Abstract
Higher whole grain cereal intakes are associated with substantially lower risks of type 2 diabetes, coronary heart disease, and hypertension. These reduced risks have been established in large prospective studies that now include millions of person-years of follow-up. We analyze the results of 11 major prospective studies to provide recommendations about whole grain consumption. The following review establishes the amount of whole grains that should ideally be consumed based on prospective evidence; defines the nature of whole grains; identifies that the whole grain evidence is robust and not due to confounding; and provides a detailed assessment of several potential mechanisms for the effect of whole grains on health. We draw the following conclusions. Firstly, to maintain health, 40 grams or more of whole grains should be consumed daily. This is about a bowl of whole grain breakfast cereal daily, but 80% of the population does not achieve this. Secondly, aleurone in bran is a critical grain component generally overlooked in favor of indigestible fiber. Live aleurone cells constitute 50% of millers’ bran. They store minerals, protein, and the antioxidant ferulic acid, and are clearly more than just indigestible fiber. Finally, we suggest potential roles for magnesium, zinc, and ferulic acid in the development of chronic disease. If the results of prospective studies were applied to the life-style practices of modern societies there exists the potential for enormous personal health and public financial benefits.
Key Terms: Whole grains, Type 2 Diabetes, Hypertension, Coronary Heart Disease, Prospective studies, Fiber, Bran, Aleurone, Ferulic Acid, Magnesium, Zinc
Health context of whole grain consumption
Higher whole grain intakes are associated with a lower incidence of chronic disease (1–12), and the reduction in risk appears substantial. This potential to alter the risk of chronic disease is critical because type 2 diabetes (T2D), coronary heart disease (CHD), and hypertension generate huge health costs. For example in the US, the incidence of diabetes rose from 4.8 cases/1000 person-years in 1997 to 9.1 in 2007(13) leading to a staggering estimated annual cost of US$85 billion, directly attributed to the disease (14). The health care costs of hypertension and CHD are similarly high (15).
An increased whole grain intake may well reduce the prevalence of chronic disease and lead to personal and public gain. To realize this potential whole grain boon we need to convince clinicians and the public alike of the benefits of whole grain consumption. This could be achieved by: demonstrating that the reduced incidence in chronic disease is causally related to whole grains; defining the “dose” of whole grain intake required for a health advantage; and by providing plausible mechanisms by which whole grains might work. These are the purposes of this review
Definitions for whole grains and cereal fiber
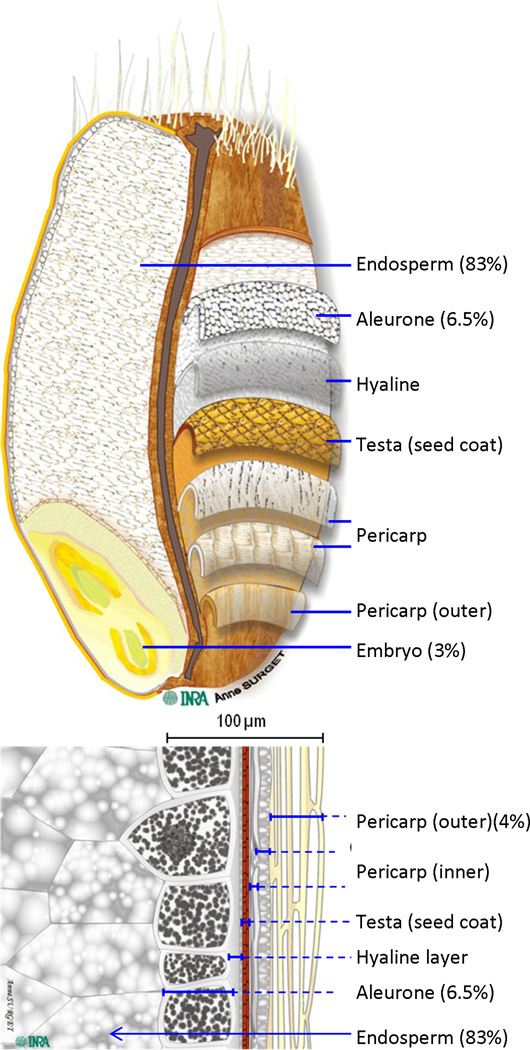
“Whole grains are cereal grains that consist of the intact, ground, cracked, or flaked kernel, which includes the bran, the germ, and the inner most part of the kernel (the endosperm).” (FDA) (16) (Figure 1). This definition still holds even if the grain components are initially separated and later recombined. However the bran, germ, and endosperm must be present in the same relative proportions as they exist in the intact grain kernel for the recombined food to be considered a whole grain food. Whole should not be confused with unbroken: a grain of polished Jasmine rice is in one piece (unbroken) but it is not a whole grain as both the bran and germ have been removed. This is not a trivial issue; the word “whole” has a very specific definition in this instance, one that is easily misunderstood by even experienced medical professionals.
Figure 1.
The physical structure of a wheat grain with the proportions w/w presented (18, 20). (Used by permission of Dr Cécile Barron, INRA - Institut National de la Recherche Agronomique (France), and Dr Jacques Potus, Editor–in-Chief Industries des Céréales).
Whole grains are well recognized as an important source of dietary fiber. The US Institute of Medicine (IOM) of the National Academies defines dietary fiber as those food components that are indigestible by human enzymes (17). The IOM recommendation for a total fiber ‘Adequate Intake’ is derived from levels of intake observed to protect against CHD. Functional fiber is a sub-category of total dietary fiber identified by three physiological effects: laxative effect, blood lipid and blood glucose lowering. The emphasis in this report is firmly on the physical properties of fiber.
Prospective studies have demonstrated a relationship between chronic disease and both cereal fiber and whole grain intake, and the two are often considered equivalent. This equivalence is true in a practical sense since IOM-defined cereal fiber is never consumed in a pure and isolated form, but typically as either whole grains or in the bran or germ fractions prepared by grain milling. It is possible therefore that in prospective studies the relationships found with cereal fiber are confounded by the effects of other components also present in whole grain foods. A focus on cereal fiber could easily dismiss contributions of other accompanying key nutrients. We aim to demonstrate the plausibility that it is key nutrients associated with fiber, not indigestible fiber itself, that principally confer the positive effects of whole grains on T2D, CHD and hypertension.
Anatomy a whole grains (wheat as the example)
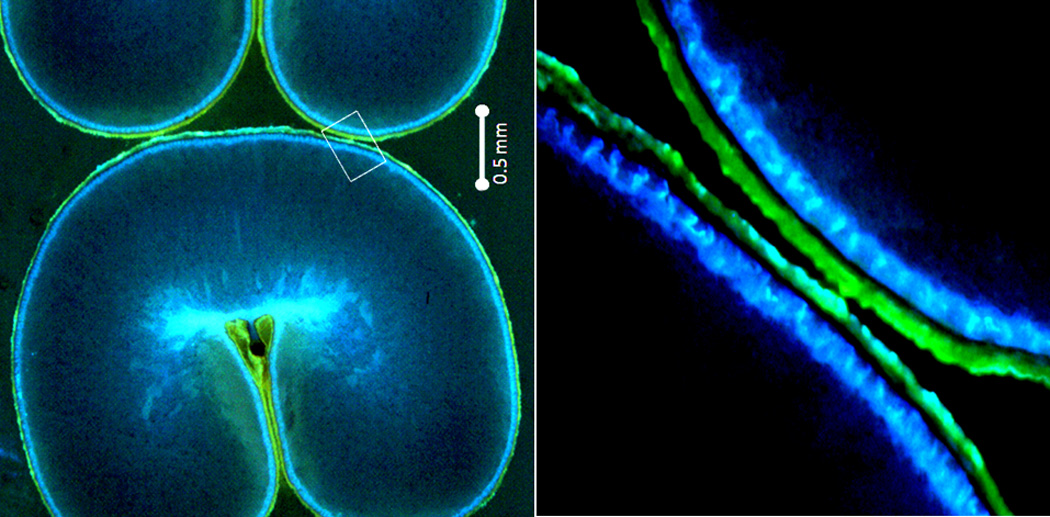
A dissected wheat grain is illustrated in Figure 1, and the relative proportions of the tissues are provided (18–20). The endosperm, aleurone and germ are composed of triploid cells that develop following pollen fertilization (i.e. they are true grain cells). The outer layers of the grain by contrast are diploid maternal tissues (21, 22). All of these tissues must be present to fulfill the FDA definition of a whole grain. The germ is the embryonic plant and remains dormant until germination. The endosperm is composed of apoptosed cells (23) containing linear (amylose) or branched (amylopectin) glucose polymers. Small amounts of cell wall also remain. The endosperm is lower in protein than the aleurone or germ, but because most of a grain is endosperm, three quarters of wheat protein resides in the endosperm (19). Aleurone cells are endosperm-derived tissues and can interconvert with endosperm cells during grain development (21, 22). However, the aleurone is highly specialized, with several critically important and distinct features. Firstly, the aleurone is tolerant of desiccation, remaining alive and metabolically active in mature grain while most of the grain tissue has apoptosed. Secondly, the aleurone is rich in inorganic nutrients, vitamins and antioxidants. Upon grain germination, the aleurone cells release metabolic machinery (e.g. amylase with Ca2+ near its active site) to digest the endosperm, and provide nutrients to the growing embryonic plant (e.g. Mg2+ for chlorophyll) (22–24). Finally, the aleurone is firmly attached to the external grain-covering layers even though it is endosperm in origin. This is clearly seen in Figure 2(25). This attachment results in the aleurone cell walls and contents partitioning physically with the bran fraction, not the endosperm/flour fraction, during milling (19, 25). The outer-most layer of the grain is the pericarp which is composed of remnant cell walls (26), and a complex intermediate layer underlies it. More than 50% of millers’ bran is aleurone cells, the remainder being pericarp and other covering tissues (20, 27).
Figure 2.

The microstructure of acid fuchin and calcofluor stained wheat bran (25). Protein appears red, β-glucan in cell walls light blue, and grain coverings (pericarp and intermediate layer) - green/yellow/brown. The importance of this photomicrograph is that wheat bran contains not only the tough outer pericarp and associated diploid maternal tissues, but also a layer of endosperm-related aleurone cells (triploid) which are still alive in the mature grain unlike the apoptosed cells of the starchy endosperm. These live cells supply the necessary biochemical machinery (such as amylase) to mobilize the endosperm for the germinating embryo, and store minerals such as magnesium which will be essential for chlorophyll function. (Used by permission of Per Åman, Professor, Swedish University of Agricultural Sciences).
The key structural molecule in cereals (wheat, corn, rice and rye) is arabinoxylan (28). Arabinoxylan (one of the hemicellulose families) is a polymer of pentose sugars formed from a linear backbone of xylose (C5H10O5) with arabinose (C5H10O5) side chains attached (29). Ferulic acid ([E]-3-[4-hydroxy-3-methoxyphenyl]prop-2-enoic acid) attaches to the arabinose side chains forming cross links between neighboring arabinoxylan polymers. This cross-linking gives grain cell walls their structural properties (26) and the degree of cross-linking is reflected in the ratio of ferulic acid dimers to xylose (20, 27, 30). Cross-linking determines the ease of access of a suite of catalytic enzymes required for complete arabinoxylan digestion (31–33), and therefore the degree of resistance to cell wall degrading enzymes from grain pathogens, or colonic micro-organisms (fermentation). Bran (i.e. the aleurone and outer layers of the grain) is the chief source of grain arabinoxylan (20). Ferulic acid cross-linking is 3 to 5 times higher in the pericarp than the aleurone (20, 27, 30), making the aleurone more fermentable than the pericarp. Cellulose and lignin, both relatively minor components of whole grains, are largely limited to the pericarp and non-aleurone parts of bran (19, 26, 28, 30, 34). They provide structural support and resistance to enzyme attack (35), combining with ferulic acid cross-linking to prevent pericarp and intermediate layer degradation. These factors suggest that the pericarp is the major stool bulking agent in whole grains, and probably explain why bran is a superior stool bulking agent compared to other fiber sources (36, 37).
β-glucan is the major structural element in oats and is also found in barley, but it is present in lesser amounts in other grains (28). It has the structure of cellulose (i.e. a glucose polymer with β-1→4 linkages) but approximately 30% of the bonds are non-cellulosic (i.e. 1→3 linkages). These 1→3 bonds create kinks in the molecule preventing alignment and fibril development, but thereby also allowing gel formation (38). The health benefits of β-glucan have been attributed to these polymer physicochemical properties (39). The human colon microbiome synthesizes glucanases (40), so that while β-glucan is not digestible in other regions of the gut, it can be fermented to cellulose tri- and tetra-saccharides (38), fermentation presumably reducing any substantial stool bulking effect.
