Abstract
Adrenocorticotropic hormone (ACTH) is among the several melanocortin peptide hormones that are derived from proopiomelanocortin (POMC). ACTH has been found to enhance osteogenesis and chondrogenesis. We show that in the presence of dexamethasone, ACTH dose-dependently increases chondrogenic nodule formation in bone marrow stromal cells (BMSC) from the Wystar Kyoto (WKY) rat. The nodules consist of condensed cells highly expressing alkaline phosphatase, Sox9 and type II collagen transcripts, and a proteoglycan rich matrix. Immunoblot analysis of crude membrane fractions was used to determine that these cells express three melanocortin receptors (MC-R); MC2-R, MC3-R and MC5-R, as well as the melanocortin 2-receptor accessory protein (MRAP). To determine which of these receptors mediate ACTH-induced effects, we used MC-R specific peptides and the known agonist profiles of the receptors. Neither α-MSH, a strong agonist of the MC5-R nor γ2-MSH, a strong agonist of the MC3-R, duplicates ACTH effects in rat BMSC. In addition, calcium flux was examined as a mechanism for ACTH action at the MC2-R. Consistent with MC2-R and MRAP expression patterns in the BMSC cultures, ACTH-induced transient increases in intracellular calcium were increased with dexamethasone treatment. Neither α-MSH nor γ2-MSH affected calcium flux. Dexamethasone increased MC2-R and MRAP expression as well as POMC peptide expression and cleavage to increase the production of the lipolytic β-LPH product. Therefore the effects of ACTH in rat BMSC enriched for mesenchymal progenitors are consistent with an MC2-R signaling mechanism and dexamethasone is capable of regulating components of the melanocortin system in these cells.
Keywords: proopiomelanocortin, osteogenesis, chondrogenesis, mesenchymal progenitors, prohormone convertases, β-lipotropic hormone (β-LPH)
1. Introduction
The precursor peptide proopiomelanocortin (POMC), derived from the pituitary gland and some peripheral tissues, is processed by tissue-specific prohormone convertases (PC) producing the melanocortin peptides. Cleavage via PC1/3 produces ACTH and β-lipotropic hormone (β-LPH), whereas PC2 is required for the generation of α-melanocyte-stimulating hormone (α-MSH), β-MSH, γ-MSH and the endorphins (Bicknell 2008; Catania 2007; Cone 2006).
Melanocortin peptides signal through a family of five G-protein-coupled receptors (GPCR) collectively termed MC-R. Melanocortin peptides bind and activate melanocortin receptors with varying but specific affinities. The primary agonist of the MC1-R is α-MSH, the MC2-R is only responsive to ACTH, the primary agonist of the MC3-R is γ-MSH and the MC4-R and MC5-R bind to ACTH and α-MSH with equal affinities.
Ligand binding to the MC-R results in the activation of second messenger pathways such as accumulation of adenylyl cyclase and the mobilization of intracellular calcium ([Ca2+]i) (Evans, et al. 2005; Gallo-Payet and Payet 2003; Hoogduijn, et al. 2002; Yang 2011). Initiation of these second messenger effector pathways is largely dependent on which G -protein subunit is activated upon ligand binding to the receptor. While activation of the Gαs subunit typically results in the activation of adenylyl cyclase and the accumulation of cAMP, the activation of Gαq/11 family of subunits largely results in the mobilization of [Ca2+]i (Neves, et al. 2002).
MC-R’s are generally expressed in a tissue-specific manner. The MC1-R is primarily expressed in skin (Le Pape, et al. 2008), with more recent data demonstrating its expression in leukocytes (Getting, et al. 2008; Kang, et al. 2006). The MC2-R is traditionally known to be expressed in the adrenal cortex (Chan, et al. 2011; Papadimitriou and Priftis 2009) and in adipocytes (Noon, et al. 2006). The MC3-R and MC4-R are for the most part expressed in brain (Butler 2006; Cone 2006; Sutton, et al. 2006) but there are reports of MC3-R expression in leukocytes (Getting, et al. 2001; Getting et al. 2008) and chondrogenic cells (Evans, et al. 2004) as well as reports of MC4-R expression in peripheral tissues (Mountjoy, et al. 2003; Siljee-Wong 2011). The MC5-R is the most ubiquitous and is highly expressed in exocrine tissues (van der Kraan, et al. 1998; Zhang, et al. 2011).
All five MCR’s are expressed by mesenchymal progenitors (Evans, et al. 2012; Evans et al. 2004; Evans et al. 2005; Isales, et al. 2010; Zaidi, et al. 2010; Zhong, et al. 2005) and previous in vitro studies have shown a direct relationship between their exposure to melanocortin peptides and their osteogenic and chondrogenic differentiation (Evans et al. 2004; Isales et al. 2010; Zaidi et al. 2010). In this study, using osteogenic conditions, we determined the effect of ACTH on proliferation, alkaline phosphatase expression, and mineralization in rat bone marrow derived stromal cells (BMSC) enriched for mesenchymal progenitors. ACTH-induced calcium flux was examined as a potential mechanism for ACTH effects in these cells. Additionally, we determined the membrane-associated expression profile of the MC-R in and the known peptide agonist profiles of the receptors were used to determine which receptor is responsible for mediating the ACTH signal. Dexamethasone (DEX) is a synthetic glucocorticoid used to enhance mineralization in mesenchymal progenitor cultures and can promote chondrogenic differentiation of human mesenchymal stem cells (Derfoul, et al. 2006; Pei, et al. 2003). Therefore, we also examined the effect of DEX on MC-R expression and calcium flux, as well as POMC and PC1/3 expression in our BMSC cultures.
2. Methods
2.1. Materials
All cell culture media, trypsin, FBS, charcoal/dextran treated FBS and antibiotic/antimycotic solutions were obtained from Invitrogen (Carlsbad, CA). The integrin β1, vimentin, fibronectin and MC-R polyclonal primary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The POMC and PC1/3 antibodies were from Novus Biologicals (Littleton, CO). The MRAP antibody was from Protein Tech (Chicago, IL). The monoclonal Na+/K+ ATPase α-1 antibody (clone C464.6) was from Millipore (Temeculae, CA) and the β-Actin monoclonal antibody from SIGMA-Aldrich (USA). The α-MSH and γ2-MSH peptides were from BACHEM (Torrance, CA). The ACTH (1–39) porcine pituitaryderived peptide and all other chemicals were from SIGMA Aldrich (USA) unless otherwise specified.
2.2. Animals
All animal protocols were approved by the Institutions Animal Care and Use Committee and adhere to the regulations outlined by the National Institutes of Health. Wistar Kyoto (WKY) rats were obtained from Taconic, North America. Animals were housed under local vivarium conditions (12h light-dark cycle) and allowed to acclimate for at least 7 days prior to experimentation. Rats were euthanized under CO2 at 8–12 weeks of age and hind limbs were removed in preparation for bone marrow isolation.
2.3. Bone marrow stromal cell isolation
Bone marrow from the hind limbs of the WKY rat was isolated as previously described (Yeh, et al. 1999). After creating a single-cell suspension, nucleated cells were counted using 3% acetic acid/trypan blue exclusion and plated in α-MEM without phenol red supplemented with 10% FBS, 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B at 107 cells per 100 mm dish. Cultures were depleted of mature macrophage by 24 h adherence depletion. Non-adherent cells were then re-plated into 100 mm dishes and allowed to adhere for 3–4 days before the first medium change. The medium was changed every 2–3 days thereafter. At 80% confluence cells were split at a ratio of 1:3 to 1:4. Cultures were used at passage 3 or 4.
2.4. Chondrogenic, Adipogenic and Osteogenic differentiation
For chondrogenic differentiation, BMSC were plated as micromass culture and maintained in α-MEM without phenol red supplemented with 5% charcoal/dextran treated FBS, 50 µg/mL L-ascorbic acid, 40 µg/mL L-proline, 25 ng/mL BMP-2, 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B for 14 days prior to staining proteoglycan enriched matrix with Alcian blue. For adipogenic differentiation, confluent BMSC cultures were incubated in α-MEM without phenol red supplemented with 10% FBS, 15 mM D-glucose, 1 µM dexamethasone, 0.5 mM IBMX, 10 µg/mL insulin 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B for 4 days. Medium was then changed to basal medium and cultures were incubated for 4 more days before staining neutral lipid accumulation using Oil-Red-O. For osteogenic differentiation confluent BMSC cultures were incubated in α-MEM without phenol red supplemented with 10% FBS, 10 mM β-glycerophosphate, 50 µg/ml ascorbic acid and 10−8 M dexamethasone, 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B for 28 days before von Kossa staining for mineral.
