Abstract
Background:
Hip arthroscopy is rapidly becoming the mainstay of treatment for femoroacetabular impingement (FAI), but remains technically demanding and has its limitations. The failures of arthroscopic FAI surgery due to inaccurate and inadequate resection are reported to be increasing. Computer-assisted surgery (CAS) can theoretically improve the accuracy and precision of the osseous resections required to treat FAI. It does so by providing a preoperative assessment tool, an intraoperative tracking device, and a robotic-assisted cutting instrument.
Questions/Purposes:
The purpose of this review is to discuss the evolution of CAS to address the current limitations of arthroscopic FAI surgery and propose the features required of the ideal CAS solution for FAI.
Methods:
A computerized keyword search of MEDLINE was performed for studies that investigated the use of computer assistance in FAI surgery. Data was collected on preoperative assessment tools, intraoperative navigation programs, and robotic-assisted execution of FAI surgery.
Results:
Sixty-one articles were identified after the keyword search. Nineteen studies met our inclusion criteria. Thirteen studies were selected to address our study questions: three studies were analyzed for preoperative planning, six for navigated osseous resection, and four for robotic-assisted execution.
Conclusion:
Navigation and robotic-assisted surgery can preoperatively plan and execute osseous resection with greater accuracy compared to freehand techniques, although the clinical success and cost-effectiveness has yet to be demonstrated. The ideal CAS solution must be able to virtually plan a resection, guide the surgeon towards accurate execution of the plan, and facilitate post-resection assessment of the adequacy of resection.
Keywords: femoroacetabular impingement, computer-assisted surgery, non-arthritic hip pain, osteochondroplasty, osseous resection
Introduction
There is little doubt that computer-assisted surgery (CAS) produces more accurate and precise results and reduces the learning curve in lower limb arthroplasty, trauma, and spine surgery [13, 33]. Efforts are now being made to incorporate computer-assisted solutions in the treatment of femoroacetabular impingement (FAI).
Clinical studies have shown that resection of the osseous abnormalities that causes FAI improves function and relieves pain, regardless of whether it is done by arthroscopic or open techniques [3, 5, 6, 19, 32, 33]. With the inherent advantages of a minimally invasive approach and a recent systematic review reporting 67–100% of patients with good to excellent outcomes from arthroscopic FAI surgery [4], it is not surprising that this form of treatment has been widely adopted in the last decade. It must be stressed however that favorable outcomes require strict attention to a thorough and accurate resection of the offending bony lesion. The most common reason for failure after arthroscopic FAI surgery is inaccurate resection [14, 25]. Despite the improvement in imaging techniques, hip arthroscopy instrumentation, and skill and experience of hip arthroscopists, the challenge of diagnosing, visualizing, and eradicating impingement lesions reliably remains a problem. The disease is often subtle, the surgery is technically demanding, and this combined with the increase in failures has in part fueled the drive towards computer-assisted solutions.
In this review, we will first outline the current limitations of arthroscopic FAI surgery. We will then discuss the evolution of CAS to address these limitations. Our final aim is to propose the features required of the ideal CAS solution for arthroscopic FAI surgery.
Current Limitations of Arthroscopic FAI Surgery
The majority of the current limitations of arthroscopic FAI surgery fall into two broad categories: preoperative planning and intraoperative execution.
In its present form, preoperative planning is based on a static anatomical model. Preoperative assessment tools provide the surgeon with a patient-specific reconstruction of the osseous anatomy. These include imaging modalities such as radiographs, computed tomography (CT) scans, and magnetic resonance imaging (MRI) scans. Imaging modalities produce a static, anatomic preoperative analysis that not only characterizes “cam” and “pincer” lesions but also other structural anomalies that can be responsible for mechanical hip pain and impingement. A recent CT-based study looking at patients with symptomatic labral tears found that 90% of patients had structural abnormalities, which included femoral retroversion or excessive anteversion, coxa valga, and acetabular dysplasia, including lateral and/or anterior undercoverage [11]. It is essential to recognize these abnormalities when planning surgery for a patient with FAI.
Although an anatomic preoperative plan provides critical information, it still fails to identify the three-dimensional (3D) bony abnormalities of the hip. Historically, an anatomic plan relies on the alpha angle [23]. This angle is drawn on MRI cuts parallel to the axis of the neck and passing through the center of the head and is defined by the axis of the femoral neck and a line connecting the center of the femoral head to the anterior extent of the concavity of the femoral neck. An angle of less than 50° is defined as normal. One goal for surgical correction has been proposed by Stahelin et al. who have recommended that an alpha angle less than 50°, or a reduction of the alpha angle by 20° (in cases of very large alpha angles), will result in satisfactory restoration of femoral head–neck offset [30]. This goal highlights many of the limitations of the alpha angle. It does not take into account the length of the cam lesion. If the “bump” is long, the resection may have to be advanced into the trochanteric fossa. Furthermore, the maximal loss of head–neck offset is present at different locations in different patients, and thus, a single value is limited in the information it provides [24]. The other issue that has been debated is what truly is a pathological value of the alpha angle? Clohisy et al. evaluated the alpha angle in patients with FAI and asymptomatic controls and found a comparable range of normal and abnormal alpha angles in the two groups [9]. They could not define an alpha angle threshold beyond which a pathological diagnosis can be considered. It has also been shown that the alpha angle, in itself, does not reliably correlate with the clinical range of motion with one study reporting that patients with insufficient offset correction showed a slightly better internal rotation than patients with satisfactory offset restoration [7].