Plant cell wall structural polysaccharides - cellulose, arabinoxylans and β-glucan from grains, and pectins and xyloglucans from fruit (35, 41) - are generally not digested by humans. Lignin, perhaps the most complex of all biopolymers, is not a polysaccharide. It renders plant tissues particularly resistant to breakdown, and is only efficiently digested by soil white-rot fungi (42). The human gut microbiome produces a wide range of polysaccharide digestive enzymes allowing many indigestible polysaccharides to be dismantled to individual monosaccharides (40, 43, 44). Monosaccharides are then metabolized by gut micro-organisms to pyruvate (C3) and lactate (C3), followed by even more energy harvesting by conversion to acetyl-CoA, acetate (C2) and butyrate(C4), or lactate condensation to propionate (C3)(45–48). Acetate comprises 60–80% of the volatile fatty acids (also called short chain fatty acids, SCFA) produced by fermentation of polysaccharides in vitro, with lesser amounts of propionate and butyrate produced (37, 49–51). These SCFA have attracted attention because of their potential benefits to the colon mucosa (52).
What emerges from this assessment is that three separate benefits can be attributed to the fiber itself. The stool bulking effect is not so much an effect of the chemical structure of the polysaccharide itself, but how tightly it is bound to other cell wall components. This tight binding regulates access by microbial digestive enzymes. The second benefit is that some cell wall polysaccharides, especially pectins and β-glucan, will create gels (see below). This principally benefits upper gastrointestinal function since the effect disappears if the polysaccharide is fermented. The third benefit is that most polysaccharides, if enzyme accessible, will be fermented to volatile fatty acids with potential direct health benefits.
As illustrated in Figure 1, aleurone cells have a distinct granular substructure. This appearance is due to aleurone cells containing large numbers of sub-cellular organelles that have an endoplasmic reticulum-derived enclosing membrane and which store proteins, inositol-6-phosphate (phytate), and essential inorganic nutrients (53–58). These organelles are variously described as protein bodies, protein storage vacuoles, and phytate globoids within the vacuoles. Phytate globoids are sufficiently rich in inorganic nutrients that high intensity x-rays can induce characteristic fluorescence (Figure 3a). Aleurone cell walls are also rich in phenolic acids, particularly ferulic acid, and they possess a characteristic natural fluorescence (Figure 3b), known to grain millers and cereal scientists for decades (59–61), though probably not to clinicians to whom it may come as a surprise. Aleurone cells therefore provide human nutrition with a major source of inorganic nutrients as well as anti-oxidants from within cell walls.
Figure 3.
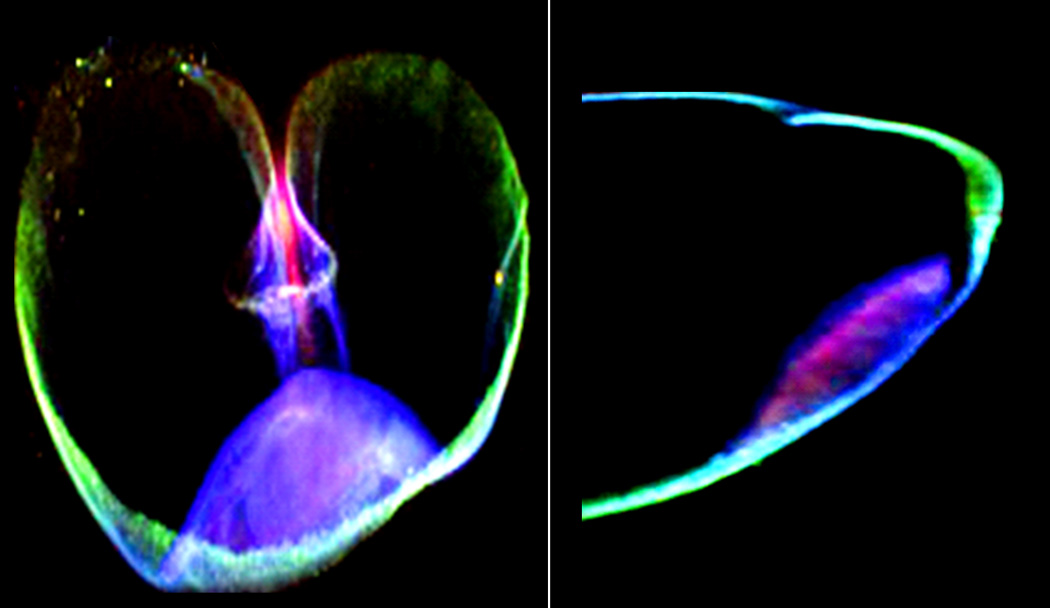
a Mineral fluorescence in transverse (left) and longitudinal (right) sections of wheat grain under x-ray illumination. In both sections, the crease region is at the top of the image and the embryo (germ) at the bottom. It is clearly evident that the central part of the grain (the endosperm) contains little to no mineral content. The minerals, here represented as iron (green), zinc (red) and manganese (blue) are distributed particularly in the phytate inclusions of the aleurone (53–56). The embryo here shows zinc and manganese whose concentration is higher in the germ (64), however due to the germ’s smaller mass w/w, the bran still contributes more zinc and manganese overall in a whole grain diet. These images were produced at the Diamond Light Source synchrotron facility on the microfocus spectroscopy beamiline I18 using 10 keV excitation.
b Native fluorescence associated with the pericarp and aleurone layers observed in sectioned wheat grain. When excited with 359 nm wavelength light, aleurone cells emit fluorescence in the 460 nm (blue) region of the spectrum due to high concentrations of hydroxycinnamic acids, particularly ferulic acid. In a similar manner, when viewed under 492 nm light, the grain coverings emit fluorescence in the 520 nm (green) region of the spectrum of uncertain origin (59–61).
This detailed discussion of grain structure demonstrates that whole grain fiber is not merely indigestible material. In fact, fiber is always associated with a host of other nutrients that probably have significant biological effects. Health advantages from whole grains are not likely to be limited to the direct or indirect effects of fiber itself. Bran, the archetypal source of dietary fiber, is always accompanied by aleurone, one of the most nutrient rich and specialized layers in a grain kernel. Whole grains and bran represent a great deal more than indigestible dietary fiber.
The advantage of prospective studies in establishing the health benefits of whole grains
Environmental and lifestyle factors, acting over decades of life, eventually lead to the development of chronic disease in susceptible individuals. Experimental evaluation of these factors with short or long term randomized nutrition trials present some practical problems. Short term nutrition trials measure markers thought to predict future disease development, and assume that the selected maker reflects the underlying chronic disease mechanism accurately. Identifying the optimal marker will depend on accurately knowing the disease pathophysiology. However, this pathophysiology may be the very knowledge being sought – mutually frustrating requirements, a “Catch-22”. Alternatively, long-term nutrition trials are logistically difficult and prohibitively expensive for dietary exposures lasting years, and are never likely to be achieved. As a result, studies performed prospectively, over protracted risk periods appropriate to chronic disease development, provide some of the richest information about contributions of habitual diet to disease development. Well designed prospective studies, where the historical dietary behavior of subjects currently developing disease is assessed, have a key role in informing us about the etiology of chronic disease.
Selecting prospective studies for an analysis of whole grain contribution to chronic disease
To examine the effect of whole grain intake on the development of T2D, CHD, and hypertension, we evaluated eleven prospective US disease studies (1–12) (Table1), correlating disease incidence to whole grain intake (grams/day). Articles were selected primarily through Medline® and met the following criteria: studies were prospective, with whole grain intake specifically documented and daily intake presented quantitatively; studies were all from within the US, providing consistent food culture and assessment methodologies. Only the most recent study update is plotted in figure 4 a–c. Three studies, the Nurses’ Health Study I (NHS1), the Nurses’ Health Study II (NHS2), and the Health Professionals Follow-up Study (HPFS) have published progressive follow-up reports (2, 4, 8, 11). A wide selection of the US population is represented in the selected studies (Table 1). As noted above, whole grain content is defined as the presence of bran, germ, and starch in the proportions that occur naturally in grains (16). Whole grain intake is estimated at the individual subject level from food questionnaire responses, and later reinterpreted in units of grams (dry weight) of whole grain/day (62, 63) (whole grain wheat flour is ~11% water (64)), or in earlier studies as whole grain serves (Jacobs serve). The NHS1 and HPFS have published results using both methods on similar subjects (1, 4, 8, 11). The median whole grain intake (dry weight) from the later study was used to analyze the earlier results for NHS1 and HPFS, but also used to develop a conversion equation for studies only reporting in Jacobs serves of whole grains. The conversion derived from regression is [Dry weight whole grain = (14.51 × Jacobs whole grain serve) + 1.13]). This provides a means to express all studies in the same units without access to original data. The benefit of the conversion is to be able to compare and combine the information in all these studies.
Table 1.
| Study | Race and Sex* |
Start Year | End year | Follow-up Years |
Final Age | Study Subjects |
Disease cases |
Person- years of Follow-up |
Incidence | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Type 2 Diabetes | ||||||||||
| Nurses' Health Study 2 (NHS2) | WF | 1991 | 2005 | 14 | 50 | 88343 | 2359 | 1210903 | 1.95 | 11 |
| Health Professionals Follow-up Study (HPFS) | WM | 1986 | 2006 | 20 | 72 | 39765 | 2648 | 702921 | 3.77 | 11 |
| Nurses' Health Study 1 (NHS1) | WF | 1984 | 2006 | 22 | 72 | 69120 | 5500 | 1404374 | 3.92 | 11 |
| Iowa Women's Health Study (IOWA) | WF | 1986 | 1992 | 6 | 68 | 35988 | 1141 | 202653 | 5.63 | 3 |
| Black Women's Health Study (BWHS)† | BW | 1995 | 2007 | 12 | 50 | 43960 | 3671 | 439048 | 8.36 | 12 |
| Coronary Heart Disease | ||||||||||
| Nurses' Health Study 1 (NHS1) | WF | 1984 | 1994 | 10 | 60 | 75521 | 761 | 729473 | 1.04 | 1 |
| Iowa Women's Health Study (IOWA) | WF | 1986 | 2003 | 17 | 78 | 27312 | 1034 | 454941 | 2.27 | 9 |
| Health Professionals Follow-up Study (HPFS) | WM | 1986 | 2000 | 14 | 67 | 42850 | 1818 | 510182 | 3.56 | 6 |
| Atherosclerosis Risk in Communities (ARIC) | Both | 1988 | 1999 | 11 | 65 | 11940 | 535 | 131340 | 4.07 | 5 |
| Hypertension | ||||||||||
| Women's Health Study (WHS) | WF | 1994 | 2004 | 10 | 66 | 28926 | 8722 | 232763 | 37.47 | 7 |
| Health Professionals Follow-up Study (HPFS) | WM | 1986 | 2004 | 18 | 71 | 31684 | 9227 | 345360 | 26.72 | 10 |
W=White, B=Black, F=Female, M=Male
T2D and whole grain data updated by personal communication – Dr Julie R. Palmer
Figure 4.
