxter Healthcare Corp., Deerfield, IL) or equal volume of NS was administered twice daily sc in the area of the upper or lower back of newborn pups for 6 days (9 AM and 5 PM starting at day 1 of life). Pups received the last sc injection on the morning of the 7th day (PD7). Injections were done using 10 μl and 100 μl Hamilton syringes (Hamilton Company, Reno, NV). Pups from both sexes were included in the study. Studies by Craft et al. (Craft et al., 1999) demonstrated that there are no sex differences in morphine’s antinociceptive effect following 1-week of twice daily 10 mg/kg sc morphine dosing in adult rats (as demonstrated by both Hot-Plate and tail withdrawal tests).
2.3. Cleaved Caspase-3 and Cell-type Immunofluorescence
A total of 19 pups were used in 3 experimental groups: naïve (n=6), normal saline control (n=5), and chronic morphine (n=8) groups. Following pharmacological treatment, animals were anesthetized, and transcardially perfused on the 7th day, 2 h after the last sc injection. A two-hour time point was selected because antinociceptive effects following systemic administration of morphine in newborn rats peak at 30 min and lasts 3 h (Thornton et al., 1997). This timing is similar to older rats (Johannesson and Becker, 1973; Spratto and Dorio, 1978). The experimental groups were matched and individuals from different groups were processed in parallel.
2.3.1. Perfusion
Each animal was deeply anesthetized with sodium pentobarbital (100 mg/kg, ip; obtained from Animal Resources at Children’s Hospital (ARCH) Facility, Boston, MA). The rat pups were perfused through the ascending aorta with 100 ml of 4% paraformaldehyde in 0.1M phosphate buffer (PB) solution. The brains were removed and stored in the same fixative solution overnight (4°C), followed by 30% sucrose solution in 0.1M PB for at least 48 h. Subsequently, the brains were frozen and 40 μm coronal sections were cut either using a freezing microtome (Leica RM 2255) or cryostat (Leica CM3050S; Leica Microsystems Inc., Buffalo Grove, IL). Free-floating sections were collected in 0.1M PB in NS; while cryostat sections were collected directly on SuperfrostR Plus microscope slides (Fisher Scientific, Pittsburg, PA). Free-floating sections produced better immunolabeling, while cryostat sections allowed better preservation of tissue quality.
2.3.2. Immunofluorescence
Tissue sections were processed for either double- or triple-immunofluorescence for a marker of apoptosis (caspase-3) with several additional markers against neurons or glia (microglia and astrocytes). Specifically, we analyzed the co-localization of cleaved caspase-3 in cells immunolabeled for Neuronal Nuclear Antigen (NeuN, marker of mature neurons), neuronal Nitric Oxide Synthase (nNOS), Glial Fibrillary Acidic Protein (GFAP, marker of astrocytes) and Ionized Calcium Binding Adaptor Molecule 1 (Iba-1, marker of microglia) to determine the cell population most sensitive to chronic administration of morphine in developing rat brain. Both primary and secondary antisera were diluted in 0.1M PB with normal saline containing 0.04% bovine serum albumin and 0.1% sodium azide. Both free floating and brain sections on the slides were incubated in primary antibodies for 2 days at 4°C, and subsequently in secondary antibodies for 1 to 2 h at room temperature. Sections were rinsed in 0.1M PB in saline (3 times for 10 min) in between immunofluorescent processing. Summary of both primary and secondary antibodies used, their concentration and source is shown in Table 1. Immunolabeling produced characteristic cell body and/or nuclear staining (e.g. NeuN is a neuronal nuclear marker). Free-floating sections were rinsed in 0.1M PB in saline solution prior to mounting on slides in 0.05M PB. Finally, the sections were mounted (if cut on a freezing microtome), dried, and the cover slip was applied with 90% glycerol solution. Cryostat sections were processed on slides in the wet chamber using the same solutions.
TABLE 1.
ANTIBODIES USED FOR IMMUNOFLUORESCENCE
| Antibody | Dilution | Catalog ID | Source | |
|---|---|---|---|---|
| PRIMARY ANTIBODIES | ||||
| 1 | Rabbit anti cleaved caspase-3 | 1:400 | No.9664, ASP175, 5A1E |
Cell Signaling Technology, Beverly, MA |
| 2 | Mouse anti neuronal nuclear protein (NeuN) conjugated biotin |
1:500 | Cat# ab77315 | Abcam, Cambridge, MA |
| 3 | Mouse anti neuronal nitric oxide synthase (nNOS) |
1:5000 | NOS1 (A-11): sc- 5302 |
Santa Cruz Biotechnology, Santa Cruz, CA |
| 4 | Mouse anti glial fibrillary acidic protein (GFAP) |
1:300 | Cat# 3670 | Cell Signaling Technology Inc., Beverly, MA |
| 5 | Goat anti-ionized calcium binding adaptor (Iba-1) |
1:500 | Cat# ab5076 | Abcam, Cambridge, MA |
| SECONDARY ANTIBODIES | ||||
| 1 | Donkey anti rabbit conjugated to Cy3 (red) |
1:200 | Cat# 711-165-152 Lot# 84873 |
Jackson ImmunoResearch Labs, Inc., West Grove, PA |
| 2 | Streptavidin conjugated to Alexa FluorR 488 (green) |
1:200 | Code# S32354 Lot# 37423A |
Molecular Probes, Eugene, OR |
| 3 | Donkey anti mouse conjugated to Alexa FluorR 488 (green) |
1:200 | Cat# A21202 Lot# 1022448 |
Invitrogen Corp., Carlsbad, CA |
| 4 | Donkey anti goat conjugated to Alexa FluorR 488 (green) |
1:200 | Cat# A11055 Lot# 41245 |
Invitrogen Corp., Carlsbad, CA |
| 5 | Donkey anti goat conjugated to Cy5-DyLight™ (far-red) |
1:200 | Code# 705-515- 003 |
Jackson ImmunoResearch Labs, Inc., West Grove, PA |
Summary of both primary and secondary antibodies used, their concentration and source. Labeling against cleaved caspase-3 was done with red fluorophore (Cy3), in combination with another label with green fluorophore (Alexa FluorR 488) for double-labeling immunofluorescence (e.g. streptavidin secondary antibody for NeuN, donkey anti-mouse for nNOS or GFAP, and donkey anti-goat for Iba-1). In case of triple-immunofluorescent labeling, additional fluorophore (far-red; Cy5) was used. Specifically, triple labeling was done for caspase-3, nNOS and Iba-1.