Intraoperative execution of a preoperative plan requires a high level of arthroscopic skills, good visualization, the identification of the margins of the osseous impingement lesion, and deciding on the adequacy of resection. The steep learning curve associated with arthroscopic FAI surgery is well documented [16]. Potential technical errors are present in almost every part of the procedure from positioning, cannulation, visualization, and osseous resection. With the increasing popularity of the procedure, it is likely that more failures due to poor visualization and hence technical error will occur. The hip arthroscopist at present combines fluoroscopy with arthroscopy to perform an intraoperative assessment of an adequate resection. The problem with this approach is that 2D modalities are being utilized to define a 3D morphology. Even in the hands of experienced hip arthroscopists, who have achieved adequate exposure, the margins of the lesion are not always obvious. With cam lesions that extend posteriorly or distally, it is not uncommon for the inexperienced arthroscopist to abort the osseous resection once an adequate image is obtained on fluoroscopy without appreciating that further internal rotation or an accessory portal may show an inadequate resection. Osseous abnormalities are commonly under-resected, and this is a common cause for revision surgery, accounting for up to 78% to 90% of all failed arthroscopic hip surgery [14, 25]. On the other hand, over-resection beyond the margins of a cam lesion can compromise the cortical bone support of the femoral neck leading to fracture [19]. Over-resection of a pincer lesion can result in acetabular undercoverage and iatrogenic dysplasia. Postoperative instability and dislocation have also been reported after hip arthroscopy and are likely linked to over-resection [20, 28].
Methods
In order to discuss the evolution of CAS to address the limitations of arthroscopic FAI surgery, a computerized keyword search of MEDLINE was performed to identify studies that investigated the use of computer assistance in FAI surgery. The keywords used in the search were (hip arthroscopy) AND (femoroacetabular impingement) AND (computer) AND (navigation) AND (robotic). The search generated 61 articles, of which 19 articles represented experiments investigating the use of CAS in FAI surgery. For the purposes of formulating a focused review, we selected 13 articles to address our study questions: three articles were analyzed for preoperative assessment tools, six for intraoperative navigated osseous resection, and four for robotic-assisted execution. These articles are summarized in Table 1.
Table 1.
Studies investigating the use of CAS in FAI surgery
| Product | Purpose | Technology/imaging modality | Function |
|---|---|---|---|
| Preoperative assessment tools | |||
| Tannast et al. (HipMotion) [32] | FAI | BrainLAB | Localize impingement zone |
| CT-based 3D | Collision detection algorithm | ||
| Kinematics | Predict improvement in ROM after virtual resection | ||
| Puls et al. [26] | FAI | Laser/CT 3D model | Localize impingement zone |
| MARVIN navigation | Collision detection algorithm | ||
| Equidistant method | Predict improvement in ROM after virtual resection | ||
| Tannast et al. (HipMotion) [17] | FAI | BrainLAB | Localize impingement zone |
| CT-based 3D | Collision detection algorithm | ||
| Kinematics | Predict improvement in ROM after virtual resection | ||
| Intraoperative navigation programs | |||
| Monahan et al. [21, 22] | FAI | Encoder-Linkage and 3D CT/MRI | Encoder: captures tool motion |
| Surgical instrument tracking | |||
| Incorporates soft tissue and bony anatomy | |||
| Brunner et al. [7] | FAI | BrainLAB and fluoroscopy | Surgical instrument tracking |
| No pre-op planning, delineation of impingement, or display of amount of resected bone during surgery | |||
| Almoussa et al. [2] | FAI | BrainLAB | Surgical instrument tracking |
| 3D CT | Pre-op plan available | ||
| Intra-op resection monitoring | |||
| Ecker et al. (HipMotion) [12] | FAI | 3D CT/MRI | Surgical instrument tracking |
| MARVIN navigation | Pre-op plan available | ||
| Intra-op resection monitoring | |||
| Comparison of post-op resection to original plan | |||
| Rudolph et al. [29] | FAI | MARVIN navigation | Surgical instrument tracking |
| Intra-op resection monitoring | |||
| Robotic-assisted execution | |||
| “da Vinci” [2] | Urological | Tele-robotic platform | Remote control of robotic arms |
| gynecological procedures | 3D view of operative site via stereostatic cameras | ||
| Kather et al. [15] | FAI | Tele-robotic platform | Remote control of robotic arms |
| 3D view of operative site via stereostatic cameras | |||
| “Brigit” bone resection instrument guide | TKA | Positioning arm | Applies pre-op defined cutting limits to intra-op multi-slot cut guide placement for accurate tool positioning |
| Acrobot [10] (“Haptics”) | TKA | Haptically-guided arm 3D CT | Uses pre-op plan to control surgeon's movement and sense of touch via forces and vibrations |
| MAKO [27] tactile guidance system | TKA/THA | Haptically-guided arm | Haptically guided bone milling |
| 3D CT | |||
Results
Preoperative Assessment Tools
As previously discussed, preoperative planning using anatomic parameters, such as the alpha angle, has its limitations. In the future, anatomic plans may be rejected in favor of pure kinematic plans or collision detection algorithms which plan virtual bony resections to eliminate mechanical impingement and improve motion, especially flexion combined with internal rotation. Although these tools have been shown to be helpful in predicting postoperative range of motion (ROM) and have been validated, they also raise as many questions as they answer. Specifically, what parameter does one use to define adequacy of osseous resection?
