Abstract
The goal of this review is to summarize our current knowledge about the helicases involved in translation initiation and their roles in both general and mRNA-specific translation. The main topics covered are the mechanisms of helicase action, with emphasis on the roles of accessory domains and proteins; the functions performed by helicases in translation initiation; and the interplay between direct and indirect effects of helicases that also function in steps preceding translation initiation. Special attention is given to the dynamics of eIF4A binding and dissociation from eIF4F during mRNA unwinding. It is proposed that DHX29, as well as other helicases and translation initiation factors could also cycle on and off the translation initiation complexes, similar to eIF4A. The evidence in favor of this hypothesis and its possible implications for the mechanisms of translation initiation are discussed.
Keywords: Translation, RNA helicase, DEAD-box, DExH-box, translation initiation factor
I. Introduction
Eukaryotic translation initiation is a multistep process involving over 10 eukaryotic initiation factors (eIFs), composed of more than 30 different polypeptides. The majority of mRNAs in the cell are capped and polyadenylated, and start codon recognition involves recruitment of the 43S pre-initiation complex (43S pre-IC) to the 5'-cap, followed by scanning. Therefore, for the purposes of this review, “general” eIFs are the proteins involved in translation initiation on capped, polyadenylated mRNAs. Certain types of mRNAs contain internal ribosome entry sites (IRESs) that require only a subset of these factors, with some only requiring the 40S subunit. In addition to the general translation factors acting on most mRNAs, there are many proteins that either stimulate or repress translation initiation on specific types of mRNAs (reviewed in [1–4]).
The 43S complex consists of the small, 40S ribosomal subunit, the eIF2•GTP•Met-tRNAi ternary complex (TC), as well as eIFs 1, 1A, 3 and 5. It is recruited to the mRNA 5'-cap by the eIF4E/4G/4A/4B complex. In higher eukaryotes, eIF4E, 4G and 4A form a stable complex called eIF4F. eIF4E is the cap-binding protein; eIF4G serves as a scaffold interacting with the other members of the complex, as well as with the poly-A binding protein (PABP) bound to the 3'-poly-A tail at the other end of the mRNA. eIF4A/DDX2 is a DEAD-box (DDX) helicase whose activity is stimulated by eIF4G and eIF4B (and also eIF4H in higher eukaryotes). 43S complex recruitment is mediated by eIF4G (possibly helped by eIF4B) and at least in higher eukaryotes involves binding to eIF3. The 43S complex then scans along the mRNA in the 3' direction until it reaches the start codon. eIF4F remains bound to the scanning 43S complex and promotes unwinding of secondary structures on its path, while its association with the 5'-cap is likely lost allowing for a new 43S complex to be recruited while the first one is still scanning [5]. eIF1 and eIF1A are important for scanning and start codon selection. eIF1 discriminates against non-AUG codons, AUG codons located too close to the 5'-end, or AUG codons in poor sequence context that does not match the so-called Kozak consensus sequence. Basepairing between the start codon and the tRNA anticodon triggers major conformational changes in the 43S complex, leading to adoption of a “closed” scanning-incompetent conformation: the 48S IC. eIF5 is required to promote GTP hydrolysis by eIF2 in the 48S IC, followed by phosphate release and displacement of eIF1 from its binding site on the 40S subunit. GTP hydrolysis lowers the affinity of eIF2 for the Met-tRNAi. eIF2•GDP dissociates and another G-protein eIF5B replaces it on the Met-tRNAi. eIF5B together with eIF1A promotes the recruitment of the large 60S ribosomal subunit. Finally, ribosomal subunit joining promotes GTP hydrolysis by eIF5B, which dissociates together with eIF1A, leaving an 80S ribosome with a Met-tRNAi in the P-site, ready for translation elongation (reviewed in [1–4]).
For a long time, eIF4A was the only helicase eIF, although yeast Ded1 was also reported to play a role in general translation initiation. This changed in recent years with the discovery that the helicase DHX29 (DExH-box protein 29) is important for scanning 5'-untranslated regions (UTRs) containing stable hairpins [6–8].
In addition to eIF4A and DHX29, the helicases Ded1/DDX3, Vasa/DDX4, RNA helicase A (RHA/DHX9), and Dhh1/RCK/DDX6 also play roles related to translation initiation (reviewed in [9–11]). Dhh1/RCK is a translational repressor that acts at least in part at the level of translation initiation. However, this helicase will not be discussed here since its functions relate mainly to translational repression and mRNA decay [12–14]. Vasa/DDX4 [15–18] and RNA helicase A (RHA/DHX9) [19–22] have been reported to stimulate translation initiation of specific mRNAs, but the molecular mechanisms of their functions remain unclear. Yeast Ded1 and its mammalian homolog DDX3 have been reported by different groups to stimulate or to repress translation and to act as general or mRNA-specific factors [23–30]. These apparently conflicting data about Ded1/DDX3 illustrate the complexity of translation initiation and the current limits of our understanding of its molecular mechanisms. To further complicate matters, Ded1/DDX3 and RHA/DHX9, and maybe also Vasa/DDX4, all appear to play roles at steps preceding translation initiation, such as transcription, pre-mRNA splicing and transport [27, 31–36], making it difficult to separate direct from indirect effects on translation initiation.
The goal of this review is to propose a framework for the analysis of the roles of helicases in translation initiation, with the hope of clarifying some apparent contradictions in the field or suggesting experimental approaches to address them. The main topics covered are: i) the mechanisms of helicase action, with emphasis on the roles of accessory domains and proteins; ii) the functions performed by helicases in translation initiation; iii) the interplay between direct and indirect effects of helicases that also function in steps preceding translation initiation; and iv) the dynamics of helicase binding and release from initiation complexes. In-depth descriptions of the structures and detailed enzymatic mechanisms of RNA helicases, which are beyond the scope of this article, can be found in a number of recent reviews [9–11, 37, 38].
II. Mechanism of action and domain structure of DEAD and DExH box helicases
A. Mechanism of helicase action
All helicases known to function in translation initiation are Superfamily 2 (SF2) helicases that belong to the DEAD-box or DExH-box families, named after the sequence of the helicase motif II [39, 40]. These helicases contain two RecA domains, often surrounded by additional accessory or regulatory domains. While in the absence of ATP and RNA, the two RecA domains are not stably bound to each other (“open” conformation), both the ATP- and the RNA-binding sites are formed by the RecA domains coming in a specific orientation (“closed”, catalytically active conformation). ATP is bound in a pocket between the two RecA domains while the RNA binds on the surface. The equilibrium between open and closed conformations is controlled by ATP and RNA binding, as well as by accessory domains and/or proteins (Fig. 1). The ADP-bound conformation (after ATP hydrolysis but before ADP release) is also “closed” but somewhat different from the ATP-bound state (reviewed in [9–11]).
Figure 1. Regulation of helicase function by accessory domains and proteins.
A. Roles of accessory domains in regulation of helicase function. The RNA is shown in red. RNA unwinding is mediated by the two RecA domains of the helicase (light and dark blue) in the presence of ATP (shown as black oval between the two RecA domains). The interaction of the RecA domains with mRNA is shown with black arrows. Interactions of accessory domains are shown with dashed arrows: blue (stimulatory) or red (inhibitory). A, activating domain; I, inhibitory domain; ssRBD, ssRNA-binding domain; dsRBD, dsRNA-binding domain. The accessory domains may be connected covalently as shown (part of the same polypeptide chain), or through protein-protein interactions (multisubunit complex). The RNA-binding domains may bind to RNA either specifically (helicase recruitment) or nonspecifically. The activating (A) domain can stimulate the helicase activity by binding both RecA domains in a closed, active conformation. The mechanism of action of the inhibitory (I) domain can be binding both RecA domains and trapping them in an open, inactive conformation and/or competing with RNA for binding to the RecA domains (see also the model for the eIF4A:eIF4G interactions in Panel B).
B. Model for the dynamics of the eIF4A:eIF4G interactions. eIF4G HEAT-1 domain (yellow) stimulates ATP binding and the helicase activity of eIF4A by simultaneous binding to both eIF4A domains in the closed ATP-bound conformation (left). eIF4G HEAT-2 domain (orange) favors the nucleotide-free state by simultaneous binding to both eIF4A domains in an open conformation (right). The interdomain linker of eIF4G (light orange) also binds eIF4A and stabilizes the complex. The arrows indicate directions of rearrangements during the ATP hydrolysis/nucleotide exchange cycle. ATP (not shown) binds at the interface between the two eIF4A domains. HEAT-2 is shown semi-transparent to emphasize that it is not required for the ATP-binding/hydrolysis cycle. Panel B is reprinted from Marintchev et al., Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation, Cell, 136, 447–460, Copyright 2009, with permission from Elsevier.
