Abstract
Recent studies have suggested that progestins play a role in the etiology of breast cancer; however, the mechanisms by which progestins promote tumor formation/progression have not been defined. Progestin action, in target tissues such as the breast, is mediated by the progesterone receptor (PR). PR signaling is complex and PR regulates transcription of target genes through a variety of mechanisms. Many cell signaling pathways are activated inappropriately in breast cancer cells and these pathways can regulate PR activity. For example, the p42/p44 MAPK pathway can regulate PR function by altering phosphorylation of PR, as well as its coregulators. We found that inhibition of the p42/p44 MAPK signaling pathway with a MEK inhibitor (U0126) impairs PR-mediated gene induction, but not gene repression. In addition, the effects of U0126 on PR-mediated gene transcription are much greater with long-term versus short-term inhibition and are gene-specific. Finally, treatment with U0126 delays phosphorylation of Ser294, but does not block phosphorylation completely, suggesting that p42/p44 MAPK kinase is not the dominant kinase responsible for phosphorylating this site. Collectively, these studies suggest that in addition to the p42/p44 MAPK pathway, other signaling pathways are also important for PR transcriptional activity in breast cancer cells. The integration of PR transcriptional effects and cell signaling pathways has implications for the initiation or progression of breast cancer. Understanding how these pathways interact may aid in the development of prevention and/or treatment strategies for the disease.
Keywords: Progesterone receptor, breast cancer, p42/p44 MAPK, phosphorylation, U0126, T47D
1. Introduction
Progesterone action, through the progesterone receptor (PR), is important for the regulation of mammary gland development and reproductive function [1-3]. In addition to its defined role in normal target tissues, studies also have suggested that synthetic progestins play a role in the development and/or progression of breast cancer [4, 5]. PR is a member of the steroid hormone receptor superfamily that includes the estrogen receptor, androgen receptor and glucocorticoid receptors. Progesterone action in target tissues is mediated by two isoforms of PR, PR-A and PR-B. The two isoforms are encoded by the same gene, but are produced from different mRNAs. They differ only by an additional 164 amino acid sequence unique to the amino-terminus of PR-B [6]. Studies have shown that isoform-specific regulation of gene expression is tissue, cell type and promoter specific [6].
PR can regulate transcription through a variety of mechanisms. In the classical mechanism of PR action, progestins diffuse across the cell membrane and bind PR inducing a conformational change in the receptor that results in dissociation of the receptor from chaperone complexes and receptor dimerization, followed by localization to the nuclear compartment and binding to progesterone-responsive elements [7]. PR regulates transcription through the dynamic and sequential recruitment of primary and secondary co-regulators that alter chromatin structure facilitating transcription. A wide variety of PR coregulators have been identified. These include the p160 steroid receptor coactivators, histone acetylases, DNA helicases, ubiquitin ligases, methylases and kinases that serve to modify histones and remodel chromatin resulting in recruitment and activation of RNA polymerase [8]. PR also can mediate gene repression by binding to promoter regions [9,10] and recruiting corepressors [8, 11, 12] or by physically interfering with the recruitment of other transcription factors [13, 14]. In addition, PR regulates transcription through alternative mechanisms that include PR interacting with and modulating other sequence-specific transcription factors such as AP1 and Sp1 [15], resulting in gene transcription in the absence of direct DNA binding. These tethering interactions may also regulate recruitment of coregulators.
PR activity is also regulated by cell signaling pathways that alter phosphorylation of PR as well as of coregulators. PR recruits cyclin A/Cdk2 [16, 17], as well as p42/p44 MAPK to chromatin [17], facilitating the phosphorylation of histones and other proteins that regulate transcription. PR also can mediate progestin activation of the Src/Ras/MAPK, PI3K/Akt, and JAK2/Stat3 signaling pathways [18]. These effects are dependent on the interaction of a proline-rich region of the amino terminus of non-nuclear PR with signaling molecules and also can lead to altered transcription of target genes [18]. Deletion of the region prevents rapid activation of kinases and some downstream targets, but does not alter induction of classical PR target genes such as serum and glucocorticoid induced kinase (SGK) [18, 19]. Some of the targets that are induced by rapid signaling may also require binding of PR to the target gene whereas others are presumably independent of such binding. In addition to PR’s ability to activate rapid signaling pathways, kinases can modulate PR function. For example, PR requires cyclin-dependent kinase 2 (Cdk2) for hormone-dependent activation of some target genes [16, 20]. Although cyclin A/Cdk2, phosphorylates PR and thus can regulate its activity directly, the activity of a PR mutant lacking all consensus Cdk2 sites (measured using a PR responsive reporter) also is enhanced by increased expression of cyclin A [16]. This suggests that there are additional effects of cyclin A/Cdk2 aside from receptor phosphorylation, including phosphorylation of SRC-1 as well as other components of the PR transcription complex [16, 20].