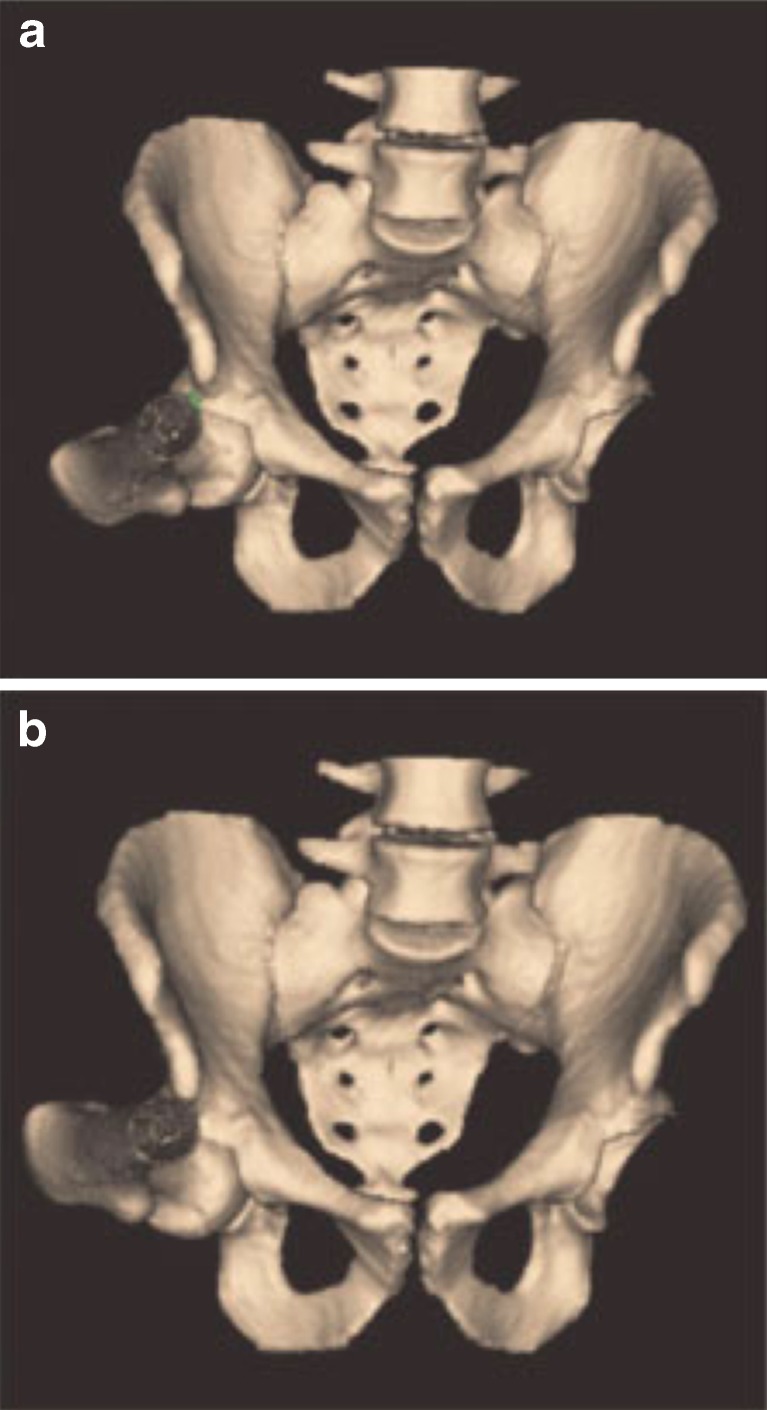
Tannast et al. designed the first comprehensive preoperative assessment tools in 2005, utilizing “HipMotion” software (Bern, Switzerland) to perform a CT-based 3D kinematics analysis of the hip joint [32]. This software uses a kinematic plan to define zones of impingement and then predict improvement in ROM after a virtual resection. It therefore addresses the need for an accurate kinematic preoperative plan but also gives enhanced visual guidance to the surgeon in executing the plan precisely. The software reconstructs a 3D model of the pelvis and femur, which is digitized and orientated to the anterior pelvic plane (APP). After localization of the hip center, the native preoperative ROM is calculated using collision algorithms which determine ROM based on points at which contact (i.e., impingement) occurs (Fig. 1). Hence, a zone of impingement is identified. A virtual surgical acetabular and femoral resection is then performed to delay impingement until later in the motion cycle. Virtual postoperative ROM is simulated by reconstructing the hip joint using the new parameters to assess the efficacy of the planned procedure (Fig. 2a, b). This program was validated by the authors by integrating it with the imageless BrainLAB (Feldkirchen, Germany) software and comparing virtual with real ROM. Perhaps the most encouraging aspect of HipMotion is that it calculates the volume of resection based on an impingement-free postoperative ROM rather than a desirable postoperative alpha angle. The limitations of the program is that it assumes the hip joint has a perfect center of rotation, thereby not accounting for the translation which occurs with weight-bearing, hip motion, and muscular activation. This issue is now being debated, and more accurate models are being proposed [26].
Fig. 1.
Image from HipMotion software of the pelvis and both hips. The acetabular and femoral location of impingement is identified for the right hip. Reprinted with permission from Tannast et al. [32] copyright 2007, with permission from John Wiley and Sons.
Fig. 2.
HipMotion 3D ROM analysis showing the beneficial effect of a virtual femoral osteochondroplasty. A native internal rotation of 11° in 90° of flexion (a) is increased to 37° (b) after the virtual offset creation. Reprinted with permission from Tannast et al. [32] copyright 2007, with permission from John Wiley and Sons.