B. Roles of accessory domains
Unlike replicative helicases, the DEAD and DExH box helicases involved in translation initiation cannot processively unwind long duplexes and appear to have a different mode of unwinding [41–44]. The helicase activity is mediated by binding to RNA in the presence of ATP, followed by cycles of ATP hydrolysis, release of the helicase from the RNA and re-binding, or partial release and sliding along the RNA. Strand separation is driven by the high affinity of the helicase for ssRNA. In crystal structures of DEAD/DExH box helicases in complex with ATP and RNA, the ssRNA is kinked, which may also promote strand separation [42]. A single cycle of ATP binding and hydrolysis is often sufficient to unwind a relatively short hairpin [45]. In some reports, ATP hydrolysis was not required for strand separation [46], whereas in others strand separation occurred after ATP hydrolysis, but before phosphate release [47]. The source for this discrepancy is not clear but it could be explained by the relative rates of ATP hydrolysis (fast), phosphate release (slow and rate-limiting), and strand separation, which can vary among different helicases. In principle, the mechanism of action of DEAD/DExH box helicases described above is consistent with the helicase binding to one RNA strand and displacing the second strand in a short duplex, or displacing a large enough portion of it to cause it to dissociate. In this scenario, ATP hydrolysis would not be required for the act of strand separation but for lowering the affinity of the helicase for RNA, leading to dissociation of the helicase and a new cycle of binding and release [10].
A helicase must only be active when bound to the right substrate at the right time, in order to perform its specific functions in the cell. This is accomplished by a combination of accessory domains and proteins that either stimulate or inhibit the helicase activity. The most common mechanisms of regulation of helicase activity are listed below and illustrated in Fig. 1A, followed by descriptions of the individual helicases involved in translation initiation.
Promotion of ATP binding and hydrolysis by stabilizing the “closed”, active conformation. This is typically achieved through binding to both RecA domains in a “closed” conformation (activating domain in Fig. 1A). Since ATP and RNA bind cooperatively, this would also promote RNA binding. For example, the HEAT-1/MIF4G domain of eIF4G stimulates the activity of eIF4A by binding to both RecA domains. The interaction with the N-terminal domain of eIF4A (eIF4A-NTD) is much weaker, acting as a soft clamp, which allows stimulation of ATP binding without trapping the enzyme in the closed conformation (Fig. 1B) [48]. eIF4B- and eIF4H-mediated stimulation of ATP binding and hydrolysis by eIF4A [49–51] likely follows a similar mechanism, since stable binding to eIF4B and eIF4H requires an ATP/transition state analog and both eIF4A domains [52–54].
Inhibition of ATP binding and hydrolysis by destabilizing the “closed” active conformation. This can be achieved allosterically through binding to both RecA domains in an “open” conformation (inhibitory domain in Fig. 1A). Since ATP and RNA bind cooperatively, RNA binding would also be inhibited. For example, the tumor suppressor protein Pdcd4 inhibits eIF4A activity by binding to both RecA domains in an inactive conformation, as well as directly blocking the RNA-biding surface of eIF4A (see Mechanism #4, below) [55, 56]. Binding at the interdomain interface, including the ATP-binding site would also lead to inhibition.
-
Stabilization of RNA binding through additional protein - RNA interactions anchoring the helicase to its RNA substrate. For example, in addition to directly stimulating eIF4A activity, eIF4B, eIF4H and eIF4G all have one or more RNA-binding regions that both increase the eIF4A affinity for RNA [9–11] and influence its substrate specificity [57–59]. The accessory RNA-binding domains/proteins may have preference for ssRNA or dsRNA (ssRBD and dsRBD in Fig. 1A). ssRNA binding domains could also promote helicase activity by binding to newly unwound regions of RNA (either the segment bound to the helicase RecA domains or the opposite RNA strand) and trapping them in single-stranded form [52]. Although this is yet to be experimentally demonstrated for proteins involved in translation initiation, ssDNA-binding proteins (SSBs) stabilize the newly-formed ssDNA behind replicative helicases and are integral part of every replication system [60].
Sequence- and structure-specific RNA-binding domains have additional functions in targeting the helicase to specific mRNAs or to the ribosome (see next section). For example, RHA is targeted to mRNAs containing the so-called post-transcriptional control element (PCE) [61, 62], and DHX29 is recruited directly to the 40S ribosomal subunit [6, 8, 63]. While targeting itself is independent of any direct effects on the helicase activity, anchoring the enzyme to the RNA would have the same stimulatory effect, whether it is sequence-specific or nonspecific in nature.
Inhibition of RNA binding. Direct inhibition is observed when the inhibitor directly competes with RNA for binding to the helicase (inhibitory domain in Fig. 1A). Inhibition of ATP binding, e.g. by stabilizing the open conformation of the helicase, also leads indirectly to inhibition of RNA binding (see Mechanism #2 above).
Promotion of phosphate release and nucleotide exchange upon ATP hydrolysis. Phosphate release and nucleotide exchange are often rate-limiting and thus likely targets for stimulation of helicase activity. For example, ADP release from Dbp5, a protein involved in mRNA export, is promoted by Nup159 [64–66]. While there is currently no solid evidence of such a process for helicases involved in translation initiation, the stimulation of eIF4A helicase activity by eIF4G and/or eIF4B most likely involves stimulation of phosphate release and/or nucleotide exchange. The eIF4G HEAT2/MA3 domain could play such a role since it lowers the affinity of eIF4A for ADP [52]. The eIF4G HEAT-1/MIF4G domain was shown to modestly accelerate phosphate release by eIF4A (the rate-limiting step), presumably by promoting a “half-open” conformation [67]. Finally, eIF4G and eIF4B synergistically stimulate the eIF4A ATPase activity, which should involve stimulation of phosphate release (assuming it is the rate-limiting step), although that was not addressed directly [53].
Trapping the helicase on RNA in an ATP-bound state, while blocking ATP hydrolysis. This mechanism exploits the high affinity of the ATP-bound helicase for RNA. Therefore, the helicase•ATP•RNA complex remains stable as long as the ATPase activity is inhibited. For example, the helicase eIF4A-3/DDX48 is trapped on mRNA within the Exon Junction Complex (EJC) and serves as a “placeholder” marking the splicing site while the mRNA is being exported from the nucleus. Subsequent ATP hydrolysis leads to dissociation of the EJC [68, 69]. To date, no examples for such a mechanism have been described for helicases directly involved in translation initiation.
The activity of most helicases involved in translation initiation is regulated in response to various stimuli in the cell. Regulation of helicase activity, e.g. by phosphorylation or dephosphorylation, would be mediated by one or more of the above mechanisms.
C. Helicases involved in translation initiation
eIF4A
The main functions of eIF4A (together with eIF4G and eIF4B) are in 43S complex recruitment and scanning. The kcat for ATP hydrolysis by free eIF4A is on the order of once per minute or slower and its rate of RNA duplex unwinding is slower than once per minute (see e.g. [41, 70, 71]). This is slower than the rates of translation initiation, which has been estimated to take typically a few seconds (reviewed in [3]), and the rates of scanning by the 43S pre-IC, estimated to be faster than 10 nt/s [5]. Therefore, the ATPase and helicase activities of free eIF4A are negligible at the time scale of translation initiation and the biologically relevant helicase is the complex of eIF4A with eIF4G and eIF4B (or its smaller homolog eIF4H). The eIF4A/4G/4B helicase complex unwinds secondary structures in the vicinity of the 5'-cap and clears the “landing pad” for the 43S complex. If the secondary structure is immediately at the 5'-end, DDX3 is also necessary for efficient translation initiation [72].
During scanning, the helicase complex could be behind (5' from) the scanning 43S and act by some other mechanism that does not involve RNA duplex unwinding [73], or in front of (3' from) the 43S complex and act in much the same way as in 43S complex recruitment [52]. A Brownian ratchet model was proposed to explain how the helicase complex can work from behind, although it was mentioned that the model could also apply if the helicase is in front of the scanning complex [74]. eIF4B binding was recently mapped to the Head of the yeast 40S subunit, near the Entry channel. eIF4B binding to the 40S subunit was shown to induce changes in the Entry channel and it was proposed that this Entry channel remodeling could promote mRNA binding. The position of eIF4B on the ribosome could also allow it to bind mRNA directly [75]. These findings suggest that eIF4A, too, may be located near the Entry channel, which would be consistent with our model that the eIF4A/eIF4G/eIF4B helicase complex unwinds mRNA in front of the scanning initiation complex [52].
eIF4G also recruits eIF4A to the 5'-cap (through interaction with eIF4E) and the 43S complex (through interaction with eIF3). Furthermore, the RNA-binding domains of eIF4G and eIF4B influence the substrate specificity of the helicase complex [58, 59]. The RNA recognition motif (RRM) domain of mammalian eIF4B was reported to bind to the 18S rRNA and thus its function was proposed to be in anchoring the helicase to the 43S complex [76]. It was recently reported that yeast eIF4B promotes recruitment of eIF4A to the 43S complex [75], as suggested for the mammalian proteins [76]. However, the RRM of yeast eIF4B was not required for binding to the 40S ribosomal subunit. Instead, deletion of the N-terminal region and the C-terminal 7-repeat region showed the greatest defects in 40S binding [75]. The source of this discrepancy is not clear, but it could reflect differences between yeast and mammalian eIF4B: while the N-terminal and the repeat regions of mammalian and yeast eIF4B most likely have a common origin, they have diverged drastically and the consensus of the repeats is clearly different [3, 75]. Remarkably, the C-terminal 7-repeat region of yeast eIF4B was shown to be most important for promoting eIF4A binding to the 43S complex [75], which is consistent with our observation that the corresponding region of eIF4H binds to eIF4A [52], despite the great degree of divergence between the two sequences.