2.5. Fluorescent Immunocytochemistry
BMSC at passage 3 or 4 were initiated in 8-well chamber slides at a density of 2500 cells per well in basal medium. After 4 days of growth, cells were fixed in 2% PFA, rinsed in PBS and blocked using 10% horse serum in PBS with 0.3% Triton X-100. Primary and appropriate secondary antibodies were diluted at 1:100 and 1:50 respectively, in a solution containing 1% BSA, 1% horse serum, 0.3% Triton x-100 and 0.01% sodium azide. Normal goat IgG was used as a control. Washes were carried out with 0.1%BSA in PBS. Slides were mounted using Vectashield mounting medium containing DAPI (Vector Labs, Burlingame, CA).
2.6. Proliferation Assay
Cell proliferation was assessed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Cells were plated in basal medium at 7500 cells per well in 96-well plates and treated with and without ACTH, α-MSH and γ2-MSH peptides at a range of doses (10−6 M – 10−8 M) for 1, 4 and 7 days. At each time point MTT was added at 500 µg/ ml and cultures were incubated for 3 hr. Precipitated formazan was solubilized with 50% DMSO in EtOH and absorbance read at 550 nm.
2.7. Alkaline phosphatase, proteoglycan matrix and mineral staining
WKY BMSC cultures were established at a density of 2.5×104 cells per well in 24-well plates in α-MEM without phenol red supplemented with 10% FBS, 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL amphotericin B until near confluence. To induce mineralization, medium was supplemented with 10 mM β-glycerophosphate, 50 µg/ml ascorbate acid and 10−8 M dexamethasone. Melanocortin peptides were added with each medium change at a range of doses (10−6 M – 10−8 M). Cells were maintained in this medium for 14 days with changes every 2–3 days and peptides added at every change. Alkaline phosphatase staining was carried out using the Leukocyte Alkaline Phosphatase Kit from Sigma-Aldrich (85L2-KT) and following the manufacturer’s instructions. To assess mineralization and proteoglycan matrix cultures were fixed in 10% phosphate buffered formalin using von Kossa stain or Alcian blue stain, respectively.
2.8. Real-Time quantitative PCR
Total RNA was extracted from WKY BMSC cultures maintained in α-MEM without phenol red supplemented with 10% FBS, 10 mM β-glycerophosphate, 50 µg/ml ascorbate acid and 10−8 M dexamethasone, 100 U/mL penicillin sodium, 100 U/mL streptomycin sulfate, and 0.25 µg/mL after 4 or 9 days of treatment with or without 10−7 M ACTH. Reverse transcription and PCR were carried out as described in (Evans, et al. 2009). Primers sequences used were: Col1-5’-AAGGCCCACGGGGACCTGTT-3’ and 5’-GGGCCAGGCACGGAAACTCC-3’, Col2a1-5’-CTACGGCGACGGCAACCTGG-3’ and 5’-TGCCCTCGGCCCTCATCTCC-3’, Sox9-5’-GGGCGAGCACTCTGGGCAAT-3’ and 5’-CGTCGCGGAAGTCGATGGGG-3’, and Gapdh-5’-GGGGCTCTCTGCTCCTCCTG-3’ and 5’-ACGGCAAAATCCGTTCACACCG-3’.
2.9. Immunoblotting
Crude membrane fractions were prepared from WKY BMSC. Cells were lysed in HES buffer (0.25 M sucrose, 5 mM EDTA, 2 mM EGTA, 20 mM HEPES) with 1 X protease inhibitor cocktail (SIGMA-Aldrich, USA, #P8340) and homogenized with a Teflon on glass homogenizer. The homogenate was then centrifuged at 760 g for 5 min and the supernatant centrifuged at 25,000 rpm for 1 h. The pellet was dissolved in modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% NP-40 (w/v), 0.25% Na-deoxycholate (w/v), 150 mM NaCl, 1 mM EDTA and 1X protease inhibitor cocktail) and protein content determined via bicinchroninic acid assay (BCA) assay (Thermo Fisher Scientific). To normalize for protein loading, membrane fractions were also probed for Na+/K+ ATPase expression.
Whole lysates were prepared from BMSC cultures that had either been left untreated or treated with dexamethasone for 2, or 5 days. Protein concentrations were determined by BCA assay. Protein samples were separated by SDS-PAGE and transferred to PVDF membrane. After blocking, membranes were incubated with either POMC or PC1/3 primary antibodies. After incubation with appropriate horseradish peroxidase (HRP)-tagged secondary antibody, bands were visualized using ECL. To normalize protein loading, blots were also probed for β-actin expression.
2.10. Calcium Measurements
Transient elevations in calcium in response to melanocortin peptides were measured as previously described (Evans et al. 2005). Briefly, at confluence WKY BMSC cultures were incubated in α-MEM supplemented with 5% charcoal/dextran treated FBS, 100 U/ml penicillin sodium, 100 U/ml streptomycin sulfate, and 0.25 µg/ml amphotericin B. Cultures were maintained in this medium for at least 5–7 days. Before assay some cultures were treated with 10−8 M dexamethasone for at least 48 hr. Single-cell suspensions were created using trypsin and collagenase, centrifuged and re-suspended in calcium loading buffer with Ca2+ (Hanks; balanced salt solution plus Ca2+ and Mg2+ supplemented with 0.05% BSA) and loaded with 1.5 mM fura-2 at room temperature. For measurements made in the absence of extracellular calcium, calcium loading buffer without Ca2+ (Hank’s balanced salt solution without Ca2+ and Mg2+ supplemented with 0.05% BSA and 80 mM MgCl) was used. All peptides were first diluted in sterile water at a stock concentration of 5 mM and stored in small aliquots at −20 °C. On the day of use, stocks were further diluted by 10X and 100X in calcium loading buffer without Ca2+ and used in experiments at the concentrations stated in the text.
2.11. Statistical Analysis
Data were analyzed using one-way ANOVA or two-way ANOVA where indicated. Post hoc test P values were adjusted using the Bonferroni correction. All tests were two-tailed and a nominal significance level of 0.05 was used.
3. Results
3.1. Third and fourth passage rat BMSC are capable of multi-lineage differentiation
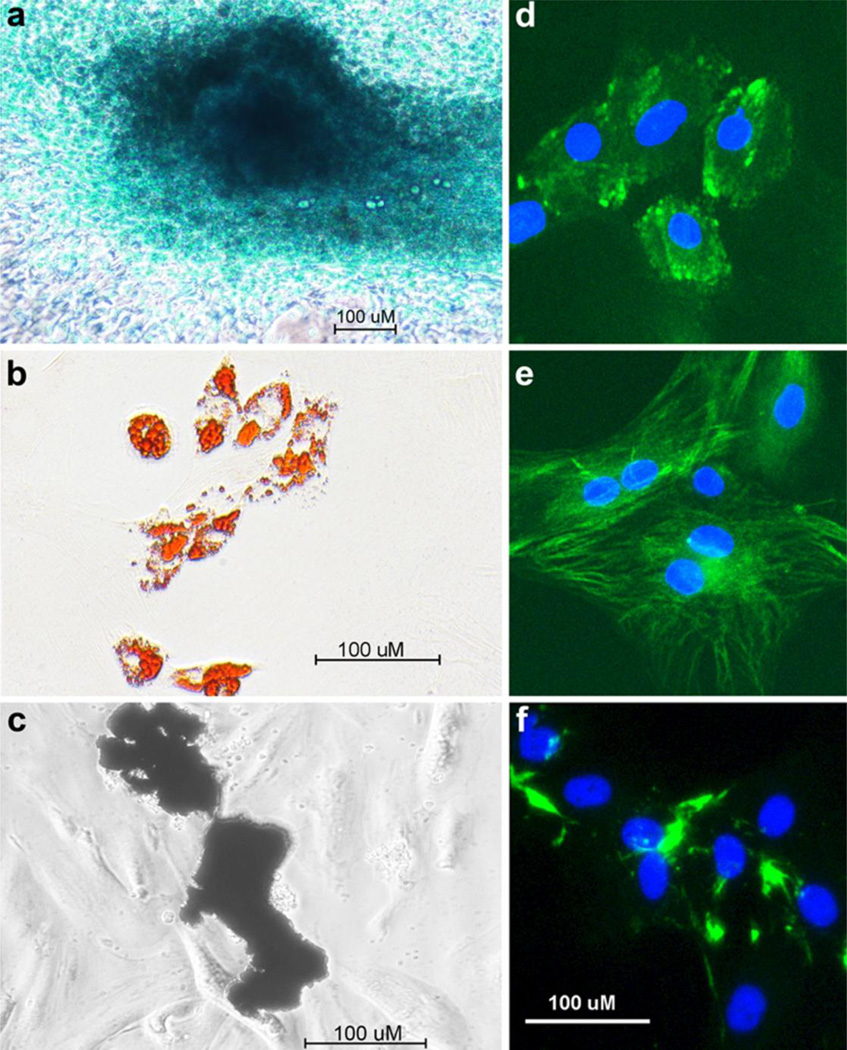
BMSC were isolated from the hind limbs of Wistar Kyoto rats and enriched for mesenchymal progenitors. After isolation, cells were first depleted of mature macrophage through 24 h adherence depletion. The 24 h non-adherent cells were subsequently plated and passaged 3 to 4 times to deplete the cultures of leukocytes and mature cells of mesenchymal lineage. To establish the mesenchymal progenitor potential of our cultures we tested their ability to differentiate into multiple lineages. After adipogenic induction, BMSC enriched for mesenchymal progenitors accumulated lipid as assessed by oil-red-O stain, after chondrogenic induction they formed Alcian blue positive colonies and after osteogenic induction they mineralized consistent with osteogenic differentiation (Fig. 1a–c). To determine the homogeneity of the enriched BMSC cultures, we examined the expression of the mesenchymal cell markers integrinβ1, fibronectin and vimentin using fluorescent immunocytochemistry. The cultures were 96% positive for integrinβ1, 98% positive for vimentin and 90% positive for fibronectin, respectively (Fig. 1d–f).