HipMotion was also used in a clinical pilot study by the same authors to compare the ROM in 28 hips with anterior FAI to a control group of 33 normal hips [17]. The hips with FAI had decreased flexion, internal rotation, abduction, and internal rotation in 90° of flexion. The zones of impingement were found anterosuperiorly and were similar in the two groups. The virtual postoperative ROM improved in all subgroups of FAI. In summary, there are various noninvasive preoperative software programs available which help the surgeon localize the zone of impingement, quantify the volume of resection, and predict postoperative ROM using both anatomic and kinematic data.
Intraoperative Navigation Programs
Navigation programs guide the surgeon intraoperatively to precisely reproduce preoperative plans. Navigation can be image-based, imageless, or fluoroscopically guided. Image-based navigation obtains registration of anatomical landmarks with the use of osseous pins. With the pelvis, for example, pins are inserted into the anterior superior iliac spines and pubic tubercles. These pins allow the digitization of the pelvis in virtual space, align it to the APP, and match it to preoperative 3D MR or CT data. Imageless navigation achieves registration by the use of optical infrared trackers mounted on the pelvis, coupled with a calibrated optical pointer to register the anterior superior iliac spine and pubic tubercle. Fluoroscopically guided navigation uses a calibrated tracker on a specially designed C-arm which takes a series of images in multiple planes to establish registration. The intraoperative images are then matched with preoperative data. Due to the complex 3D osseous morphology in FAI, most navigation programs use 3D CT-based technology with either direct bony registration or 3D to 2D registration with specialized fluoroscopy. Once the patient's anatomy and spatial position has been registered by the computer, the instruments are registered, allowing the visualization and real-time tracking of the surgical instrumentation in relation to the virtual representation of the patient as defined by the preoperative imaging.
One of the first groups to intraoperatively track instruments during hip arthroscopy was from Pittsburgh (USA), who developed an encoder linkage system to track surgical instruments [21]. This eliminates the problem of occlusion with standard optical tracking systems. An encoder is a device which captures tool motion and orientation. The setup consists of a chain of rotational encoders connecting a surgical instrument to a reference point on the patient's pelvis. A similar chain is attached between the arthroscope and the pelvis. The encoder linkages are calibrated with preoperative, patient-specific 3D CT or MR imaging data so the position of the surgical tools can be verified with respect to patient anatomy. This system is unique in that it incorporates soft tissue as well as bony anatomy and therefore also serves as a useful aid for safe portal placement. The software therefore can warn a surgeon when a surgical instrument has moved too close to a neurovascular structure for example. This system has been tested by performing a user study where ten participants completed a simple navigation task with and without the aid of the system. The computer-aided system resulted in a 38% reduction in operative time and 78% reduction in tool path length [22].
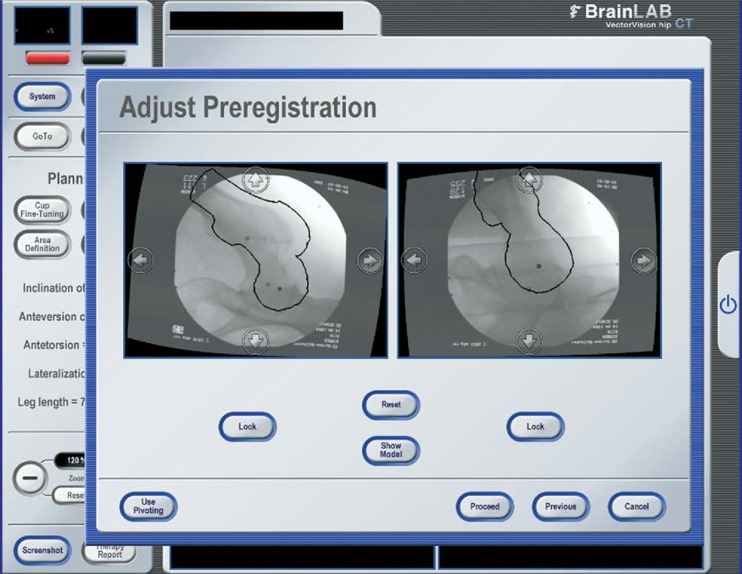
Brunner et al. performed a prospective study looking at the clinical outcomes and head–neck offset correction in patients with cam impingement in both navigated and non-navigated groups [7]. Fifty patients were randomized to receive navigated or “freehand” arthroscopic cam decompression. A 3D CT-based navigation system was used to upload a preoperative CT scan of the pelvis and crossmatch this with intraoperative fluoroscopy (Fig. 3). This system gives the surgeon real-time information about the position of the surgical instruments in relation to the femoral neck. The study found no significant difference in femoral offset correction with 24% of subjects in both the navigated and non-navigated groups having an inadequate reduction of the alpha angle. Both groups showed significant improvements in ROM and non-arthritic hip scores, but with no demonstrable difference between the groups. This study once again illustrates the limitations of using the alpha angle as an outcome measure and emphasizes the importance of measuring clinical outcomes.
Fig. 3.
Fluoroscopically-guided navigation using ‘cross-matching’ of online fluoroscopy and preoperative computed tomography data. Reprinted from Journal of Arthroscopy, 25(4), Brunner A, Horisberger M, Herzog RF, Evaluation of a Computed Tomography-Based Navigation System Prototype for Hip Arthroscopy in the Treatment of Femoroacetabular Cam Impingement, 382-391, 2009 with permission from Elsevier.