Since eIF4G and eIF4B synergistically stimulate eIF4A activity, functionally the eIF4A/eIF4G/eIF4B (or eIF4H) complex is the bona fide helicase in vivo, even though this complex is rather dynamic [53]. Whereas the studies employing free eIF4A or subcomplexes offer insights into the functions of individual proteins, only the helicase activity and specificity of the eIF4A/4G/4B and eIF4A/4G/4H holoenzymes are biologically relevant when studying the role of eIF4A in translation initiation.
DHX29
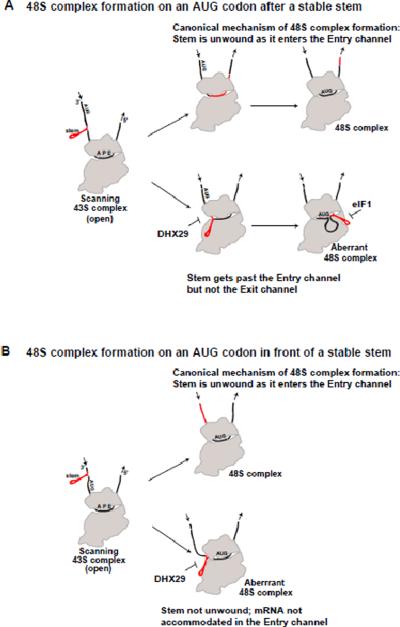
The role of DHX29 is to help the scanning 43S complex unwind stable RNA hairpins in the 5'-UTR and ensure base-by-base inspection, as well as correct positioning of mRNA in the ribosome Entry channel (Fig. 2). It is also important for translation initiation on some viral mRNAs. DHX29 acts by binding directly to the 40S subunit and remodeling the Entry channel. It is not clear whether or not it also interacts directly with the mRNA [6–8].
Figure 2. Role of DHX29 in ensuring base-by-base inspection of 5'-UTRs by scanning 43S complexes.
Models for 48S complex formation on an AUG codon after (3' from) a stable stem (A) and in front of (5' from) a stable stem (B). The canonical initiation with unwinding of the stem is shown on the top in each panel. The stem in the mRNA is shown in red, whether intact or unwound. Suppression of stem bypass by DHX29 and of aberrant 48S complexes with a stem near the Exit channel by eIF1 is indicated by “ ”. The positions of the ribosomal A, P and E sites and the 5'/3' polarity of mRNA are shown in the left panels. The direction of movement of mRNA with respect to the ribosome is shown with arrows. The start codon position is labeled with “AUG” (please, note that the letters in the label are in the opposite order compared to the bases in the AUG codon, with respect to the 5'/3' polarity of the mRNA). Adapted from Abaeva et al., Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning, EMBO J, 30, 115–129, Copyright 2011, with permission from Nature Publishing Group.
”. The positions of the ribosomal A, P and E sites and the 5'/3' polarity of mRNA are shown in the left panels. The direction of movement of mRNA with respect to the ribosome is shown with arrows. The start codon position is labeled with “AUG” (please, note that the letters in the label are in the opposite order compared to the bases in the AUG codon, with respect to the 5'/3' polarity of the mRNA). Adapted from Abaeva et al., Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning, EMBO J, 30, 115–129, Copyright 2011, with permission from Nature Publishing Group.
Unlike eIF4A, DHX29 is a large multidomain helicase that carries its accessory domains with it, forming an amazingly complex regulatory network [63]. Similar to eIF4A, free DHX29 has very low basal NTPase activity, which is greatly stimulated upon binding to the 43S complex [8]. Like a number of other DExH-box helicases, the C-terminal region of DHX29 consists of a winged helix (WH) domain, a ratchet domain; and an oligonucleotide/oligosaccharide-binding fold (OB) domain. The region N-terminal from the RecA domains contains a dsRNA-binding domain (dsRBD) and an Ubiquitin-associated (UBA) domain. The second RecA domain (RecA-2) contains a β-hairpin and a large insertion loop ([63] and AM, unpublished observations). As shown in a recent study by Pestova and co-authors, both the dsRBD and the WH domain bind to the 40S subunit. The dsRBD mediates 40S-stimulated NTPase activity. The OB domain mediates RNA-stimulated NTPase activity and inhibits basal NTPase activity in the absence of RNA. Finally, the RecA-2 insertion segment also serves to inhibit basal NTPase activity. The β-hairpin in RecA-2 is also important for activity and likely coordinates regulation with other domains. The function of the UBA domain has not been investigated, but it likely plays a regulatory role, since deletion of only the dsRBD had greater effect on ribosome binding than deletion of the entire N-terminal region of DHX29 (including both the dsRBD and UBA domains) [63]. It is not known whether other eIFs regulate DHX29 activity, recruitment, or dissociation from 43S complexes.
RNA helicase A (RHA)/DHX9
RHA plays roles at multiple stages of mRNA metabolism, including translation initiation [19, 20, 22, 31–33]. The mechanism of action of RHA in translation initiation is not well understood. It stimulates translation of mRNAs containing the post-transcriptional control element (PCE) [61, 62], to which it is recruited directly by specific binding of its N-terminal dsRBDs to the PCE [20]. It is also recruited to other groups of mRNAs indirectly via protein-protein interactions [77, 78]. No direct interactions between RHA and components of the translation initiation complex have been reported.
The C-terminal two-thirds of RHA are homologous to the corresponding region of DHX29, which includes the two helicase domains, the WH domain, the ratchet domain, and the OB domain. Therefore, many of the regulatory properties described above for C-terminal domains of DHX29 may apply also to the corresponding RHA domains. The N-termini of the two proteins are more diverse, with RHA containing two dsRBDs [79], instead of one, and lacking an obvious UBA domain. Furthermore, the RHA dsRBDs interact specifically with the PCE-containing mRNAs [20], instead of the 40S subunit. There is no data about whether RHA helicase activity is regulated by other proteins.
Vasa/DDX4
Vasa stimulates translation of a set of mRNAs encoding proteins that function in embryonal development and the microRNA (miRNA) pathway [16, 18, 80]. It is known to interact with eIF5B and mutations that abolish eIF5B binding also affect its ability to stimulate translation initiation. It was suggested that Vasa acts by recruiting eIF5B [15, 16]. However, it is not clear why eIF5B would not be recruited to these mRNAs in the same way as to all other mRNAs, and why it would need Vasa's help. Alternative mechanisms for Vasa action can also be envisioned. During transport, the mRNA should be translationally repressed. The ATPase activity of Vasa is likely inhibited as well (see also Section V below). Thus, the Vasa – eIF5B interaction could be necessary to relieve translational repression, e.g. by activating Vasa, remodeling the mRNP, or release of a protein from the mRNA.
In addition to the two RecA domains, Vasa contains an N-terminal Arg-Gly-Gly (RGG) repeat region, as well as a short C-terminal extension. Vasa binds specifically to a U-rich element in the 3'-UTR of certain mRNAs, like the mRNA for Mei-P26, a protein that represses miRNA activity. The interaction is likely mediated by the N-terminal RGG repeat segment [18]. The eIF5B-binding site of Vasa was mapped to the second RecA domain [16]. Vasa also interacts with Oskar [81] and Gustavus [80], which are involved in translation regulation during embryonal development. Gustavus was reported to bind near the N-terminus of Vasa [80]. It is presently not known whether these or other proteins regulate Vasa's helicase activity.
DDX3 and Ded1
Mammalian DDX3 and its yeast ortholog Ded1 play roles in the export and translation initiation of mRNAs, including a number of viral intron-less mRNAs [23–30] and act as translational repressors [29, 30, 82]. It has been proposed that these two proteins also function in general translation initiation [5, 6, 24, 26, 28, 30, 83]. The evidence in favor of a general translation function is much stronger for Ded1, which was shown to be important for translation of mRNAs with long 5'-UTRs in vivo and translation in yeast cell lysates [5, 24, 26, 30]. Ded1 also stimulated 48S complex assembly on mRNAs with moderately stable hairpins in the 5'-UTR, together with eIF4A/4G/4B in an in vitro reconstituted mammalian system. DDX3 was inactive under the same conditions [6].
In addition to the two RecA domains, DDX3 and Ded1 contain N- and C-terminal extensions, which appear to mediate interactions with other proteins and possibly mRNA [29, 30]. Both the mammalian and yeast proteins were reported to bind to eIF4G and PABP [30, 72]. DDX3 also binds to eIF4E [29] and was reported to co-immunoprecipitate with eIF3; however it was not determined whether the DDX3:eIF3 interaction was direct or mediated by other molecules [28]. These interactions could play multiple roles: i) eIF4F recruitment by Ded1/DDX3 to specific mRNAs; ii) Ded1 recruitment to mRNA-bound eIF4F; and/or iii) translational repression, since the eIF4G-interacting region of Ded1 was found to be important for both translational repression and stimulation [30].
