Abstract
Background
Previous studies have demonstrated the distinct advantages of thoracoscopically assisted spinal fusion compared to traditional open thoracotomy. However, these techniques are limited by a steep learning curve, prolonged operative time, and lack of three-dimensional visualization of the surgical field.
Objective
The objective of this study was to describe our initial experience with an adaptation of the extreme lateral interbody fusion (XLIF) technique allowing access to the anterior aspect of the thoracic and thoracolumbar spine with specific reference to (1) early pulmonary complications, (2) non-pulmonary complications, and (3) ability of this technique to successfully achieve spinal decompression and fusion at the operative level.
Methods
Clinical and radiographic data were reviewed for the entire perioperative period. A total of 18 patients (72% females; mean age, 56.8 years) underwent a thoracic XLIF procedure for spinal pathologies including disc herniation, fracture, tumor, pseudoarthrosis, and proximal junctional kyphosis. A total of 32 levels were treated, with the majority located at the thoracolumbar junction. Twelve of the procedures were done as part of a combined anterior/posterior surgery.
Results
The mean estimated blood loss was 577 ml and the mean length of stay was 12 days. At a mean follow-up of 14 months, all patients except for one (who died of widely metastatic disease) had achieved radiographic evidence of fusion. Two patients developed pulmonary effusions requiring medical intervention. Six patients had seven non-pulmonary complications: incidental durotomy (two), infection (one), instrumentation pullout (one), cardiac arrhythmia (two), and death from metastatic disease (one).
Conclusions
The XLIF technique can be utilized for access to the anterior column of the thoracic and thoracolumbar spine. The advantages of this minimally invasive technique include avoidance of the need for an access surgeon and for lung deflation during surgery as well as excellent visualization of the spinal pathology.
Keywords: spine, thoracolumbar spine, XLIF, lateral access surgery
Introduction
Multiple minimally invasive approaches have been described to access the pathology of the anterior column of the thoracic and thoracolumbar spine, including video-assisted thorascopic surgery and lateral extracavitary approaches [6, 7, 11, 13, 15, 17]. In general, the development of these techniques has been motivated by the disadvantages of open thoracotomy, including decreased pulmonary function, increased blood loss, the need for postoperative monitoring in an intensive care unit, longer chest tube times, more postoperative pain, longer hospital stays, and the need for an access surgeon [2, 6, 10, 12, 16, 21].
The extreme lateral interbody fusion (XLIF) technique was initially described by Ozgur et al. [18] to address disease in the lumbar spine. The traditional XLIF technique allows for access to the anterior and middle columns of the lumbar spine via a small incision (approximately 3–4 cm) with subsequent dissection through the retroperitoneal space and psoas muscle. There is limited information in the literature regarding the application of the XLIF technique to the thoracic or thoracolumbar spine [8].
In this report, we present our technique and initial outcomes using a modification of the XLIF technique to address disease in the thoracic and thoracolumbar spine, including traumatic, osteoporotic, and pathologic fractures; pseudoarthrosis; junctional kyphosis; and scoliosis. The specific aims of the study were to report (1) early pulmonary complications, (2) non-pulmonary complications, and (3) ability of this technique to successfully achieve spinal decompression and fusion at the operative level.
Patients and Methods
Following Institutional Review Board approval, we retrospectively reviewed all patients treated at our institution by the senior author (RCH) using a modified XLIF technique in the thoracic or thoracolumbar spine from 2007 to 2009. Outpatient and inpatient medical records were reviewed. Patient demographic factors that were analyzed included age; gender; body mass index (BMI); indication for the index procedure; and associated comorbidities including smoking, obesity, and osteoporosis. The operative indication was confirmed by a review of relevant imaging studies including standing long-cassette (14 × 36 in.) anteroposterior (AP) and lateral scoliosis films as well as MRI studies performed with a 1.5-T MRI (HDx, GE Healthcare) using an eight-channel cervicothoracolumbar coil by the senior author (RCH).
