Abstract
The marine environment has been a source of more than 20,000 inspirational natural products discovered over the past 50 years. From these efforts, 9 approved drugs and 12 current clinical trial agents have been discovered, either as natural products or molecules inspired from the natural product structure. To a significant degree, these have come from collections of marine invertebrates largely obtained from shallow water tropical ecosystems. However, there is a growing recognition that marine invertebrates are oftentimes populated with enormous quantities of ‘associated’ or symbiotic microorganisms, and that microorganisms are the true metabolic sources of these most valuable of marine natural products. Also, because of the inherently multidisciplinary nature of this field, a high degree of innovation is characteristic of marine natural product drug discovery efforts.
Keywords: Marine microorganisms, pharmaceuticals, anticancer agents, symbiosis, drug discovery
A. Introduction
The field of marine natural products chemistry is a young pursuit, dating back only to the 1960s, and in earnest with a drug discovery focus to the 1980s. Initial studies largely focused on the most conspicuous and collectable organisms, namely intertidal and shallow subtidal macroalgae and invertebrates. With the advent of SCUBA, and subsequently application of deep sea diving vessels to this pursuit, progressively deeper water organisms have been collected and screened for their biomedically-significant secondary metabolites. From these efforts, more than 22,000 discrete marine metabolites have been isolated and structurally characterized, and a significant percentage has been evaluated for some level of biological activity, such as antimicrobial effects or mammalian cell toxicity [1]. However, the range of biological assays is so enormous that it is fair and accurate to say that this field of investigation has only plucked the ‘low hanging fruit’ at this point, and many exciting discoveries await a more comprehensive biological evaluation of these known compounds as well as those still left to discover.