The role of DDX3 in translation initiation was recently elucidated by Ohlmann and co-authors. It was shown that DDX3 is required for translation initiation on viral and cellular mRNAs with secondary structure at the 5'-end. Both DDX3 and eIF4A were required for efficient translation of DDX3-dependent mRNAs. Since DDX3 was found to interact with eIF4F and PABP, and nonspecifically with the mRNA itself, all these interactions could mediate its binding to the target mRNAs [72]. The authors also showed that DDX3 is not important for translation initiation on mRNAs, whose 5'-UTRs contained secondary structures 15 or more nt from the 5'-end and it is unclear whether DDX3 remains associated with the scanning 43S complex [72].
These results raise the question whether DDX3 and Ded1 are true orthologs:
-
-
Ded1 acts as a general translation initiation factor [5, 24, 26, 30], whereas DDX3 does not [72].
-
-
Ded1 is important for initiation on mRNAs with long and structured 5'-UTRs in yeast [5], as well as in a heterologous mammalian in vitro reconstituted system [6], whereas DDX3 is not, unless the secondary structure is at the 5'-end [6, 72].
Nevertheless, the mouse PL10 protein, a close DDX3 homolog, is able to functionally replace yeast Ded1 [24].
It is not presently known whether eIF4G or other binding partners also affect the Ded1/DDX3 helicase activity. It would be important to test the role of Ded1 and its interplay with eIF4A/4G/4B in a yeast in vitro reconstituted system on mRNAs with structured 5'-UTRs, unstructured 5'-UTRs, as well as mRNAs to which Ded1 is known to bind in vivo during mRNA export. Similarly, further insights into the function of DDX3 could be gained by studying its potential roles in mRNA-specific translation in an in vitro reconstituted system, since this would allow discrimination between direct and indirect effects on translation initiation.
III. Functions of helicases in translation initiation
Helicases are known to have multiple roles in translation initiation, summarized below.
Clearing the “landing pad” for the 43S Pre-IC on mRNA by unwinding of secondary structures in the vicinity of the 5'-cap of mRNA. This is accomplished by eIF4A with the help of eIF4E, eIF4G and eIF4B [9–11]. In mammals, DDX3 is also required for this process if the secondary structures are at the 5'-end [72].
-
Unwinding secondary structures in the 5'-UTR during scanning. This function is performed by eIF4A/4G/4B, which accompanies the 43S complex during scanning [9–11]. In yeast, the two related helicases Ded1 and Dbp1 are also involved in this process [5, 24, 26, 84].
As discussed above, efficient scanning on 5'-UTRs with stable secondary structures requires the DExH-box helicase DHX29 [8]. Instead of directly unwinding mRNA, DHX29 acts by dynamically remodeling the Entry channel of the 40S subunit (likely opening and closing). It prevents mRNA from slipping out of the Entry channel and disrupts 48S initiation complexes, where the mRNA downstream from the start codon has slipped out of the channel. As a result, DHX29 prevents bypassing of intact RNA stem-loops by the scanning ribosomal complex (Fig. 2) [6].
mRNA-specific regulation of translation initiation. mRNA-specific helicases are recruited to the mRNA via direct binding to a sequence or structure motif in the mRNA itself or indirectly by interaction with another protein. The functions of the helicase can be both stimulation and inhibition of translation. The stimulation can be mediated by unwinding or remodeling of secondary structures in the 5'-UTR or recruitment of the initiation complex preferentially to this mRNA. Recruitment of the IC may be independent of the helicase activity of the protein [9–11]. A well-characterized example is the recruitment of the 43S pre-IC to type 1 and type 2 IRESs. The process is mediated by eIF4G/4A/4B, often together with a set of IRES trans-acting factors (ITAFs) [2]. As this example illustrates, a general factor like eIF4A can also have mRNA-specific functions. Translational repression often involves binding to the 5'-UTR and may not require the helicase activity, as is the case with Ded1 [30].
The fact that the unique function of DHX29 in translation initiation was only recently discovered suggests that helicases could have additional roles that are yet to be discovered.
IV. General vs. mRNA-specific helicases
In terms of their roles in translation initiation, proteins involved in the process can be subdivided into two major groups: eIFs with functions in “general” translation initiation and mRNA-specific factors. Obviously, the same distinction applies to helicases as well. A “general” eIF must:
Have cytoplasmic concentration comparable to the concentrations of total translated mRNA and 43S preinitiation complexes. For example, eIF4A, at least in yeast, has concentration even higher than those of mRNA and 43S complexes [85].
Associate with the “general” translation initiation complexes in order to be recruited to most mRNAs: either with the cap-binding complex or with the 43S complex. While in a test tube, a helicase can unwind any appropriate substrate, without being specifically recruited to it, this is neither desirable, nor possible in the cell. It is undesirable because this process would consume prohibitively large amounts of energy and many of the random unwinding events would likely have deleterious effects on the cell. And it is impossible because the amount of total RNA would require enormous helicase concentrations in order to randomly bind to and unwind over and over again all available RNA duplexes. For example, eIF4A is recruited to the 5'-cap of mRNA via interactions with eIF4G. DHX29 is recruited to the 43S preinitiation complex via direct interaction with the 40S ribosomal subunit [8].
If both of these criteria are fulfilled for a given protein, it is all but certain to be a general eIF. A general eIF may only have a peripheral or regulatory function and need not be essential. For instance, not all of the yeast eIF3 subunits are essential[86], and neither is yeast eIF5B [87], despite its well defined role in subunit joining.
In contrast to “general” (eIF) helicases, an mRNA-specific helicase is recruited to a group of mRNAs via specific interactions with either the mRNA itself or with proteins bound to that mRNA. An mRNA-specific helicase may interact directly with IC components, but it does not have to, since it gets recruited to the mRNA itself. If the helicase does interact with components of the IC, it could serve additional “non-helicase” roles in IC recruitment to the specific group of mRNAs.
The cellular concentration of an mRNA-specific helicase would typically be comparable to that of its target mRNAs and much lower than that of total translated mRNA. From the above discussion of “general” eIFs, it becomes clear that the boundary between general and mRNA-specific factors can become blurred. Knowledge of the cellular concentration of the protein can often provide a clear indication whether or not it could have a role in general translation. Unfortunately, such information is usually scarce.
Of the helicases known to be involved in translation initiation, eIF4A clearly satisfies both criteria for a general eIF. While the cytoplasmic concentration of DHX29 is unknown, it can also be designated as general eIF: because it specifically binds to the 40S ribosomal subunit and because there is no evidence for specific recruitment of DHX29 to the mRNAs with structured 5'-UTRs on which it is required. According to the above classification, a general helicase does not have to be required for initiation on all mRNAs, as long as it is present in the initiation complexes. For instance, DHX29 is not needed on mRNAs whose 5'-UTRs do not have stable secondary structures [6, 8], and even eIF4A is not needed for translation initiation on mRNAs whose 5'-UTRs are completely devoid of secondary structures [88], yet both eIF4A and DHX29 are recruited to such mRNAs as part of the initiation complex.
Yeast Ded1 and its mammalian homolog DDX3 have been shown to bind to eIF4F [29, 30, 72], which fulfills the second criterion for a general eIF. However, they have also been shown to stimulate translation of specific mRNAs [34, 89–91] and could be recruiting eIF4F to these mRNAs, instead of being recruited to all capped mRNAs. Of course, the mRNA-specific and “general” roles are not mutually exclusive. If the protein is abundant enough, it should be possible to saturate its binding sites on the “specific” mRNAs and the remaining portion could be recruited to any capped mRNA (this rationale of course relies on the assumption that the binding interfaces between Ded1/DDX3 and eIF4F are not obstructed). It is thus essential to determine the cytoplasmic concentrations of Ded1 and DDX3.
A number of fungi, including S. cerevisiae, lack a DHX29 ortholog. In these species either there has been selective pressure against stable secondary structures in the 5'-UTR requiring DHX29 or another protein has at least partially filled the void. Ded1 appears to be a candidate for such a role. Therefore, it would be interesting to compare 5'-UTRs and scanning efficiencies between DHX29-containing fungi like S. pombe and fungal species like S. cerevisiae that lack DHX29.
RHA/DHX9 and Vasa/DDX4 are known to regulate translation initiation on specific mRNAs and there have been no reports about any role for these proteins in general translation initiation. Therefore, it should be fairly safe to consider them exclusively mRNA-specific factors, unless their cytoplasmic concentrations turn out to be unusually high for such a role. It should be noted that high cytoplasmic concentration is only a necessary, but not a sufficient condition for a protein to have a role in general translation, because if its affinity for the target mRNAs is low, the high concentration may be required to ensure efficient binding.