Fig. 1.
BMSC enriched for mesenchymal progenitors are capable of multi-lineage differentiation and are positive for mesenchymal stem cell (MSC) markers. Under chondrogenic conditions these cells were capable of producing a proteoglycan matrix as assessed by Alcian blue stain (a), capable of adipogenic differentiation with induction as assessed by oil-red-O stain (b) and capable of osteogenic differentiation as assessed by von Kossa stain for mineral(c). Cultures were also highly positive for integrinβ1 (d), vimentin (e) and fibronectin (f) as assessed by fluorescent immunocytochemistry
3.2. Rat BMSC enriched for mesenchymal progenitors express MC2-R and its accessory protein MRAP, as well as MC3-R and MC5-R; Expression of MC2-R and MRAP are regulated by dexamethasone
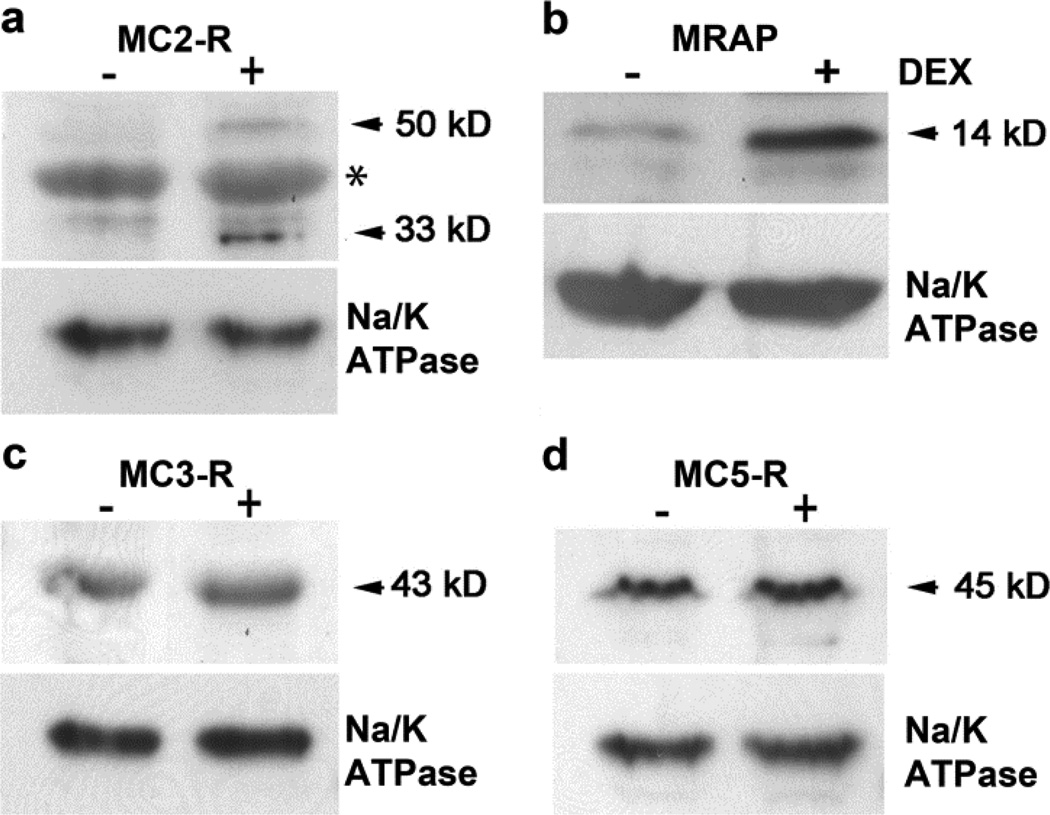
Several works have described the gene and protein expression of MC-R in progenitor cell populations (Evans et al. 2004; Evans et al. 2005; Isales et al. 2010; Zaidi et al. 2010; Zhong et al. 2005). We used crude membrane preparations to confirm that the MC-R expressed by mesenchymal progenitor enriched BMSC are membrane associated and therefore functional. We also determined if glucocorticoid regulates their expression. Cultures were left untreated or treated with 10−8 M dexamethasone for 48 h. Enriched rat BMSC expressed the MC2-R, MC3-R and the MC5-R. The MC4-R was not detected (data not shown).
There were no notable changes in MC3-R and MC5-R expression with dexamethasone treatment (Fig. 2c&d). However expression of the MC2-R was up-regulated by treatment with dexamethasone (Fig. 2a). The MC2-R was expressed as its 33 kD immature and 50 kD mature forms and expression of both was increased with dexamethasone treatment.
Fig. 2.
The MC2-R (a), MRAP (b), MC3-R (c) and MC5-R (d) proteins are expressed in rat BMSC enriched for mesenchymal progenitors and expression of MC2-R (a) and MRAP (b) are regulated by dexamethasone. Cultures were left untreated (−) or were treated with 10−8 M dexamethasone for 48 h (+Dex). MC-R’s and MRAP were detected by immunoblot in crude membrane fraction lysates (50 µg per lane). Na/K ATPase was used to normalize loading. Asterisk (*) denotes a non-specific band
Recent data have revealed that the MC2-R requires the MC2-R accessory protein (MRAP) to reach the cell surface (Sebag and Hinkle 2007; Webb and Clark 2010). Therefore we examined the expression of MRAP in membrane fractions of rat MSC to confirm MC2-R cell surface expression and function. MRAP was detected as a 14 kD band and increased with dexamethasone treatment (Fig. 2b).
3.3. ACTH increases proliferation in rat BMSC enriched for mesenchymal progenitors in a manner dependent on dose; α-MSH and γ-MSH do not duplicate this effect
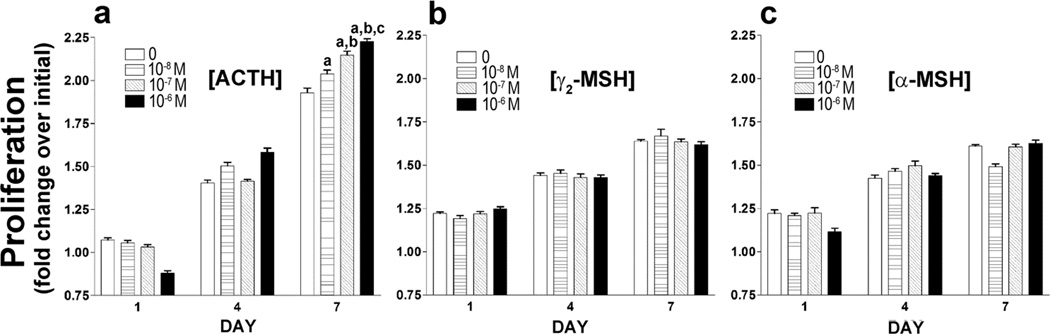
The MTT assay was used to determine the effects of ACTH treatment on cell proliferation in BMSC cultures. By day 7 ACTH increased proliferation in a manner consistent with increasing dose (Fig. 3a); proliferation in the BMSC cultures was increased by 5.65% at 10−8 M, 11.35% at 10−7 M and 15.4% at 10−6 M ACTH. Results of two-way ANOVA, with time and dose of ACTH as factors, demonstrate a significant interaction effect [F(6,84) = 61.53, P < 0.0001]. These data confirm an increase in proliferation with increasing ACTH dose over time. Results of proliferation assays using γ2-MSH, a primary agonist of the MC3-R, and α-MSH, a primary agonist of the MC5-R do not show a significant proliferation effect and do not duplicate the pattern observed when BMSC are treated with ACTH (Fig. 3b & 3c).