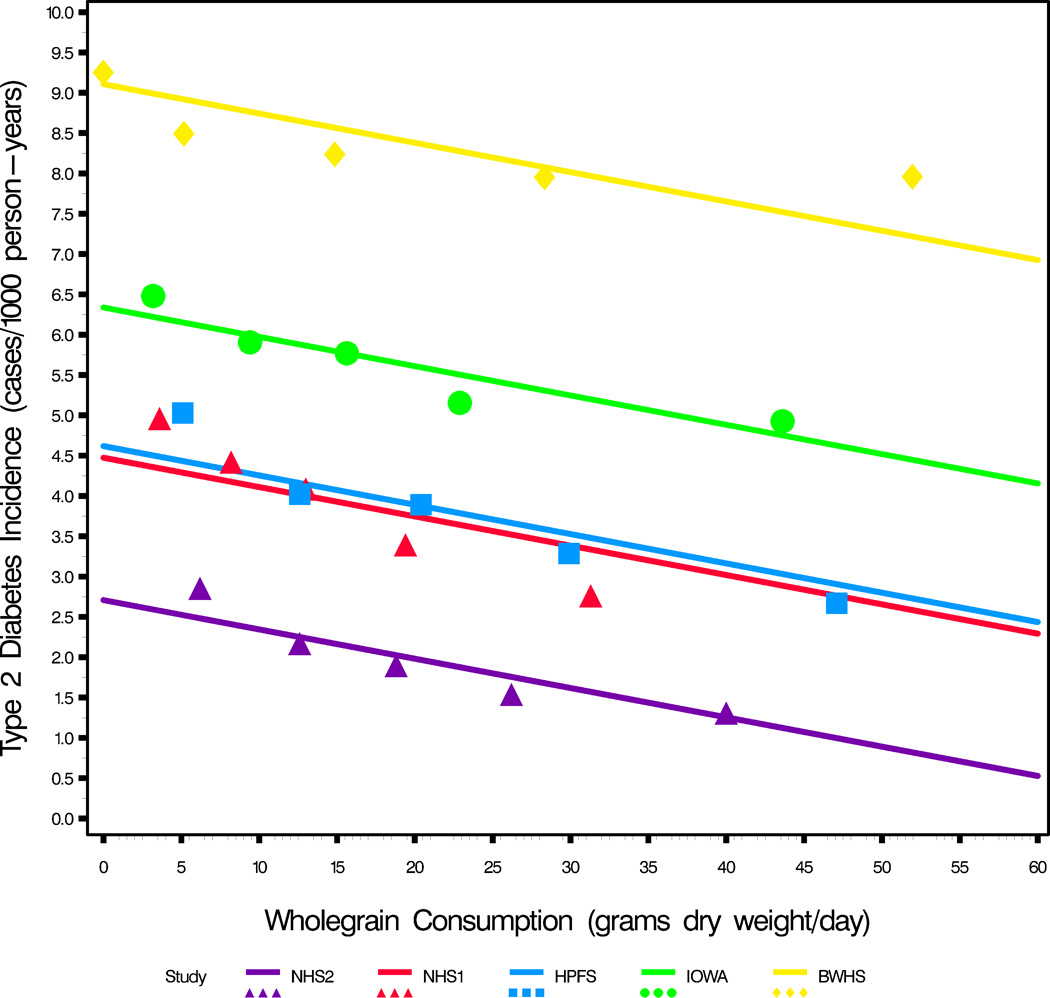
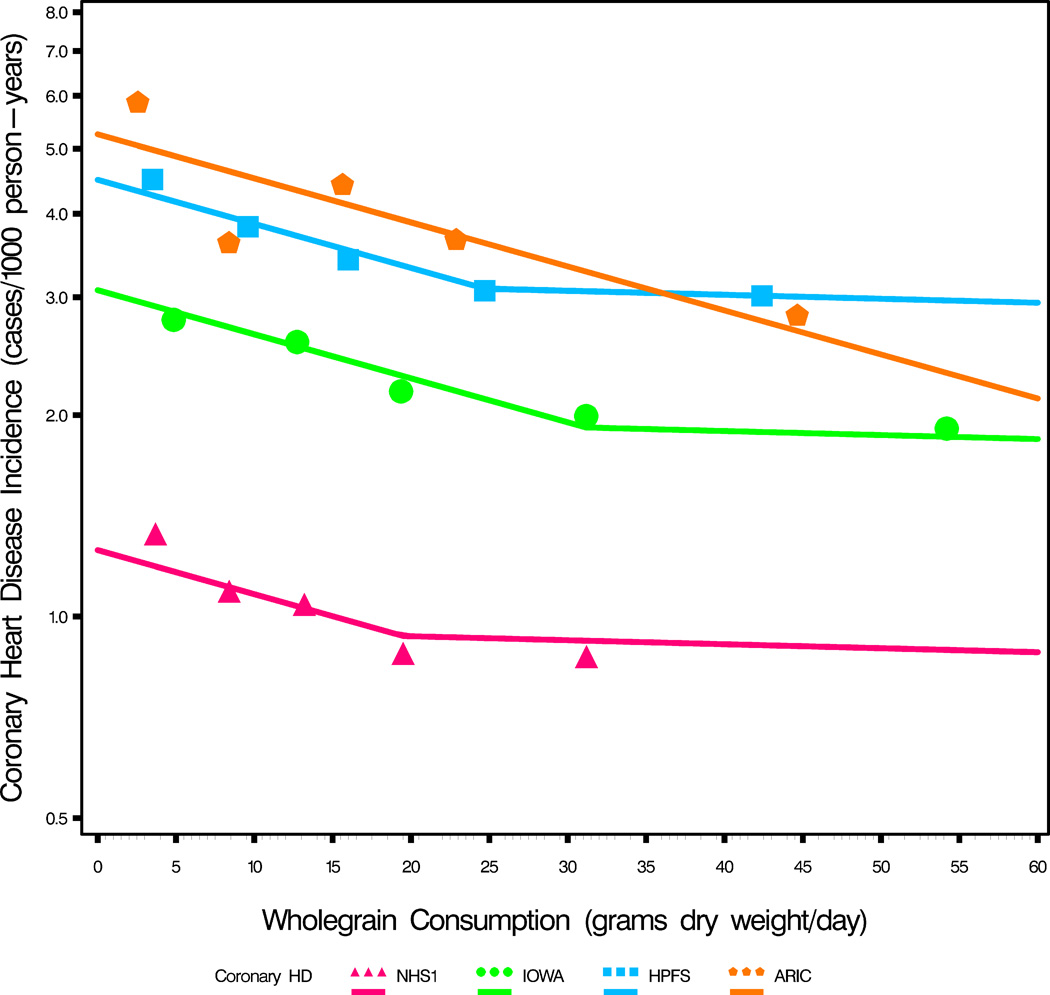
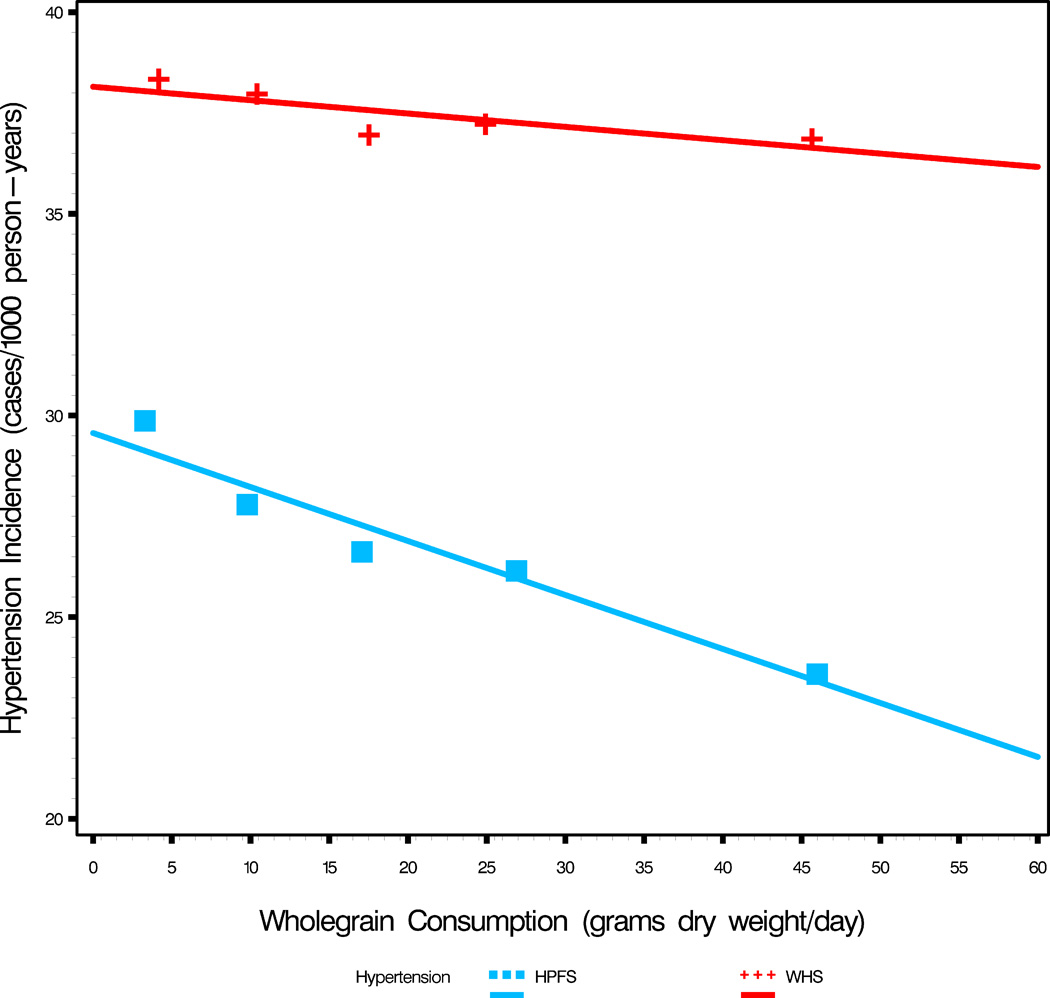
New onset disease (Incidence) by whole grain intake (in grams dry-weight per day) (see Table 1).
a. Whole grain intake and new onset T2D (see Table 1). Common slope is −0.039 ±0.005 (slope units: 1 case/1000 person years (incidence) per 1 gram of whole grain (dietary intake)). That is, the incidence of T2D drops by 1.57 cases/1000 person years (95% CI 1.14–1.99) for an increase of 40 grams of whole grains per day.
(Analysis of covariance regression model: (incidence= whole_grain [continuous variable] + study [class variable] + whole_grain × study [interaction effect]). For BWHS the data are more accurately described by an inverse function (r2=0.99) than linear negative (r2=0.62, p=0.11) with the effect of whole grains appearing to flattening out at higher whole grain consumption).
b. New onset of CHD by quintiles of whole_grain intake (see Table 1) (note the logarithmic scale). The CHD incidence drops 26% (95% CI 19–33%) for a 20 gram daily increase in whole grain intake (linear portions of regressions). The CHD incidence drops 36% (95% CI 27–45%) for a 30 gram daily increase in whole grain intake, but this may only apply to those populations where the slopes plateau at ≥30 grams per day (For ARIC, quintile person-years are calculated from total person-years).
(Analysis of covariance regression model with spline, as graphed: (Log_incidence=whole_grain [continuous variable] + study [class variable] + spline × (whole_grain - elbow) [spline effect variable (where spline=0 below elbow and 1 above elbow (in grams) and elbow=point of the bend)]). For the linear parts of Figure 4b; Analysis of covariance regression model: (Log_incidence=whole_grain [continuous variable] + study [class variable] + whole_grain × study [interaction effect]). For Log_Incidence v. whole_grain, p<0.0001, for interaction effect p=0.70 i.e. slopes are not different and regression solution obtained ignoring any interaction effect therefore).
c. New onset Hypertension (Incidence) by quintiles of whole grain intake (see Table 1). The slopes are significantly different (p<0.006). Ignoring this difference, the common slope by analysis of covariance is= −0.085 (p<0.007) (for reference, the risk ratio for women with multivariate adjustment was 0.89, p=0.007 in the original publication(7)). For men (10), in Figure 4c the slope= −0.134 ± 0.019 (p=0.006). A 40 gram daily increase of whole grains reduces the hypertension incidence by 5.4 cases/1000 person-years (95% CI 3.8–6.9), a drop of 20% (95% CI 14–26%).
Estimating the ‘dose’ of whole grains required for positive health outcomes
For T2D, CHD, and hypertension, the raw disease incidence calculated as cases/person-years is plotted against the mean or median of quintiles of whole grain intake in Figures 4 a–c. For T2D the relationships are parallel for NHS2, HPFS, and the Iowa Womens’ Health Study (IOWA) (p=0.46 different slopes test) (3, 11). The slope for NHS1 in figure 4a is steeper, a change from earlier NHS1 follow-up reports (2, 8, 11). Indeed there was a significant steepening of this slope of incidence/whole grain intake with follow-up in NHS1, NHS2 and HPFS studies (p<0.003) (2, 4, 8, 11). This shows that the whole grain effect on T2D has become stronger in subsequent years of follow-up in these studies, indicating that whole grain intake continues to be important as the population ages. The common slope in figure 4a, derived from analysis of covariance for all 5 studies, is −0.039 ±0.005 (cases/1000 person-years) per 1 gram increase in whole grain consumption (p<0.0001). For a T2D incidence of 3.87 (the mean incidence for these 5 studies), this equates to a 30% drop in diabetes incidence for a 30 gram/day increase in whole grain intake (95% confidence interval (CI) 22–39%), and a 40% drop (95% CI 29–52%) for a 40 gram daily increase in intake. These are very large potential changes if achievable in the general population.
A strong negative relationship between the incidence and whole grain intake and CHD is also apparent (p<0.0001). For CHD the trends are parallel (logarithmic scale linear portions, p=0.70 for different slopes test) but reach a plateau (in three of the studies). Based on the collective regression slope (and its standard error) a 20 gram/day increase in whole grain intake provides a 26% drop in CHD incidence (95% CI 19–33%), and a 30 gram/day increase in whole grain intake equates to a 36% drop in CHD incidence (95% CI 27–45%).