The focus of the perspective is to share the excitement that surrounds this quest for new pharmaceuticals from the sea, some of the success stories that validate this pursuit, and suggest some future research areas that are on the horizon. Despite general excitement for the field and successful products that have been developed, most research in marine natural products drug discovery is occurring in the public sector as changes in the private sector have altered enthusiasm for these “higher risk” activities. But is it accurate to characterize marine natural products drug discovery efforts as high risk? Perhaps, but this is a matter of perspective. For example, the number of marine-derived chemical entities that need to be encountered and evaluated in some respect for their useful properties and then result in an approved drug is considerably less than from other sources. A widely held perception is that it requires the evaluation of 15,000 traditionally sourced compounds to advance a single agent to the clinic [2]; however, from the marine environment there are 9 approved drugs, which roughly translates into one drug entity for every 2,500 characterized natural products, a 6-fold improvement over other sources.
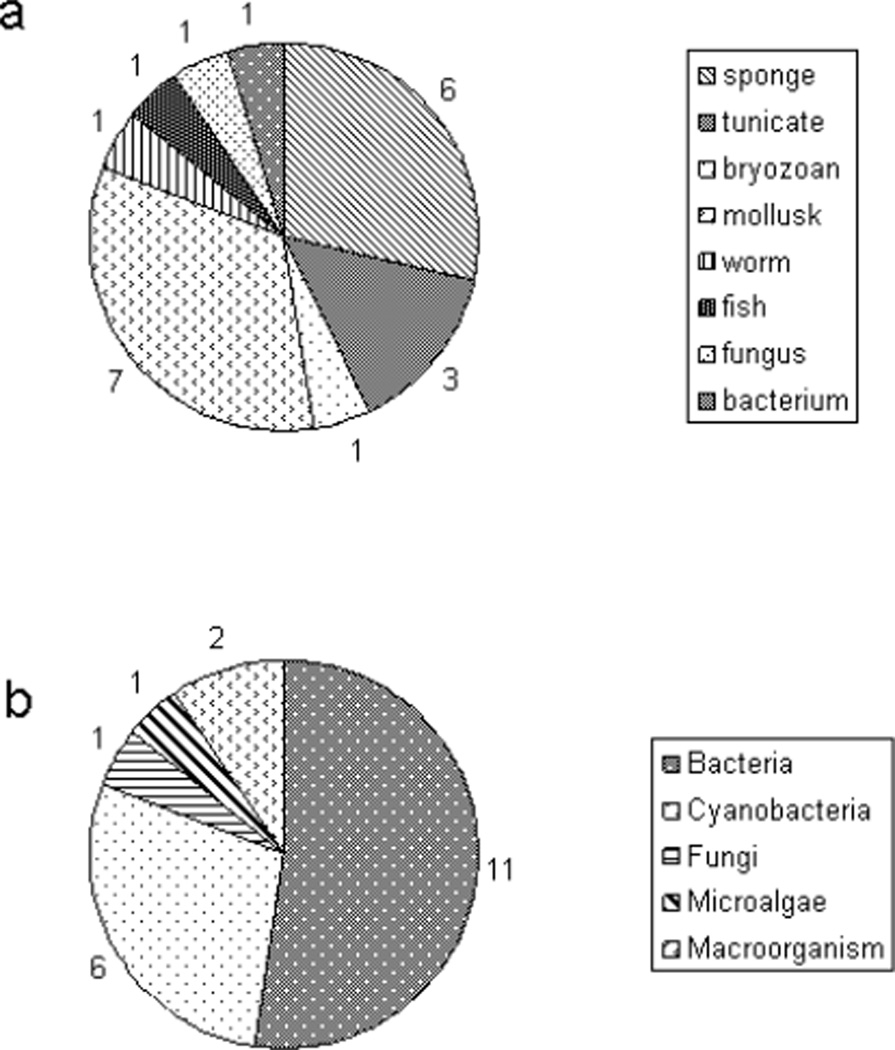
It is of great interest to query those compounds which have gone into the clinic, either as natural products or analogs, as to the type of organism from which they derive. At the same time, there has been a growing perception that many of the compounds found originally from collected biomass of macroorganisms, such as sponges, tunicates, opistobranch mollusks and the like, are actually being produced by symbiotic or associated microorganisms, or derive from a diet of prokaryotic microorganisms. Indeed, in several cases experimental or circumstantial evidence supports this conclusion. Figure 1a provides a pie chart perspective on the marine natural products in the clinic today, or in clinical trial presently or recently, tallied by their collected sources [3,4]. Clearly, marine invertebrates have indeed provided the majority of these (18 of 21, 86%). However, a tally of the proven or hypothesized biosynthetic sources of these same compounds is given in figure 1b, and clearly reveals that it is the metabolic processes of the marine microbes that are truly the treasure troves of new marine pharmaceuticals.
Fig 1.
Pie charts showing a) the collected sources of the 21 marine derived or inspired agents in the clinic or clinical trial, presently or recently, and b) the same agents with their demonstrated or predicted metabolic sources
B. A Selection of Marine Natural Product Success Stories
1. Ecteinascidin from the tunicate Ecteinascidia turbinata
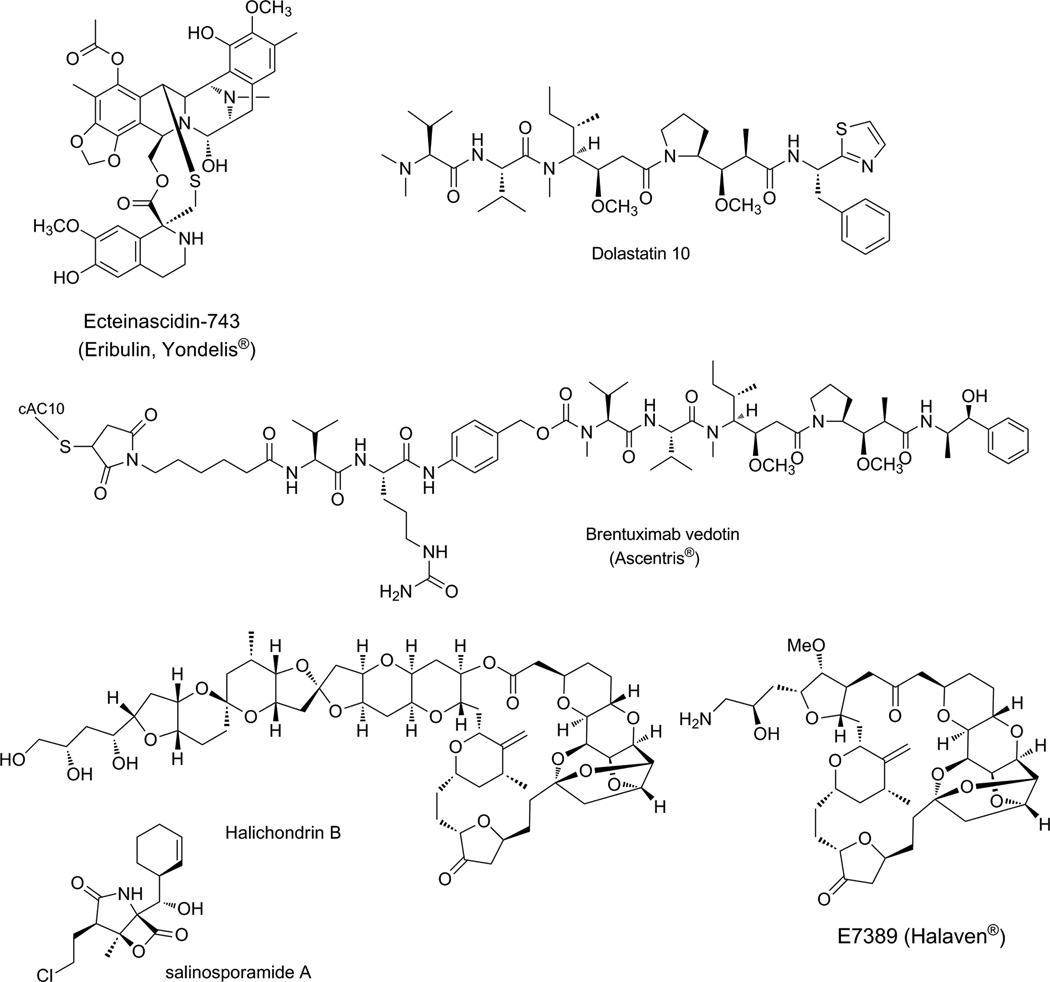