An apparent contradiction exists between the ability of a protein to bind specifically to a secondary structure element (e.g. a stem-loop) in the mRNA 5'-UTR and to promote its unwinding, since specific binding is expected to stabilize the target structure. A recent study on the function of eIF4H may shed light into how that can be accomplished. eIF4H was shown to bind strongly to loops in the RNA, which would leave the base of the stem-loop free and accessible for unwinding [59].
V. Direct vs. indirect effects of mRNA-specific helicases on translation initiation
Certain mRNA-specific helicases are already bound to their cognate mRNAs as they are being exported from the nucleus. Such helicases could play roles in one or more of the processes preceding translation initiation (e.g. mRNA splicing, export from the nucleus, transport, etc.). Alternatively they could only be “riding along” and have no nuclear function, or they could participate in both translation initiation and in earlier stages. It is often difficult to distinguish in such cases between direct and indirect effects on translation initiation. While a detailed discussion of processes preceding translation initiation lies beyond the scope of this review, their impact on translation initiation needs to be considered. Any proteins that remain associated with the mRNA at the time of initiation could affect the process, e.g. by recruiting other proteins or modulating their activity. If bound to the 5'-UTR, they would need to be displaced during the pioneer round of translation. More importantly, the process of mRNA transport and localization often involves maintaining the mRNA in a translationally repressed state as part of a ribonucleoprotein complex (mRNP), followed by activation (or derepression) of translation.
Translational repression and activation are intrinsically interrelated. Repression can be achieved at various stages of initiation. The same proteins are often responsible, at different times, for recruitment of IC components to the mRNA, repression, and derepression. Depending on the stage at which initiation is blocked, a number of initiation complex components may be present in the translationally repressed mRNP that is being transported or stored. Helicases, by virtue of their ATPase activity and ability to undergo major conformational changes, are often involved in mediating both the trapping of the mRNPs in a repressed state and the activation of translation by remodeling the mRNPs in response to the appropriate stimuli. This high degree of complexity is probably among the reasons why helicases such as Ded1 and DDX3 have been reported to stimulate translation by some groups and to inhibit translation by others (see above). For instance, overexpression of a “dual-role” helicase could easily yield different conclusions than depletion or inhibition of the same protein. Another critical factor to consider is whether the observed effects are direct or indirect. Changes in splicing, export, and/or translation of specific mRNAs may ultimately affect the abundance and/or activity of individual translation factors or the metabolic program of the cell as a whole. Thus, depletion or inhibition experiments lasting several hours or days cannot distinguish between direct and indirect effects [92]. In vitro experiments with depleted cell lysates suffer from similar limitations, unless it can be shown that adding back the depleted factor restores normal levels of translation.
As far as helicases are concerned, an additional consideration during mRNA transport is the functional state of the helicase itself. If an mRNA-associated helicase remains active in ATP hydrolysis during transport, it would be wasting energy and may also affect the stability of the mRNP. Therefore, it is safe to assume that, at least for the most part, mRNP-associated helicases are kept inactive during mRNP transport and storage. A helicase can be trapped in an ATP- and RNA-bound state by inhibiting its ATPase activity. A well known example for such a mechanism from mRNA splicing is locking eIF4A-3 onto mRNA within the exon junction complex near the splice site [68, 69]. Alternatively, a helicase can be kept inactive in a nucleotide-free state. A possible example of such a mechanism is yeast Ded1, which was recently shown to bind mRNA and inhibit its translation in an ATP-independent manner. While mutations affecting ATP binding or hydrolysis abolished the Ded1 ability to stimulate translation initiation, such Ded1 mutants were even more potent translation repressors than WT Ded1 [30].
VI. Dynamics of helicase association with the initiation complex
In yeast, eIF4A is present in significant excess over other initiation factors [85] and suprastoichiometric eIF4A concentrations are needed for efficient translation initiation in an in vitro reconstituted mammalian system [93]. The apparent excess of eIF4A is probably related to its rather dynamic association with eIF4G. Free eIF4A exchanges with eIF4F-bound eIF4A [94]. eIF4A mutants defective in ATP hydrolysis are dominant negative, blocking cycling of eIF4A in and out of eIF4F and inhibiting eIF4F helicase activity, and leading to drastic translational repression. Accordingly, adding eIF4F was much more effective in relieving inhibition by the eIF4A mutants than eIF4A alone [95]. Thus, it is widely accepted that recycling of eIF4A through eIF4F is required for translation initiation [95] (Fig. 3). If eIF4A is continuously dissociating and re-binding to the initiation complex, its rate of binding is directly dependent on the free eIF4A concentration in the cell. Up- or down-regulation of eIF4A in the cell is correlated with changes in translation efficiency, particularly on mRNAs with structured 5'-UTRs that show stronger dependence on eIF4A activity (reviewed in [1–3, 52]).
Figure 3. Model for dynamic association of helicases with the initiation complex.

Cycling of eIF4A on and off the initiation complex is shown with a double arrow. Proposed cycling on and off the ribosome for DHX29 is shown with a dashed double arrow with a question mark. The position of DHX29 near the mRNA Entry channel of the 40S subunit is based on [8]. eIF4G is placed on the back (solvent-exposed) surface of the 40S [73, 100]. eIF4A is placed in contact with both eIF4G and the mRNA in front of the scanning complex [52] but could also be behind it [73]. The stem in the mRNA is shown in red. The positions of the ribosomal A, P and E sites and the 5'/3' polarity of mRNA are shown. The direction of movement of mRNA with respect to the ribosome is shown with arrows. The start codon is labeled with “AUG” (please, note that the letters in the label are in the opposite order compared to the bases in the AUG codon, with respect to the 5'/3' polarity of the mRNA). The hypothesis for dynamic association of helicases illustrated here could also apply to other helicases (e.g. Ded1) and other translation factors.
A similar pattern is starting to emerge for DHX29. While there is no data about the relative abundance and regulation of DHX29, it was reported that only ~1/10 of 40S complexes isolated from rabbit reticulocyte lysates (RRL) contained DHX29 [8] and that its depletion inhibits translation and cancer cell growth [7]. In an in vitro reconstituted system, optimal 48S complex formation was achieved by sub-stoichiometric amounts of DHX29, indicating that DHX29 participates in multiple rounds of initiation and raising the possibility that it may dissociate early in the scanning process [8]. Therefore like eIF4A, DHX29 could also cycle on and off the ribosomal complexes on the time scale of translation initiation (Fig. 3).
An argument can be made that the conditions of the in vitro reconstituted system may have overemphasized the ability of DHX29 to participate in multiple rounds of 48S complex formation, since it would have more time to do so than in vivo. Translation initiation is known to be highly processive, with negligible frequency of dropoff, where virtually every scanning 43S complex results in translation of a polypeptide [5]. Therefore, it is generally assumed that eIFs are stably bound to the IC and dissociate only after they have performed their functions. However, in light of some recent reports [8, 96–98], this notion may need to be reevaluated.
So, what happens if there is not enough of a certain eIF? The answer depends on the specific eIF in question. If the eIF is critical for 43S complex assembly, then there would be fewer 43S complexes. For other 43S components, there would be just as many 43S complexes as when the concentration of the given component is higher, but at any given time not all of them may have the complete set of eIFs bound. Two scenarios can then be envisioned:
A portion of the complexes are missing the eIF throughout the translation initiation process and thus have different properties.
The eIF may reversibly bind to, and dissociate from the initiation complex, causing the same initiation complex to contain the given eIF only during a portion of the translation initiation process.
While the first scenario is more in line with the conventional views about translation initiation, there is increasing evidence in favor of the second one. The recently discovered mechanisms of autoregulation of eIF1 and eIF5 translation provide strong evidence that the association of eIF1 and eIF5 with the scanning initiation complex may be dynamic. Translation efficiency of the mRNAs with a start codon in a poor context is regulated by the relative abundance of eIF1 and eIF5 in the cell, with eIF1 increasing, and eIF5 decreasing the stringency of start codon selection. This phenomenon is exploited for regulation of the steady-states of eIF1 and eIF5 themselves [96–98]. eIF1 is translated from an AUG codon in poor context whose translation efficiency is inhibited by high eIF1 concentrations [96, 98] and stimulated by high eIF5 concentrations [97]. The mRNA encoding eIF5 has three upstream open reading frames (uORFs) with AUG in poor context and increased efficiency of initiation at these uORFs lowers the translation initiation at the start codon of the eIF5 ORF, leading to opposite effects of eIF1 and eIF5 concentrations [97]. These results are difficult to explain with the first scenario above, postulating that a subset of the initiation complexes lack a given eIF from the beginning to the end. A scanning 43S complex lacking eIF1 or eIF5 would not just show lower or higher stringency of start codon selection, respectively. Instead, the defects would be expected to be much more severe. eIF1 plays a critical role in scanning and in its absence the scanning complex often gets stuck at a non-AUG codon near the 5'-end of mRNA [88]. Similarly, in the absence of eIF5, the 48S initiation complex would be stuck at the start codon fully blocking subunit joining [7]. Thus, it appears highly unlikely for a 48S complex that lacks eIF5 from the beginning to the end, to be able to initiate translation at an appreciable rate, irrespective of whether it is assembled on a start codon in poor or perfect context. eIF1 dissociates pre-formed 48S complexes (but not 80S initiation complexes) assembled on an AUG codon in poor complex [88]; and eIF5 promotes GTP hydrolysis by eIF2 on pre-formed 48S complexes, allowing ribosomal subunit joining to occur [7]. Therefore, the rate of eIF5 binding to an eIF5-less 48S complex stuck at the start codon would be proportional to the concentration of free eIF5, thus promoting subunit joining and limiting the time available for eIF1 to “challenge” the complex if it is assembled on a start codon in poor context. Likewise, the likelihood of eIF1 being bound to a scanning 43S complex and its rate of binding to an eIF1-less 48S complex would depend on the concentration of free eIF1. Therefore, eIF1 and eIF5 would need to have non-negligible rates of dissociation and re-binding to the initiation complex on the time frame of scanning and 48S complex formation, in order for their cellular concentrations to modulate the stringency of start codon selection.