Fig. 3.
ACTH promotes cell proliferation of rat BMSC enriched for mesenchymal progenitors in a dose-dependent manner (a). γ2–MSH (b) and α-MSH (c) do not exhibit this effect. Proliferation in response to melanocortin peptides was assessed using MTT assay. The fold change in OD of formazan product from 24 h post-plating (day 0) is shown. Data are presented as means ± SD, n = 8. Data are representative of 3 separate experiments with similar results. Individual comparisons were made after a significant two-way ANOVA (day×ACTH dose) using the Bonferroni correction. a-significantly different from 0 nM, b-significantly different from 0 and 10−8 M, c-significantly different from 0, 10−8 and 10−7 nM
3.4. ACTH dose-dependently increases chondrocyte-like nodule formation in rat BMSC enriched for mesenchymal progenitors in a manner characteristic of MC2-R signaling
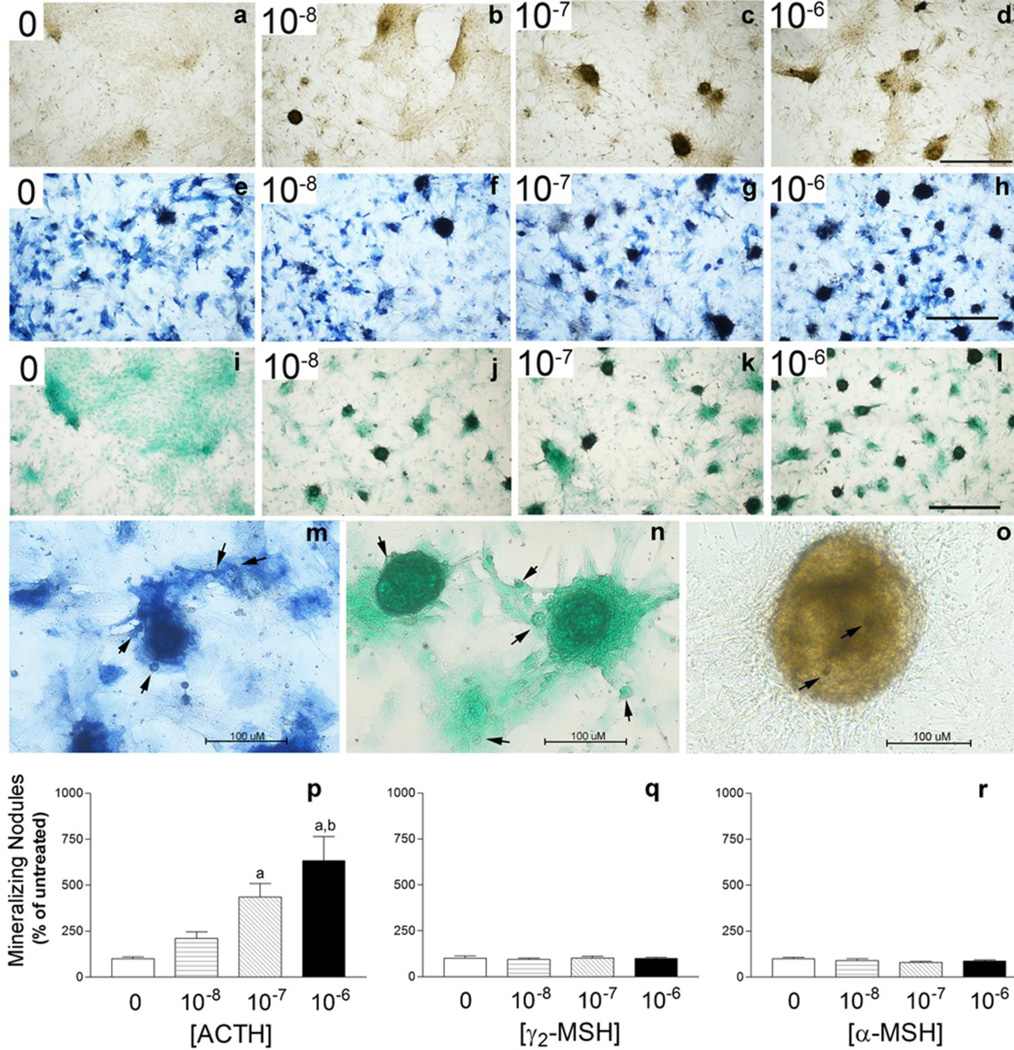
Previous studies have demonstrated an ACTH effect on chondrogenic differentiation (Evans et al. 2004) as well as osteogenesis and mineralization in human mesenchymal stem cell (MSC) cultures (Zaidi et al. 2010). Here we demonstrate that ACTH dose-dependently increases the formation of mineralizing nodule formation (Fig. 4) in rat BMSC enriched for mesenchymal progenitors when grown under typical osteogenic conditions for 14 days. The mineralizing nodules consist of condensed mesenchymal cells consistent with a chondrogenic differentiation program. The nodules are also highly positive for alkaline phosphatase and reside in a proteoglycan-rich matrix (Fig. 4a–o). One way ANOVA results revealed a significant ACTH dose effect on nodule formation, P < 0.0001 and as with the proliferation study, treatment with γ2-MSH or α-MSH does not duplicate ACTH effects (Fig. 4p–r).
Fig. 4.
ACTH promotes chondrogenic mineralized nodule formation in rat BMSC enriched for mesenchymal progenitors in a dose-dependent manner. γ2–MSH and α-MSH do not exhibit this effect. Cultures were stained for mineral (a–d), stained for alkaline phosphatase (e–h) or stained for proteoglycan matrix (i–k) after 14 days of osteogenic culture in the presence or absence of ACTH at a range of doses (10−8 to 10−6 M). Photomicrographs were taken using bright field under scanning power (40X total magnification) to emphasize differences in nodule numbers with ACTH treatment, Bar = 500 µM. Photomicrographs in m–o were taken using bright field (200X total magnification) to emphasize the multi-cellular alkaline phosphatase (m), proteoglycan rich (n), mineralizing nodules (o) as well as the condensed cells in the periphery. Bar = 100 µM. Arrowheads indicate condensed cells in the periphery of the nodules as well as chondrocytes in lacunae. In p–r, the number of mineralized nodules per well in ACTH treated cultures as well as α-MSH and γ2-MSH cultures are presented as % of untreated control (0) ± SEM. Data are representative of 3 separate experiments with each treatment group having an n = 3–5 wells. Individual comparisons were made after a significant one-way ANOVA using the Bonferroni correction. a-significantly different from 0, 10−8 M, b-significantly different from 0, 10−8, and 10−7 M.