2.3.3. Mapping and Cell Counts
Apoptotic cells were photographed and analyzed with a fluorescent microscope (Olympus IX81; Olympus America Inc. Melville, NY, USA) equipped with a camera and digital microscopy software (Slidebook version 4.2, Olympus). We performed quantitative analysis of caspase-3 immunoreactive cells density in the cortical area corresponding to PD7 coronal Figures 58 and 59 in the neonatal rat brain atlas by Ramachandra and Subramanian (Ramachandra and Subramanian, 2011). This area includes the amygdala, hippocampus, and hypothalamus (Fig. 2). The area of the cortex included the visual, parietal association cortex, somatosensory and auditory cortex, as well as the piriform cortex. The regions of hypothalamus included both posterior hypothalamic area and ventromedial hypothalamic nucleus. Finally, the area of the periaqueductal gray of the midbrain corresponds to Figures 19 and 20 of the neonatal rat brain (Ramachandra and Subramanian, 2011) and includes both the dorsolateral and ventrolateral subdivisions of the periaqueductal gray that are divided by horizontal line through the center of the cerebral aqueduct (Beitz, 1995) (Fig. 2). The number of caspase-3 immunoreactive cells was counted bilaterally for all the regions analyzed in 4-10 sections/brain by an observer blind to the treatment group. Single and double-labeled caspase-3 immunoreactive cells were manually counted during microscopy using a 10× microscope objective by visualizing the individual and merged images of each fluorophore. Schematic drawings in Fig. 2 illustrate the anatomical areas of analysis and a representative topographical distribution of caspase-3 immunoreactive cells. Drawings were made using Adobe Illustrator CS5 Graphic Suite version 13.0 (Adobe Systems Incorporated) on a Mac computer from a montage made from individual photomicrographs done using a 10× microscope objective and digital microscopy software. The photomicrographs were digitally adjusted only for the brightness and contrast.
Figure 2. Anatomical Regions of Analysis in PD7 Rats.
Representative coronal forebrain (A) and brainstem (B) sections of newborn rat at the postnatal day (PD) 7 schematically illustrate anatomical regions of interest. Sections were drawn from a representative animal that was treated with morphine for 6 ½ days. Solid lines outline the brain sections, while dashed lines illustrate approximate location of anatomical landmarks. Dots illustrate distribution of caspase-3 immunoreactive cells. Density of caspase-3 immunoreactive cells was analyzed in anatomical regions shown in grey. These areas include: (1) cortex, (2) hippocampus (Hipp), (3) amygdala (Amyg), (4) hypothalamus (Hypoth), and (5) periaqueductal gray of the midbrain. Region of cortex included visual cortex (VCtx), somatosensory cortex (SCtx), auditory (AuCtx) and piriform (Pir) cortex at the level of the thalamus (Th). Hypothalamus included both posterior hypothalamic area (PH) and ventromedial hypothalamic nucleus (VMH). Periaqueductal gray included both of its divisions: ventrolateral (vlPAG) and dorsolateral (dlPAG). Abbreviations: IC, inferior colliculus; PnO, pontine reticular nucleus, oral part; py, pyramidal tract; scp, superior cerebellar peduncle. Abbreviations were adapted from The Rat Brain Atlas (Paxinos and Watson, 1998).
2.4. Statistical Analysis
2.4.1. Body Mass Data
The average body mass (g) per certain age (day) were compared between two treatment groups (normal saline control and morphine group) using independent t-test. Two-tailed p value of 0.05 was considered statistically significant.
2.4.2. Density of Caspase-3 Immunoreactive Cells
We calculated the mean density of apoptotic cells (cleaved-caspase-3 immunolabeled profiles/section/brain) ± SD in each anatomical region for all experimental groups. Since the density of double-labeled profiles was small (< 3 double-labeled profiles/section/brain), we did not include a quantitative analysis of the mean density of double-labeled profiles. The mean density of caspase-3 immunolabeled profiles was compared among the three different treatment groups (naïve, normal saline control, and morphine group) using one-way ANOVA. Significant F-tests were followed by post-hoc Tukey HSD comparisons for pairwise tests. This test was selected to protect against false positive results (type I error). Two-tailed p value less than 0.05 with Tukey correction was considered statistically significant. Statistical analysis was performed using the SPSS software package, v.16.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Body Mass in a Model of Repeated Morphine Exposure
We used a model of repeated morphine exposure that was associated with development of morphine dependence and antinociceptive tolerance in PD7 rats (Barr and Wang, 1992; Jones and Barr, 1995; Zhu and Barr, 2003). The latter was also confirmed in out lab (Bajic et al., 2012)Prolonged morphine administration in neonatal rats (PD1-PD7) of twice daily 10 mg/kg sc was associated with slower body mass gain (Fig. 1).