A similar phenomenon has been observed for bacterial IF3, which like eIF1, promotes higher stringency of start codon selection. IF3 translation is initiated from a non-cognate AUU codon and is autoregulated by IF3 concentration (reviewed in [99]). While reversible binding and re-binding is easier to accept for IF3, the notion that the same applies to eIF1 and eIF5 is in line with the observations that overexpressing mutant eIFs with lower affinity for the initiation complex, (e.g. eIF1 mutants with reduced 40S binding) suppresses their defects (reviewed in [86]).
If proteins like eIF1 and eIF5, which are integral parts of the initiation complex and are involved in multiple interactions, can cycle on and off the IC, then the same should be considered possible for other initiation factors as well. This is particularly relevant to helicases because of their dynamic nature, with constantly changing conformations and affinities. In view of the mechanism of DHX29 action in ensuring base-by-base inspection as well as dissociating aberrant initiation complexes [6, 8], this opens a wide range of possible regulatory mechanisms. For instance, on mRNAs with structured 5'-UTRs requiring DHX29, translation efficiency will depend on the fraction of 43S complexes that contain an active DHX29 at any given time. It remains to be seen whether the binding of other helicases to the ICs is also dynamic.
VII. Unanswered questions and outlook
In summary, a lot has been learned in recent years about the functions of helicases in translation initiation, but many important questions remain unanswered. Even for the eIF4A/4G/4B helicase complex, we do not have a comprehensive understanding of its mechanisms of action, e.g. whether it operates in the same way before and after 43S recruitment, or whether it works “from behind” during scanning, without directly unwinding mRNA hairpins. Even less clear are the mechanisms of stimulation of translation by other helicases, such as Vasa/DDX4, or the mechanisms of translational repression by Ded1/DDX3. It will be important to identify the target mRNAs for individual helicases. It is also important to know what accessory proteins regulate the helicase activity, how the helicase is kept inactive in its ground state, and how it is activated at the right place and time.
Further progress will require a combination of experimental approaches. Properly controlled ribosome profiling experiments can elucidate the immediate effects of depletion or inhibition of a helicase on the rates of translation of thousands of individual mRNAs. It is critical to know the cytoplasmic concentrations of the helicases and their accessory proteins, as this will determine the range of possible functions they can perform. This should help with interpretation of in vitro experiments in lysates depleted of endogenous mRNAs, especially where the lysates are supplemented with purified helicase. We need in vitro reconstituted systems for mRNA-specific regulation (e.g. of Vasa-specific mRNAs) with a complete set of factors that reproduce what is known about regulation in vivo. Combining results from such systems with quantification of in vivo protein concentrations would allow elucidating the mechanisms of action of mRNA-specific helicases and distinguishing between direct and indirect effects on translation initiation. Studying the dynamic interactions of helicases with the initiation complexes would be much more challenging and would require kinetic experiments on the time scale of translation initiation, and possibly single-molecule studies.
Highlights
The roles of accessory domains and proteins in helicase function are discussed.
Helicases act as either general or mRNA-specific translation initiation factors.
Direct and indirect effects of helicases on translation initiation are discussed.
The helicase eIF4A cycles on and off the initiation complexes.
DHX29 and other proteins could also cycle on and off the initiation complexes
Acknowledgements
The author thanks Boriana Marintcheva, Katherine Edmonds and Tatyana Pestova for helpful discussions.
Financial Support This work was supported by a NIH grant R01 GM095720.
Abbreviations
- eIF
eukaryotic translation initiation factor
- IC
initiation complex
- TC
ternary complex
- EJC
Exon Junction Complex
- PABP
poly-A binding protein
- SF2
Superfamily 2
- DDX
DEAD-box protein
- DHX
DExH-box protein
- RHA
RNA helicase A
- UTR
untranslated region
- PCE
post-transcriptional control element
- IRES
Internal Ribosome Entry Site
- ORF
open reading frame
- uORF
upstream ORF
- WT
wild type
- mRNA
messenger RNA
- mRNP
mRNA ribonucleoprotein
- tRNA
transfer RNA
- Met-tRNAi
initiator methionyl-tRNA
- ssRNA
single-stranded RNA
- dsRNA
double-stranded RNA
- ssDNA
single-stranded DNA
- SSB
ssDNA-binding protein
- OB
oligonucleotide/oligosaccharide binding fold domain
- dsRBD
dsRNA-binding domain
- WH
winged helix domain
- RRM
RNA recognition motif domain
- UBA
Ubiquitin-associated domain
- miRNA
microRNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hinnebusch AG, Lorsch JR. The mechanism of eukaryotic translation initiation: new insights and challenges. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011544. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marintchev A, Wagner G. Translation initiation: structures, mechanisms and evolution. Q Rev Biophys. 2004;37:197–284. doi: 10.1017/S0033583505004026. [DOI] [PubMed] [Google Scholar]
- [4].Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berthelot K, Muldoon M, Rajkowitsch L, Hughes J, McCarthy JE. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol Microbiol. 2004;51:987–1001. doi: 10.1046/j.1365-2958.2003.03898.x. [DOI] [PubMed] [Google Scholar]
- [6].Abaeva IS, Marintchev A, Pisareva VP, Hellen CU, Pestova TV. Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning. EMBO J. 2011;30:115–129. doi: 10.1038/emboj.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Parsyan A, Shahbazian D, Martineau Y, Petroulakis E, Alain T, Larsson O, Mathonnet G, Tettweiler G, Hellen CU, Pestova TV, Svitkin YV, Sonenberg N. The helicase protein DHX29 promotes translation initiation, cell proliferation, and tumorigenesis. Proc Natl Acad Sci U S A. 2009;106:22217–22222. doi: 10.1073/pnas.0909773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5'UTRs requires DExH-box protein DHX29. Cell. 2008;135:1237–1250. doi: 10.1016/j.cell.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, Sonenberg N. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol. 2011;12:235–245. doi: 10.1038/nrm3083. [DOI] [PubMed] [Google Scholar]
- [10].Linder P, Jankowsky E. From unwinding to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell Biol. 2011;12:505–516. doi: 10.1038/nrm3154. [DOI] [PubMed] [Google Scholar]
- [11].Andreou AZ, Klostermeier D. The DEAD-box helicase eIF4A: Paradigm or the odd one out? RNA Biol. 2012;10 doi: 10.4161/rna.21966. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Minshall N, Kress M, Weil D, Standart N. Role of p54 RNA helicase activity and its C-terminal domain in translational repression, P-body localization and assembly. Mol Biol Cell. 2009;20:2464–2472. doi: 10.1091/mbc.E09-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fischer N, Weis K. The DEAD box protein Dhh1 stimulates the decapping enzyme Dcp1. EMBO J. 2002;21:2788–2797. doi: 10.1093/emboj/21.11.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Carrera P, Johnstone O, Nakamura A, Casanova J, Jackle H, Lasko P. VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol Cell. 2000;5:181–187. doi: 10.1016/s1097-2765(00)80414-1. [DOI] [PubMed] [Google Scholar]
- [16].Johnstone O, Lasko P. Interaction with eIF5B is essential for Vasa function during development. Development. 2004;131:4167–4178. doi: 10.1242/dev.01286. [DOI] [PubMed] [Google Scholar]
- [17].Liang L, Diehl-Jones W, Lasko P. Localization of vasa protein to the Drosophila pole plasm is independent of its RNA-binding and helicase activities. Development. 1994;120:1201–1211. doi: 10.1242/dev.120.5.1201. [DOI] [PubMed] [Google Scholar]
- [18].Liu N, Han H, Lasko P. Vasa promotes Drosophila germline stem cell differentiation by activating mei-P26 translation by directly interacting with a (U)-rich motif in its 3' UTR. Genes Dev. 2009;23:2742–2752. doi: 10.1101/gad.1820709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bolinger C, Sharma A, Singh D, Yu L, Boris-Lawrie K. RNA helicase A modulates translation of HIV-1 and infectivity of progeny virions. Nucleic Acids Res. 2010;38:1686–1696. doi: 10.1093/nar/gkp1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ranji A, Shkriabai N, Kvaratskhelia M, Musier-Forsyth K, Boris-Lawrie K. Features of double-stranded RNA-binding domains of RNA helicase A are necessary for selective recognition and translation of complex mRNAs. J Biol Chem. 2011;286:5328–5337. doi: 10.1074/jbc.M110.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Short JD, Pfarr CM. Translational regulation of the JunD messenger RNA. J Biol Chem. 2002;277:32697–32705. doi: 10.1074/jbc.M204553200. [DOI] [PubMed] [Google Scholar]