The third group to investigate intraoperative tracking is an Ottawa-based group that used an improved version of the software of Brunner et al. [2]. They tracked bony resection for cam impingement and assessed the adequacy of resection when comparing surgeons of varying experience which included a surgeon specializing in surgical correction of FAI deformities, a surgeon not specializing in surgical correction of FAI deformities, and a fellow. A preoperative plan was generated for all cases from CT scans and the BrainLAB navigation system (Feldkirchen, Germany). An alpha angle of 45° was selected as an indicator of adequate resection. Real-time tracking could be performed by the surgeon using a pointer with marker arrays to ensure resection was performed according to the preoperative plan. Postoperative CT scans were performed to assess the resections. Similar post-resection alpha angles were observed between all three surgeons. This nicely demonstrated how CAS could minimize the learning curve in FAI surgery and permit the less-experienced surgeon to perform bony resections equivalent to an experienced surgeon.
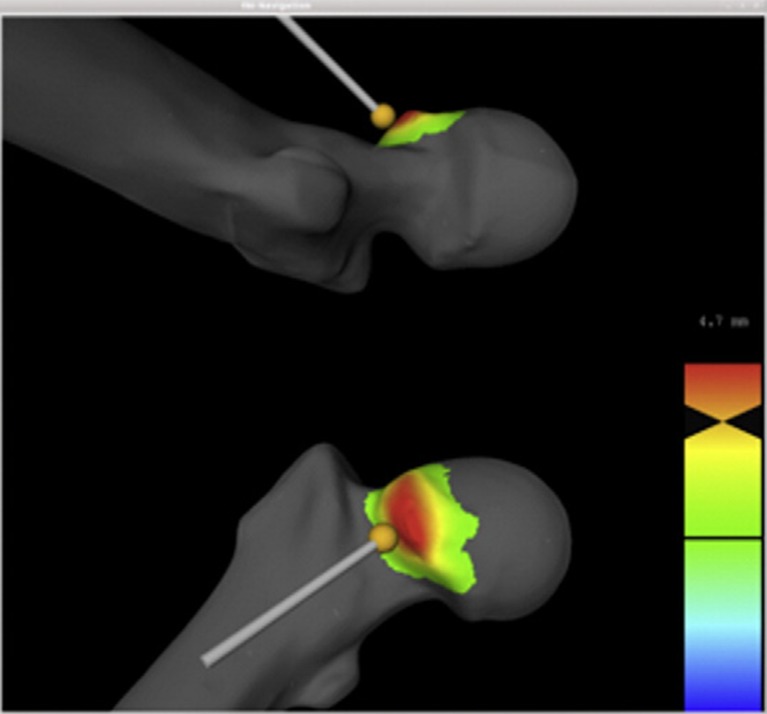
Most recently, Tannast's group have published the follow-up paper to their previous paper on ROM analysis in FAI [12]. Their current software combines their previous collision detection and ROM analysis technology [32] with a color-coded intraoperative map to guide resection in real time. The navigation application is based on the MARVIN application framework [29] where a 3D model of the patient's pelvis is derived from MR or CT. Preoperative ROM analysis is performed to define a zone of impingement. This is followed by a virtual cam decompression according to the preoperative plan to ensure an improved postoperative ROM without impingement. A superimposed translucent sphere prevents excessive resection, ensures sphericity, and depicts pre- and postoperative femoral morphology. The pre-planned resection area is highlighted on the screen as color-coded distance map with a red color indicating the pre-reamed state and a change to green when the reamer is within 1 mm of the resection goal (Fig. 4).
Fig. 4.
The volume of resection is highlighted as a color-coded distance map according to the preoperative collision detection and ROM analysis. The burr is tracked in real time and color changes on the map indicate the proximity of the depth of resection to the pre-planned goal. Reprinted from The Journal of Arthroplasty, 27(2), Ecker TM, Puls M, Steppacher SD, Bastian JD, Keel MJB, Siebenrock KA, Tannast M, Computer Assisted Femoral Head-Neck Osteochondroplasty Using a Surgical Milling Device, 122-131, 2012, with permission from Elvesier.
The feasibility and accuracy of this navigation device was tested using 3D models of 18 identical sawbone femurs. Postoperative models were created to compare with preoperative plans. Two surgeons performed three different osteochondroplasties on three occasions and demonstrated excellent intraobserver and interobserver agreement with the mean distance between planned and actual reamed surface at the femoral neck of 0.41 mm. The discrepancy between planned and actual reaming was consistently less than 1 mm in all 18 sawbone operations. These results show beyond doubt the accuracy of this planning and tracking system. It seems to address the limitations of all the previous applications presented. The next test is going to be its applicability to an actual intraoperative arthroscopic setting.
Robotic-Assisted Execution
Robotic-assisted surgery is the most recent development in CAS and translates the quantitative assessment produced by navigation into an automated mechanical action. It moves one step beyond preoperative planning and navigation with even greater accuracy [28]. Surgical instruments are mounted on a robotic arm, which may partially or completely automate the entire surgical procedure, based on a preoperative plan. Robotic surgery provides a greater level of dexterity and precision, and even allows for unmanned or remote surgery [1].
The most widely used robotic surgical system in use today is the “da Vinci” tele-robotic platform. It was licensed in 2001 for urological procedures and in 2005 for gynecological procedures by the US Food and Drug Administration. This system allows the surgeon to sit remotely at a console and control the movements of several robotic arms while viewing the operative site in three dimensions using stereotactic cameras. Currently, it is being used in procedures such as hysterectomies, prostatectomies, and gastric bypass.