The history of the discovery of ecteinascidin as a powerful new antineoplastic agent is long and involved many different laboratories. The extract of the Caribbean tunicate Ecteinascidia turbinata was first studied by the physician M. Sigel [5] for its cancer curative properties detected as an in vivo activity. However, the quantity of active compound and its chemical complexity rendered its chemical structure intractable in the 1970s. Subsequently, two laboratories, that of Amy Wright at the then Harbor Branch Oceanographic Institute and Ken Rinehart at the University of Illinois nearly simultaneously solved the structure of the active metabolite, ecteinascidin-743 [6,7]. At the time, this was one of the most complex alkaloidal structures ever to be determined by spectroscopic means and was a considerable intellectual achievement (Figure 2).
Fig 2.
Marine natural products and/or their clinically useful derivatives.
Development of the drug occurred by the Spanish company Pharmamar and initially there were significant problems with establishing a commercial supply [8]. Natural stocks of the producing tunicate were insufficient to provide a reliable supply, and aquaculture in the sea or seawater ponds or total chemical synthesis were all too expensive [9]. Ultimately, a portion of the metabolite, namely cyanosafracin B, could be obtained from fermentation of the terrestrially-derived microbe Pseudomonas fluorescens, and this was synthetically modified into ecteinascidin-743 [10]. That related alkaloids were known products of microbial metabolism was a first clue that microorganisms were likely the ultimate source of the ecteinascidins, but this was difficult to establish by experimental methods. Recently, a metagenomic approach to isolating the biosynthetic gene cluster for ecteinascidin production successfully characterized a 35 kB DNA sequence as that responsible for producing the required 25 biosynthetic enzymes [11]. Heterologous expression of some of these enzymes helped confirm that this was indeed the correct biosynthetic gene cluster, and sequence analysis identified the uncultured symbiotic bacterium Endoecteinascidia frumentensis as the ultimate metabolic source of these genes. Yondelis®, the drug name for ecteinascidin-743, was approved in 2007 in Europe for treating soft tissues sarcomas, and trials against other cancer types such as ovarian, prostate and breast cancer are in progress [12].