Hypertension incidence rates in relation to whole grain intake differed between men and women (p<0.006, slopes test). The common slope, by analysis of covariance, is −0.085 cases/1000 person-yrs (p<0.007). In men the slope of the 5 quintile points in figure 4c is −0.134 ± 0.019. For this relationship in men, an increase of 30 grams of whole grain/day reduces the hypertension incidence by 4.0 cases/1000 person-years (95% CI 2.9– 5.2), a 15% drop (95% CI 11– 19) from the average incidence in that study (Table 1). A 40 gram increase in whole grain intake drops the incidence by 5.4 cases/1000 person-years (95% CI 3.8–6.9), a 20% (95% CI 14–26%) drop in incidence. This is a substantial effect.
Similar striking effects of whole grain consumption have been noted in other studies. Sun et al. have estimated that replacing 50 grams per day of cooked white rice with 50 grams of whole grains (dry weight) might lower the risk of T2D by over 35% (11). In the Physicians’ Health Study, eating whole grain breakfast cereal equivalent to at least once daily, was associated with nearly a 50% drop in T2D incidence(65) and a 20% drop in hypertension(66). These studies indicate a potential for remarkable health benefits if whole grain consumption levels increased. It is of concern that 80% (4 quintiles) of the US population have suboptimal whole grain intakes. Increasing whole grain consumption to 40 grams, and ideally 50 grams daily (approximately one bowl of whole grain breakfast cereal daily) could have major public health benefits.
The whole grain health effects are not due to confounding
The actual cause of disease may be correlated with obvious traits that are easily measured. As a result, an obvious trait may be misidentified as causing the disease, while the true cause remains obscure. Such confounding of hidden traits hampers correlative studies, such as those being considered here. There are foods that some may believe are more important than wholegrains in the development of chronic disease. Wholegrain intake will therefore be regarded as merely a confounder, a confusing quirk of information, obscuring the true relationships of food intake and T2D, CHD, and hypertension. What follows is an attempt to demonstrate that wholegrain intake is the key relationship with chronic disease and not some other nutrient intake. In every published prospective study used in this analysis (Figures 4 a–c) the whole grain effect remained significant after multivariate adjustments including physical activity (data not shown) (1–11). Therefore, wholegrain intake is unlikely to be a marker of an underlying effect of physical activity on chronic disease onset.
Obesity is a known risk factor for CHD, hypertension, and T2D and a high whole grain intake is associated with lower weight ((67)(3 large prospective studies)). This relationship is interpreted to mean that obesity might mediate some of the effects of whole grains (10). It seems unlikely that obesity in itself leads to low whole grain consumption.
A high consumption of fruit and vegetables suggests an intention to eat healthily. The intake of these foods, however, has not been shown to influence T2D onset (except for a possible beneficial effect of green leafy vegetables (68)) and therefore an underlying effect of fruit and vegetable intake cannot explain the whole grain effect. Whole grains may well contribute to a low glycemic index (GI) of a food product by gel formation, or physically restricting access to absorptive surfaces. Whole grains therefore, might form part of the mechanism by which GI predicts the onset of T2D (69). Whole grains represent much more than white flour with added fiber, and the effect of whole grains on T2D incidence, not unexpectedly, appears more marked than the effect of GI (11, 70).
The reduced risk of CHD associated with whole grain intake is similar to, or better than, the benefit of a fruit and vegetable intake of >5 servings daily (70, 71). Even so, the beneficial effect of whole grain on CHD (6, 9), and the beneficial effect of cereal fiber on CHD (72, 73), are independent of the intake of fruit and vegetables or their fiber. There is some doubt about the role of saturated fat intake in CHD over very long periods, and the whole grain effect is probably not explained by a hidden low saturated fat intake (74, 75). Sodium intake and health has been extensively studied. Surprisingly, a higher sodium intake (assessed by urine measurement) more often than not predicts a lower cardiovascular disease mortality (76). In the presence of pre-existing heart disease or diabetes, there is a biphasic, ‘J-shaped’ relationship between cardiovascular mortality and sodium with a very steep increase in mortality at low sodium intakes in some groups (76–78). We conclude that the effect of whole grain intake on heart disease is not due to a hidden effect of fruit and vegetable intake, saturated fat intake, or sodium intake.
Numerous nutritional factors relating to hypertension development have been evaluated in prospective studies. In Europeans, sodium intake does not predict the development of clinical hypertension in spite of the interest in developing such evidence (79–81), and the effect of whole grain intake on the development of hypertension in men is independent of sodium intake (10). Whole grain intake is therefore not a marker for a putative effect of low sodium intake on the development of hypertension. Prospective studies have linked both alcohol (82) and meat intake (83) (WHS (7)) with new onset hypertension. Others studies have noted a prospective negative relationship with an increased intake of bioflavonoid rich foods (84–86) (e.g. cocoa, blueberries, strawberries, nuts) and low fat dairy products, calcium, and vitamin D (87) (WHS (7)). Whether or not these dietary components could result in confounding between whole grains and hypertension is uncertain.
The effect of whole grains is very consistent across multiple studies (Figure 4a–c) suggesting that the effect is not due to confounding factors. We present evidence that the relationship of whole grain intake to the onset of T2D and CHD is not due to the underlying effects of other commonly recognized healthy eating behaviors or exercise. For hypertension and whole grains we can at least exclude sodium intake as the reason for the whole grain effect. We conclude, therefore, that a higher intake of whole grain is a specific and reproducible predictor of the later onset of chronic disease and is potentially involved in the etiology of these diseases.
Two diseases not considered elsewhere in this paper deserve mention. Whole grain intake is associated with a reduced incidence of bowel cancer (88). In colorectal cancer, the effects of whole grains are both quantitatively greater and statistically stronger than are the individual effects of fruit fiber, vegetable fiber, or cereal fiber on the disease (88). In addition to the effects of minerals and antioxidants discussed below, the effects of fermentable fiber and the bulking effect of cereal fiber, singly or in combination, may benefit colon health (36, 37). Secondly, whole grain intake is associated with a reduced incidence of inflammation associated diseases (9) and this interesting and important finding deserves further investigation, with antioxidant effects of whole grains of particular interest as the mechanism.
The bran component of whole grains appears to contain the protective effect
Bran or germ can be added to the diet independently of whole grain content, for example as bran breakfast cereal which is commonly recommended for its laxative effect. It is possible in prospective studies therefore to account for individual effects of whole grain components. It has been observed that the bran component of whole grains confers the beneficial effect on chronic disease (5, 6, 10, 11, 89). It is critically important to note that the corollary is not true: refined grain intake has seldom been demonstrated to associate with disease in prospective studies ((90), white rice and T2D an exception (11, 91)). This suggests that it is not the presence of refined flour, but the absence of whole grain bran, that leads to chronic disease. This suggestion has major implications for public health messages. Processed white flour and its products need not be avoided, but rather bran needs to be included. We do not recommend excluding processed grain products from the diet, but rather that we consume more complete grain products.
Bran contents that have health implications
The Fiber Hypothesis – dietary fiber encourages a healthy colon - was spurred by the observations of diet and health in Africa by the Irish born, Scottish-trained surgeon Sir Denis Burkitt (92). Whatever the exact mechanism of the effect, this hypothesis signaled an important shift in thinking in Western medical practice: fiber was no longer merely inconvenient waste. The IOM (17) defines fiber by its lack of digestibility and this concept of the nature of fiber is clearly influenced by the changes in thinking that Burkitt inspired. Focusing on the physicochemical properties of fiber such as water retention, gel formation, and stool bulking, or on the fermentation products of cereal fiber breakdown, has produced promising lines of research. However, both lines of investigation also discount much of what we suggest is important about cereal fiber - the aleurone layer and its contents of inorganic nutrients and antioxidants. Since these components are potentially important in human health, this broader view of whole grains will be considered below.
Fiber from both fruit and grain is derived from the structural polysaccharides of plant cell walls. However, the two are not equivalent. Lignin and cellulose are key components in fruit fiber, with pectins and xyloglucans providing the main fermentable polysaccharides (34, 93–95). The salient distinguishing feature of all plant polysaccharides is the linear backbone of the polymer. Glucose provides the backbone for cellulose, β-glucan, and xyloglucan, in grains and fruit, xylose the backbone for arabinoxylan in grains, and galacturonic acid (C6H10O7) the backbone for pectins in fruit (35). Pectins are recognized and exploited for their gelling properties and may be beneficial in slowing glucose absorption in the upper gastrointestinal tract (96), but since they are highly fermentable (37, 49–51) they do not contribute to stool bulk. A role for fruit fiber could be inferred indirectly from the inconsistent and small effect of fruit juice consumption on T2D incidence (12, 97, 98), and the possible physical effects of removing fiber on glycemia and satiety (99). However, fruit and vegetable fiber is rarely eaten on its own, thus it is not possible to separate fiber benefits from those due to the rest of the fruit and vegetables. There are two other key differences between fruit fiber and cereal fiber. The antioxidant ferulic acid that forms cross-linking in cereal fiber is lacking from fruit fiber. However, fruit is generally rich in other antioxidants (100), such as anthocyanins that give them their rich colors, and whole grains are easily matched by the in vitro anti-oxidant properties of fruit and vegetables. Finally, the mineral rich aleurone accompanying cereal fiber is absent in fruit. Our contention is that it is the aleurone, rather than the indigestible fiber, which presents the most potent element influencing chronic disease.
Whole grains contain a vast array of compounds (101), and much of these are removed during the processing of grains to white flour (USDA (64)). Comparison of the nutrient contents of ‘wheat flour, whole grain’ and ‘wheat flours, bread, unenriched’ (USDA (64)) indicates an 80–90% drop in thiamine, niacin and vitamin B6, a 70–80% drop in magnesium, iron, phosphorus, potassium and manganese, and a 50–70% drop in calcium, zinc, copper and riboflavin in processed white flour. Of the components removed from whole grains during processing it appears to be the bran fraction, rather than the germ that contains the whole grain effect as noted above (5, 6, 10, 11, 89). The only caution to add is that bran is >80% of what is removed during grain processing (Figure 1) so the bran effect will dominate any germ effect if both are contributing to health benefits. We have compared ‘wheat bran, crude’ with ‘wheat germ, crude’ in the USDA database (64). Accounting for the relative proportions of bran and germ in a wheat grain (3.1% germ, 13.7% bran) (20), it is possible to estimate the relative contributions to whole grains of the bran and germ. Bran carries 11-fold more magnesium, 7 to 9-fold more niacin (B3), iron, and calcium, and 4 to 6-fold more phosphorus, potassium, copper, selenium, riboflavin (B2) and vitamin B6, and 2- to 4-fold more zinc and manganese than germ. Bran also contains 90% of whole grain phenolic acids (principally ferulic acid) and 93% of the readily accessed phenolic acids in whole grains, while germ has 1% (calculated from (20) and based on the ferulic acid dimer content, see above). Alkylresorcinols are located in bran but not the germ though their contribution to overall antioxidant effects is probably only 10% of that of the phenolic acids (see below). Finally inositol-6-phosphate (phytate) is enriched around 10-fold in wheat and rice bran compared to germ (102, 103) (though not in maize). Based on these and other lines of evidence, the most relevant components in bran to examine for possible roles in the protective effects of whole grains are magnesium, zinc, phytate, and ferulic acid. A discussion of the potential effect of the removal of these four components from whole grains in the development of T2D, CHD, and hypertension, follows. This is not to be taken, however, as an exhaustive discussion of role of whole grain contents and their effects on chronic disease.
Whole grains and minerals
The grain aleurone is sufficiently rich in minerals that these can be observed by x-ray fluorescence. In Figure 3a, three minerals -iron, zinc, and manganese - are shown. Minerals are mainly located in the globoid component of the protein storage vacuoles of the aleurone cells. They are stored in large quantities chelated by inositol-6-phosphate (phytate). Minerals are complexed by phosphate groups and twelve charge binding sites are available on phytate. These sites appear saturated with mineral charges in a whole grain (58).
Magnesium has a key role in animal physiology where Mg2+ is the second most common intracellular cation to K+, and where the actual substrate for P donation is MgATP2−. In plants, one critical role for Mg2+ is as the metal ion in chlorophyll. Wheat bran is a rich source of magnesium at 611mg/100g (7mg/g dry mass), compared to spinach at 87mg/100g (10mg/g dry mass) (64), most of it lost, however, during processing to white flour (64). Magnesium intake predicts the onset of T2D in prospective studies (104). Tissue insulin resistance is a predictor of T2D development and a key component of the disease. Insulin sensitivity, the converse, correlates with intracellular red blood cell Mg2+ (105) and magnesium intake (106–109). Insulin resistance decreases with Mg2+ supplements (110). Since magnesium has such an apparent key role in insulin sensitivity and resistance, and zinc a potential role in insulin secretion (see below), a reduced whole grain intake potentially assails both pillars of glucose homeostasis.