- [22].Hartman TR, Qian S, Bolinger C, Fernandez S, Schoenberg DR, Boris-Lawrie K. RNA helicase A is necessary for translation of selected messenger RNAs. Nat Struct Mol Biol. 2006;13:509–516. doi: 10.1038/nsmb1092. [DOI] [PubMed] [Google Scholar]
- [23].Leroy P, Alzari P, Sassoon D, Wolgemuth D, Fellous M. The protein encoded by a murine male germ cell-specific transcript is a putative ATP-dependent RNA helicase. Cell. 1989;57:549–559. doi: 10.1016/0092-8674(89)90125-6. [DOI] [PubMed] [Google Scholar]
- [24].Chuang RY, Weaver PL, Liu Z, Chang TH. Requirement of the DEAD-Box protein ded1p for messenger RNA translation. Science. 1997;275:1468–1471. doi: 10.1126/science.275.5305.1468. [DOI] [PubMed] [Google Scholar]
- [25].de la Cruz J, Iost I, Kressler D, Linder P. The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1997;94:5201–5206. doi: 10.1073/pnas.94.10.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iost I, Dreyfus M, Linder P. Ded1p, a DEAD-box protein required for translation initiation in Saccharomyces cerevisiae, is an RNA helicase. J Biol Chem. 1999;274:17677–17683. doi: 10.1074/jbc.274.25.17677. [DOI] [PubMed] [Google Scholar]
- [27].Lai MC, Lee YH, Tarn WY. The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol Biol Cell. 2008;19:3847–3858. doi: 10.1091/mbc.E07-12-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee CS, Dias AP, Jedrychowski M, Patel AH, Hsu JL, Reed R. Human DDX3 functions in translation and interacts with the translation initiation factor eIF3. Nucleic Acids Res. 2008;36:4708–4718. doi: 10.1093/nar/gkn454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shih JW, Tsai TY, Chao CH, Wu Lee YH. Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene. 2008;27:700–714. doi: 10.1038/sj.onc.1210687. [DOI] [PubMed] [Google Scholar]
- [30].Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol Cell. 2011;43:962–972. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nakajima T, Uchida C, Anderson SF, Lee CG, Hurwitz J, Parvin JD, Montminy M. RNA helicase A mediates association of CBP with RNA polymerase II. Cell. 1997;90:1107–1112. doi: 10.1016/s0092-8674(00)80376-1. [DOI] [PubMed] [Google Scholar]
- [32].Anderson SF, Schlegel BP, Nakajima T, Wolpin ES, Parvin JD. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet. 1998;19:254–256. doi: 10.1038/930. [DOI] [PubMed] [Google Scholar]
- [33].Gatfield D, Le Hir H, Schmitt C, Braun IC, Kocher T, Wilm M, Izaurralde E. The DExH/D box protein HEL/UAP56 is essential for mRNA nuclear export in Drosophila. Curr Biol. 2001;11:1716–1721. doi: 10.1016/s0960-9822(01)00532-2. [DOI] [PubMed] [Google Scholar]
- [34].Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT. Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell. 2004;119:381–392. doi: 10.1016/j.cell.2004.09.029. [DOI] [PubMed] [Google Scholar]
- [35].Schroder M, Baran M, Bowie AG. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yajima M, Wessel GM. The multiple hats of Vasa: its functions in the germline and in cell cycle progression. Mol Reprod Dev. 2011;78:861–867. doi: 10.1002/mrd.21363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Henn A, Bradley MJ, De La Cruz EM. ATP utilization and RNA conformational rearrangement by DEAD-box proteins. Annu Rev Biophys. 2012;41:247–267. doi: 10.1146/annurev-biophys-050511-102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Byrd AK, Raney KD. Superfamily 2 helicases. Front Biosci. 2012;17:2070–2088. doi: 10.2741/4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gorbalenya AE, Koonin EV, Donchenko AP, Blinov VM. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, Schnier J, Slonimski PP. Birth of the D-E-A-D box. Nature. 1989;337:121–122. doi: 10.1038/337121a0. [DOI] [PubMed] [Google Scholar]
- [41].Rogers GW, Jr., Richter NJ, Merrick WC. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J Biol Chem. 1999;274:12236–12244. doi: 10.1074/jbc.274.18.12236. [DOI] [PubMed] [Google Scholar]
- [42].Sengoku T, Nureki O, Nakamura A, Kobayashi S, Yokoyama S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell. 2006;125:287–300. doi: 10.1016/j.cell.2006.01.054. [DOI] [PubMed] [Google Scholar]
- [43].Yang Q, Del Campo M, Lambowitz AM, Jankowsky E. DEAD-box proteins unwind duplexes by local strand separation. Mol Cell. 2007;28:253–263. doi: 10.1016/j.molcel.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [44].Yang Q, Jankowsky E. The DEAD-box protein Ded1 unwinds RNA duplexes by a mode distinct from translocating helicases. Nat Struct Mol Biol. 2006;13:981–986. doi: 10.1038/nsmb1165. [DOI] [PubMed] [Google Scholar]
- [45].Chen Y, Potratz JP, Tijerina P, Del Campo M, Lambowitz AM, Russell R. DEAD-box proteins can completely separate an RNA duplex using a single ATP. Proc Natl Acad Sci U S A. 2008;105:20203–20208. doi: 10.1073/pnas.0811075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu F, Putnam A, Jankowsky E. ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proc Natl Acad Sci U S A. 2008;105:20209–20214. doi: 10.1073/pnas.0811115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Henn A, Cao W, Licciardello N, Heitkamp SE, Hackney DD, De La Cruz EM. Pathway of ATP utilization and duplex rRNA unwinding by the DEAD-box helicase, DbpA. Proc Natl Acad Sci U S A. 2010;107:4046–4050. doi: 10.1073/pnas.0913081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Oberer M, Marintchev A, Wagner G. Structural basis for the enhancement of eIF4A helicase activity by eIF4G. Genes Dev. 2005;19:2212–2223. doi: 10.1101/gad.1335305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abramson RD, Dever TE, Lawson TG, Ray BK, Thach RE, Merrick WC. The ATP-dependent interaction of eukaryotic initiation factors with mRNA. J Biol Chem. 1987;262:3826–3832. [PubMed] [Google Scholar]
- [50].Abramson RD, Dever TE, Merrick WC. Biochemical evidence supporting a mechanism for cap-independent and internal initiation of eukaryotic mRNA. J Biol Chem. 1988;263:6016–6019. [PubMed] [Google Scholar]
- [51].Bi X, Ren J, Goss DJ. Wheat germ translation initiation factor eIF4B affects eIF4A and eIFiso4F helicase activity by increasing the ATP binding affinity of eIF4A. Biochemistry. 2000;39:5758–5765. doi: 10.1021/bi992322p. [DOI] [PubMed] [Google Scholar]
- [52].Marintchev A, Edmonds KA, Marintcheva B, Hendrickson E, Oberer M, Suzuki C, Herdy B, Sonenberg N, Wagner G. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell. 2009;136:447–460. doi: 10.1016/j.cell.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nielsen KH, Behrens MA, He Y, Oliveira CL, Jensen LS, Hoffmann SV, Pedersen JS, Andersen GR. Synergistic activation of eIF4A by eIF4B and eIF4G. Nucleic Acids Res. 2011;39:2678–2689. doi: 10.1093/nar/gkq1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rozovsky N, Butterworth AC, Moore MJ. Interactions between eIF4AI and its accessory factors eIF4B and eIF4H. RNA. 2008;14:2136–2148. doi: 10.1261/rna.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, Liu D, Song H. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J. 2009;28:274–285. doi: 10.1038/emboj.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, Wagner G. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci U S A. 2008;105:3274–3279. doi: 10.1073/pnas.0712235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mayberry LK, Allen ML, Dennis MD, Browning KS. Evidence for variation in the optimal translation initiation complex: plant eIF4B, eIF4F, and eIF(iso)4F differentially promote translation of mRNAs. Plant Physiol. 2009;150:1844–1854. doi: 10.1104/pp.109.138438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Rajagopal V, Park EH, Hinnebusch AG, Lorsch JR. Specific domains in yeast translation initiation factor eIF4G strongly bias RNA unwinding activity of the eIF4F complex toward duplexes with 5'-overhangs. J Biol Chem. 2012;287:20301–20312. doi: 10.1074/jbc.M112.347278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sun Y, Atas E, Lindqvist L, Sonenberg N, Pelletier J, Meller A. The eukaryotic initiation factor eIF4H facilitates loop-binding, repetitive RNA unwinding by the eIF4A DEAD-box helicase. Nucleic Acids Res. 2012;40:6199–6207. doi: 10.1093/nar/gks278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chase JW, Williams KR. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- [61].Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- [62].Zanotti KJ, Lackey PE, Evans GL, Mihailescu MR. Thermodynamics of the fragile X mental retardation protein RGG box interactions with G quartet forming RNA. Biochemistry. 2006;45:8319–8330. doi: 10.1021/bi060209a. [DOI] [PubMed] [Google Scholar]