Figure 1. Chronic Morphine Administration in Newborn Rats.
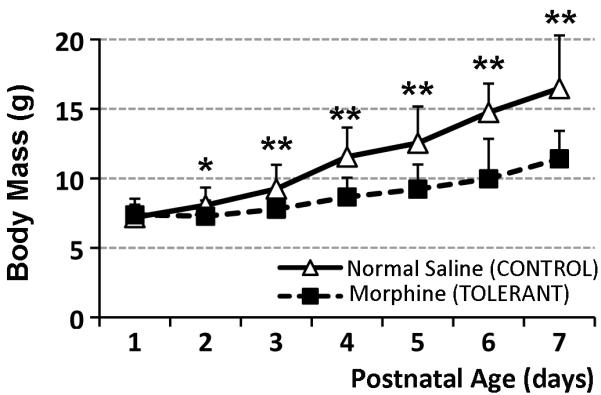
Graph illustrates the effect of repeated morphine exposure on the body mass of newborn rats. Day of birth was considered day 0 of life. Morphine sulfate (10 mg/kg sc twice-daily) or equivalent volume of normal saline was injected for 6 ½ days starting on the postnatal day (PD) 1. Systemic morphine exposure (n=17) significantly decreased body mass (± SD) in newborn rats in comparison to normal saline control group (n=30). There were no differences between naïve (n=6) and normal saline groups (not shown). Two-tailed t-Test: *, p<0.05; **, p<0.01.
3.2. Density of Apoptotic Cells with Morphine Administration
The density of apoptotic cells was analyzed in total of 5 different anatomical regions of interest that included: cortex, amygdala, hippocampus, and hypothalamus of the forebrain (Fig. 2A), as well as the periaqueductal gray of the midbrain (Fig. 2B). Representative illustrations demonstrate the distribution of apoptotic cells following prolonged morphine administration inselected regions. Quantitative analysis of caspase-3 immunoreactive density showed no differences in density between naïve and control groups in any of the regions analyzed (Fig. 3). The highest density of baseline caspase-3 immunoreactive labeling was found in the region of cortex. In addition, only selected brain areas (cortex and amygdala) demonstrated increased density of apoptotic cells following morphine administration. Specifically, the density of apoptotic cells (#caspase-3 cells/section/brain) ± SD was statistically different among treatment groups in the cortex (F(2,15)=5.82; p=0.013) and amygdala (F(2,13)=5.84; p=0.006). Pairwise comparisons with Tukey post-hoc test of all groups in the region of cortex showed a significant increase in density of caspase-3 immunoreactive cells in the chronic morphine group (129.71 ± 65.95) compared to naïve (59.33± 5.26; p<0.05), and control group (61.60 ± 8.22; p<0.05). Similarly, in amygdala the morphine group (29.97 ± 8.44) was significantly different in comparison to naïve (19.82 ± 1.3; p<0.05), and control (18.95 ± 2.52; p<0.01) groups. We found no statistical difference in density of apoptotic cells in hippocampus (F(2,13)=0.47, p=0.63), hypothalamus (F(2,10)=1.68; p=0.23), or periaqueductal gray (F(2,12)=0.34, p=0.71) among three different groups.
Figure 3. Density of Caspase-3 Immunoreactive Cell in Newborn Rat at Postnatal Day 7 (PD7).
Graph illustrates average density of apoptotic cells (#caspase-3 cell/section/brain) ± SD among three treatment groups (naïve, normal saline (control), and morphine (tolerant) group) in five different anatomical regions of interest. There are no differences in density between naïve and control groups in any of the regions analyzed. Morphine treatment was associated with increased density of apoptotic cells only in the cortex (F(2,15)=5.82; p=0.013) and amygdala (F(2,13)=5.84; p=0.006) of newborn rats. Pairwise comparisons of all groups showed statistically significant increase in density of caspase-3 immunoreactive cells in tolerant group compared to naïve (p<0.05) and control groups (p<0.05 for cortex; p<0.01 for amygdala). We found no statistical difference in density of apoptotic cells in hippocampus (F(2,13)=0.47, p=0.63), hypothalamus (F(2,10)=1.68; p=0.23), and periaqueductal gray (PAG; F(2,12)=0.34, p=0.71) among different treatments. For schematic representation of anatomical regions of analysis, refer to Fig. 2. One-way ANOVA with Tukey post-hoc test. Asterisks (*) denote statistically significant increase in values.