There has been a preliminary attempt to apply the “da Vinci” surgical system to hip arthroscopy. By only using the instrumentation available to them, Kather et al. attempted to perform a hip arthroscopy on two fresh frozen cadavers [15]. They were able to resect the acetabular labrum with a hook knife and scissors. However, they had difficulty accessing the posterior or posteroinferior labrum, and the medial and posteromedial femoral head. This would currently limit the “da Vinci” system's applicability to FAI surgery, although it must be noted that the instrumentation being used was not specialized for the demands of arthroscopic surgery. This study shows that remote robotic hip arthroscopy is in its infancy but with time and appropriate instrumentation, robotic technology has the potential to allow orthopedic surgeons to perform complex procedures in very restricted spaces from a remote location.
There are already a number of robotic surgery systems in use in orthopedic surgery, especially in total hip and knee arthroplasty. The “Brigit” Bone Resection Instrument Guide applies preoperatively defined cutting limits to an intraoperative rigid multi-slot guide to assist the surgeon in accurate tool positioning. An advancement of this principle is the use of “Haptic” technology. “Haptics” is a tactile feedback technology that utilizes a preoperative plan to control the operator's movement and sense of touch by applying forces and vibrations. A haptically guided semi-active robot can therefore add virtual safety barriers based on patient-specific templates or preoperative plans to control the movements of the surgeon and his/her instruments. Haptically guided robotic technology has already demonstrated success in orthopedics. The group of Cobb et al. in London, UK, has used the Acrobot haptic-guided unicondylar knee replacement system (Acrobot, London, UK) to improve implant-positioning precision [10]. In a prospective randomized controlled trial of 28 knees, they found that the tibiofemoral alignment of all the robotically assisted knees was within 2° of the planned position, whereas only 40% of the conventional group achieved this accuracy.
A system similar to the Acrobot has been developed in the USA: The Tactile Guidance System (MAKO Surgical, Fort Lauderdale, FL, USA). This system is currently being used to perform partial knee replacement and total hip arthroplasty. The senior author of this review has conducted a study on robotic-assisted femoral osteochondroplasty for FAI [27]. Sixteen identical sawbone models with a cam deformity were treated by a single surgeon simulating an open FAI procedure. Eight of the procedures were performed using a freehand technique, and eight were performed using robotic assistance with the MAKO system (Fig. 5). For the models that used robotic assistance, a 3D haptic volume was defined by the desired postoperative morphology. After resection, all the sawbones were scanned, and post-resection measurements of the arc of resection, volume of bone removed, and resection depth were obtained and compared to the preoperative plan. The desired arc of resection was 117.7° starting at −1.8° and ending at 115.9°. The models resected using a freehand technique produced an average arc resection error of 42.0 ± 8.5°. Those that were resected using robotic assistance produced an average arc resection error of 1.2 ± 0.7°, which was significantly lower than the freehand group (p < 0.0001). The average cutting time for a robotic-assisted resection was 210 s, which was significantly less than 303 s seen in the freehand group (p < 0.001). This study shows that robotic assistance is significantly more accurate and precise than freehand techniques. The precision and accuracy that CAS offers over freehand surgery has also been proven in other well-constructed experimental models [8].
Fig. 5.

Photograph of the MAKO robotic arm being used to perform femoral osteochondroplasty in sawbone models.
Discussion
Arthroscopic FAI surgery remains challenging and continues to become more complex as our understanding of the mechanical sources of non-arthritic hip pain increases. A multitude of deformities can accompany a cam or pincer lesion and contribute to the stresses placed on the native hip joint. Despite the development of better instrumentation and increased surgical experience, inaccurate and inadequate resection persists as a problem. The majority of revision hip arthroscopies are currently being performed for inadequate resection, and unless the quality of the surgery improves, clinical results are likely to decline. CAS is an attractive proposition, as it not only improves our ability to diagnose FAI but it also helps to plan and execute osseous resection with greater accuracy. It is perhaps ambitious to believe that the anatomical and surgical complexity of FAI pathology can be treated with intraoperative surgical judgment alone.
The future of CAS in arthroscopic FAI surgery will depend on its ability to address all the current limitations of freehand surgery. The latest study of Tannast et al. is perhaps the best evidence to the significant advances that have been made in this field in a short space of time, as it integrates a kinematic preoperative plan with intraoperative tracking and a virtual postoperative assessment. The combination of these features with robotic assistance is awaited.
There are, of course, limitations to CAS as well. The prototypes discussed in this review have shown encouraging results in vitro, but clinical success and commercial viability is yet to be demonstrated. CAS has its own learning curve, and increased surgical times with navigation and robotics in surgery have long been a concern. Image-based navigation may require CT scans, and this contributes to extra radiation exposure for the patient. Finally, the accuracy of the computer-assisted process depends on the accuracy of the anatomical registration, and therefore, mistakes made at an early stage of the operation will be compounded as it proceeds.