Other kinases such as p42/p44 MAPK have also been shown to regulate PR action. EGF treatment, which activates p42/p44 MAPK, enhances hormone-dependent PR activity [21]. Inhibition of MAP kinase kinase (MEK) upstream of p42/p44 MAPK, results in impaired transcription of reporter [17, 22, 23] and endogenous genes [23-28]. Phosphorylation of PR by p42/p44 MAPK has been proposed to explain the observed effect of inhibition of this signaling pathway on gene transcription of selected MAPK-sensitive endogenous genes [29]. MAPK activation by progestins, however, also leads to activation and recruitment of kinases that phosphorylate histone H3, ultimately resulting in nucleosome remodeling and target gene induction [17]. These models may not be mutually exclusive and highlight the convergence of genomic and non-genomic mechanisms of PR action to regulate transcriptional activation. Although it is clear that p42/p44 MAPK plays a role in gene induction, there is no information regarding the requirement for p42/p44 MAPK in PR-mediated repression. Moreover, the direct contribution of p42/p44 MAPK versus indirect effects secondary to changes in downstream proteins as a result of long term inhibition of MEK has not been examined. To address these questions, we have studied the effect of inhibition of the p42/p44 MAPK signaling pathway on endogenous target gene induction and repression, as well as on PR phosphorylation in T47D breast cancer cells.
2. Experimental
2.1 Materials
Cell culture reagents were obtained from Invitrogen (Carlsbad, CA). The MEK inhibitor U0126 was purchased from Promega (Madison, WI). Dimethyl sulfoxide (DMSO) was obtained from Sigma (St. Louis, MO). R5020 (Promegestone) was from Perkin Elmer (Boston, MA). Epidermal growth factor (EGF) was from Sigma (St. Louis, MO).
2.2 Cell culture
T47D cells were obtained from the American Type Culture Collection (Manassas, VA) and were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS) and 5 μg/ml insulin at 37°C with 5% CO2. For gene expression analysis, cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO vehicle or 10 μM U0126 for 1 hour, followed by treatment with ethanol vehicle or 10 nM R5020 for 6 hours or 24 hours. In parallel, samples were harvested for Western blot analysis of total PR levels. For analysis of PR phosphorylation, cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or 10 μM U0126 for 1 hour or overnight, followed by treatment with ethanol or 10 nM R5020 for 30 minutes or 2 hours. To determine the effect of treatment on p42/p44 MAPK phosphorylation, cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or 10 μM U0126 for 1 hour, followed by treatment with ethanol or 10 nM R5020 for 5 minutes, 30 minutes, 2 hours, or 24 hours or 50 ng/ml EGF for 10 minutes.
2.3 Real-time quantitative PCR
RNA was isolated using Trizol (Invitrogen, Grand Island, NY) and reverse transcribed using amfiRivert Platinum cDNA Synthesis Master Mix from GenDEPOT (Barker, TX). Real-time quantitative PCR was performed using SYBR Green PCR Master Mix using standard conditions on a StepOnePlus™ real-time PCR machine (Applied Biosystems, Carlsbad, CA). Primers for 18S (previously described in [30]), SGK and cyclin D1 (CCND1) (previously described in [20]), FK506 binding protein 5 (FKBP5) (sense 5′-GGATATACGCCAACATGTTCAA-3′, antisense 5′-CCATTGCTTTATTGGCCTCT-3′), N-myc downstream regulated 1 (NDRG1) (sense 5′-CTACCATGACATCGGCATGAA-3′, antisense 5′-CGTGGCAGACGGCAAAGT-3′), and interleukin 1 receptor, type 1 (IL1R1) (sense 5′-AATTGATGAAGATGACCCAGTGC-3′, antisense 5′-GCAGGATTTTCCACACTGTAATAGTCT-3′) were purchased from Sigma-Genosys (The Woodlands, TX). The relative mRNA expression of each gene was determined using a standard curve and normalizing to 18S mRNA expression.