2. Development of a Dolastatin 10 Analog into a Useful Antineoplastic Agent
In 1987, Bob Pettit, then at Arizona State University, reported the results of a several-year effort to characterize a very minor but highly cytotoxic product from a collection of the sea hare Dolabella auricularia [13]. The cytotoxin, called dolastatin 10, was found to be a linear Non-Ribosomal Peptide Synthetase-Polyketide Synthase (NRPS-PKS) hybrid natural product with a number of novel features, including a methoxystatin residue, an amino terminus composed of an N,N-dimethylvaline, and a carboxyl terminus composed of a thiazole ring (Figure 2). Dolastatin 10 shows extraordinary antiproliferative properties by virtue of its anti-tubulin mechanism of action, and was one of several compounds that revealed the presence of a drug-binding site on tubulin that overlapped that of the vinca alkaloids [14]. It was suggested early on, based on the low yield of dolastatin 10 from the sea hare, and its structural resemblance to other cyanobacterial natural products, that this metabolite likely derived from metabolic pathways in filamentous marine cyanobacteria, and that the latter were fed upon by grazing sea hares that assimilated these diet-derived natural products. A few years later, efforts in the R.E. Moore laboratory confirmed this suspicion with the isolation of the closely related symplostatins, and subsequently dolastatin 10 itself, directly from field collection of the marine cyanobacterium Symploca sp. [15].
Dolastatin 10 and related analogs were evaluated in a number of clinical trials for their antiproliferative properties, but in all cases were found to be too toxic, cause peripheral neuropathy, and lack efficacy in the treatment of cancer [16]. However, in 2010, a different approach was attempted, namely to attach dolastatin 10 (actually, a close analog was used, auristatin PE), to a monoclonal antibody which would target the delivery of this warhead molecule directly to tumor cells exhibiting the appropriate cancer cell epitope. Moreover, by attaching a cleavable linker between the drug and the targeting antibody, a higher level of efficacy was observed. This may be because some of the drug is released at the cell surface and diffuses into the cell as the active substance, or that the entire conjugate is endocytosed and cleaved in endosomes to the active drug or homolog. In either case, the active molecule does find its tubulin target and results in a powerful cytotoxic effect. To date, this conjugated drug, brentuximab vendotin, is marketed as Ascentris®, and has been approved for treating anaplastic large cell lymphoma (ALCL) and Hodgkin’s lymphoma [17].
3. The Long Road from Discovery of a Sponge Cytotoxin to the Useful Drug Halaven®
A listing of the milestones of achievement in marine natural products drug discovery must certainly include the amazing work of the Uemura laboratory to first characterize the halichondrins, isolated from the Japan Sea sponge Halichondria okadai (Figure 2) [18]. These extraordinarily complex structures are also some of the most potent cancer cell cytotoxins known, and also work through an antitubulin mechanism. A shortage of supply curtailed their immediate development, but this need was subsequently partially met by finding a population of New Zealand deepwater sponges that also contained the lead compound in the series, halichondrin B [19]. The latter source led to evaluations by the National Cancer Institute in the US and fueled a high level of enthusiasm for this structurally complex yet biologically valuable natural product. Total chemical synthesis efforts in the Kishi laboratory at Harvard revealed that approximately only one half of the molecule was required for activity [20], and this helped spawn an industrial effort by Eisai, Inc. Ultimately, the activity of the halichondrin B fragment was found to be enhanced by replacing a labile ester bond with a simple ketone, thus stabilizing the molecule and improving its potency. For Phase I trials, a commercial synthesis of this derivative, known as eribulin, was developed and required some 67 separate synthetic steps [21]; clearly, this drug is produced by the most complex process developed to date for reliable supply of a drug molecule! Nevertheless, the drug, sold as Halaven®, is showing good efficacy against a range of tumor types, especially breast carcinoma [22].
A specific study of the biosynthesis and metabolic origin of halichondrin B has not yet occurred, however, it is relatively certain that a microorganism is responsible for the production of this elaborate natural product. The overall architecture of the molecule is diagnostic for its biosynthesis via a PKS pathway. The long linear carbon chain, deriving from polymerization of acetate units, is decorated with remnant oxidations, mostly occurring at predicted C-1 (carbonyl) sites of acetate, and with methyl groups at C-2 sites deriving from SAM methylation or incorporation of propionate at these positions. Alternate sites of oxygenation at C-2 carbons are predicted to be introduced by P-450 oxidations whereas methylations at C-1 carbons are hallmarks of beta-branch reactions involving HMGCoA synthase-like reactions [23]. These fundamental pathways and unique modifications are hallmarks of microbial metabolism, and thus it is quite certain that the halichondrins derive from sponge-associated or symbiotic microorganisms.