A higher zinc intake predicts a lower risk of T2D (111). Zinc is found in wheat germ and bran at 7–12mg/100g (64), which is comparable to another important source viz. beef (5–7mg/100g) (64). However, ~70% of zinc is removed in processing to white flour (64). Zinc accumulates in high concentration in the insulin storage granules of the beta–cell where it crystallizes with insulin (112) and is co-released with insulin (113). In animal models disruption of the zinc transporter protein Znt8 in beta-cells severely disrupts insulin production (114, 115), while global Znt8 loss is associated with obesity, hyperglycemia, and hyperinsulinemia. Mutations in Znt8 are associated with T2D in humans (116). It seems possible that a mild depletion of zinc, over decades, might contribute to the onset of T2D by a chronic subtle reduction in insulin secretion, or in one of zinc’s many other functions, especially when combined with a marginal intake of magnesium or other whole grain element.
Magnesium intake is linked to the development of hypertension through its activity as a vasodilator and through other vascular effects (117). Both systolic and diastolic blood pressure correlate well with intracellular free magnesium (118). Experimentally imposed magnesium deficiency decreases arteriolar luminal diameter and raises arterial blood pressure (119). Magnesium intake predicts the onset of new hypertension in women (independent of sodium intake) (120) (WHS (7)), and also in men (dependent on total dietary fiber) (121). Magnesium supplementation decreases blood pressure, especially in cross-over trials or where supplementation is higher (122). In population studies, serum magnesium (a more crude measure than intracellular Mg2+) has been shown to predict increments in ventricular mass (123), and stroke incidence (124). The mechanism of action of magnesium deficiency in the heart and blood vessels goes well beyond simple competition between calcium and magnesium. In cardiac or vascular tissues, magnesium deficiency up-regulates sphingolipid (ceremide) synthesis, elevates the production of multiple cytokines (including IL-1α, RANTES, TNF-α) and activates Nf-κB (125). This has potentially wide-ranging effects on vessel structure, cell differentiation, atherogenesis and hypertension. Since magnesium intake in western diets appears commonly to be inadequate (126), we conclude that increasing the dietary supply of magnesium through whole grain consumption has the potential to help prevent hypertension and other cardiovascular disorders.
In summary we conclude that the widespread removal of minerals from grains during processing to white flour might contribute to the development of T2D, CHD, and hypertension. This is not the same as the overt lack seen in severe deficiencies, but a chronic low grade effect matching the slow onset of chronic disease.
Whole grains and Inositols
Concerns are frequently expressed about the benefits of whole grains since they contain an abundance of phytate. It is feared that this will reduce mineral absorption. In wheat, about 90% of phytate is found in the aleurone while only a little is found in the germ (see above). Phytate is inositol-6-phosphate (inositol hexakisphosphate, IP6) and is a member of a well studied family of intracellular signaling molecules which includes the ubiquitous inositol-3-phosphate that triggers calcium signaling(127). IP6 is now recognized as having key health benefits such as anticancer effects (128) and renal stone protection (129). Concerns about phytate arise because it is an efficient metal ion chelator and assumed therefore to prevent trace mineral absorption. These concerns are almost certainly exaggerated. Detailed grain analyses confirm that phytate is the means by which aleurone cells store minerals (53–56). IP6 has six phosphate groups contributing twelve negative charges (130), and can therefore bind 12 monovalent or 6 divalent ions, and also trivalent iron (131). From an analysis of phytate globoids from wheat (58) we estimate there are 1.85 mineral charges per phosphate. This suggests that the globoid phytates are saturated with inorganic ions. The presence of phytate in whole grains is, therefore, a signature for the presence of minerals. Given that whole grain flour has 5.5-fold more magnesium, 4.0-fold more iron, 3.6-fold more potassium, and 3.1-fold more zinc than white flour (64), and that up to 50% of phytate or even more, is degraded in the human gut (132, 133), rather than removing minerals from the diet, phytate is actually supplying minerals.
Animal studies support this view that phytate signals the availability of minerals (134–136). Whole grain consumption in rats reduced the proportion of zinc that was absorbed from the diet. However whole grains supplied so much more zinc than white flour that the whole grain diet easily compensated for this decreased bio-availability (134). Likewise, the absorption of magnesium and iron increased substantially with a whole grain diet in spite of the increased phytate intake (134). In a different study the zinc absorbed was 10-fold greater on bran diets compared to white flour diets (136).An increased absorption of magnesium, zinc, and iron, in humans, is also possible on a whole grain diet, in spite of the presence of phytate, because a major portion of phytate is degraded in the human gut (132, 133). This degradation may also be inducible in humans just as it appears to be in rats (133, 137). This data illustrates that a focus on the bioavailability of minerals will not provide an adequate view of the quantity of minerals that will be absorbed from whole grains. Therefore, whole grains should be regarded a potentially important source of dietary minerals.
Whole grains and anti-oxidants
It is generally poorly appreciated by clinicians and public alike that grains accumulate antioxidants and phytochemicals to levels comparable to that in fruits and vegetables (100). This antioxidant effect of whole grains appears principally due to ferulic acid in aleurone cells (138). Ferulic acid (C10H10O4), a phenolic ring with a 3 carbon carboxylic acid substitution, possesses intrinsic antioxidant activity (139), but also activates cellular antioxidant systems. Ferulic acid is present as a key structural component of grain cell walls forming cross-links to strengthen the walls. About 25% of the ferulic acid is probably tightly bound and not available, but this leaves 75% of ferulic acid accessible to the gastro-intestinal tract (calculated from (20)). In support of this gastro-intestinal availability, conjugated ferulic acid is present in blood and urine after a bran or aleurone meal, with levels depending on the degree of processing of the foodstuff (140–145).
An additional grain antioxidant source in wheat and rye, though not other grains, are the alkylresorcinols, phenolic rings with a lipid-like side chain. These are found especially in the testa. Since alkylresorcinols are in lower concentrations than phenolic acids in whole grains (20, 146) and possess lower antioxidant activity (147), we calculate that their contribution to the antioxidant effects of grains may be only 10% of the phenolic acids (20, 146, 147).
We conclude that whole grains should now be considered along with fruit and vegetables, nuts and berries, as antioxidant food. This antioxidant status for whole grains has received less attention from clinicians or those promoting public health than it deserves. In the following section we develop these links between whole grains and antioxidants further.
Roles for oxidation in type 2 diabetes, coronary heart disease and hypertension
There is a potential link between whole grain antioxidant actions and chronic disease. The production of reactive oxidant species (ROS) and the resulting oxidative stress has been widely implicated in disease pathogenesis (148–151), including roles in T2D, CHD, and hypertension. The pancreatic beta-cell is especially prone to ROS damage because of low inherent anti-oxidant activity (150–152). In the vascular system, endothelial cell nitric oxide is a key vasodilator (153), but it is also readily oxidized and inactivated (154), leading to endothelial dysfunction. The oxidation of lipoproteins in blood vessel walls forms part of the cascade to atherosclerosis (155). Not surprisingly there has been considerable interest in the use of antioxidants to prevent heart disease. However, supplementation with vitamin E (tocopherols) or vitamin A (retinols) may actually increase mortality and vitamin C (ascorbate) has no effect (156). While this has been disappointing, these therapeutic attempts reflect a far too superficial view on how physiology manages and controls reactive oxygen and other reactive species.
Physiology has an elaborate system to defend against uncontrolled oxidation from ROS. The major antioxidant defense in cells is glutathione, a cysteine-containing tri-peptide that can reach concentrations of 10 mM in cells (157). Glutathione synthesis enzymes and a host of other antioxidant proteins (157, 158), are induced by the same promoter sequence, the “Antioxidant Response Element” (ARE) (159). Increased production of these proteins can provide a key defense against oxidative stress. Ferulic acid has been demonstrated to increase the production of key antioxidant proteins by activating ARE dependent transcription (160).
Ferulic acid has direct antioxidant properties (139), but with typical plasma levels ≤1μM (140–144) there is insufficient ferulic acid on its own to have a major direct ROS scavenging effect (148, 149). However, animal studies demonstrate that ferulic acid, when administered with an oxidant stress, reduces ROS and lipid peroxidation, reduces oxidant tissue damage, and prevents the fall of glutathione and antioxidant enzymes. This is achieved by activating the ARE via the transcription factor Nrf2 (160). These animal studies have been done with a wide range of pro-oxidant stressors including alloxan, nicotine, acetaminophen, radiation, u/v light, carcinogens, and exercise (160–171). While human studies are lacking, this animal work suggests that ferulic acid could serve as a key anti-oxidant in human physiology also.
The failure of clinical trials testing anti-oxidant vitamins in CHD should not detract from the potential of ferulic acid to provide protection against chronic disease by anti-oxidant mechanisms. The reasons for the failure of vitamin clinical trials are quite likely related to the high levels of anti-oxidants that would be required to match the reaction rates and amounts of ROS present at key sites such as the endothelial cell (148, 149). The plasma concentrations of vitamin E (~30 μM) or vitamin C (~37 μM) (172, 173) are at least an order of magnitude lower than glutathione, and glutathione is just one of the many anti-oxidant peptides defending against ROS (148, 149, 158). In addition, the reaction rate of vitamin E and superoxide is 6 orders of magnitude slower than the reaction rate of superoxide and nitric oxide (148), leaving vitamin E inadequate to defend nitric oxide from damage. Vitamin E and vitamin C, therefore, would need to be at physiologically impossible concentrations to be fully effective as direct ROS scavenging agents. Since ferulic acid activates the endogenous anti-oxidant defense system, and vitamin E and vitamin C probably do not (174–176), it is possible that ferulic acid could fulfill a powerful role as an anti-oxidant agent where vitamins E and C have failed.
We do not know if the apparent protective effects of whole grains in T2D, CHD, and hypertension are mediated by ferulic acid and its antioxidant effects. However the above discussion points to a potential role for reducing oxidant stress by whole grains and points to potentially productive areas for further investigation.
Other actions of ferulic acid of potential importance to T2D, CHD and Hypertension
Other observations regarding ferulic acid may also be relevant to the prevention or treatment of T2D, CHD, and hypertension. Many of these studies were performed at supra-physiological doses however, although ferulic acid does seem to be relatively non-toxic. A contribution to this research interest is that ferulic acid is found in some Chinese herbal remedies, for example derivatives of Angelica sinensis (Danggui) (177). In relation to T2D physiology, ferulic acid has the following effects: it increases glucose uptake 3-fold in myotubes and potentiates the effect of known anti-diabetic agents to increase glucose uptake 5-fold (178); it increases insulin secretion 2-fold in cultured beta cells; stimulates insulin secretion 3-fold in the perfused rat pancreas; is acutely hypoglycemic in Wistar rats; and acutely releases insulin in vivo these rats (179); it is an α-glucosidase inhibitor and hence slows maltose and sucrose digestion (180) and the rise of blood glucose at meal time, mimicking the currently used anti-diabetic agent Acarbose (Bayer Pharmaceuticals); finally ferulic acid administration increases the exercise endurance of mice two fold (170, 181) and if this also applied to humans it might assist in weight regulation. In relation to coronary heart disease and hypertension, ferulic acid has the following effects: it acutely decreases cholesterol levels in the stroke prone rat (182); it reduces atherosclerotic plaque in rabbits (183); it reduces aortic fatty plaque in apoE deficient mice (169); it increases iNOS activity in cardiomyocytes (184); it is acutely hypertensive in 2 different hypertensive rat models (182, 185); it is acutely additive to other antihypertensive agents and contributes to chronic blood pressure reduction (185); and it relaxes aortic smooth muscle in 2 different rat models (186, 187).
These remarkable effects of ferulic acid, albeit often from pharmacological doses, suggest that a chronic exposure to ferulic acid from whole grains consumption might contribute to the observed reductions in chronic disease. There is the potential here for productive research in our effort to understand how the natural product ferulic acid works, what else in grains are providing health benefits, and how further the benefits of whole grains can be extended.
The effects of whole grain components in combination - Synergism
We have highlighted three constituents of whole grains that might contribute to the observed beneficial effects of whole grains on chronic disease. These constituents - magnesium, zinc, and ferulic acid are consumed together in whole grains, along with many other nutrients, and any effects are always combined. Synergy can occur when different nutrients affect different pathways converging on the same disease process, or alternatively, when different nutrients produce similar effects and these effects are summed. For example, in T2D, it is possible that zinc intake subtly improves insulin secretion, ferulic acid defends against beta cell stressors, magnesium improves insulin action, and the result is that glucose levels remain normal. In hypertension, magnesium may help in vessel dilation, while ferulic acid helps prevent NO destruction. Alternatively, summed effects could occur between the host of natural chemicals in whole grains which include iron, selenium, manganese, zinc, magnesium, B vitamins, tocotrienols, carotenoids, anthocyanins, alkylresorcinols, betaine, choline, fermentable polysaccharides, et cetera (101).Both individual and combined effects need to be considered if we are to understand the benefits of whole grains better. In addition, given the years of exposure that precedes chronic disease, even subtle alterations in function could contribute to a large cumulative effect, leading in turn to overt disease in those with a reduced whole grain intake.