2.4 Western blot
Cells were lysed in lysis buffer containing 10 mM Tris-HCl pH 7.7 with 1 mM EDTA, 12 mM monothioglycerol and 0.4 M NaCl plus cocktail protease and phosphatase inhibitors purchased from GenDEPOT (Barker, TX) using three freeze-thaw cycles. Proteins were separated using SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Membranes were blocked with 5% milk in TBST (10 mM Tris HCl, 150 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 hour followed by addition of primary antibody and incubation overnight at 4°C. Primary antibodies used were PR 1294 (previously described in [31]), PR phosphorylation-specific Ser294 (previously described in [32]), phospho-p42/p44 and p42/p44 (Cell Signaling, Danvers, MA) and tubulin (Upstate, Lake Placid, NY). Membranes were washed with TBST and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (GenDEPOT, Barker, and TX) or HRP-conjugated donkey anti-rabbit IgG (GE Healthcare, Buckinghamshire, England) secondary antibodies in TBST for 1 hour. Proteins were detected using Enhanced Chemiluminescence (ECL) detection reagent (GE Healthcare, Buckinghamshire, England) or ECL 2 Western Blotting Substrate (Pierce, Rockland, IL).
2.5 Statistical Analysis
Experiments were performed at least three times. Representative examples are shown for Western blot analyses. For real-time RT-PCR, bars represent the average ± standard error of the mean (SEM) of biological triplicates of a representative experiment. Statistical differences between treatment groups were analyzed by two-way ANOVA with Bonferonni’s post-hoc test. Tests were performed using GraphPad Prism 5 software (La Jolla, CA) and p-values <0.05 were considered statistically significant.
3. Results
3.1 PR-mediated gene repression is unaffected by U0126 treatment
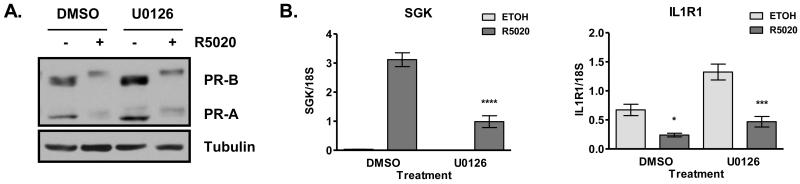
T47D cells were treated with the indicated combinations of vehicle, 10 μM U0126, and 10 nM R5020 for 24 hours (Fig. 1). To rule out the possibility that any observed effects of U0126 on PR transcriptional activity were due to loss of PR protein expression, we performed a Western blot analysis and found that U0126 does not decrease PR-B or PR-A expression either in the absence or presence of R5020 (Fig. 1A). Furthermore, there is a reduction in PR mobility upon hormone treatment in the absence or presence of U0126, indicative of total PR phosphorylation occurring in both treatment groups (Fig. 1A). As expected, 24 hours of R5020 treatment down-regulates expression of both receptor isoforms. This hormone-mediated degradation appears to be slightly reduced in the presence of U0126 (Fig. 1A).
Figure 1. PR-mediated gene repression is unaffected by U0126 treatment.
A) T47D cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or U0126 (10 μM) for 1 hour, followed by treatment with ethanol or R5020 (10 nM) for an additional 24 hours. PR and tubulin protein expression levels were determined by Western blot analysis. A representative experiment is presented. U0126 does not decrease PR expression levels in the absence or presence of R5020, but may inhibit ligand-dependent downregulation of PR. B). In parallel, relative SGK and IL1R1 mRNA expression levels were determined by real-time RT-PCR and normalized to 18S rRNA levels. SGK gene induction was robustly decreased with U0126, but IL1R1 gene repression was not affected by U0126. **** p<0.0001 compared to DMSO R5020, * p<0.05 compared to DMSO ETOH and *** p<0.001 compared to U0126 ETOH. All bars represent the average ± SEM of triplicate samples from a representative experiment.