3.5. ACTH increases early gene markers of mesenchymal progenitor chondrogenic differentiation
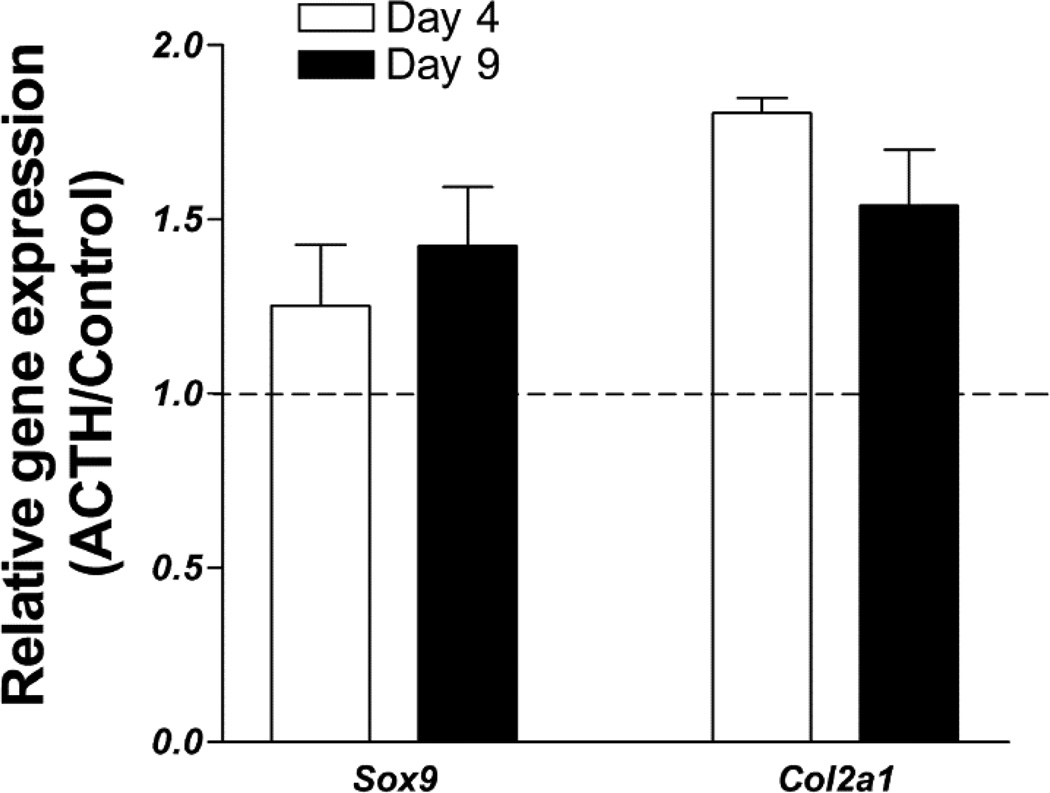
Alkaline phosphatase and mineralization are both late markers of mesenchymal progenitor cell differentiation along the chondrogenic pathway (Minashima, et al.) but could also indicate osteogenic differentiation. To confirm that the ACTH-induced increase in mineralizing nodules reflects an increase in chondrogenic differentiation we examined the expression of the Sox9 chondrogenic transcription factor as well as the early marker of chondrogenic differentiation, type II collagen (Col2a1). ACTH increases the expression of the Sox9 transcription factor by 1.25-fold at day 4 and by 1.42-fold at day 9 of culture. Expression of the Col2a1 is also increased by ACTH exposure by 1.80-fold at day 4 and by 1.54-fold at day 9 (Fig. 5).
Fig. 5.
ACTH enhances early gene markers of chondrogenic differentiation in rat BMSC enriched for mesenchymal progenitors. Real-time reverse transcription PCR was carried out on cultures after 4 or 9 days of culture in the presence of 10−7 M ACTH. The graph shows expression of Sox9 and Col2a1 transcripts in ACTH cultures relative to control. Expression in both groups was first normalized to a GAPDH internal control. Data are presented as mean ± SD, n = 3.
3.6. ACTH induces transient elevations in intracellular calcium in rat BMSC enriched for mesenchymal progenitors
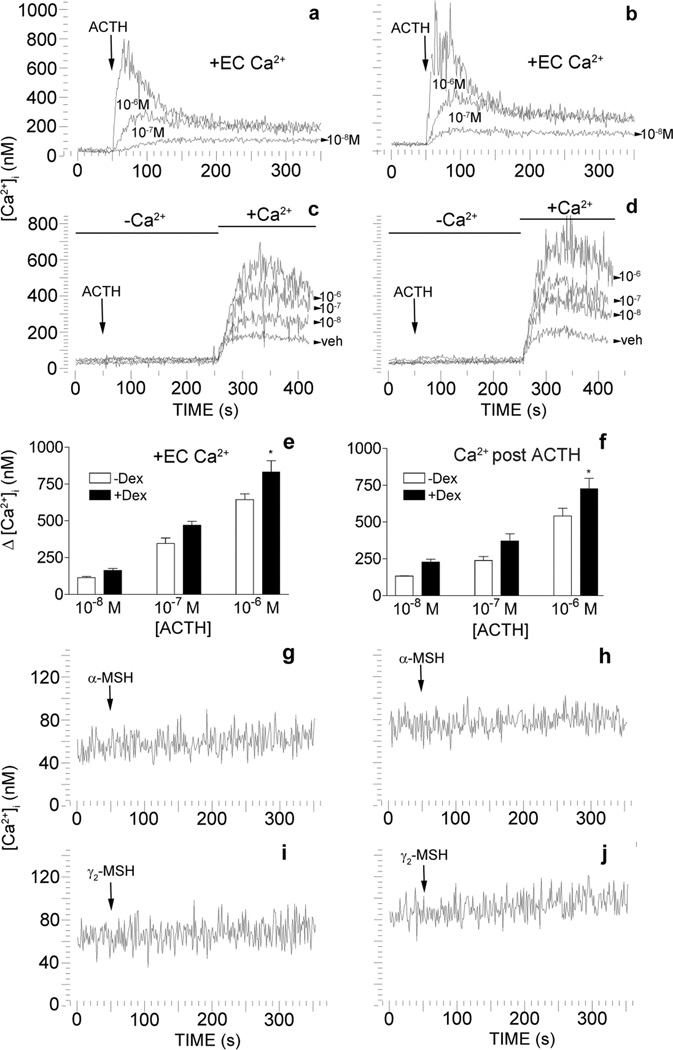
Melanocortin peptides are known to initiate both the cAMP and Ca2+ mediated second-messenger pathways. In chondrogenic cells, ACTH induces calcium flux in a phospholipase-C dependent manner (Evans et al. 2005). Therefore to delineate possible intracellular pathways that are activated by ACTH in rat BMSC, we used the fluorescent Ca2+ indicator, fura-2, to examine intracellular Ca2+ flux. In the presence of extracellular Ca2+, ACTH induces transient increases in [Ca2+]i in a manner dependent on dose. These transient elevations increase when the cells are primed with dexamethasone (Fig. 6a,b&e). Results of two-way ANOVA reveal a significant dose effect of ACTH, [F(2,12) = 111.68, P = 0.0001] and dexamethasone priming [F(1,12) = 13.14, P = 0.0035]. Neither γ2-MSH nor α-MSH induce transient elevations in [Ca2+]i in untreated or dexamethasone treated rat BMSC (Fig. 6g–j).
Fig. 6.
ACTH induces transient elevations in [Ca2+]i through an influx of extracellular calcium in rat BMSC enriched for mesenchymal progenitors. This influx is enhanced with dexamethasone priming. In the presence of extracellular calcium (EC Ca2+), ACTH induces transient increases in [Ca2+]i in a manner consistent with dose in BMSC left untreated (a & e). When rat MSC are treated with 10−8 M dexamethasone for 48 h (+Dex) these increases are enhanced (b & e). Some cultures, left untreated (c & f) or treated with 10−8 M dexamethasone (d & f), were re-suspended in buffer without Ca2+ and exposed to ACTH over a range of doses (10−8 M to 10−6 M). After ~3 min Ca2+ (1.8 mM) was added back to the medium and Ca2+ influx was measured. Times of Ca2+ addition are indicated by the lines above the traces. The dose of ACTH in moles/L is indicated under or next to the corresponding trace. In e & f are bar graphs of the results shown in a–d and indicate the Δ in [Ca2+]i from baseline. Data are presented as mean ± SD, n = 3. Individual comparisons were made after a significant two-way ANOVA using the Bonferroni correction. * -significantly different from DEX treated counterpart. Neither α-MSH (10−6 M) nor γ2–MSH (10−6 M) elicit changes in [Ca2+]i in untreated control (g & i) or dexamethasone treated (h & j) cultures. Arrows indicate time of ACTH, α-MSH or γ2–MSH addition. α-MSH or γ2–MSH were added at 10−6 M. Traces are representative of 3 separate experiments.