Conclusions
Based upon observing consistent positive effects of whole grain intake across eleven major prospective studies, we provide compelling evidence that increasing whole grain intake in modern societies is a pressing public health concern. In many people’s mind, whole grain intake is equated with fiber intake, and fiber is regarded as inert material not much different to wood. Talk of increasing whole grain intake therefore is uninspiring for most hearers. In contrast, when consuming whole grain we get aleurone. Aleurone is live plant tissue, and the most highly concentrated source of nutrients in cereals! That bran is alive will surprise most consumers. To illustrate how poorly the dietary richness of whole grains has penetrated thinking, we searched for “aleurone” in titles, abstracts, and keywords, in clinical nutrition journals. In over 63,000 articles there were just 16 hits - 13 from one journal, the British Journal of Nutrition, with some highly acclaimed journals having no hits at all. It is vital that health care providers, from dieticians to physicians, gain a much more comprehensive view of the structure and nature of whole grains and bran, and of the possible mechanisms by which the components of the aleurone might exert an effect. And the consumer needs access to whole grain content on package labels. Hopefully these measures will make the presentation of the importance of whole grains to patients and populations alike more interesting and therefore more convincing. Moreover, compared to fruit and vegetables, whole grains are in plentiful supply, store easily, and are relatively inexpensive. Therefore public health practitioners need to raise the profile of whole grains at least as high as that of sodium, fruit, and vegetables, if not higher.
Acknowledgments
Funding Sources
The Black Women’s Health Study is funded by National Cancer Institute Grant CA58420 and National Institute of Diabetes, Digestive, and Kidney Disease Grant DK068738.
Disclosures
Linda Tapsell’s research unit has received grants from Go Grains Health & Nutrition Ltd, Cereal Partners Australia Pty Ltd and related companies, and Horticulture Australia Ltd.
There are no financial interest to declare by the authors, their spouses, partners, or children
LT and DRJ are on the scientific advisory board of the California Walnut Commission.
Rothamsted Research receives grant in aid from the United Kingdom’s Biotechnology and Biological Science Research Council. Use of the Diamond Light Source synchrotron facility is supported by the Science and Technology Facilities Council.
Footnotes
Disclosure Summary: The Authors have nothing to disclose.
References
- 1.Liu S, Stampfer MJ, Hu FB, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses' Health Study. Am. J. Clin. Nutr. 1999;70:412–419. doi: 10.1093/ajcn/70.3.412. [DOI] [PubMed] [Google Scholar]
- 2.Liu s, Manson JE, stampfer M, et al. A Prospective Study of Whole-Grain Intake and Risk of Type 2 Diabetes Mellitus in US Women. Am. J. Public Health. 2000;90:1409–1415. doi: 10.2105/ajph.90.9.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer K, Kushi L, Jacobs DJ, et al. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 4.Fung T, Hu F, Pereira M, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am. J. Clin. Nutr. 2002;76:535–540. doi: 10.1093/ajcn/76.3.535. [DOI] [PubMed] [Google Scholar]
- 5.Steffen LM, Jacobs DR, Jr, Stevens J, et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003;78:383–390. doi: 10.1093/ajcn/78.3.383. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MK, Koh-Banerjee P, Hu FB, et al. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am. J. Clin. Nutr. 2004;80:1492–1499. doi: 10.1093/ajcn/80.6.1492. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Gaziano JM, Liu S, et al. Whole- and refined-grain intakes and the risk of hypertension in women. Am. J. Clin. Nutr. 2007;86:472–479. doi: 10.1093/ajcn/86.2.472. [DOI] [PubMed] [Google Scholar]
- 8.de Munter JS, Hu FB, Spiegelman D, et al. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs DR, Jr, Andersen LF, Blomhoff R, et al. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women's Health Study. Am. J. Clin. Nutr. 2007;85:1606–1614. doi: 10.1093/ajcn/85.6.1606. [DOI] [PubMed] [Google Scholar]
- 10.Flint AJ, Hu FB, Glynn RJ, et al. Whole grains and incident hypertension in men. Am. J. Clin. Nutr. 2009;90:493–498. doi: 10.3945/ajcn.2009.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Q, Spiegelman D, van Dam RM, et al. White Rice, Brown Rice, and Risk of Type 2 Diabetes in US Men and Women. Arch. Intern. Med. 2010;170:961–969. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer JR, Boggs DA, Krishnan S, et al. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch. Intern. Med. 2008;168:1487–1492. doi: 10.1001/archinte.168.14.1487. (updated data on the consumption of whole-grains by personal communication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. State-specific incidence of diabetes among adults--participating states, 1995-1997 and 2005-2007. MMWR - Morbidity & Mortality Weekly Report. 2008;57:1169–1173. [PubMed] [Google Scholar]
- 14.American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. [erratum appears in Diabetes Care. 2008 Jun;31(6): 1271] Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics-2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 16.FDA. [Accessed October 8 2012]; http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM152011.pdf. Published May 6 2009.
- 17.Lupton J, Miller S. The Institute of Medicine (IOM) of the National Academy of Sciences, Food and Nutrition Board. Chapter 7. 2002. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; pp. 339–421. (Chairpersons) [Google Scholar]
- 18.Surget A, Barron C. Histologie du grain de blé. Industrie des Céréales. 2005;145:3–7. [Google Scholar]
- 19.Brouns F, Hemery Y, Price R, Anson NM. Wheat Aleurone: Separation, Composition, Health Aspects, and Potential Food Use. Crit. Rev. Food Sci. Nutr. 2012;52:553–568. doi: 10.1080/10408398.2011.589540. [DOI] [PubMed] [Google Scholar]
- 20.Barron C, Surget A, Rouau X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007;45:88–96. [Google Scholar]
- 21.Gillies SA, Futardo A, Henry RJ. Gene expression in the developing aleurone and starchy endosperm of wheat. Plant Biotechnol. J. 2012;10:668–679. doi: 10.1111/j.1467-7652.2012.00705.x. [DOI] [PubMed] [Google Scholar]
- 22.Becraft PW, Yi GB. Regulation of aleurone development in cereal grains. J. Exp. Bot. 2011;62:1669–1675. doi: 10.1093/jxb/erq372. [DOI] [PubMed] [Google Scholar]
- 23.Sabelli PA. Replicate and die for your own good: Endoreduplication and cell death in the cereal endosperm. J. Cereal Sci. 2012;56:9–20. [Google Scholar]
- 24.Ritchie S, Swanson SJ, Gilroy S. Physiology of the aleurone layer and starchy endosperm during grain development and early seedling growth: new insights from cell and molecular biology. Seed Sci. Res. 2000;10:193–212. [Google Scholar]
- 25.Kamal-Eldin A, Lærke HN, Knudsen K-EB, et al. Physical, microscopic and chemical characterisation of industrial rye and wheat brans from the Nordic countries. Food & Nutrition Research. 2009 doi: 10.3402/fnr.v53i0.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saulnier L, Guillon F, Chateigner-Boutin A-L. Cell wall deposition and metabolism in wheat grain. J. Cereal Sci. 2012;56:91–108. [Google Scholar]
- 27.Parker ML, Ng A, Waldron KW. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum. L. cv. Avalon) grains. J. Sci. Food Agric. 2005;85:2539–2547. [Google Scholar]
- 28.Collins HM, Burton RA, Topping DL, et al. Variability in Fine Structures of Noncellulosic Cell Wall Polysaccharides from Cereal Grains: Potential Importance in Human Health and Nutrition. Cereal Chem. 2010;87:272–282. [Google Scholar]
- 29.Izydorczyk MS, Biliaderis CG. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995;28:33–48. [Google Scholar]
- 30.Antoine C, Peyron S, Mabille F, et al. Individual contribution of grain outer layers and their cell wall structure to the mechanical properties of wheat bran. J. Agric. Food Chem. 2003;51:2026–2033. doi: 10.1021/jf0261598. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen LE, Xu C, Sørensen JF, et al. Enzyme kinetics and identification of the rate-limiting step of enzymatic arabinoxylan degradation. Biochem. Eng. J. 2012;69:8–16. [Google Scholar]
- 32.Sunna A, Antranikian G. Xylanolytic Enzymes from Fungi and Bacteria. Crit. Rev. Biotechnol. 1997;17:39–67. doi: 10.3109/07388559709146606. [DOI] [PubMed] [Google Scholar]
- 33.Damen B, Verspreet J, Pollet A, et al. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011;55:1862–1874. doi: 10.1002/mnfr.201100377. [DOI] [PubMed] [Google Scholar]
- 34.Bunzel M, Schussler A, Saha GT. Chemical Characterization of Klason Lignin Preparations from Plant-Based Foods. J. Agric. Food Chem. 2011;59:12506–12513. doi: 10.1021/jf2031378. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar P, Bosneaga E, Auer M. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J. Exp. Bot. 2009;60:3615–3635. doi: 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- 36.Cummings JH, Branch W, Jenkins DJA, et al. Colonic response to Dietary Fiber from Carrot, Cabbage, Apple, Bran, and Guar Gum. Lancet. 1978;1:5–9. doi: 10.1016/s0140-6736(78)90357-4. [DOI] [PubMed] [Google Scholar]
- 37.Bourquin LD, Titgemeyer EC, Fahey GC. Fermentation of various dietary fiber sources by human fecal bacteria. Nutrition Research. 1996;16:1119–1131. [Google Scholar]
- 38.Burton RA, Fincher GB. (1,3;1,4)-beta-D-Glucans in Cell Walls of the Poaceae, Lower Plants, and Fungi: A Tale of Two Linkages. Mol. Plant. 2009;2:873–882. doi: 10.1093/mp/ssp063. [DOI] [PubMed] [Google Scholar]
- 39.Daou C, Zhang H. Oat Beta-Glucan: Its Role in Health Promotion and Prevention of Diseases. Comprehensive Reviews in Food Science and Food Safety. 2012;11:355–365. [Google Scholar]
- 40.Tasse L, Bercovici J, Pizzut-Serin S, et al. Functional metagenomics to mine the human gut microbiome for dietary fiber catabolic enzymes. Genome Res. 2010;20:1605–1612. doi: 10.1101/gr.108332.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 42.Wong DWS. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009;157:174–209. doi: 10.1007/s12010-008-8279-z. [DOI] [PubMed] [Google Scholar]
- 43.Martens EC, Lowe EC, Chiang H, et al. Recognition and Degradation of Plant Cell Wall Polysaccharides by Two Human Gut Symbionts. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chassard C, Delmas E, Robert C, Bernalier-Donadille A. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol. Ecol. 2010;74:205–213. doi: 10.1111/j.1574-6941.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 45.Bourriaud C, Robins RJ, Martin L, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005;99:201–212. doi: 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- 46.Duncan SH, Barcenilla A, Stewart CS, et al. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison DJ, Mackay WG, Edwards CA, et al. Butyrate production from oligofructose fermentation by the human faecal flora: what is the contribution of extracellular acetate and lactate? Br. J. Nutr. 2006;96:570–577. [PubMed] [Google Scholar]
- 48.Seeliger S, Janssen PH, Schink B. Energetics and kinetics of lactate fermentation to acetate and propionate via methylmalonyl-CoA or acrylyl-CoA. FEMS Microbiol. Lett. 2002;211:65–70. doi: 10.1111/j.1574-6968.2002.tb11204.x. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen D, Gidley MJ, Williams BA. In vitro fermentation of bacterial cellulose composites as model dietary fibers. Journal of Agricultural and Food Chemis ry. 2011;59:4025–4032. doi: 10.1021/jf104855e. [DOI] [PubMed] [Google Scholar]
- 50.Jonathan MC, van den Borne JJGC, van Wiechen P, et al. In vitro fermentation of 12 dietary fibres by faecal inoculum from pigs and humans. Food Chem. 2012;133:889–897. [Google Scholar]
- 51.Nordlund E, Aura A-M, Mattila I, et al. Formation of Phenolic Microbial Metabolites and Short-Chain Fatty Acids from Rye, Wheat, and Oat Bran and Their Fractions in the Metabolical in Vitro Colon Model. J. Agric. Food Chem. 2012;60:8134–8145. doi: 10.1021/jf3008037. [DOI] [PubMed] [Google Scholar]
- 52.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- 53.Prom-U-Thai C, Huang L, Rerkasem B, et al. Distribution of protein bodies and phytate-rich inclusions in grain tissues of low and high iron rice genotypes. Cereal Chem. 2008;85:257–265. [Google Scholar]
- 54.Moore KL, Zhao F-J, Gritsch CS, et al. Localisation of iron in wheat grain using high resolution secondary ion mass spectrometry. J. Cereal Sci. 2012;55:183–187. [Google Scholar]