4. Salinosporamide A, a Clinical Trial Agent for Multiple Myeloma
A last example of a clinically used microbial marine natural product is salinosporamide A, which has been in recent clinical trials alone and in combination with other agents for cancer indications [24]. Whereas in the previous cases microbes living in association with macro-organisms, or being preyed upon, were the metabolic sources of the biologically active natural products, in this case it is a cultured marine microbe. The source organism belongs to the recently described and exclusively marine genus of streptomycete bacteria known as Salinispora, of which three species are recognized and all inhabit marine sediments. Fermentation cultures of S. tropica were found to produce a powerful cancer cell toxin, which was defined as a unique beta-lactone called salinosporamide A (Figure 2). Because this structure closely resembled that of another microbial metabolite, namely omuralide, it was reasoned that it had a similar mechanism of action, namely inhibition of the 20S proteasome through a covalent modification of the active site threonine residue. This was indeed shown to be the case through X-ray crystallography, and moreover, revealed that a pendant chlorine atom plays a role in the mechanism of the drug through a secondary tetrahydrofuran ring formation which in turn blocks the approach of water and stabilizes the new ester bond with the enzyme [25]. Proteasome inhibitors, such as the currently approved Bortezomib (trade name Velcade®), show a particular utility in treating multiple myeloma, and thus salinosporamide has been advanced to Phase II clinical trials for this indication by the San Diego company Nereus Pharmaceuticals.
C. Future Trends
As the pie charts above in Figure 1 suggest, the available evidence suggests that the real wealth in bioactive marine natural products rests within the metabolic capabilities of marine microbes. As a result of this growing recognition, a number of marine natural product discovery programs have come to focus on marine microbiology. Sub-specialization within this general area is occurring with various researchers examining marine sediments, seawater itself, various classes of marine invertebrates and vertebrates, marine algal associated microbes including endophytic fungi, and other specialized niches. However, new methods are ardently needed for the efficient culture of these organisms, taking into account their unique nutrient and physical requirements, and in some cases, growth at low cell densities [26]. Alternately, culture independent methods are being developed at the present time wherein informatic analyses of metagenomes can be used to identify unique secondary metabolite gene clusters, and these genes can subsequently be synthesized and introduced into expression hosts [27]. While in principle a generic solution, it has been difficult to enact in more than a few model cases, and technological knowledge of the pathways and their operation is still fragmentary and in need of additional research.
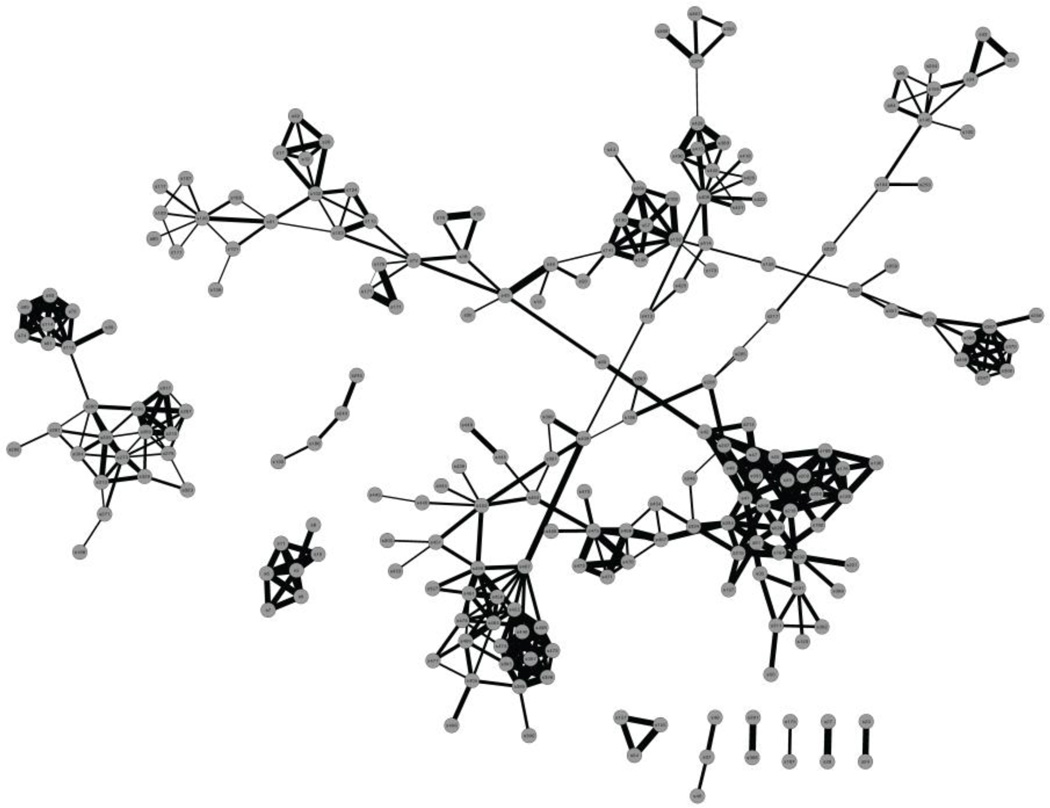