3.3. Cell Type of Caspase-3 Immunoreactive Cells: Double-Labeling Immunofluorescence
3.3.1. Neuronal Apoptosis
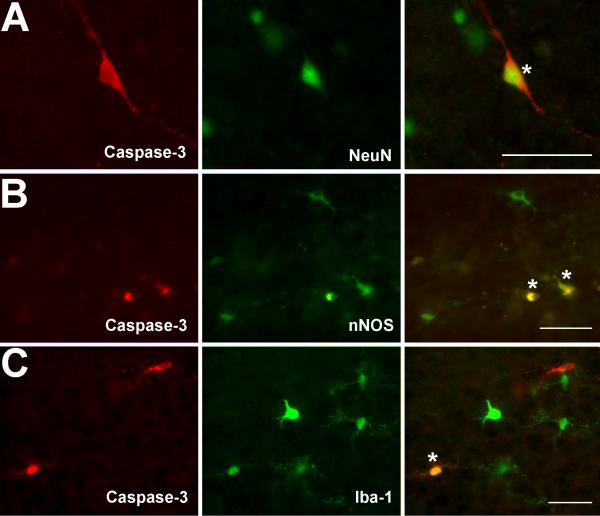
Caspase-3 immunoreactive cells exhibited morphology analogous to different neuronal cell types. In the cortex, caspase-3 immunoreactive cells were typically located in the superficial granular layer (Layer 2) (Vernon, 1998) and corresponded to smaller pyramidal neurons. Caspase-3 immunoreactive cells of the internal pyramidal layer (Layer 5) (Vernon, 1998) exhibited morphology of large neurons. Caspase-3 immunoreactive cells in the cortical multiform layer (Layer 6) (Vernon, 1998) and hippocampus exhibited morphology of stellate or basket neurons. The neuronal nature of caspase-3 immunoreactive cells was confirmed by double-labeling immunofluorescence for neuronal nuclear stain (NeuN; Fig. 4A), as well as nNOS (Fig. 4B). Since the density of apoptotic nNOS neurons was small (0-3 double-labeled neuron/section/brain) in each treatment group, no quantitative analysis was done. In addition, clusters of caspase-3 immunoreactive cells without a defined morphology were also double labeled with nNOS (Fig. 5A; see below for details on caspase-3 immunoreactive clusters). Since it is a specific neuronal marker, nNOS cells did not co-localized with any of the glial labeling.
Figure 4. Identity of Apoptotic Cells in PD7 Rats.
Photomicrographs show double-labeling of caspase-3 immunoreactive cells with neuronal nuclear label (NeuN; A), neuronal nitric oxide synthase (nNOS; B), and microglial label (Iba-1; C). Individual double-labeled cells were marked with asterisks. Scale Bars = 50 μm.
Figure 5. Clusters of Caspase-3 Immunoreactive Cells.
Photomicrographs show double-labeling of sporadic clusters of caspase-3 immunoreactive cells with neuronal nitric oxide synthase (nNOS; A), and label for astrocytes (GFAP; B). Clusters of caspase-3 immunoreactive cells that were losing morphological features were noted only in morphine group in the regions of cortex and hippocampus. Scale Bars = 50 μm.
3.3.2. Glial Apoptosis
Few microglial cells (0-2 double-labeled microglial cell/section/brain) were double-labeled with caspase-3 and Iba-1 immunohistochemistry in each treatment group (Fig. 4C). In addition, the specific marker of microglia used in this study (Iba-1) failed to detect activation of microglia in any of the treatment groups. In other words, there was no difference in morphological appearance of Iba-1 labeled microglia between the treatment groups in any of the regions analyzed (not shown). Furthermore, we did not identify any isolated GFAP-immunoreactive astrocytes that were double-labeled with caspase-3 throughout the forebrain or midbrain in any of the treatment groups. In addition, morphological characteristics of GFAP-immunoreactive astrocytes did not differ between the treatments in any of the regions analyzed (not shown). One exception was the presence of caspase-3 immunoreactive cells in sparce clusters that were losing their morphological features. Clusters were noted to be double-labeled with GFAP, while GFAP labeling showed characteristics of astrocytic activation, such as cellular hypertrophy (Fig. 5B). These sparse clusters of caspase-3 immunoreactive cells were found only in the cortex and hippocampus of the morphine group. Since the density of identified caspase-3 immunoreactive clusters were small (0-3 /tolerant brain), no quantitative analysis was done.
4. Discussion
In the present study, we used previously published model of morphine dependence and antinociceptive tolerance in newborn rats (Barr and Wang, 1992; Jones and Barr, 1995; Zhu and Barr, 2003). The latter was also confirmed in our recent report (Bajic et al., 2012). Our current findings demonstrate that prolonged morphine administration in the neonatal rat (PD1-7) is associated with an increased density of apoptotic cells in selected brain regions. Statistically significant increase in the number of cleaved caspase-3 positive cells was only present in the cortex and amygdala, while no changes were found in hippocampus, hypothalamus orperiaqueductal gray. Additionally, most of caspase-3 immunoreactive cells were neurons, with very few double-labeled for nNOS or microglial marker (Iba-1). These findings implicate susceptibility of selective supraspinal regions (cortex and amygdala) to apoptotic effects of chronic morphine involving primarily neurons instead of glia of neonatal rat.
The association of rat and human developmental stages depends upon several endpoints such as the number of brain cells, degree of myelination, brain growth rate, synaptogenesis, as well as measures related to more contemporary neuroinformatics (Clancy et al., 2001; Clancy et al., 2007). In rodents, this critical period of neuronal differentiation and synaptic development is limited to a time window up to a fourth postnatal week (De Felipe et al., 1997; Micheva and Beaulieu, 1996, 1997). In humans, described brain growth spurt characterized with synaptogenesis and accompanied by dendritic and axonal growth, as well as myelination of the subcortical white matter extends from the last trimester of pregnancy up to the first few years of postnatal life (Huttenlocher and Dabholkar, 1997). In fact, the newborn rat model at PD7 has been extensively used in relation to early (premature, neonatal, and infant) development in humans (McCann and Soriano, 2009). Thus, considering comparative developmental trajectory of rats (days to weeks) in comparison to humans (years) (Andersen, 2003; Andersen and Navalta, 2004), we refer to 6 ½ days of treatment as prolonged (chronic).