To examine the source of the Ca2+ during the ACTH-induced transient increase in [Ca2+]i, we examined Ca2+ flux in the absence of calcium. When cells were exposed to ACTH over a range of doses (10−8 M to 10−6 M) in the absence of extracellular Ca2+, no substantial changes in [Ca2+]i were observed . When Ca2+ was added back to the medium 3 minutes after stimulation with ACTH, transient increases of [Ca2+]i were consistent with increasing dose of ACTH (Fig. 6c&f). Treatment with dexamethasone increased the ACTH-induced Ca2+ influx above that observed in untreated control cultures (Fig. 6d–f). Again, results of two-way ANOVA reveal a significant dose effect of ACTH [F(2,12) = 57.34, P = 0.0001] and dexamethasone priming [F(1,12) = 14.83, P = 0.0023].
3.7. Dexamethasone regulates POMC and PC1/3 expression in rat BMSC enriched for mesenchymal progenitors
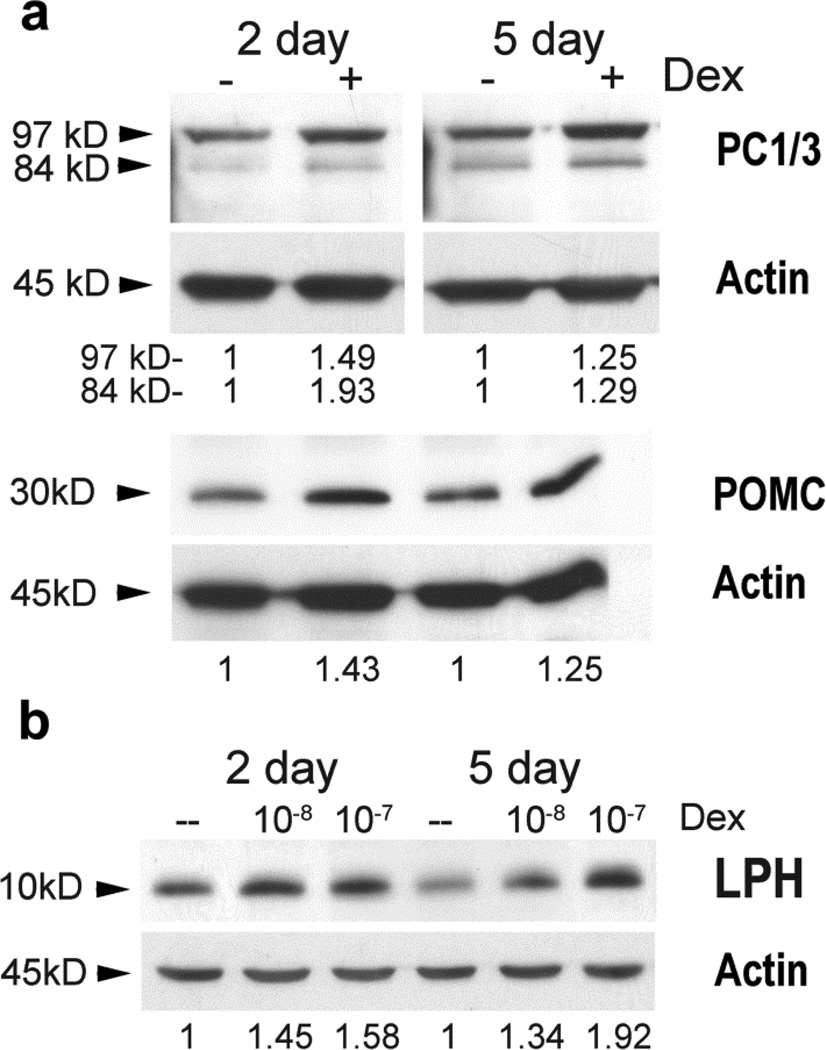
POMC and PC1/3 are expressed in peripheral tissues (Bicknell 2008; Harbour, et al. 1987; Rajora, et al. 1996; Star, et al. 1995) and dexamethasone is used to enhance mineralization during osteogenic differentiation of progenitor cells (Bruedigam, et al. 2011; Mostafa, et al. 2011). Dexamethasone also promotes chondrogenic differentiation of human mesenchymal stem cells (Derfoul et al. 2006). Therefore we examined the expression of POMC and PC1/3 in rat BMSC and the effects of dexamethasone on these proteins. POMC, its derivatives and the POMC cleavage enzyme PC1/3 are expressed in rat BMSC (Fig. 7). PC1/3 is expressed as its inactive 97 kD form and its partially active 84 kD form. After 2 and 5 days of dexamethasone treatment, expression of POMC was increased 1.43 and 1.25 fold, respectively. Expression of the 97 kD form of PC1/3 was increased 1.49 and 1.25 fold, respectively while expression of the 84 kD form was increased by 1.93 and 1.29 fold, respectively (Fig. 7a).
Fig. 7.
Dexamethasone increases the expression of POMC, PC1/3 and the POMC cleavage product, β-LPH, in rat BMSC cultures enriched for mesenchymal progenitors. In (a) cultures were left untreated (−) or treated with 10−8 M dexamethasone (+) for 2 and 5 days. Protein lysates were subjected to SDSPAGE (50 µg/lane). β-actin was used to normalize loading. Densitometry measurements are indicated below the corresponding lanes. Band intensities were normalized to the loading control and are presented as fold change from the untreated (−) cultures. In (b) whole protein lysates were prepared from cultures untreated (--) or treated with 10−8 M or 10−7 M dexamethasone and subjected to SDS-PAGE (30 µg/lane). β-actin was used to normalize loading. Densitometry measurements are indicated below the corresponding lanes. Band intensities were normalized to the loading control and are presented as fold change from the untreated (--) cultures.
If expression of PC1/3 was indeed increased then we expected this to be reflected in increased processing of the POMC precursor. Therefore we also examined the level of the β-lipotropin hormone (β-LPH) cleavage product after cells were treated with dexamethasone. Using a 15% PAGE gel and the same POMC antibody which detects the C-terminal epitope of POMC, we were able to determine that β-LPH (~10 kD) levels increased with exposure to dexamethasone consistent with increased PC1/3 activity (Fig. 7b). After 5 days of treatment with 10−8 M or 10−7 M dexamethasone, β-LPH expression increased by 1.34 and 1.92 fold, respectively.
4. Discussion
Previous work has introduced the presence of a local melanocortin system in bone and cartilage (Evans et al. 2004; Isales et al. 2010; Zaidi et al. 2010; Zhong et al. 2005). These studies have documented gene and protein expression of the MC2-R and other MC-R in human bone and mesenchymal cells and in mouse bone tissues (Zaidi et al. 2010; Zhong et al. 2005). In this work, we detected the MC2-R, MC3-R and MC5-R proteins in membrane fractions of rat BMSC enriched for mesenchymal progenitors suggesting that these receptors are functional in these cells.
ACTH, a potent agonist at all three of these receptors, enhanced chondrogenic mineralizing nodule formation of our mesenchymal progenitor enriched BMSC cell population in a dose-dependent manner. Therefore we sought to identify the MC-R through which ACTH exerts these effects in these cells. We present evidence that identify the MC2-R as the mediator of ACTH-induced effects in rat BMSC. Neither γ2-MSH, a potent MC3-R agonist, nor α-MSH, a potent MC5-R agonist, duplicates the effects of ACTH. The MC2-R accessory protein is also expressed in rat BMSC confirming the capacity for MC2-R cell surface expression and function. Moreover, results of MC2-R expression studies and calcium flux studies are consistent; DEX treatment up regulates MC2-R expression as well as increases ACTHinduced calcium flux. Again neither γ2-MSH nor α-MSH have an effect on [Ca2+]i in these cells. More studies are necessary to fully delineate the MC2-R pathway as well as determine the role(s) for the MC3-R and MC5-R in these cells. ACTH is known to increase the proliferation of BMSC as measured by 3H-thymidine incorporation (Evans et al. 2004) and in the present study we confirm this effect and show its dependence on dose by measuring mitochondrial activity (Fig. 3). Additionally, previous studies have demonstrated that under chondrogenic conditions ACTH enhances the development of the chondrogenic phenotype among rat BMSC (Evans et al. 2004) while other data show ACTH enhances osteoblast development in murine derived BMSC (Zaidi et al. 2010). In the current study rat BMSC were exposed to ACTH under osteogenic conditions with the inclusion of dexamethasone. Under these conditions, ACTH increases the development of mineralizing nodules consistent with chondrogenic differentiation. The nodules consist of condensed mesenchymal cells which is an essential step in chondrogenic differentiation (Ghosh, et al. 2009). They are also positive for alkaline phosphatase and a proteoglycan rich matrix consistent with terminally differentiating chondrocytes (Minashima, et al. 2012). Moreover, ACTH increases expression of the chondrogenic transcription factor, Sox9 and the early chondrocyte marker gene Col21a. Therefore our results indicate that under typical osteogenic conditions, ACTH promotes a chondrogenic phenotype in rat BMSC.