Although total PR levels are unchanged in the presence of U0126, R5020-mediated transcription of the PR target gene SGK is greatly reduced upon treatment with the inhibitor (Fig. 1B). On the other hand, U0126 did not inhibit R5020-mediated repression of IL1R1 (Fig. 1B). Note that basal expression of IL1R1 is increased in the presence of U0126 indicating that U0126 is not an inhibitor of overall transcription. Western blot analysis confirms that p42/p44 MAPK activity is inhibited under these conditions (Fig. 2C).
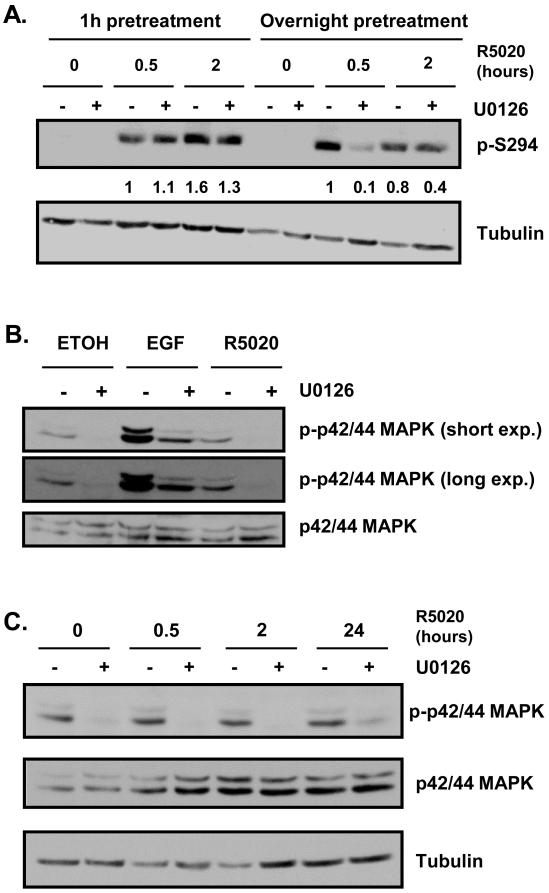
Figure 2. Ser294 phosphorylation is not affected by short-term inhibition of p42/p44 MAPK.
A) T47D cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or U0126 (10 μM) for 1 hour or overnight, followed by treatment with ethanol or R5020 for an additional 30 minutes or 2 hours. Phosphorylated Ser294 and tubulin expression levels were determined by Western blot analysis. A representative experiment is presented. Densitometry was performed on scanned films using ImageJ software and numerical data is presented. Pretreatment with U0126 for 1 hour does not significantly affect Ser294 phosphorylation at either hormone time point. Overnight pretreatment with U0126 decreases phosphorylation of Ser294, especially after 30 minutes of hormone treatment. B) T47D cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or U0126 (10 μM) for 1 hour, followed by treatment with ethanol or R5020 (10nM) for 5 minutes or EGF (50 ng/mL) for 10 minutes. Phosphorylated p42/p44 MAPK and total p42/p44 MAPK levels were assessed by Western blot analysis. A representative experiment is shown. U0126 pretreatment for 1 hour decreases phosphorylation of p42/p44 MAPK under basal, R5020 treatment, and EGF treatment conditions. C) T47D cells were cultured and pretreated as in panel A, followed by treatment with ethanol or R5020 for an additional 30 minutes, 2 hours, or 24 hours. Phosphorylated p42/p44 MAPK, total p42/p44 MAPK and tubulin levels were assessed by Western blot analysis. A representative experiment is shown. Inhibition of p42/p44 MAPK phosphorylation is sustained up to 24 hours of R5020 treatment.
3.2 Ser294 phosphorylation is not affected by short-term inhibition of p42/p44 MAPK
In order to determine whether the effects of U0126 on PR-mediated gene expression are due to p42/p44-mediated phosphorylation of the receptor we performed Western blot analysis of phosphorylated Ser294 levels. Only PR-B is shown because, as we have shown previously [32], PR-A is a poor substrate for phosphorylation at this site in T47D cells. As expected, there is no Ser294 phosphorylation in the ethanol control. With short-term (1 hour) U0126 pretreatment, there is no decrease in phosphorylated Ser294 expression in the presence of R5020 for 30 minutes or 2 hours (Fig. 2A). In contrast, there is an effect of long-term (overnight) U0126 pretreatment on Ser294 phosphorylation. Ser294 phosphorylation is greatly reduced by U0126 at 30 minutes of R5020 treatment, but is less affected at 2 hours (Fig. 2A).