4.1. Chronic Morphine Administration and Apoptosis
The mechanisms and sequelae of morphine-induced apoptosis remain unclear, and are unknown in the case of developing brain. Our results are consistent with accumulating evidence from in vitro studies that demonstrated morphine-enhanced apoptosis (Goswami et al., 1998; Singhal et al., 2002; Singhal et al., 2000; Singhal et al., 1999; Singhal et al., 1998; Tegeder et al., 2003; Yin et al., 2000). Spinal cord dorsal horn apoptosis was reported in adult rats made tolerant to morphine administered through either intrathecal boluses or continuous infusion (Mao et al., 2002). In addition, caspase-3 inhibitors that block apoptosis also prevented the development of morphine antinociceptive tolerance in adult rats (Hassanzadeh et al., 2011; Lim et al., 2005; Mao and Mayer, 2001; Mao et al., 2002). Our study implicates susceptibility of selective supraspinal regions to apoptotic effects of morphine that involve the cortex and amygdala, known to be important for sensory and memory of emotional processing, respectively (see review by Davidson (Davidson, 2002)). In contrast, a forebrain region important for learning and memory (hippocampus) (Leuner and Gould, 2010) was not affected by prolonged morphine administration in our study. Studies in adult mice using chronic opioid-treatment paradigm (6 days) demonstrated apoptotic cell death that was accompanied by up-regulation of the pro-apoptotic proteins in the cortex and hippocampus (Emeterio et al., 2006). Interestingly, authors did not detect any base-line apoptosis under control conditions and they described that apoptotic cells were detected “diffusely throughout the brain sections, without any evident regional preference.” Differences between our results and the report by Emeterio et al. (2006) (Emeterio et al., 2006) could be explained by gender differences (mice vs. rat), age (adult vs. PD7), pharmacological treatment (sustained-release preparation of morphine vs. bolus sc injections), or immuno-labeling technique (TUNEL staining vs. cleaved caspase-3 immunofluorescence). Finally, we report that brain regions important for autonomic and nociceptive processing (hypothalamus and periaqueductal gray) (Leone et al., 2006) did not demonstrate change in apoptosis following morphine administration from PD1-7. This is despite our recent report that acute morphine activates neurons in the ventrolateral periaqueductal gray, and moreover, that chronic morphine exposure is associated with plasticity in the same region at this early age (Bajic et al., 2012).
4.2. Morphine Induces Neuronal Apoptosis and Oxidative Stress
Our study provided evidence that some apoptotic cells are neurons (as demonstrated by NeuN immunofluorescence). This is supported by both in vitro (Hu et al., 2002) and in vivo (Emeterio et al., 2006) studies. The former study demonstrated morphine-enhanced apoptosis in a concentration-dependent manner, and that neurons were more sensitive than glia to morphine’s effect on apoptosis (Hu et al., 2002). Furthermore, our study also showed that only a few apoptotic cells were nNOS immunoreactive irrespective of the treatment. It is known that nitric oxide is upregulated as a part of oxidative stress mechanism associated with chronic opioid exposure in adult rats (Babey et al., 1994), which in turn may lead to neuronal apoptosis (Salvemini, 2009). High concentrations of nitric oxide (initial signaling mediator of nitro-oxidative stress) was associated with neuronal necrosis (Virag et al., 2003), while exposure to lower concentrations of reactive oxygen species leads to delayed, apoptotic neuronal death (Niles et al., 2006). Given these reports and our observation of negligible number of nNOS neurons undergoing apoptosis, the role of nNOS in developing brain with chronic morphine administration, in particular within the cortex and amygdala, remains to be investigated.
4.3. Glial Role with Morphine
In the current study, only a small number of caspase-3 immunoreactive cells were identified as microglia (Iba-1 immunoreactive) irrespective of the treatment while no individual astrocytes (GFAP-immunoreactive) were found to undergo apoptosis. These findings are consistent with reported in vitro study, which demonstrated that, in contrast to microglia and neurons, astrocytes were completely resistant to morphine-induced apoptosis (Hu et al., 2002). However, this is not in agreement with report by Emeterio et al. (2006) (Emeterio et al., 2006) that showed induction of apoptosis in adult mouse astrocytes following chronic morphine treatment. Considering glial distinctly unique morphology during early brain development accompanied by significantly higher expression of many cytokines when compared to the adult brain (see Review (Schwarz and Bilbo, 2012)), future studies should investigate the ontogeny of morphine-induced sensitivity of astrocytes to apoptosis. Furthermore, we did not identify morphological characteristics of microglial activation following repeated morphine administration. Few researchers have noted that the invasion of microglia within the developing brain coincides with naturally occurring cell death during early brain development (Ashwell, 1990, 1991; Perry et al., 1985). Very sparse astrocytic activation was noted only after repeated chronic treatment (Fig. 5B), which is in contrast to the striking astrocytic activation observed in adult rats (Bajic and Commons, unpublished observations). Since astrocytic activation is closely linked to its role in the repair and scarring process of the brain (Pekny and Nilsson, 2005), it remains to be investigated if observed sparse clusters of hypertrophied astrocytes have detrimental or beneficial effects for overall neuroplasticity in cortex and hippocampus. Glial activation has been implicated in mediating negative behavioral effects of chronic morphine administration in adult rodents (viz., antinociceptive tolerance, opioid dependence) via Toll-like receptor 4 (Hutchinson et al., 2007; Hutchinson et al., 2012; Watkins et al., 2005; Watkins et al., 2009; Watkins and Maier, 2003). Thus, future pharmacological studies should also elucidate age-dependent role of glia in chronic morphine effects.