The discrepancy between this study and the study that reveals ACTH as an osteogenic facilitator (Zaidi et al. 2010) is likely due to species differences and/or the presence of dexamethasone in the cultures. There can be significant variation in bone marrow composition between species of animals (Elmore 2006) and this could easily lead to differences in the progenitor potential of isolated stromal populations. Additionally, the studies using mouse bone marrow were performed in the absence of dexamethasone and ACTH-induced enhanced osteogenic differentiation was only observed during the latest stages of differentiation (Zaidi et al. 2010). Our rat BMSC cultures were grown in the presence of dexamethasone which has been shown to promote chondrogenic differentiation in human mesenchymal stem cell cultures (Derfoul et al. 2006). Under these conditions, ACTH-induced increases in nodule formation could be observed using light microscopy as early as 9 days post treatment.
The MC-Rs belong to a family of G-protein coupled receptors and activate both Gαs and Gαq proteins traditionally resulting in increased cAMP accumulation or transient fluxes in [Ca2+]i respectively (Evans et al. 2005; Gallo-Payet and Payet 2003; Hoogduijn et al. 2002). Factors that increase the differentiation of both chondrocytes and osteoblasts from progenitors also increase [Ca2+]i, (Shin, et al. 2008; Sun, et al. 2007; Wang, et al. 1998; Zayzafoon 2006). In fact calcium ionophore induced elevations in [Ca2+]i can promote chondrogenesis though a calcineurin/nuclear factor of activated T-cells (NFAT) axis (Tomita, et al. 2002). Therefore we investigated the ability of ACTH to induce transient elevations in [Ca2+]i and raise basal [Ca2+]i in rat BMSC which would provide a mechanism for ACTH effects. In these cells, ACTH-induces transient elevations in [Ca2+]i through an influx of extracellular Ca2+. No appreciable transient increases of calcium were observed in the absence of extracellular Ca2+. The extracellular influx in rat BMSC, through activation of the MC2-R, thus appears to be a receptor operated calcium entry mechanism. These data contrast with previous work with committed chondroprogenitor populations as well as aorta-derived mesenchymal progenitor populations, where ACTH-induces the mobilization of calcium from intracellular stores as well as the influx of extracellular calcium (Evans et al. 2012; Evans et al. 2005). As cells differentiate, basal cytosolic calcium and calcium in intracellular stores increases. The handling of those stores in response to ACTH stimulation likely varies according to lineage commitment as well as stage of differentiation. It must be noted that ACTH-induced changes in [Ca2+]i were measured in trypsin detached, suspended, progenitor enriched BMSC cultures and the effects of this treatment must be considered. More studies examining this effect in adherent cultures are therefore warranted.
Dexamethasone is often used to promote mineral accumulation in mesenchymal progenitor cultures (Bruedigam et al. 2011; Mostafa et al. 2011). The mechanisms through which dexamethasone promotes chondrogenic and osteogenic differentiation and mineralization are still being investigated. In osteogenesis some data suggests that dexamethasone up regulation of osteoblast-specific transcription factors such as Cbfa1 and Osterix (Osx) (Igarashi, et al. 2004; Mikami, et al. 2011) while others have found it can also up regulate chondrogenic transcription factors such as Sox-9 (Derfoul et al. 2006). Our data suggest that dexamethasone may also influence osteogenic/chondrogenic differentiation and mineralization in culture through regulation of the melanocortin system. Dexamethasone increases the expression of the MC2-R, the melanocortin pre-cursor peptide, POMC, as well as the POMC cleavage enzyme, PC1/3. Activity of the PC1/3 enzyme is also increased as evidenced by the increase in the β-LPH cleavage product. β-LPH is a known lipolytic (Halabe Bucay 2008) and therefore its up-regulation in response to dexamethasone exposure is fitting, as glucocorticoids are known to mobilize lipid. The possibility exists that through the local up-regulation of β-LPH, dexamethasone promotes lypolysis of adipogenic cells in BMSC thereby favoring osteogenic/chondrogenic differentiation. Although our cultures are enriched for mesenchymal progenitors, the precise cell type expressing β-LPH is yet to be determined. Our cultures were significantly homogeneous for mesenchymal markers pointing to the likely expression by mesenchymal cells but their precise developmental stage and lineage commitment needs to be clarified.
Some have hypothesized β-LPH supports hematopoietic stem cells by mobilizing local adipocytic energy reserves (Halabe Bucay 2008) and the pituitary has thus far been considered the major source of β-LPH. Here we present evidence that this hormone is likely produced locally by cells of the bone marrow and potentially participates in release of lipids. Peripheral expression of POMC peptides is not unprecedented. We have detected expression of POMC as well as PC1/3 in mesenchymal progenitors derived from mouse aorta (Evans et al. 2012) and immune cells produce α-MSH and ACTH (Harbour et al. 1987; Rajora et al. 1996; Star et al. 1995). Moreover recent data demonstrate β-LPH expression in many tissues as well as its localization in peroxisomes (Hoftberger, et al. 2010). However there is little data regarding the function of β-LPH beyond its capacity for lipid mobilization. The receptor mediating β-LPH actions is also not clearly defined, although the MC5-R is a potential candidate (Boston 1999). More studies are needed to explore the paracrine roles of these peptides in tissues outside of the CNS as well as to fully define their receptors and the pathways they mediate.
The present study demonstrates that under typical osteogenic culture conditions exposure to ACTH enhances the development of mineralizing chondrogenic nodules in rat BMSC cultures enriched for MSC. ACTH also initiates a MC2-R mediated pathway that induces transient increases in [Ca2+]i and elevates basal [Ca2+]i in these cells. Additionally, dexamethasone can regulate components of this pathway, namely expression of the MC2-R and its accessory protein, MRAP as well as cleavage of the POMC precursor to produce β-LPH. These data provide further evidence for an active local melanocortin system in bone and cartilage.
Acknowledgments
Acknowledgements/Grants
This work was supported by a grant from the NIH/NHLBI, K99HL091116-02
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
Contributor Information
Jodi F. Evans, Email: jevans@winthrop.org.
Sylvana Rodriguez, Email: srodriguez@molloy.edu.
Louis Ragolia, Email: lragolia@winthrop.org.