Since U0126 is an inhibitor of MEK (upstream of p42/p44 MAPK) we wanted to ensure that 1 hour of treatment is long enough to effectively inhibit p42/p44 MAPK activity. Because phosphorylation of p42/p44 MAPK is required for activity, this phosphorylation can be used as a surrogate for inhibition of activity. Using western blotting, we found that short-term (1 hour) U0126 pretreatment reduced phosphorylation of p42/p44 MAPK under basal (ethanol) or hormone (R5020) conditions greater than 90% relative to pretreatment with DMSO (Fig. 2B). In contrast to short-term treatment (10 minute) with EGF, a known stimulator of p42/p44 phosphorylation, we did not observe increased phosphorylation with short-term (5 minute) R5020 treatment under our culture conditions (Fig. 2B). Even in the presence of EGF, short-term U0126 inhibits activity of p42/p44 MAPK by about 70% (Fig. 2B). In addition, activity was still inhibited after treatment with R5020 for 30 minutes, 2 hours, and 24 hours (Fig. 2C). These results suggest that pretreatment with U0126 for 1 hour is sufficient to effectively inhibit p42/p44 MAPK activity up to at least 24 hours of hormone treatment.
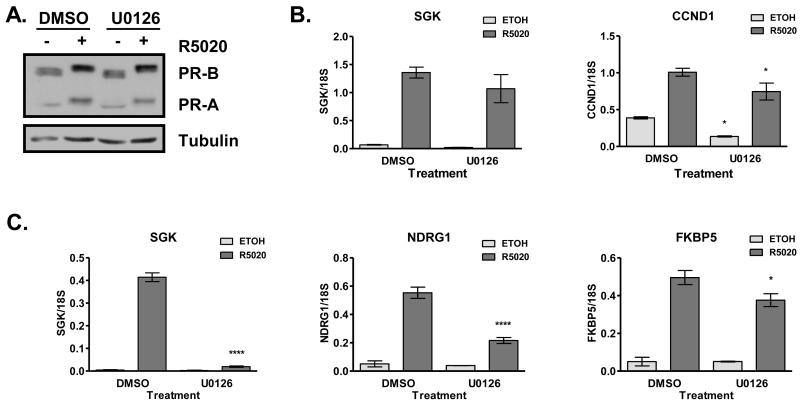
3.3 Effects of MEK inhibition on PR-mediated gene transcription are much greater at 24 hours than at 6 hours
We observed that Ser294 phosphorylation is delayed by long-term, but not short-term, pretreatment with U0126, suggesting that there are indirect effects of p42/p44 MAPK signaling on PR function. To further investigate this, we analyzed PR target gene induction with 6 hours versus 24 hours of R5020 treatment. PR expression levels are not decreased by U0126 pretreatment in the absence or presence of hormone for 6 hours (Fig. 3A) or 24 hours (Fig.1A). Hormone-mediated SGK gene expression is not significantly affected by U0126 with 6 hours of hormone treatment (Fig. 3B). Basal and hormone-mediated CCND1 expression is modestly decreased upon pretreatment with U0126 followed by 6 hours of hormone treatment (Fig. 3B). In contrast to results obtained after 6 hours of R5020, R5020-mediated SGK gene expression is significantly reduced with long-term U0126 treatment (Fig. 3C and Fig. 1B). This dramatic inhibition of PR activity is gene-specific. Similar to SGK, induction of NDRG1 is sensitive to U0126, but induction of FKBP5 is relatively insensitive (Fig. 3C). This gene-specific effect suggests that the role of p42/p44 and downstream substrates in regulating PR-mediated gene expression is dependent on promoter context.
Figure 3. Effects of MEK inhibition on PR-mediated gene transcription are much greater at 24 hours than at 6 hours.