- 55.Heard PJ, Feeney KA, Allen GC, Shewry PR. Determination of the elemental composition of mature wheat grain using a modified secondary ion mass spectrometer (SIMS) Plant J. 2002;30:237–245. doi: 10.1046/j.1365-313x.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- 56.Regvar M, Eichert D, Kaulich B, et al. New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. J. Exp. Bot. 2011;62:3929–3939. doi: 10.1093/jxb/err090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibl V, Stoger E. The formation, function and fate of protein storage compartments in seeds. Protoplasma. 2012;249:379–392. doi: 10.1007/s00709-011-0288-z. [DOI] [PubMed] [Google Scholar]
- 58.Bohn L, Josefsen L, Meyer AS, Rasmussen SK. Quantitative analysis of phytate globoids isolated from wheat bran and characterization of their sequential dephosphorylation by wheat phytase. J. Agric. Food Chem. 2007;55:7547–7552. doi: 10.1021/jf071191t. [DOI] [PubMed] [Google Scholar]
- 59.Fulcher RG, O'Brien TP, Lee JW. Studies on the Aleurone Layer I. Conventional and Fluorescence Microscopy of the Cell Wall With Emphasis on Phenol-Carbohydrate Complexes in Wheat. Aust. J. Biol. Sci. 1972;25:23–34. [Google Scholar]
- 60.Harris PJ, Hartley RD. Detection of Bound Ferulic Acid in Cell-Walls of Gramineae by Ultraviolet Fluorescence Microscopy. Nature. 1976;259:508–510. [Google Scholar]
- 61.Dexter JE, Wood PJ. Recent applications of debranning of wheat before milling. Trends Food Sci. Technol. 1996;7:35–41. [Google Scholar]
- 62.Franz M, Sampson L. Challenges in developing a whole grain database: Definitions, methods and quantification. J. Food Compos. Anal. 2006;19:S38–S44. [Google Scholar]
- 63.HSPH Harvard School of Public Health (HSPH) Nutrition Department - Nutrient Tables. [Accessed February 15 2012]. https://regepi.bwh.harvard.edu/health/nutrition.html. Updated 2011. [Google Scholar]
- 64.USDA. [Accessed October 2012];National Nutrient Database-Release 24. SR24 - Reports by Single Nutrients. 2012 http://www.ars.usda.gov/
- 65.Kochar J, Djousse L, Gaziano JM, et al. Breakfast cereals and risk of type 2 diabetes in the Physicians' Health Study I. Obesity. 2007;15:3039–3044. doi: 10.1038/oby.2007.362. [DOI] [PubMed] [Google Scholar]
- 66.Kochar J, Gaziano JM, Djoussé L. Breakfast cereals and risk of hypertension in the Physicians’ Health Study I. Clin. Nutr. 2012;31:89–92. doi: 10.1016/j.clnu.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mozaffarian D, Hao T, Rimm EB, et al. Changes in Diet and Lifestyle and Long-Term Weight Gain in Women and Men. N. Engl. J. Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carter P, Gray LJ, Troughton J, et al. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. Br. Med. J. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong J-Y, Zhang L, Zhang Y-H, Qin L-Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br. J. Nutr. 2011;106:1649–1654. doi: 10.1017/S000711451100540X. [DOI] [PubMed] [Google Scholar]
- 70.Ye EQ, Chacko SA, Chou EL, et al. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012;142:1304–1313. doi: 10.3945/jn.111.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He FJ, Nowson CA, Lucas M, MacGregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J. Hum. Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 72.Rimm EB, Ascherio A, Giovannucci E, et al. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 73.Mozaffarian D, Kumanyika SK, Lemaitre RN, et al. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA. 2003;289:1659–1666. doi: 10.1001/jama.289.13.1659. [DOI] [PubMed] [Google Scholar]
- 74.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 75.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alderman MH, Cohen HW. Dietary Sodium Intake and Cardiovascular Mortality: Controversy Resolved? Am. J. Hypertens. 2012;25:727–734. doi: 10.1038/ajh.2012.52. [DOI] [PubMed] [Google Scholar]
- 77.O'Donnell Mj YSMA, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 78.Thomas MC, Moran J, Forsblom C, et al. The Association Between Dietary Sodium Intake, ESRD, and All-Cause Mortality in Patients With Type 1 Diabetes. Diabetes Care. 2011;34:861–866. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meneton P, Galan P, Bertrais S, et al. High plasma aldosterone and low renin predict blood pressure increase and hypertension in middle-aged Caucasian populations. J. Hum. Hypertens. 2008;22:550–558. doi: 10.1038/jhh.2008.27. [DOI] [PubMed] [Google Scholar]
- 80.Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons.[see comment] N. Engl. J. Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 81.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. Fatal and Nonfatal Outcomes, Incidence of Hypertension, and Blood Pressure Changes in Relation to Urinary Sodium Excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 82.Taylor B, Irving HM, Baliunas D, et al. Alcohol and hypertension: gender differences in dose–response relationships determined through systematic review and meta-analysis. Addiction. 2009;104:1981–1990. doi: 10.1111/j.1360-0443.2009.02694.x. [DOI] [PubMed] [Google Scholar]
- 83.Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. J. Hypertens. 2008;26:215–222. doi: 10.1097/HJH.0b013e3282f283dc. [DOI] [PubMed] [Google Scholar]
- 84.Desch S, Schmidt J, Kobler D, et al. Effect of Cocoa Products on Blood Pressure: Systematic Review and Meta-Analysis. Am. J. Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 85.Cassidy A, O'Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Djousse L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in US male physicians. Clin. Nutr. 2009;28:10–14. doi: 10.1016/j.clnu.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Manson JE, Buring JE, et al. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51:1073–1079. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 88.Aune D, Chan DSM, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Br. Med. J. 2011:343. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koh-Banerjee P, Franz M, Sampson L, et al. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. The American Journal of Clinical Nutrition. 2004;80:1237–1245. doi: 10.1093/ajcn/80.5.1237. [DOI] [PubMed] [Google Scholar]
- 90.Williams PG. Evaluation of the evidence between consumption of refined grains and health outcomes. Nutr. Rev. 2012;70:80–99. doi: 10.1111/j.1753-4887.2011.00452.x. [DOI] [PubMed] [Google Scholar]
- 91.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. Br. Med. J. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28:3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 93.Arnous A, Meyer AS. Quantitative Prediction of Cell Wall Polysaccharide Composition in Grape (Vitis vinifera L.) and Apple (Malus domestica) Skins from Acid Hydrolysis Monosaccharide Profiles. J. Agric. Food Chem. 2009;57:3611–3619. doi: 10.1021/jf900780r. [DOI] [PubMed] [Google Scholar]
- 94.Bunzel M, Seiler A, Steinhart H. Characterization of dietary fiber lignins from fruits and vegetables using the DFRC method. Journal of Agricultural and Food Chemis ry. 2005;53:9553–9559. doi: 10.1021/jf0520037. [DOI] [PubMed] [Google Scholar]
- 95.Nunan KJ, Sims IM, Bacic A, et al. Isolation and characterization of cell walls from the mesocarp of mature grape berries (Vitis vinifera) Planta. 1997;203:93–100. [Google Scholar]
- 96.Jenkins DJ, Leeds AR, Gassull MA, et al. Decrease in postprandial insulin and glucose concentrations by guar and pectin. Ann. Intern. Med. 1977;86:20–23. doi: 10.7326/0003-4819-86-1-20. [DOI] [PubMed] [Google Scholar]
- 97.de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am. J. Clin. Nutr. 2011;93:1321–1327. doi: 10.3945/ajcn.110.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan A, Malik VS, Schulze MB, et al. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. The American Journal of Clinical Nutrition. 2012;95:1454–1460. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haber GB, Murphy D, Heaton KW, Burroughs LF. Depletion and Disruption of Dietary Fiber - Effects on Satiety, Plasma-Glucose, and Serum-Insulin. Lancet. 1977;2:679–682. doi: 10.1016/s0140-6736(77)90494-9. [DOI] [PubMed] [Google Scholar]
- 100.Liu R. Whole grain phytochemicals and health. J. Cereal Sci. 2007;46:207–219. [Google Scholar]
- 101.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr. Res. Rev. 2010;23:65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 102.O'Dell BL, de Boland AR, Koirtyohann SR. Distribution of Phytate and Nutritionally Important Elements Among Morphological Components of Cereal Grains. J. Agric. Food Chem. 1972;20 718-&. [Google Scholar]
- 103.Hemery Y, Lullien-Pellerin V, Rouau X, et al. Biochemical markers: Efficient tools for the assessment of wheat grain tissue proportions in milling fractions. J. Cereal Sci. 2009;49:55–64. [Google Scholar]
- 104.Schulze MB, Schulz M, Heidemann C, et al. Fiber and magnesium intake and incidence of type 2 diabetes - A prospective study and meta-analysis. Arch. Intern. Med. 2007;167:956–965. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 105.Barbagallo M, Dominguez LJ, Tagliamonte MR, et al. Effects of glutathione on red blood cell intracellular magnesium: relation to glucose metabolism. Hypertension. 1999;34:76–82. doi: 10.1161/01.hyp.34.1.76. [DOI] [PubMed] [Google Scholar]
- 106.Rumawas ME, McKeown NM, Rogers G, et al. Magnesium intake is related to improved insulin homeostasis in the framingham offspring cohort. J. Am. Coll. Nutr. 2006;25:486–492. doi: 10.1080/07315724.2006.10719563. [DOI] [PubMed] [Google Scholar]
- 107.Kim DJ, Xun P, Liu K, et al. Magnesium Intake in Relation to Systemic Inflammation, Insulin Resistance, and the Incidence of Diabetes. Diabetes Care. 2010;33:2604–2610. doi: 10.2337/dc10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song Y, Manson JE, Buring JE, Liu S. Dietary Magnesium Intake in Relation to Plasma Insulin Levels and Risk of Type 2 Diabetes in Women. Diabetes Care. 2004;27:59–65. doi: 10.2337/diacare.27.1.59. [DOI] [PubMed] [Google Scholar]
- 109.Huerta MG, Roemmich JN, Kington ML, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28:1175–1181. doi: 10.2337/diacare.28.5.1175. [DOI] [PubMed] [Google Scholar]
- 110.Paolisso G, Sgambato S, Pizza G, et al. Improved Insulin Response and Action by Chronic Magnesium Administration in Aged NIDDM Subjects. Diabetes Care. 1989;12:265–269. doi: 10.2337/diacare.12.4.265. [DOI] [PubMed] [Google Scholar]
- 111.Sun Q, van Dam RM, Willett WC, et al. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32:629–634. doi: 10.2337/dc08-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baker EN, Blundell TL, Cutfield JF, et al. The Structure of 2Zn Pig Insulin Crystals at 1.5A Resolution. Philos. T. Roy. Soc. B. 1988;319:369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- 113.Michael DJ, Ritzel RA, Haataja L, Chow RH. Pancreatic β-Cells Secrete Insulin in Fast- and Slow-Release Forms. Diabetes. 2006;55:600–607. doi: 10.2337/diabetes.55.03.06.db05-1054. [DOI] [PubMed] [Google Scholar]
- 114.Wijesekara N, Dai FF, Hardy AB, et al. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53:1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hardy AB, Wijesekara N, Genkin I, et al. Effects of high-fat diet feeding on Znt8-null mice: differences between β-cell and global knockout of Znt8. Am. J. Physiol.-Endoc. M. 2012;302:E1084–E1096. doi: 10.1152/ajpendo.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 117.Yogi A, Callera GE, Antunes TT, et al. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circulation Journal. 2011;75:237–245. doi: 10.1253/circj.cj-10-1021. [DOI] [PubMed] [Google Scholar]
- 118.Resnick LM, Gupta RK, Laragh JH. Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6511–6515. doi: 10.1073/pnas.81.20.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altura B, Altura B, Gebrewold A, et al. Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 120.Song YQ, Sesso HD, Manson JE, et al. Dietary magnesium intake and risk of incident hypertension among middle-aged and older US women in a 10-year follow-up study. Am. J. Cardiol. 2006;98:1616–1621. doi: 10.1016/j.amjcard.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 121.Ascherio A, Rimm EB, Giovannucci EL, et al. A prospective study of nutritional factors and hypertension among US men. Circulation. 1992;86:1475–1484. doi: 10.1161/01.cir.86.5.1475. [DOI] [PubMed] [Google Scholar]
- 122.Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur. J. Clin. Nutr. 2012;66:411–418. doi: 10.1038/ejcn.2012.4. [DOI] [PubMed] [Google Scholar]