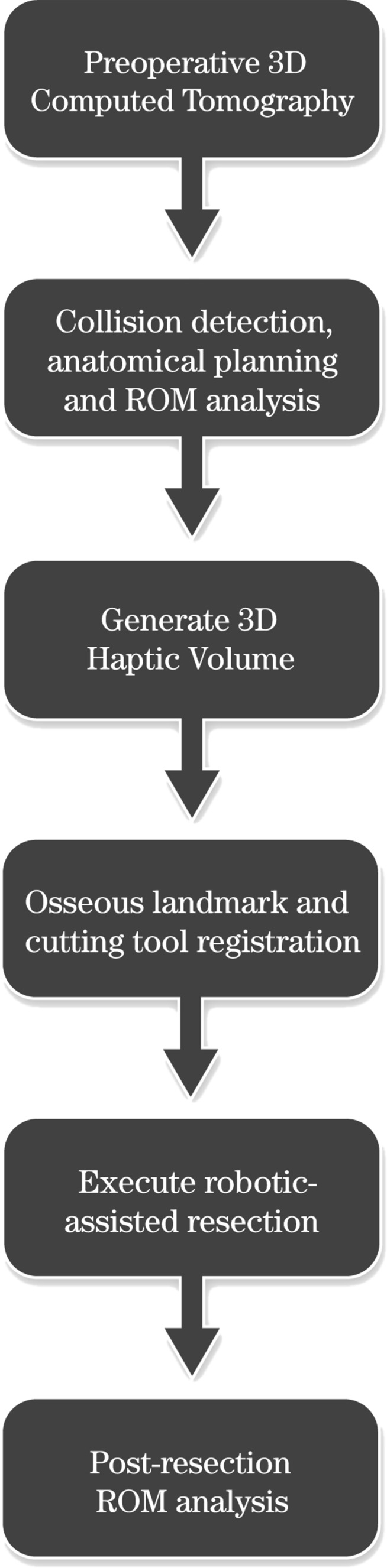
In conclusion, the ideal CAS solution for arthroscopic FAI surgery needs to define the zone of impingement preoperatively, plan the bony resection based on a virtual impingement-free ROM, track arthroscope and instrument movement intraoperatively, guide the surgeon towards accurate resection with haptic barriers, and facilitate dynamic intraoperative assessment of ROM to ensure adequacy and precision of resection (Fig. 6). CAS must also reduce technical difficulty, decrease operative time, minimize costs, demonstrate safety, and eventually improve patient outcomes. Although the ideal CAS solution is currently not available, the latest research suggests that it may be attainable.
Fig. 6.
Surgical strategy flow diagram.
Conflict-of-Interest:
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, and patent/licensing arrangements) that might pose a conflict of interest in connection with the submitted article. One or more of the authors have or may receive payments or benefits from a commercial entity that may be perceived as a potential conflict of interest.
Human/Animal Rights: N/A.
Informed Consent: N/A.
References
- 1.Advincula AP. Surgical Techniques: Robot-Assisted Laparoscopic Hysterectomy with the da Vinci Surgical System. International Journal of Medical Robotics and Computer Assisted Surgery. 2006;2:305–311. doi: 10.1002/rcs.111. [DOI] [PubMed] [Google Scholar]
- 2.Almoussa S, Barton C, Speirs AD, Gofton W, Beaule PE. Computer-assisted correction of cam-type femoracetabular impingement. A sawbones study. J Bone Jt Surg Am. 2011;93(Suppl 2):70–75. doi: 10.2106/JBJS.J.01706. [DOI] [PubMed] [Google Scholar]
- 3.Beaule PE, Le Duff MJ, Zaragoza E. Quality of life following femoral head-neck osteochondroplasty for femoroacetabular impingement. J Bone Joint Surg Am. 2007;89:773–9. doi: 10.2106/JBJS.F.00681. [DOI] [PubMed] [Google Scholar]
- 4.Bedi A, Chen N, Robertson W, Kelly BT. The management of labral tears and femoroacetabular impingement of the hip in the young, active patient. Arthroscopy. 2008;24:1135–45. doi: 10.1016/j.arthro.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(Suppl):20–28. doi: 10.1177/0363546511412734. [DOI] [PubMed] [Google Scholar]
- 6.Botser IB, Smith TW, Jr, Nasser R, Domb BG. Open Surgical Dislocation Versus Arthroscopy for Femoroacetabular Impingement: A Comparison of Clinical Outcomes. Arthroscopy. 2011;27(2):270–278. doi: 10.1016/j.arthro.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Brunner A, Horisberger M, Herzog RF. Evaluation of a Computed Tomography-Based Navigation System Prototype for Hip Arthroscopy in the Treatment of Femoroacetabular Cam Impingement. Journal of Arthroscopy. 2009;25(4):382–391. doi: 10.1016/j.arthro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Cartiaux O, Paul L, Docquier P, Raucent B, Dombre E, Banse X. Computer-Assisted and Robot-Assisted Technologies to Improve Bone-Cutting Accuracy when Integrated with a Freehand Process Using an Oscillating Saw. J Bone Joint Surg Am. 2010;92:2076–82. doi: 10.2106/JBJS.I.00457. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy JC, Nunley RM, Otto RJ, Schoenecker PL. The frog-leg lateral radiograph accurately visualized hip cam impingement abnormalities. Clin Orthop Relat Res. 2007;462:115–121. doi: 10.1097/BLO.0b013e3180f60b53. [DOI] [PubMed] [Google Scholar]
- 10.Cobb J, Henckel J, Gomes P, et al. Handson robotic unicompartmental knee replacement: a prospective, randomized controlled study of the acrobat system. J Bone Joint Surg Br. 2006;88:188–197. doi: 10.1302/0301-620X.88B2.17220. [DOI] [PubMed] [Google Scholar]
- 11.Dolan MM, Heyworth BE, Bedi A, Duke G, Kelly BT. CT reveals a high incidence of osseous abnormalities in hips with labral tears. Clin Orthop Relat Res. 2011;469(3):831–8. doi: 10.1007/s11999-010-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ecker TM, Puls M, Steppacher SD, Bastian JD, Keel MJB, Siebenrock KA, Tannast M. Computer Assisted Femoral Head-Neck Osteochondroplasty Using a Surgical Milling Device. The Journal of Arthroplasty. 2012;27(2):310–316. [DOI] [PubMed]