- [63].Dhote V, Sweeney TR, Kim N, Hellen CU, Pestova TV. Roles of individual domains in the function of DHX29, an essential factor required for translation of structured mammalian mRNAs. Proc Natl Acad Sci U S A. 2012;109:E3150–3159. doi: 10.1073/pnas.1208014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hodge CA, Tran EJ, Noble KN, Alcazar-Roman AR, Ben-Yishay R, Scarcelli JJ, Folkmann AW, Shav-Tal Y, Wente SR, Cole CN. The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1. Genes Dev. 2011;25:1052–1064. doi: 10.1101/gad.2041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Montpetit B, Thomsen ND, Helmke KJ, Seeliger MA, Berger JM, Weis K. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472:238–242. doi: 10.1038/nature09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Noble KN, Tran EJ, Alcazar-Roman AR, Hodge CA, Cole CN, Wente SR. The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1. Genes Dev. 2012;25:1065–1077. doi: 10.1101/gad.2040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hilbert M, Kebbel F, Gubaev A, Klostermeier D. eIF4G stimulates the activity of the DEAD box protein eIF4A by a conformational guidance mechanism. Nucleic Acids Res. 2011;39:2260–2270. doi: 10.1093/nar/gkq1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861–869. doi: 10.1038/nsmb990. [DOI] [PubMed] [Google Scholar]
- [69].Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 2000;19:6860–6869. doi: 10.1093/emboj/19.24.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lorsch JR, Herschlag D. The DEAD box protein eIF4A. 1. A minimal kinetic and thermodynamic framework reveals coupled binding of RNA and nucleotide. Biochemistry. 1998;37:2180–2193. doi: 10.1021/bi972430g. [DOI] [PubMed] [Google Scholar]
- [71].Rozen F, Edery I, Meerovitch K, Dever TE, Merrick WC, Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990;10:1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Soto-Rifo R, Rubilar PS, Limousin T, de Breyne S, Decimo D, Ohlmann T. DEAD-box protein DDX3 associates with eIF4F to promote translation of selected mRNAs. EMBO J. 2012;31:3745–3756. doi: 10.1038/emboj.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Siridechadilok B, Fraser CS, Hall RJ, Doudna JA, Nogales E. Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science. 2005;310:1513–1515. doi: 10.1126/science.1118977. [DOI] [PubMed] [Google Scholar]
- [74].Spirin AS. How does a scanning ribosomal particle move along the 5'-untranslated region of eukaryotic mRNA? Brownian Ratchet model. Biochemistry. 2009;48:10688–10692. [Google Scholar]
- [75].Walker SE, Zhou F, Mitchell SF, Larson VS, Valasek L, Hinnebusch AG, Lorsch JR. Yeast eIF4B binds to the head of the 40S ribosomal subunit and promotes mRNA recruitment through its N-terminal and internal repeat domains. RNA. 2012 doi: 10.1261/rna.035881.112. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Methot N, Pickett G, Keene JD, Sonenberg N. In vitro RNA selection identifies RNA ligands that specifically bind to eukaryotic translation initiation factor 4B: the role of the RNA remotif. RNA. 1996;2:38–50. [PMC free article] [PubMed] [Google Scholar]
- [77].Jin J, Jing W, Lei XX, Feng C, Peng S, Boris-Lawrie K, Huang Y. Evidence that Lin28 stimulates translation by recruiting RNA helicase A to polysomes. Nucleic Acids Res. 2011;39:3724–3734. doi: 10.1093/nar/gkq1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Manojlovic Z, Stefanovic B. A novel role of RNA helicase A in regulation of translation of type I collagen mRNAs. RNA. 2012;18:321–334. doi: 10.1261/rna.030288.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Gibson TJ, Thompson JD. Detection of dsRNA-binding domains in RNA helicase A and Drosophila maleless: implications for monomeric RNA helicases. Nucleic Acids Res. 1994;22:2552–2556. doi: 10.1093/nar/22.13.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Styhler S, Nakamura A, Swan A, Suter B, Lasko P. vasa is required for GURKEN accumulation in the oocyte, and is involved in oocyte differentiation and germline cyst development. Development. 1998;125:1569–1578. doi: 10.1242/dev.125.9.1569. [DOI] [PubMed] [Google Scholar]
- [81].Breitwieser W, Markussen FH, Horstmann H, Ephrussi A. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 1996;10:2179–2188. doi: 10.1101/gad.10.17.2179. [DOI] [PubMed] [Google Scholar]
- [82].Beckham C, Hilliker A, Cziko AM, Noueiry A, Ramaswami M, Parker R. The DEAD-box RNA helicase Ded1p affects and accumulates in Saccharomyces cerevisiae P-bodies. Mol Biol Cell. 2008;19:984–993. doi: 10.1091/mbc.E07-09-0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Geissler R, Golbik RP, Behrens SE. The DEAD-box helicase DDX3 supports the assembly of functional 80S ribosomes. Nucleic Acids Res. 2012;40:4998–5011. doi: 10.1093/nar/gks070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jamieson DJ, Beggs JD. A suppressor of yeast spp81/ded1 mutations encodes a very similar putative ATP-dependent RNA helicase. Mol Microbiol. 1991;5:805–812. doi: 10.1111/j.1365-2958.1991.tb00753.x. [DOI] [PubMed] [Google Scholar]
- [85].von der Haar T, McCarthy JE. Intracellular translation initiation factor levels in Saccharomyces cerevisiae and their role in cap-complex function. Mol Microbiol. 2002;46:531–544. doi: 10.1046/j.1365-2958.2002.03172.x. [DOI] [PubMed] [Google Scholar]
- [86].Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol Mol Biol Rev. 2011;75:434–467. doi: 10.1128/MMBR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Choi SK, Lee JH, Zoll WL, Merrick WC, Dever TE. Promotion of met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science. 1998;280:1757–1760. doi: 10.1126/science.280.5370.1757. [DOI] [PubMed] [Google Scholar]
- [88].Pestova TV, Kolupaeva VG. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 2002;16:2906–2922. doi: 10.1101/gad.1020902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ariumi Y, Kuroki M, Abe K, Dansako H, Ikeda M, Wakita T, Kato N. DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. J Virol. 2007;81:13922–13926. doi: 10.1128/JVI.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Noueiry AO, Chen J, Ahlquist P. A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc Natl Acad Sci U S A. 2000;97:12985–12990. doi: 10.1073/pnas.240460897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. Cellular cofactors affecting hepatitis C virus infection and replication. Proc Natl Acad Sci U S A. 2007;104:12884–12889. doi: 10.1073/pnas.0704894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pisarev AV, Unbehaun A, Hellen CU, Pestova TV. Assembly and analysis of eukaryotic translation initiation complexes. Methods Enzymol. 2007;430:147–177. doi: 10.1016/S0076-6879(07)30007-4. [DOI] [PubMed] [Google Scholar]
- [94].Yoder-Hill J, Pause A, Sonenberg N, Merrick WC. The p46 subunit of eukaryotic initiation factor (eIF)-4F exchanges with eIF-4A. J Biol Chem. 1993;268:5566–5573. [PubMed] [Google Scholar]
- [95].Pause A, Methot N, Svitkin Y, Merrick WC, Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994;13:1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ivanov IP, Loughran G, Sachs MS, Atkins JF. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1) Proc Natl Acad Sci U S A. 2010;107:18056–18060. doi: 10.1073/pnas.1009269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Loughran G, Sachs MS, Atkins JF, Ivanov IP. Stringency of start codon selection modulates autoregulation of translation initiation factor eIF5. Nucleic Acids Res. 2012;40:2898–2906. doi: 10.1093/nar/gkr1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Martin-Marcos P, Cheung YN, Hinnebusch AG. Functional elements in initiation factors 1, 1A, and 2beta discriminate against poor AUG context and non-AUG start codons. Mol Cell Biol. 2011;31:4814–4831. doi: 10.1128/MCB.05819-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Petrelli D, LaTeana A, Garofalo C, Spurio R, Pon CL, Gualerzi CO. Translation initiation factor IF3: two domains, five functions, one mechanism? EMBO J. 2001;20:4560–4569. doi: 10.1093/emboj/20.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yu Y, Abaeva IS, Marintchev A, Pestova TV, Hellen CU. Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucleic Acids Res. 2011;39:4851–4865. doi: 10.1093/nar/gkr045. [DOI] [PMC free article] [PubMed] [Google Scholar]