A) T47D cells were cultured in serum-free medium for 24 hours before pretreatment with DMSO or U0126 (10 μM) for 1 hour, followed by treatment with ethanol or R5020 (10 nM) for 6 hours. PR and tubulin protein expression levels were determined by Western blot analysis. A representative experiment is shown. U0126 does not affect PR expression levels in the absence or presence of R5020. B) In parallel to the experiments in panel A, relative SGK and CCND1 mRNA expression was determined using real-time qPCR and normalized to 18S rRNA expression. SGK gene induction was insensitive to U0126, while CCND1 basal and hormone-mediated gene expression was decreased with U0126. * p<0.05 compared to respective treatment in DMSO group. C) T47D cells were cultured and pretreated as in panel A, followed by treatment with ethanol or R5020 (10 nM) for 24 hours. Relative SGK, NDRG1 and FKBP5 mRNA expression was determined using real-time qPCR and normalized to 18S rRNA expression. SGK gene induction was abrogated by U0126, while NDRG1 gene induction was robustly decreased by U0126. In contrast, FKBP5 gene induction was relatively insensitive to U0126. * p<0.05 compared to DMSO R5020. ****p<0.0001 compared to DMSO R5020.
4. Discussion
PR transcriptional regulation is a complex process involving many proteins and the activity of many signaling pathways that regulate PR phosphorylation as well as the phosphorylation of coregulators. Although the majority of phosphorylation sites in PR contain Ser-Pro motifs suggesting phosphorylation by cyclin dependent kinase and/or p42/p44 MAPK, for the most part the kinases responsible for PR phosphorylation have not been identified. Many of the sites are substrates for Cyclin A/Cdk2 in vitro [33, 34]. Overexpression of this kinase enhances basal phosphorylation of Ser190, but has no effect on hormone-dependent phosphorylation [35]. Ser294 resides in a consensus MAPK phosphorylation site. We have shown previously that activation of p42/p44 MAPK by EGF resulted in phosphorylation of this site and that phosphorylation was blocked by U0126 in T47D cells [36]. However, the same three hour pretreatment with U0126 did not block R5020-induced Ser294 phosphorylation. In an earlier study using T47D Y-B cells, Qiu et al [37] also found that short term (30 min) pretreatment with U0126 failed to inhibit R5020 induced Ser294 phosphorylation. Ser345 also resides in a consensus MAPK site. Phosphorylation of this site is not induced by EGF in the absence of R5020. [25]. However, R5020-induced phosphorylation is delayed, but not completely blocked by U0126 [25], suggesting that p42/p44 MAPK is one of the kinases that phosphorylates this site, and that PR is a substrate only in the presence of R5020. In this study, we saw no effect of U0126 on Ser294 phosphorylation at early time points, but a substantial delay in the rate of phosphorylation and a modest decrease in extent of phosphorylation after 24 hours of treatment with U0126 (Fig. 2). This suggests that p42/p44 MAPK can contribute to the phosphorylation of Ser294, but is not the dominant kinase.
Elegant work from Beato’s group showed that PR interacts with p42/p44 MAPK and the downstream kinase Msk1 and can recruit these kinases to promoters resulting in phosphorylation of serine 10 in histone H3 of nucleosome B of the MMTV promoter in T47D cells containing an integrated MMTV promoter/reporter [17]. Moreover treatment with PD98059, a MEK inhibitor, reduced hormone dependent induction of MMTV-luciferase and a subset of PR responsive genes exhibited reduced activity at 6 hours. Our studies show very little, if any, effect on SGK induction at 6 hours, but as previously reported [22, 38] basal and hormone-mediated CCND1 induction is sensitive to U0126 (Fig. 3B). The effects of MEK inhibition are more substantial after 24 hours. Hormone-mediated induction of SGK and NDRG1 is sensitive to U0126, but induction of FKBP5 is minimally affected (Fig. 3D). In contrast, IL1R1 was strongly repressed by R5020 at 24 hours, both in the absence and in the presence of U0126 (Fig. 1B). A previous study demonstrated that CCND1 is not induced with longer hormone treatment [19]; therefore, we did not analyze expression of this gene with 24 hours of hormone treatment. Similarly, since there were only modest effects of U0126 on NDRG1 and FKBP5 gene induction with 24 hours of hormone, we did not analyze the effect of U0126 on the expression of these genes with the shorter (6 hour) hormone treatment. These time-dependent and gene-specific effects suggest that the role of p42/p44 MAPK in regulating PR activity is complex and that many of the observed effects are secondary to actions of downstream substrates of the kinases or to other changes as a result of long term inhibition. Although PR can recruit p42/p44 MAPK to promoters, induction of SGK at 6 hours apparently does not require recruitment of the active kinase. The variation in sensitivity of PR mediated gene induction to U0126 treatment at 24 hours suggests that the requirements for PR mediated gene expression differ extensively and that the contribution of proteins regulated by MEK/MAPK signaling to PR mediated gene regulation also differ substantially. The p42/p44 MAPK signaling cascade has been implicated in a variety of processes that can affect gene transcription, including regulation of chromatin modification and of nuclear import machinery [39]. The reduction in the rate and extent of Ser294 phosphorylation at 24 hours of treatment suggests that the activity of other kinases also may be reduced. Impairment of PR shuttling has been suggested to explain the effect of p42/p44 MAPK inhibition on PR-mediated gene transcription in T47D-Y cells that stably express PR-B [37]. In our study in T47D cells, the effects on PR activity are gene specific and thus presumably include changes in other proteins required for regulation of specific target genes as well as any changes in PR shuttling caused by inhibition of MAPK.