4.4. Behavioral Significance
Our results implicate region-specific sensitivity to chronic morphine enhanced apoptosis in a newborn rat. Future parallel study in older rats should address if newborn period (PD1-7) is particularly critical for prolonged morphine induced apoptosis in cortex and amygdala. Although current model of prolonged morphine administration is associated with development of antinociceptive tolerance and dependence in newborn rats (Bajic et al., 2012; Barr and Wang, 1992; Jones and Barr, 1995; Zhu and Barr, 2003), future studies should investigate long-term behavioral sequelae of demonstrated increased apoptosis in cortex and amygdala. Despite documented adverse neurological impact of untreated neonatal pain in animals (Anand et al., 2007) and humans (Fitzgerald and Walker, 2009), only a few recent animal studies examined the long-term effects of preemptive antinociception in early neonatal age. Specifically, neonatal morphine treatment in mice produced long-lasting behavioral effects such as reduced adult arousal sufficient to alter learning later in life (Boasen et al., 2009). Similar treatment in rat pups also impaired the adult cognitive functioning (McPherson et al., 2007) as demonstrated by (1) impaired passive avoidance learning (test classically used to assess learning and memory mediated by amygdala), (2) conditioned place preference (mediated by the mesolimbic dopamine system (Prus et al., 2009)), and (3) the forced swimming learning paradigms (which involve serotonergic system (Petit-Demouliere et al., 2005)). Early life-prolonged opioid exposure is also associated with long-term effect such as: decreased analgesia, increased pain sensitivity (Zhang and Sweitzer, 2008), and hypersensitivity to adverse/stress conditions (Anderson et al., 2012). However, these studies did not investigate underlying cellular neuroplasticity that might explain reported behavioral changes. Damage to the amygdala in humans and animals results in a profound impairment in learning ability, especially in those tasks that require the subject to make a connection between environmental stimuli and strong emotional responses (Adolphs et al., 1995; Davis, 1992, 1994; LaBar et al., 1995; Maren et al., 1996). In addition to mediating emotional memory (Phelps and LeDoux, 2005), amygdala as a complex structure is also involved in pain pathways and emotional-affective dimension of pain (Neugebauer, 2006; Neugebauer et al., 2009; Neugebauer et al., 2004; Simons et al., 2012), addiction (Koob, 2009) and possibly in drug-associated memory and drug seeking behavior (Milton et al., 2008), processing of mood (Seymour and Dolan, 2008), and fear (Pape and Pare, 2010). It was also reported that prenatal morphine exposure leads to sex differences in mu opioid receptor densities in rat amygdala(Vathy et al., 2003). Thus, future studies should also investigate ontogeny of possible sex differences of presented findings.
5. Conclusions
Prolonged neonatal opioid exposure has been associated with long-term neurodevelopmental consequences both in rats (Boasen et al., 2009; McPherson et al., 2007) and humans (Anand et al., 2004; Ferguson et al., 2012; McGlone et al., 2009). Our results support the hypothesis that apoptotic cell death is increased following morphine administration associated with development of antinociceptive tolerance in newborn rat. A significant increase in density of apoptotic neurons was found only in cortex and amygdala known to be important for sensory and emotional memory processing, respectively (Davidson, 2002). However, irrespective of the treatment, only a few individual microglia cells were caspase-3 positive. In contrast to the adult rat (Hutchinson et al., 2007; Watkins et al., 2005; Watkins et al., 2009; Watkins and Maier, 2003), astrocytic activation was sparce in the newborn rat following repeated morphine administration, suggesting an age-dependent role of glia in mediating morphine effects.
HIGHLIGHTS.
Repeated morphine administration leads to antinociceptive tolerance in newborn rats.
It is associated with increased density of apoptotic cells on postnatal day 7.
Significant increase in density of apoptotic cells is found in the cortex and amygdala.
Apoptotic cells exhibited morphology analogous to neurons.
Only a very few individual microglia but not astrocytes were caspase-3 positive.
Astrocytic activation following chronic morphine administration was sparse in PD7 rat.
Acknowledgements
This work was supported by the Foundation for Anesthesia Education and Research (FAER), Endo Pharmaceuticals, and NIH R03 DA030874 (D.B.), NIH R01 DA021801 (K.G.C.) and the Boston Children’s Hospital Endowed Chair in Neuroanesthesia Grant (S.G.S.).