It has become quite clear that efforts to date in marine natural products chemistry have largely focused on major metabolites of those organisms most easily collected. And while there has been a recent shift to microorganisms, as detailed above, minor metabolites present in very small quantities are a challenge for analytical and biological evaluations. However, technological advances are being made in various sectors to improve access to minor metabolites, such as the increasingly widespread use of NMR microcryogenic and capillary flow-probes, biological assays in increasingly smaller volumes such as in 384- and 1534-well plate formats, and perhaps most powerfully, enhancements to the methods as well as informatics associated with mass spectrometry. In the later regard, a very powerful technology called molecular networking has recently been applied to construct a global view of the secondary metabolome of a given organism (Figure 3) [28]. This method gives essentially equal weight or ability to visualize minor metabolites as well as major metabolites, and it also provides an immediate indication of the structural relatedness to other metabolites in the extract, or libraries of compounds that have been added to the dataset so as to provide critical reference points. As the method becomes better recognized, its utility is certain to expand to many other dimensions of natural products investigation, but already this approach is having pronounced impact on a) the detection and recognition of minor analogs in an extract that are related to a compound class of interest, b) detection and recognition of fundamentally new molecular diversity that is unrelated to any known compounds, c) identification of co-metabolites which give insights into biosynthetic processes, and d) newly expressed metabolites under altered growth or elicitation conditions.
Fig 3.
A spectral network of a marine cyanobacterial extract displaying many distinct natural products grouped into clusters which reveal their chemical relatedness
A third area where we can predict dramatic improvements in marine natural products drug discovery programs is in the biological assay of extracts, fractions and pure compounds. As great as the molecular diversity of marine natural products is, biological assay space is certainly as large if not larger. The enormous range of biological targets, from enzyme to receptor to DNA or RNA, some modulated singly and others in concert with one another to varying degrees, some at active sites whereas others at allosteric sites, combines to create a staggering range of potential biological activities. Thus, it has been interesting to see a trend towards the use of phenotypic screens that evaluate rather broadly the ability of a test material to alter a cellular phenotype in a desired fashion, especially those that are run with high content methods of evaluation [29]. Moreover, in assay formats involving cellular systems, one also gains a keen insight into pharmacodynamic properties of a molecule, such as cell penetrance and stability. Of course, the phenotypic assay approach leaves open the question of biochemical mechanism, but again, there are a growing number of powerful approaches and methods for determining the mechanism or target of a new bioactive compound, so this should not be a major hurdle [30]. Rather, these assay formats will expand the range of potential targets that marine natural products are evaluated against for potentially useful biological effects, and this is a needed and important area of growth in the discipline.