Recent studies have illustrated the integration of PR and cell signaling pathways. Progestin/PR signaling has been implicated in breast cancer development and/or progression [4, 5]. Although the exact mechanisms have yet to be defined, PR can drive breast cancer cell proliferation in early stages of the disease [40] and also can regulate mammary stem cell function [41]. Protein kinases have been reported to be upregulated and inappropriately activated in breast cancer [29]. Thus, the integration of these signaling pathways has implications for the initiation or progression of breast cancer and highlights the need to understand how these pathways interact in order to develop prevention and/or treatment strategies for the disease.
Highlights.
Progesterone receptor-mediated gene repression is not affected by MEK inhibition.
Ser294 phosphorylation is not affected by short-term MEK inhibition.
Effects of long-term MEK inhibition on gene transcription are gene-specific.
Acknowledgements
The authors would like to thank the Baylor College of Medicine Department of Molecular and Cellular Biology Tissue Culture Core for technical assistance and the Proteomics Shared Resource of the NCI supported Dan L. Duncan Cancer Center (P30CA125123) for production of the monoclonal antibodies to PR. This study was supported by NIH grants R01CA57539 (NLW, DPE) T32CA090221 (LST) and F32GM103080 (LST).
Abbreviations
- PR
progesterone receptor
- MAPK
mitogen-activated protein kinase
- Cdk2
cyclin-dependent kinase 2
- EGF
epidermal growth factor
- DMSO
dimethyl sulfoxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lydon JP, Sivaraman L, Conneely OM. A reappraisal of progesterone action in the mammary gland. J Mammary Gland Biolo Neo. 2010;5:325–338. doi: 10.1023/a:1009555013246. [DOI] [PubMed] [Google Scholar]
- [2].Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- [3].Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol. 2012;357:4–17. doi: 10.1016/j.mce.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- [5].Beral V, Million Women Study Collaborators Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- [6].Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357:18–29. doi: 10.1016/j.mce.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li X, O’Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- [8].Scarpin KM, Graham JD, Mote PA, Clarke CL. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal. 2009:7. doi: 10.1621/nrs.07009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pauklin S, Petersen-Mahrt SK. Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol. 2009;183:1238–1244. doi: 10.4049/jimmunol.0803915. [DOI] [PubMed] [Google Scholar]
- [10].Thackray VG, Hunnicutt JL, Memon AK, Ghochani Y, Mellon PL. Progesterone inhibits basal and gonadotropin-releasing hormone induction of luteinizing hormone beta-subunit gene expression. Endocrinology. 2009;150:2395–2403. doi: 10.1210/en.2008-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dong X, Yu C, Shynlova O, Challis JR, Rennie PS, Lye SJ. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1) Mol Endocrinol. 2009;23:1147–1160. doi: 10.1210/me.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Amicis F, Zupo S, Panno ML, Malivindi R, Giordano F, Barone I, et al. Progesterone receptor B recruits a repressor complex to a half-PRE site of the estrogen receptor alpha gene promoter. Mol Endocrinol. 2009;23:454–465. doi: 10.1210/me.2008-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Buser AC, Gass EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, et al. Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the beta-casein gene in mammary epithelial cells. Mol Endocrinol. 2007;21:106–125. doi: 10.1210/me.2006-0297. [DOI] [PubMed] [Google Scholar]