- 13.Haaker RGA, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl J. Comparison of Conventional versus Computer-Navigated Acetabular Component Insertion. Journal of Arthroplasty. 2007;22(2):151–159. doi: 10.1016/j.arth.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and Intraoperative Findings in Revision Hip Arthroscopy. Arthroscopy. 2007;23:1295–1302. doi: 10.1016/j.arthro.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Kather J, Hagen ME, Morel P, Fasel J, Markar S, Schueler M. Robotic Hip Arthroscopy in Human Anatomy. International Journal of Medical Robotics and Computer Assisted Surgery. 2010;6:301–305. doi: 10.1002/rcs.332. [DOI] [PubMed] [Google Scholar]
- 16.Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon's learning experience. J Bone Joint Surg Am. 2011;93(Suppl 2):52–6. doi: 10.2106/JBJS.J.01587. [DOI] [PubMed] [Google Scholar]
- 17.Kubiak-Langer M, Tannast M, Murphy SB, Siebenrock KA, Langlotz F. Range of motion in anterior femoroacetabular impingement. Clin Orthop Relat Res. 2007;458:117–124. doi: 10.1097/BLO.0b013e318031c595. [DOI] [PubMed] [Google Scholar]
- 18.Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcome measures. Arthroscopy. 2008;24:540–546. doi: 10.1016/j.arthro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Mardones RM, Gonzalez C, Chen Q, Zobitz M, Kaufman KR, Trousdale RT. Surgical Treatment of Femoroacetabular Impingement: Evaluation of the Effect of the Size of the Resection. Journal of Bone and Joint Surgery Am. 2006;87(2):273–279. doi: 10.2106/JBJS.D.01793. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25:400–404. doi: 10.1016/j.arthro.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Monahan E, Shimada K. Computer-aided navigation for arthroscopic hip surgery using encoder linkages for position tracking. Int J Med Robot. 2006;2:271–278. doi: 10.1002/rcs.100. [DOI] [PubMed] [Google Scholar]
- 22.Monahan E, Shimada K. Verifying the effectiveness of a computer-aided navigation system for arthroscopic hip surgery. Stud Health Technol Inform. 2008;132:302–307. [PubMed] [Google Scholar]
- 23.Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The Contour of the Femoral Head-Neck Junction as a Predictor for the Risk of Anterior Impingement. Journal of Bone and Joint Surgery Br. 2002;84-B(4):556–560. doi: 10.1302/0301-620X.84B4.12014. [DOI] [PubMed] [Google Scholar]
- 24.Pfirrmann CW, Mengiardi B, Dora C, Kalberer F, Zanetti M, Hodler J. Cam and pincer femoroacetabular impingement: Characteristic MR arthrographic findings in 50 patients. Radiology. 2006;240:778–785. doi: 10.1148/radiol.2403050767. [DOI] [PubMed] [Google Scholar]
- 25.Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision Hip Arthroscopy. Am J Sports Med. 2007;35:1918–1921. doi: 10.1177/0363546507305097. [DOI] [PubMed] [Google Scholar]
- 26.Puls M, Ecker TM, Tannast M, Steppacher SD, Siebenrock KA, Kowal JH. The equidistant method—a novel hip joint simulation algorithm for detection of femoroacetabular impingement. Comput Aided Surg. 2010;15(4–6):75–82. doi: 10.3109/10929088.2010.530076. [DOI] [PubMed] [Google Scholar]
- 27.Ranawat A, Kang H, Kasodekar S, Nortman S, Jones JA, Conditt MA. Accuracy of Robotic-Assisted Femoral Osteochondroplasty for Treatment of FAI. [Accepted as podium presentation at Orthopedic Research Society 2012 Annual Conference, San Francisco, USA].
- 28.Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone and Joint Surg Am. 2009;91:192–7. doi: 10.2106/JBJS.G.01367. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph T, Puls M. MARVIN: a Medical Research Application Framework Based on Open Source Software. Computed Methods Programs Biomed. 2008;91:165. doi: 10.1016/j.cmpb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Stahelin L, Stahelin T, Jolles BM, Herzog RF. Arthroscopic Offset Restoration in Femoroacetabular Cam Impingement: Accuracy and Early Clinical Outcome. Arthroscopy. 2008;24(1):51–57. doi: 10.1016/j.arthro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Sussman PS, Ranawat AS, Lipman J, Lorich DG, Padgett DE, Kelly BT. Arthroscopic Versus Open Osteoplasty of the Head-Neck Junction: A Cadaveric Investigation. Arthroscopy. 2007;23:1257–1264. doi: 10.1016/j.arthro.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Tannast M, Kubiak-Langer M, Langlotz F, Puls M, Murphy SB, Siebenrock KA. Noninvasive Three-Dimensional Assessment of Femoroacetabular Impingement. Journal of Orthopaedic Research. 2006;25(1):122–131. doi: 10.1002/jor.20309. [DOI] [PubMed] [Google Scholar]
- 33.Weng YJ, Hsu RW, Hsu WH. Comparison of Computer-Assisted Navigation and Conventional Instrumentation for Bilateral Total Knee Arthroplasty. J Arthroplasty. 2009;24(5):668–673. doi: 10.1016/j.arth.2008.03.006. [DOI] [PubMed] [Google Scholar]