Footnotes
Abbreviations: GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule 1; ip; intraperitoneal; NeuN, neuronal nuclear antigen; nNOS, neuronal nitric oxide synthase (NOS1); NS, normal saline; PB, phosphate buffer; PD, postnatal day; sc, subcutaneous.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J. Neurosci. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch. Pediatr. Adolesc. Med. 2001;155:173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr. Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C. Tolerance and Withdrawal From Prolonged Opioid Use in Critically Ill Children. Pediatrics. 2010;125:1208–1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int. J. Dev. Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Neubert JK, Caudle RM. Long-term changes in reward-seeking following morphine withdrawal are associated with altered N-methyl-D-aspartate receptor 1 splice variants in the amygdala. Neuroscience. 2012;223:45–55. doi: 10.1016/j.neuroscience.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res. Dev. Brain Res. 1990;55:219–230. doi: 10.1016/0165-3806(90)90203-b. [DOI] [PubMed] [Google Scholar]
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res. Dev. Brain Res. 1991;58:1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Babey AM, Kolesnikov Y, Cheng J, Inturrisi CE, Trifilletti RR, Pasternak GW. Nitric oxide and opioid tolerance. Neuropharmacology. 1994;33:1463–1470. doi: 10.1016/0028-3908(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Bajic D, Berde CB, Commons KG. Periaqueductal gray neuroplasticity following chronic morphine varies with age: role of oxidative stress. Neuroscience. 2012;226:165–177. doi: 10.1016/j.neuroscience.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. Eur. J. Pharmacol. 1992;215:35–42. doi: 10.1016/0014-2999(92)90605-4. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. Periaqueductal gray. In: Paxinos G, editor. The Rat Nervous System. Academic Press; New York: 1995. pp. 173–182. [Google Scholar]
- Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N. Engl. J. Med. 2002;347:1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- Besunder JB, Blumer JL. Neonatal drug withdrawal syndromes. In: Koren G, editor. Maternal–Fetal Toxicology. Marcel Dekker, Inc.; New York: 1990. pp. 161–177. [Google Scholar]
- Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- Boasen JF, McPherson RJ, Hays SL, Juul SE, Gleason CA. Neonatal stress or morphine treatment alters adult mouse conditioned place preference. Neonatology. 2009;95:230–239. doi: 10.1159/000165379. [DOI] [PubMed] [Google Scholar]
- Booze RM, Mactutus CF. Experimental design considerations: a determinant of acute neonatal toxicity. Teratology. 1985;31:187–191. doi: 10.1002/tera.1420310203. [DOI] [PubMed] [Google Scholar]
- Chambliss CR, Anand KJ. Pain management in the pediatric intensive care unit. Curr. Opin. Pediatr. 1997;9:246–253. doi: 10.1097/00008480-199706000-00011. [DOI] [PubMed] [Google Scholar]
- Che Y, Sun H, Tan H, Peng Y, Zeng T, Ma Y. The effect of prenatal morphine exposure on memory consolidation in the chick. Neurosci. Lett. 2005;380:300–304. doi: 10.1016/j.neulet.2005.01.061. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ. Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology (Berl) 1999;143:1–7. doi: 10.1007/s002130050911. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol. Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends. Pharmacol. Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in emotional learning. Int. Rev. Neurobiol. 1994;36:225–266. doi: 10.1016/s0074-7742(08)60305-0. [DOI] [PubMed] [Google Scholar]
- De Felipe J, Marco P, Fairen A, Jones EG. Inhibitory synaptogenesis in mouse somatosensory cortex. Cereb. Cortex. 1997;7:619–634. doi: 10.1093/cercor/7.7.619. [DOI] [PubMed] [Google Scholar]
- de Graaf J, van Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, Roofthooft DW, Groot Jebbink LJ, Veenstra RR, Tibboel D, van Dijk M. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152:1391–1397. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Emeterio EP, Tramullas M, Hurle MA. Modulation of apoptosis in the mouse brain after morphine treatments and morphine withdrawal. J. Neurosci. Res. 2006;83:1352–1361. doi: 10.1002/jnr.20812. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: Effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol. Teratol. 2012;34:47–55. doi: 10.1016/j.ntt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat. Clin. Pract. Neurol. 2009;5:35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- Fonsmark L, Rasmussen YH, Carl P. Occurrence of withdrawal in critically ill sedated children. Crit. Care Med. 1999;27:196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J. Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh K, Habibi-Asl B, Farajnia S, Roshangar L. Minocycline Prevents Morphine-Induced Apoptosis in Rat Cerebral Cortex and Lumbar Spinal Cord: A Possible Mechanism for Attenuating Morphine Tolerance. Neurotox. Res. 2011;19:649–659. doi: 10.1007/s12640-010-9212-0. [DOI] [PubMed] [Google Scholar]
- He X, Bao Y, Li Y, Sui N. The effects of morphine at different embryonic ages on memory consolidation and rewarding properties of morphine in day-old chicks. Neurosci. Lett. 2010;482:12–16. doi: 10.1016/j.neulet.2010.06.074. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum. Dev. 2008;84:29–35. doi: 10.1016/j.earlhumdev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johannesson T, Becker BA. Morphine analgesia in rats at various ages. Acta Pharmacol. Toxicol. (Copenh) 1973;33:429–441. doi: 10.1111/j.1600-0773.1973.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Barr GA. Ontogeny of morphine withdrawal in the rat. Behav. Neurosci. 1995;109:1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit. Care Med. 1994;22:763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Konijnenberg C, Melinder A. Prenatal exposure to methadone and buprenorphine: a review of the potential effects on cognitive development. Child. Neuropsychol. 2011;17:495–519. doi: 10.1080/09297049.2011.553591. [DOI] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain. Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone M, Proietti Cecchini A, Mea E, Tullo V, Curone M, Bussone G. Neuroimaging and pain: a window on the autonomic nervous system. Neurol. Sci. 2006;27(Suppl 2):S134–137. doi: 10.1007/s10072-006-0588-9. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010;61:111–140. C111–113. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G, Wang S, Lim JA, Mao J. Activity of adenylyl cyclase and protein kinase A contributes to morphine-induced spinal apoptosis. Neurosci. Lett. 2005;389:104–108. doi: 10.1016/j.neulet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann. N Y. Acad. Sci. 2001;933:175–184. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J. Neurosci. 2002;22:7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Retrograde abolition of conditional fear after excitotoxic lesions in the basolateral amygdala of rats: absence of a temporal gradient. Behav. Neurosci. 1996;110:718–726. doi: 10.1037//0735-7044.110.4.718. [DOI] [PubMed] [Google Scholar]