D. Conclusions
As detailed above in summary form as well as a few select examples, the marine environment has been a wonderfully successful environment in which to prospect for new potential drug leads, especially so in the search for new anticancer agents. It may be that this exceptional level of success in the discovery of new anticancer-type scaffolds reflects the highly competitive environment of the shallow water marine ecosystem, or alternatively, is a result of the major stakeholders funding marine discovery research (e.g. the National Cancer Institute in the United States). In support of the first possibility, the marine metabolites or their derivatives which have been developed for their clinical utility are largely toxins in their natural context and have been ascribed a role of chemical defense against predation, overgrowth, or mediating other types of competitive interactions [31]. Thus, the ironical adage that “the difference between a pharmaceutical and a poison being a matter of dose” is exceptionally well applied in this case!
Another conclusion to be drawn from this review is the high level of multidisciplinarity and innovation that permeates this research discipline. Involving such disparate fields as natural history, microbiology, taxonomy, ecology, analytical chemistry, computer science, synthetic organic chemistry, biochemistry, molecular biology, and pharmacology, there is an inherent drawing on and utilization of techniques and knowledge from all of these fields of study. Ultimately, this makes not only for more interesting scientific studies, but also helps to drive innovation in that techniques are shuttled between these different disciplines with great frequency, finding new utilities and applications. This type of innovation, borrowing from one field and applying to another in a new and unanticipated way, has the exceptional feature of being potentially revolutionary. Indeed, the development of molecular networks from MS2 data may just be one such revolutionary advance in our search of marine microbes for their pharmaceutically useful natural products.
Acknowledgements
Research in the author’s laboratory is supported by NIH grants CA100851, TW006634, CA108874, and NS053398. We thank an anonymous reviewer for their helpful comments.
References
- 1.Blunt J, Munro M, Upjohn M. The role of databases in marine natural products research. In: Fattorusso E, Gerwick WH, Taglialatela-Scafati O, editors. Handbook of Marine Natural Products. 1st edn. Dordrecht: Springer; 2012. pp. 389–421. [Google Scholar]
- 2.Gerwick WH, Moore BS. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol. 2012;19:85–98. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM. Natural products derivatives and mimics as antitumor agents. In: Wrigley S, Thomas R, Nicholson N, Bedford C, editors. Functional Molecules from Natural Sources. Cambridge, England: RSC Publishing; 2010. pp. 3–36. [Google Scholar]
- 5.Lichter W, Lopez DM, Wellham LL, Sigel MM. Ecteinascidia turbinata extracts inhibit DNA synthesis in lymphocytes after mitogenic stimulation by lectins. Proc Soc Exp Biol Med. 1975;150:475–478. doi: 10.3181/00379727-150-39059. [DOI] [PubMed] [Google Scholar]
- 6.Rinehart KL, Holt TG, Fregeau NL, Stroh JG, Keifer PA, Sun F, Li LH, Martin DG. Ecteinascidins729 743 745 759A 759B and 770: potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinate. J Org Chem. 1990;55:4512–4215. [Google Scholar]
- 7.Wright AE, Forleo DA, Gunawardana GP, Gunasekera SP, Koehn FE, McConnell OJ. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinate. J Org Chem. 1990;55:4508–4512. [Google Scholar]
- 8.Mendola D, Naranjo L, Santiago A, Duckworth AR, Osinga R. The promise of aquaculture for delivering sustainable supplies of new drugs from the sea: examples from in-sea and tank-based invertebrate culture projects from around the world. Frontiers Mar Biotech. 2006:21–72. [Google Scholar]
- 9.Corey EJ, Gin DY, Kania RS. Enantioselective total synthesis of ecteinascidin 743. J Am Chem Soc. 1996;118:9202–9203. [Google Scholar]
- 10.Cuevas C, Pérez M, Martín MJ, Chicharro JL, Fernández-Rivas C, Flores M, Francesch A, Gallego P, Zarzuelo M, de la Calle F, García J, et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org Lett. 2000;2:2545–2548. doi: 10.1021/ol0062502. [DOI] [PubMed] [Google Scholar]