- [14].Buser AC, Obr AE, Kabotyanski EB, Grimm SL, Rosen JM, Edwards DP. Progesterone receptor directly inhibits β-casein gene transcription in mammary epithelial cells through promoting promoter and enhancer repressive chromatin modifications. Mol Endocrinol. 2011;25:955–968. doi: 10.1210/me.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Daniel AR, Knutson TP, Lange CA. Signaling inputs to progesterone receptor gene regulation and promoter selectivity. Mol Cell Endocrinol. 2009;308:47–52. doi: 10.1016/j.mce.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vicent GP, Ballaré C, Nacht AS, Clausell J, Subtil-Rodriguez A, Quiles I, et al. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- [18].Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- [19].Boonyaratanakornkit V, McGowan E, Sherman L, Mancini M, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- [20].Moore NL, Weigel NL. Regulation of progesterone receptor activity by cyclin dependent kinases 1 and 2 occurs in part by phosphorylation of the SRC-1 carboxyl-terminus. Int J Biochem Cell Biol. 2011;43:1157–1167. doi: 10.1016/j.biocel.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Daniel AR, Qiu M, Faivre EJ, Ostrander JH, Skildum A, Lange CA. Linkage of progestin and epidermal growth factor signaling: phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen activated protein kinases. Mol Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- [23].Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombès M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol. 2011;25:1710–1724. doi: 10.1210/me.2011-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu J, Brandt S, Hyder SM. Ligand- and cell-specific effects of signal transduction pathway inhibitors on progestin-induced vascular endothelial growth factor levels in human breast cancer cells. Mol Endocrinol. 2005;19:312–326. doi: 10.1210/me.2004-0252. [DOI] [PubMed] [Google Scholar]
- [25].Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22:823–837. doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Holley AK, Kiningham KK, Spitz DR, Edwards DP, Jenkins JT, Moore MR. Progestin stimulation of manganese superoxide dismutase and invasive properties in T47D human breast cancer cells. J Steroid Biochem Mol Biol. 2009;117:23–30. doi: 10.1016/j.jsbmb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wade HE, Kobayashi S, Eaton ML, Jansen MS, Lobenhofer EK, Lupien M, et al. Multimodal regulation of E2F1 gene expression by progestins. Mol Cell Biol. 2010;30:1866–1877. doi: 10.1128/MCB.01060-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knutson TP, Daniel AR, Fan D, Silverstein KA, Covington KR, Fuqua SA, et al. Phosphorylated and sumoylation-deficient progesterone receptors drive proliferative gene signatures during breast cancer progression. Breast Cancer Res. 2012;14:R95. doi: 10.1186/bcr3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hagan CR, Daniel AR, Dressing GE, Lange CA. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2011;357:43–49. doi: 10.1016/j.mce.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WEI, Erdem H, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- [31].Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, et al. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids. 2002;67:799–813. doi: 10.1016/s0039-128x(02)00039-9. [DOI] [PubMed] [Google Scholar]
- [32].Clemm DL, Sherman L, Boonyaratanakornkit V, Schrader WT, Weigel NL, Edwards DP. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Beck CA, Poletti A, Clement JP, Prendergast P, Yip T-T, et al. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–832. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- [34].Knotts TA, Orkiszewski RS, Cook RG, Edward DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276:8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- [35].Moore NL, Narayanan R, Weigel NL. Cyclin dependent kinase 2 and the regulation of human progesterone receptor activity. Steroids. 2007;72:202–209. doi: 10.1016/j.steroids.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005;25:2885–2898. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qiu M, Olsen A, Faivre E, Horwitz KB, Lange CA. Mitogen activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003;17:628–642. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- [38].Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- [40].Horwitz KB. The year in basic science: update of estrogen plus progestin therapy for menopausal hormone replacement implicating stem cells in the increased breast cancer risk. Mol Endocrinol. 2008;22:2743–2750. doi: 10.1210/me.2008-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Axlund SD, Sartorius CA. Progesterone regulation of stem and progenitor cells in normal and malignant breast. Mol Cell Endocrinol. 2012;357:71–79. doi: 10.1016/j.mce.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]