- McCann ME, Soriano SG. Is anesthesia bad for the newborn brain? Anesthesiol Clin. 2009;27:269–284. doi: 10.1016/j.anclin.2009.05.007. [DOI] [PubMed] [Google Scholar]
- McGlone L, Mactier H, Weaver LT. Drug misuse in pregnancy: losing sight of the baby? Arch. Dis. Child. 2009;94:708–712. doi: 10.1136/adc.2008.156851. [DOI] [PubMed] [Google Scholar]
- McPherson RJ, Gleason C, Mascher-Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology. 2007;92:33–41. doi: 10.1159/000100084. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J. Comp. Neurol. 1996;373:340–354. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can. J. Physiol. Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J. Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Kazeminezhad B, Dargahi L, Ahmadiani A. Maternal oral consumption of morphine increases Bax/Bcl-2 ratio and caspase 3 activity during early neural system development in rat embryos. J. Mol. Neurosci. 2010;41:156–164. doi: 10.1007/s12031-009-9312-6. [DOI] [PubMed] [Google Scholar]
- Nasiraei-Moghadam S, Sherafat MA, Safari MS, Moradi F, Ahmadiani A, Dargahi L. Reversal of Prenatal Morphine Exposure-Induced Memory Deficit in Male But Not Female Rats. J. Mol. Neurosci. 2012 doi: 10.1007/s12031-012-9860-z. doi: 10.1007/s12031-012-9860-z. [DOI] [PubMed] [Google Scholar]
- Neugebauer V. Subcortical processing of nociceptive information: basal ganglia and amygdala. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology. Elsevier; Amsterdam: 2006. pp. 141–158. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res. Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Niles JC, Wishnok JS, Tannenbaum SR. Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide. 2006;14:109–121. doi: 10.1016/j.niox.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu. Rev. Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed Academic Press; Orlando, FL: 1998. [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosecrans JA. Conditioned Place Preference. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton: 2009. [PubMed] [Google Scholar]
- Rabinowicz T, de Courten-Myers GM, Petetot JM, Xi G, de los Reyes E. Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J. Neuropathol. Exp. Neurol. 1996;55:320–328. [PubMed] [Google Scholar]
- Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur. J. Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Ramachandra R, Subramanian T. Atlas of the Neonatal Rat Brain. CRC Press; Boca Raton: 2011. [Google Scholar]
- Sadraie SH, Kaka GR, Sahraei H, Dashtnavard H, Bahadoran H, Mofid M, Nasab HM, Jafari F. Effects of maternal oral administration of morphine sulfate on developing rat fetal cerebrum: a morphometrical evaluation. Brain. Res. 2008;1245:36–40. doi: 10.1016/j.brainres.2008.09.052. [DOI] [PubMed] [Google Scholar]
- Salvemini D. Peroxynitrite and opiate antinociceptive tolerance: a painful reality. Arch. Biochem. Biophys. 2009;484:238–244. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. The functional role of microglia and immune molecules in neurodevelopment. In: Schwarz JM, Bilbo SD, editors. The Immune System and the Developing Brain. Morgan & Claypool Life Sciences; San Francisco: 2012. [Google Scholar]
- Seatriz JV, Hammer RP., Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res. Bull. 1993;30:523–527. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Simons L, Moulton E, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: Evidence from neuroimaging. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22199. doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J. Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Kapasi AA, Franki N, Reddy K. Morphine-induced macrophage apoptosis: the role of transforming growth factor-beta. Immunology. 2000;100:57–62. doi: 10.1046/j.1365-2567.2000.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Kapasi AA, Reddy K, Franki N, Gibbons N, Ding G. Morphine promotes apoptosis in Jurkat cells. J. Leukoc. Biol. 1999;66:650–658. doi: 10.1002/jlb.66.4.650. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J. Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- Slamberova R, Riley MA, Vathy I. Cross-generational effect of prenatal morphine exposure on neurobehavioral development of rat pups. Physiol. Res. 2005;54:655–660. [PubMed] [Google Scholar]
- Spratto GR, Dorio RE. Effect of age on acute morphine response in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1978;19:23–36. [PubMed] [Google Scholar]
- Tegeder I, Grosch S, Schmidtko A, Haussler A, Schmidt H, Niederberger E, Scholich K, Geisslinger G. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003;63:1846–1852. [PubMed] [Google Scholar]
- Thornton SR, Wang AF, Smith FL. Characterization of neonatal rat morphine tolerance and dependence. Eur. J. Pharmacol. 1997;340:161–167. doi: 10.1016/s0014-2999(97)01434-9. [DOI] [PubMed] [Google Scholar]
- Vathy I, Slamberova R, Rimanoczy A, Riley MA, Bar N. Autoradiographic evidence that prenatal morphine exposure sex-dependently alters mu-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:381–393. doi: 10.1016/S0278-5846(02)00355-X. [DOI] [PubMed] [Google Scholar]
- Vernon BM. Perceptual neuroscience: the cerebral cortex. 1st ed Harvard University Press; Boston: 1998. [Google Scholar]
- Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol. Lett. 2003;140-141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, Siqveland T, Fjell AM, Bjornebekk A, Smith L. Effects of prenatal opiate exposure on brain development--a call for attention. Nat. Rev. Neurosci. 2009;10:390. doi: 10.1038/nrn2598-c1. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J. Exp. Med. 2000;191:1423–1428. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Sweitzer SM. Neonatal morphine enhances nociception and decreases analgesia in young rats. Brain Res. 2008;1199:82–90. doi: 10.1016/j.brainres.2007.12.043. [DOI] [PubMed] [Google Scholar]
- Zhu H, Barr GA. Ontogeny of NMDA receptor-mediated morphine tolerance in the postnatal rat. Pain. 2003;104:437–447. doi: 10.1016/S0304-3959(03)00051-4. [DOI] [PubMed] [Google Scholar]