- 123.Reffelmann T, Dörr M, Ittermann T, et al. Low serum magnesium concentrations predict increase in left ventricular mass over 5 years independently of common cardiovascular risk factors. Atherosclerosis. 2010;213:563–569. doi: 10.1016/j.atherosclerosis.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 124.Nie ZL, Wang ZM, Zhou B, et al. Magnesium intake and incidence of stroke: Meta-analysis of cohort studies. Nutrition, Metabolism and Cardiovascular Diseases. doi: 10.1016/j.numecd.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Altura BM, Shah NC, Shah G, et al. Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: cross-talk among cytokines, Mg2+, NF-κB, and de novo ceramide. Am. J. Physiol.-Heart C. 2012;302:H319–H332. doi: 10.1152/ajpheart.00453.2011. [DOI] [PubMed] [Google Scholar]
- 126.Ford ES, Mokdad AH. Dietary Magnesium Intake in a National Sample of U.S. Adults. J. Nutr. 2003;133:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 127.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 128.Vucenik I, Shamsuddin AM. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer. 2006;55:109–125. doi: 10.1207/s15327914nc5502_1. [DOI] [PubMed] [Google Scholar]
- 129.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary Factors and the Risk of Incident Kidney Stones in Younger Women: Nurses's Health Study II. Arch. Intern. Med. 2004;164:885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 130.Persson H, Turk M, Nyman M, Sandberg AS. Binding of Cu2+, Zn2+ and Cd2+ to inositol tri-, tetra-, penta, and hexaphosphates. J. Agric. Food Chem. 1998;46:3194–3200. [Google Scholar]
- 131.Crea F, De Stefano C, Milea D, Sammartano S. Formation and stability of phytate complexes in solution. Coord. Chem. Rev. 2008;252:1108–1120. [Google Scholar]
- 132.McCance RA, Widdowson EM. Phytin in human nutrition. The Biochemical journal. 1935;29:2694–2699. doi: 10.1042/bj0292694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Joung H, Jeun BY, Li SJ, et al. Fecal phytate excretion varies with dietary phytate and age in women. J. Am. Coll. Nutr. 2007;26:295–302. doi: 10.1080/07315724.2007.10719614. [DOI] [PubMed] [Google Scholar]
- 134.Levrat-Verny MA, Coudray C, Bellanger J, et al. Wholewheat flour ensures higher mineral absorption and bioavailability than white wheat flour in rats. Br. J. Nutr. 1999;82:17–21. doi: 10.1017/s0007114599001075. [DOI] [PubMed] [Google Scholar]
- 135.Callegaro MGK, Diettrich T, Alves E, et al. Supplementation with fiber-rich multimixtures yields a higher dietary concentration and apparent absorption of minerals in rats. Nutrition Research. 2010;30:615–625. doi: 10.1016/j.nutres.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Maziya-Dixon BB, Klopfenstein CF. Nutritional Properties of Hard White and Hard Red Winter Wheats and Oatmeal. II. Effects on Fecal Water Holding Capacity and Loss of protein, Ash, Calcium, and Zinc in Cholesterol-Fed Rats. Cereal Chem. 1994;71:544–547. [Google Scholar]
- 137.Lopez HW, Vallery F, Levrat-Verny MA, et al. Dietary phytic acid and wheat bran enhance mucosal phytase activity in rat small intestine. J. Nutr. 2000;130:2020–2025. doi: 10.1093/jn/130.8.2020. [DOI] [PubMed] [Google Scholar]
- 138.Mateo Anson N, van den Berg R, Havenaar R, et al. Ferulic Acid from Aleurone Determines the Antioxidant Potency of Wheat Grain (Triticum aestivum L.) J. Agric. Food Chem. 2008;56:5589–5594. doi: 10.1021/jf800445k. [DOI] [PubMed] [Google Scholar]
- 139.Nenadis N, Zhang H-Y, Tsimidou MZ. Structure–Antioxidant Activity Relationship of Ferulic Acid Derivatives: Effect of Carbon Side Chain Characteristic Groups. J. Agric. Food Chem. 2003;51:1874–1879. doi: 10.1021/jf0261452. [DOI] [PubMed] [Google Scholar]
- 140.Hamill L, Keaveney E, Price R, et al. Absorption of ferulic acid in human subjects after consumption of wheat-bran and wheat-aleurone fractions. Proc. Nutr. Soc. 2008;67:E255. [Google Scholar]
- 141.Mateo Anson N, Aura A-M, Selinheimo E, et al. Bioprocessing of Wheat Bran in Whole Wheat Bread Increases the Bioavailability of Phenolic Acids in Men and Exerts Antiinflammatory Effects ex Vivo. The Journal of Nutrition. 2011;141:137–143. doi: 10.3945/jn.110.127720. [DOI] [PubMed] [Google Scholar]
- 142.Kern SM, Bennett RN, Mellon FA, et al. Absorption of Hydroxycinnamates in Humans after High-Bran Cereal Consumption. J. Agric. Food Chem. 2003;51:6050–6055. doi: 10.1021/jf0302299. [DOI] [PubMed] [Google Scholar]
- 143.Price RK, Welch RW, Lee-Manion AM, et al. Total phenolics and antioxidant potential in plasma and urine of humans after consumption of wheat bran. Cereal Chem. 2008;85:152–157. [Google Scholar]
- 144.Rondini L, Peyrat-Maillard M-N, Marsset-Baglieri A, et al. Bound ferulic acid from bran is more bioavailable than the free compound in rat. J. Agric. Food Chem. 2004;52:4338–4343. doi: 10.1021/jf0348323. [DOI] [PubMed] [Google Scholar]
- 145.Zhao Z, Moghadasian MH. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008;109:691–702. doi: 10.1016/j.foodchem.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 146.Barron C, Samson MF, Lullien-Pellerin V, Rouau X. Wheat grain tissue proportions in milling fractions using biochemical marker measurements: Application to different wheat cultivars. J. Cereal Sci. 2011;53:306–311. [Google Scholar]
- 147.Parikka K, Rowland IR, Welch RW, Wahala K. In vitro antioxidant activity and antigenotoxicity of 5-n-alkylresorcinols. J. Agric. Food Chem. 2006;54:1646–1650. doi: 10.1021/jf052486e. [DOI] [PubMed] [Google Scholar]
- 148.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 150.Robertson RP. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet Beta Cells in Diabetes. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 151.Harmon JS, Bogdani M, Parazzoli SD, et al. β-Cell-Specific Overexpression of Glutathione Peroxidase Preserves Intranuclear MafA and Reverses Diabetes in db/db Mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 153.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gryglewski RJ, Palmer RMJ, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 155.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 156.Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database of Systematic Reviews. 2012;3:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 158.Lee J-M, Calkins MJ, Chan K, et al. Identification of the NF-E2-related Factor-2-dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 159.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid. Redox Signal. 2010;13:1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 160.Ma Z-C, Hong Q, Wang Y-G, et al. Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol. Pharm. Bull. 2010;33:29–34. doi: 10.1248/bpb.33.29. [DOI] [PubMed] [Google Scholar]
- 161.Ramar M, Manikandan B, Raman T, et al. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012;690:226–235. doi: 10.1016/j.ejphar.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 162.Sudheer AR, Muthukumaran S, Devipriya N, et al. Influence of ferulic acid on nicotine-induced lipid peroxidation, DNA damage and inflammation in experimental rats as compared to N-acetylcysteine. Toxicology. 2008;243:317–329. doi: 10.1016/j.tox.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 163.Shanthakumar J, Karthikeyan A, Bandugula VR, Rajendra Prasad N. Ferulic acid, a dietary phenolic acid, modulates radiation effects in Swiss albino mice. Eur. J. Pharmacol. 2012;691:268–274. doi: 10.1016/j.ejphar.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 164.Baskaran N, Manoharan S, Balakrishnan S, Pugalendhi P. Chemopreventive potential of ferulic acid in 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis in Sprague-Dawley rats. Eur. J. Pharmacol. 2010;637:22–29. doi: 10.1016/j.ejphar.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 165.Alias LM, Manoharan S, Vellaichamy L, et al. Protective effect of ferulic acid on 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino mice. Exp. Toxicol. Pathol. 2009;61:205–214. doi: 10.1016/j.etp.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 166.Balakrishnan S, Menon VP, Manoharan S. Ferulic acid inhibits 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. J. Med. Food. 2008;11:693–700. doi: 10.1089/jmf.2007.0103. [DOI] [PubMed] [Google Scholar]
- 167.Pluemsamran T, Onkoksoong T, Panich U. Caffeic Acid and Ferulic Acid Inhibit UVA-Induced Matrix Metalloproteinase-1 through Regulation of Antioxidant Defense System in Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012;88:961–968. doi: 10.1111/j.1751-1097.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- 168.Krishnan D, Prasanna N, Sabina E, Rasool M. Hepatoprotective and antioxidant potential of ferulic acid against acetaminophen-induced liver damage in mice. Comp Clin Pathol. 2012:1–5. [Google Scholar]
- 169.Kwon EY, Do GM, Cho YY, et al. Anti-atherogenic property of ferulic acid in apolipoprotein E-deficient mice fed Western diet: comparison with clofibrate. Food Chem. Toxicol. 2010;48:2298–2303. doi: 10.1016/j.fct.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 170.You Y, Park J, Yoon H-G, et al. Stimulatory effects of ferulic acid on endurance exercise capacity in mice. Biosci. Biotechnol. Biochem. 2009;73:1392–1397. doi: 10.1271/bbb.90062. [DOI] [PubMed] [Google Scholar]
- 171.Yeh C-T, Ching L-C, Yen G-C. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. Journal of Nutritional Biochemistry. 2009;20:163–171. doi: 10.1016/j.jnutbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 172.Mayer-Davis EJ, Costacou T, King I, et al. Plasma and Dietary Vitamin E in Relation to Incidence of Type 2 Diabetes. Diabetes Care. 2002;25:2172–2177. doi: 10.2337/diacare.25.12.2172. [DOI] [PubMed] [Google Scholar]
- 173.Ros MM, Bueno-de-Mesquita HB, Kampman E, et al. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. The American Journal of Clinical Nutrition. 2012;96:902–910. doi: 10.3945/ajcn.111.032920. [DOI] [PubMed] [Google Scholar]
- 174.Li G, Lee M-J, Liu AB, et al. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free Radic. Biol. Med. 2012;52:1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 175.Lu Y, Gong P, Cederbaum AI. Pyrazole induced oxidative liver injury independent of CYP2E1/2A5 induction due to Nrf2 deficiency. Toxicology. 2008;252:9–16. doi: 10.1016/j.tox.2008.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lu Y, Zhang XH, Cederbaum AI. Ethanol Induction of CYP2A5: Role of CYP2E1-ROS-Nrf2 Pathway. Toxicol. Sci. 2012;128:427–438. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chao W-W, Lin B-F. Bioactivities of major constituents isolated from Angelica sinensis (Danggui) Chinese Medicine. 2011;6:29. doi: 10.1186/1749-8546-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16:1119–1126. doi: 10.1016/j.phymed.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 179.Adisakwattana S, Moonsan P, Yibchok-Anun S, et al. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008;56:7838–7844. doi: 10.1021/jf801208t. [DOI] [PubMed] [Google Scholar]
- 180.Adisakwattana S, Chantarasinlapin P, Thammarat H, Yibchok-Anun S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009;24:1194–1200. doi: 10.1080/14756360902779326. [DOI] [PubMed] [Google Scholar]
- 181.You Y, Kim K, Yoon H-G, et al. Chronic effect of ferulic acid from Pseudosasa japonica leaves on enhancing exercise activity in mice. Phytother. Res. 2010;24:1508–1513. doi: 10.1002/ptr.3152. [DOI] [PubMed] [Google Scholar]
- 182.Ardiansyah, Ohsaki Y, Shirakawa H, et al. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008;56:2825–2830. doi: 10.1021/jf072896y. [DOI] [PubMed] [Google Scholar]
- 183.Wang B, Ouyang J, Liu Y, et al. Sodium ferulate inhibits atherosclerogenesis in hyperlipidemia rabbits. J. Cardiovasc. Pharmacol. 2004;43:549–554. doi: 10.1097/00005344-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 184.Chen HP, Liao ZP, Huang QR, et al. Sodium ferulate attenuates anoxia/reoxygenation-induced calcium overload in neonatal rat cardiomyocytes by NO/cGMP/PKG pathway. European Journal of Pharmacology. 2009;603:86–92. doi: 10.1016/j.ejphar.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 185.Suzuki A, Kagawa D, Ochiai R, et al. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertension Research - Clinical & Experimental. 2002;25:99–107. doi: 10.1291/hypres.25.99. [DOI] [PubMed] [Google Scholar]
- 186.Suzuki A, Yamamoto M, Jokura H, et al. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007;20:508–513. doi: 10.1016/j.amjhyper.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 187.Mudnic I, Modun D, Rastija V, et al. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010;119:1205–1210. [Google Scholar]