- 11.Rath CM, Janto B, Earl J, Ahmed A, Hu FZ, Hiller L, Dahlgren M, Kreft R, Yu F, Wolff JJ, et al. Meta-omic characterization of the marine invertebrate microbial consortium that produces the chemotherapeutic natural product ET-743. ACS Chem Biol. 2011;6:1244–1256. doi: 10.1021/cb200244t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scotto KW. ET-743: more than an innovative mechanism of action. Anti-Cancer Drugs. 2002;13:S3–S6. [PubMed] [Google Scholar]
- 13.Pettit GR, Kamano Y, Herald CL, Tuinman AA, Boettner FE, Kizu H, Schmidt JM, Baczynskyj L, Tomer KB, Bontems RJ. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J Am Chem Soc. 1987;109:6883–6885. [Google Scholar]
- 14.Hamel E. Potent new antimitotic natural products from marine animals which act in the vinca domain of tubulin. Cell Pharm. 1993;1:S47–S52. [Google Scholar]
- 15.Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J Nat Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 16.Pitot HC, McElroy EA, Jr, Reid JM, Windebank AJ, Sloan JA, Erlichman C, Bagniewski PG, Walker DL, Rubin J, Goldberg RM, et al. Phase I trial of dolastatin-10 (NSC 376128) in patients with advanced solid tumors. Clin Cancer Res. 1999;5:525–531. [PubMed] [Google Scholar]
- 17.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotech. 2012;30:631–637. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- 18.Hirata Y, Uemura D. Halichondrins-antitumor polyether macrolides from a marine sponge. Pure Appl Chem. 1986;58:701–710. [Google Scholar]
- 19.Munro MHG, Blunt JW, Dumdei EJ, Hickford SJH, Lill RE, Li S, Battershill CN, Duckworth AR. The discovery and development of marine compounds with pharmaceutical potential. Prog Indus Micro. 1999;35:15–25. doi: 10.1016/s0168-1656(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 20.Yu MJ, Zheng W, Seletsky BM, Littlefield BA, Kishi Y. Case history: discovery of eribulin (HALAVEN) a halichondrin B analogue that prolongs overall survival in patients with metastatic breast cancer. Ann Rep Med Chem. 2011;46:227–241. [Google Scholar]
- 21.Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, et al. A phase I study of eribulin mesylate (E7389) a mechanistically novel inhibitor of microtubule dynamics in patients with advanced solid malignancies. Clin Cancer Res. 2009;15:4207–4212. doi: 10.1158/1078-0432.CCR-08-2429. [DOI] [PubMed] [Google Scholar]
- 22.Towle MJ, Nomoto K, Asano M, Kishi Y, Yu MJ, Littlefield BA. Broad spectrum preclinical antitumor activity of eribulin (Halaven(R)): optimal effectiveness under intermittent dosing conditions. Anticancer Res. 2012;32:1611–1619. [PubMed] [Google Scholar]
- 23.Jones AC, Monroe EA, Eisman E, Gerwick L, Sherman DH, Gerwick WH. The unique mechanistic transformations involved in the biosynthesis of modular natural products from marine cyanobacteria. Nat Prod Rep. 2010;27:1048–1065. doi: 10.1039/c000535e. [DOI] [PubMed] [Google Scholar]
- 24.Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, Chandra J, Chauhan D, Cusack JC, Jr, Fenical W, et al. Marizomib a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groll M, Huber R, Potts BCM. Crystal structures of salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of β-lactone ring opening and a mechanism for irreversible binding. J Am Chem Soc. 2006;128:5136–5141. doi: 10.1021/ja058320b. [DOI] [PubMed] [Google Scholar]
- 26.Fenical W, Jensen PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 27.Chang F-Y, Brady SF. Cloning and characterization of an environmental DNA-derived gene cluster that encodes the biosynthesis of the antitumor substance BE-54017. J Am Chem Soc. 2011;133:9996–9999. doi: 10.1021/ja2022653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guthals A, Watrous JD, Dorrestein PC, Bandeira N. The spectral networks paradigm in high throughput mass spectrometry. Mol BioSys. 2012;8:2535–2544. doi: 10.1039/c2mb25085c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 30.Penrod NM, Cowper-Sal-lari R, Moore JH. Systems genetics for drug target discovery. Trends Pharm Sci. 2011;32:623–630. doi: 10.1016/j.tips.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawlik JR. The chemical ecology of sponges on Caribbean reefs: natural products shape natural systems. BioScience. 2011;61:888–898. [Google Scholar]