References
- Bicknell AB. The tissue-specific processing of pro-opiomelanocortin. J Neuroendocrinol. 2008;20:692–699. doi: 10.1111/j.1365-2826.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- Boston BA. The role of melanocortins in adipocyte function. Ann N Y Acad Sci. 1999;885:75–84. doi: 10.1111/j.1749-6632.1999.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Bruedigam C, Driel M, Koedam M, Peppel J, van der Eerden BC, Eijken M, van Leeuwen JP. Basic techniques in human mesenchymal stem cell cultures: differentiation into osteogenic and adipogenic lineages, genetic perturbations, and phenotypic analyses. Curr Protoc Stem Cell Biol. 2011;Chapter 1 doi: 10.1002/9780470151808.sc01h03s17. Unit1H 3. [DOI] [PubMed] [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- Chan LF, Metherell LA, Clark AJ. Effects of melanocortins on adrenal gland physiology. Eur J Pharmacol. 2011;660:171–180. doi: 10.1016/j.ejphar.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells. 2006;24:1487–1495. doi: 10.1634/stemcells.2005-0415. [DOI] [PubMed] [Google Scholar]
- Elmore SA. Enhanced histopathology of the bone marrow. Toxicol Pathol. 2006;34:666–686. doi: 10.1080/01926230600939971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JF, Fernando A, Ragolia L. Functional melanocortin-2 receptors are expressed by mouse aorta-derived mesenchymal progenitor cells. Mol Cell Endocrinol. 2012 doi: 10.1016/j.mce.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JF, Lee JH, Ragolia L. Ang-II-induced Ca(2+) influx is mediated by the 1/4/5 subgroup of the transient receptor potential proteins in cultured aortic smooth muscle cells from diabetic Goto-Kakizaki rats. Mol Cell Endocrinol. 2009;302:49–57. doi: 10.1016/j.mce.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Evans JF, Niu QT, Canas JA, Shen CL, Aloia JF, Yeh JK. ACTH enhances chondrogenesis in multipotential progenitor cells and matrix production in chondrocytes. Bone. 2004;35:96–107. doi: 10.1016/j.bone.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Evans JF, Shen CL, Pollack S, Aloia JF, Yeh JK. Adrenocorticotropin evokes transient elevations in intracellular free calcium ([Ca2+]i) and increases basal [Ca2+]i in resting chondrocytes through a phospholipase C-dependent mechanism. Endocrinology. 2005;146:3123–3132. doi: 10.1210/en.2004-1612. [DOI] [PubMed] [Google Scholar]
- Gallo-Payet N, Payet MD. Mechanism of action of ACTH: beyond cAMP. Microsc Res Tech. 2003;61:275–287. doi: 10.1002/jemt.10337. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Allcock GH, Flower R, Perretti M. Natural and synthetic agonists of the melanocortin receptor type 3 possess anti-inflammatory properties. J Leukoc Biol. 2001;69:98–104. [PubMed] [Google Scholar]
- Getting SJ, Riffo-Vasquez Y, Pitchford S, Kaneva M, Grieco P, Page CP, Perretti M, Spina D. A role for MC3R in modulating lung inflammation. Pulm Pharmacol Ther. 2008;21:866–873. doi: 10.1016/j.pupt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Laha M, Mondal S, Sengupta S, Kaplan DL. In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials. 2009;30:6530–6540. doi: 10.1016/j.biomaterials.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabe Bucay A. The role of lipotropins as hematopoietic factors and their potential therapeutic use. Exp Hematol. 2008;36:752–754. doi: 10.1016/j.exphem.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Harbour DV, Smith EM, Blalock JE. Novel processing pathway for proopiomelanocortin in lymphocytes: endotoxin induction of a new prohormone-cleaving enzyme. J Neurosci Res. 1987;18:95–101. doi: 10.1002/jnr.490180116. [DOI] [PubMed] [Google Scholar]
- Hoftberger R, Kunze M, Voigtlander T, Unterberger U, Regelsberger G, Bauer J, Aboul-Enein F, Garzuly F, Forss-Petter S, Bernheimer H, et al. Peroxisomal localization of the proopiomelanocortin-derived peptides beta-lipotropin and beta-endorphin. Endocrinology. 2010;151:4801–4810. doi: 10.1210/en.2010-0249. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, McGurk S, Smit NP, Nibbering PH, Ancans J, van der Laarse A, Thody AJ. Ligand-dependent activation of the melanocortin 5 receptor: cAMP production and ryanodine receptor-dependent elevations of [Ca(2+)](I) Biochem Biophys Res Commun. 2002;290:844–850. doi: 10.1006/bbrc.2001.6283. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kamiya N, Hasegawa M, Kasuya T, Takahashi T, Takagi M. Inductive effects of dexamethasone on the gene expression of Cbfa1, Osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004;35:3–10. doi: 10.1023/b:hijo.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann N Y Acad Sci. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- Kang L, McIntyre KW, Gillooly KM, Yang Y, Haycock J, Roberts S, Khanna A, Herpin TF, Yu G, Wu X, et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukoc Biol. 2006;80:897–904. doi: 10.1189/jlb.1204748. [DOI] [PubMed] [Google Scholar]
- Le Pape E, Wakamatsu K, Ito S, Wolber R, Hearing VJ. Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigment Cell Melanoma Res. 2008;21:477–486. doi: 10.1111/j.1755-148X.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami Y, Lee M, Irie S, Honda MJ. Dexamethasone modulates osteogenesis and adipogenesis with regulation of osterix expression in rat calvaria-derived cells. J Cell Physiol. 2011;226:739–748. doi: 10.1002/jcp.22392. [DOI] [PubMed] [Google Scholar]
- Minashima T, Small W, Moss SE, Kirsch T. Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes. J Biol Chem. doi: 10.1074/jbc.M111.297861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minashima T, Small W, Moss SE, Kirsch T. Intracellular modulation of signaling pathways by annexin A6 regulates terminal differentiation of chondrocytes. J Biol Chem. 2012 doi: 10.1074/jbc.M111.297861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa NZ, Fitzsimmons R, Major PW, Adesida A, Jomha N, Jiang H, Uludag H. Osteogenic Differentiation of Human Mesenchymal Stem Cells Cultured with Dexamethasone, Vitamin D3, Basic Fibroblast Growth Factor, and Bone Morphogenetic Protein-2. Connect Tissue Res. 2011 doi: 10.3109/03008207.2011.611601. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Jenny Wu CS, Dumont LM, Wild JM. Melanocortin-4 receptor messenger ribonucleic acid expression in rat cardiorespiratory, musculoskeletal, and integumentary systems. Endocrinology. 2003;144:5488–5496. doi: 10.1210/en.2003-0570. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Noon LA, Bakmanidis A, Clark AJ, O'Shaughnessy PJ, King PJ. Identification of a novel melanocortin 2 receptor splice variant in murine adipocytes: implications for post-transcriptional control of expression during adipogenesis. J Mol Endocrinol. 2006;37:415–420. doi: 10.1677/jme.1.02023. [DOI] [PubMed] [Google Scholar]
- Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–271. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- Pei W, Yoshimine Y, Heersche JN. Identification of dexamethasone-dependent osteoprogenitors in cell populations derived from adult human female bone. Calcif Tissue Int. 2003;72:124–134. doi: 10.1007/s00223-001-2052-4. [DOI] [PubMed] [Google Scholar]
- Rajora N, Ceriani G, Catania A, Star RA, Murphy MT, Lipton JM. alpha-MSH production, receptors, and influence on neopterin in a human monocyte/macrophage cell line. J Leukoc Biol. 1996;59:248–253. doi: 10.1002/jlb.59.2.248. [DOI] [PubMed] [Google Scholar]
- Sebag JA, Hinkle PM. Melanocortin-2 receptor accessory protein MRAP forms antiparallel homodimers. Proc Natl Acad Sci U S A. 2007;104:20244–20249. doi: 10.1073/pnas.0708916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MK, Kim MK, Bae YS, Jo I, Lee SJ, Chung CP, Park YJ, Min do S. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell Signal. 2008;20:613–624. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Siljee-Wong JE. Melanocortin MC receptor expression sites and local function. Eur J Pharmacol. 2011;660:234–240. doi: 10.1016/j.ejphar.2010.10.104. [DOI] [PubMed] [Google Scholar]
- Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 1995;92:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Liu Y, Lipsky S, Cho M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007;21:1472–1480. doi: 10.1096/fj.06-7153com. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or-4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M, Reinhold MI, Molkentin JD, Naski MC. Calcineurin and NFAT4 induce chondrogenesis. J Biol Chem. 2002;277:42214–42218. doi: 10.1074/jbc.C200504200. [DOI] [PubMed] [Google Scholar]
- van der Kraan M, Adan RA, Entwistle ML, Gispen WH, Burbach JP, Tatro JB. Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology. 1998;139:2348–2355. doi: 10.1210/endo.139.5.6008. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhong S, Ouyang J, Jiang L, Zhang Z, Xie Y, Luo S. Osteogenesis of electrically stimulated bone cells mediated in part by calcium ions. Clin Orthop Relat Res. 1998:259–268. [PubMed] [Google Scholar]
- Webb TR, Clark AJ. Minireview: the melanocortin 2 receptor accessory proteins. Mol Endocrinol. 2010;24:475–484. doi: 10.1210/me.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. Structure, function and regulation of the melanocortin receptors. Eur J Pharmacol. 2011;660:125–130. doi: 10.1016/j.ejphar.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JK, Evans JF, Chen MM, Aloia JF. Effect of hypophysectomy on the proliferation and differentiation of rat bone marrow stromal cells. Am J Physiol. 1999;276:E34–E42. doi: 10.1152/ajpendo.1999.276.1.E34. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 2010;107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li WH, Anthonavage M, Pappas A, Rossetti D, Cavender D, Seiberg M, Eisinger M. Melanocortin-5 receptor and sebogenesis. Eur J Pharmacol. 2011;660:202–206. doi: 10.1016/j.ejphar.2010.10.100. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Sridhar S, Ruan L, Ding KH, Xie D, Insogna K, Kang B, Xu J, Bollag RJ, Isales CM. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36:820–831. doi: 10.1016/j.bone.2005.01.020. [DOI] [PubMed] [Google Scholar]