A total of 18 patients (13 females, 5 males) underwent a modified XLIF procedure during the time frame of the study (Table 1). The mean age was 56.8 years (range, 19–88 years). The mean BMI was 25.6 (range, 16.8–35.4). The indications for surgery included: thoracic compression fracture from osteoporosis or malignancy causing painful kyphosis (five patients), herniated nucleus pulposus causing compression of the spinal cord (four patients), thoracolumbar burst fracture (three patients), proximal junctional kyphosis (two patients), pseudoarthrosis (two patients), Chance fracture with kyphotic malunion (one patient), and degenerative scoliosis (one patient). The majority of involved levels were at the thoracolumbar junction (Fig. 1). Twelve of the 18 procedures were done as the anterior component of a combined anterior and posterior procedure. The mean operative time was 2.7 h (range, 1.7–4.0 h) for the anterior procedure, whether isolated or as a component of a staged procedure.
Table 1.
Summary of cohort demographics and surgical outcomes
| Case no. | Age/sex | BMI | Indication | XLIF levels | Additional instrumentation/staged | Bone graft | EBL (cc) | LOS/ICU (days) | LOF (m) | Radiographic fusion | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78/F | 19.3 | T12 burst fracture/kyphosis and myelopathy | T12 corpectomy/Synex | SPO T11-L1, PSFI T11-L1 | BMP, DBM, rib | 600 | 13/7 | 13 | Yes | Delayed pleural effusion requiring chest tube reinsertion, pullout of instrumentation requiring reoperation 1 month postoperatively |
| 2 | 78/F | 21.0 | T11 compression fracture and instrumentation breakage | T11 corpectomy/Synex | PSFI T4-Pelvis | BMP, DBM, rib | 300 | 16/4 | 12 | Yes | Incidental durotomy during posterior procedure |
| 3 | 78/F | 16.8 | T12 compression fracture/painful kyphosis | T12 corpectomy/Synex, T8-9, discectomy/Nuvasive PEEK | PSFI T8-L4 | BMP, DBM, rib | 100 | 10/3 | 6 | Yes | None |
| 4 | 50/F | 18.3 | T3-4 proximal junctional kyphosis | T3-4 discectomy/Nuvasive PEEK | Revision PSFI T3-Pelvis | BMP, DBM, rib | 300 | 21/3 | 36 | Yes | None |
| 5 | 44/F | 21.5 | T11-12 proximal junctional kyphosis | T11-12 discectomy/Nuvasive PEEK | Revision PSFI T11-L5, XLIF L3-4/Nuvasive | BMP | 800 | 13/1 | 27 | Yes | None |
| 6 | 64/F | 20.7 | T11-12 and L1-2 pseudoarthrosis | T11-12 and L1-2 discectomy/Nuvasive PEEK | PSO L3, ALIF L5-S1, TLIF L2-3, revision PSFI T11-S1 | BMP, DBM | 1500 | 14/4 | 32 | Yes | Sacral fracture 6 weeks postoperative requiring extension of PSF to the pelvis |
| 7 | 55/F | 21.8 | Degenerative scoliosis | T11-L5 discectomies/Nuvasive PEEK | PLIF L5-S1, PSFI T11-Pelvis | BMP, DBM | 800 | 8/2 | 6 | Yes | None |
| 8 | 75/F | 27.2 | T11 compression fracture/kyphosis and myelopathy | T12 corpectomy/Nuvasive Elixis | PSFI T11-L1 | BMP, DBM | 250 | 4/2 | 12 | Yes | None |
| 9 | 23/M | 23.5 | T11-12 pseudoarthrosis | T11-L1 discectomy/Nuvasive PEEK, Synthes TSLP | Revision PSFI T1-L1 | BMP, DBM, rib | 1000 | 8/1 | 7 | Yes | Delayed pleural effusion POD 3 requiring transfer to ICU for monitoring |
| 10 | 24/M | 24.9 | T11-L1 HNPs/myelomalacia | T11-L1 discectomy/Nuvasive PEEK, Synthes TSLP | None | DBM, rib | 900 | 6/1 | 12 | Yes | None |
| 11 | 71/F | 30.3 | T12 compression fracture/painful kyphosis | T11-12 discectomy/Nuvasive PEEK | PSFI T11-12 | Cancellous allograft, marrow aspirate, rib | 800 | 5/3 | 6 | Yes | None |
| 12 | 53/M | 35.1 | T11-12 HNP/myelomalacia | T11-12 discectomy/Nuvasive PEEK, Synthes TSLP | None | Rib | 1400 | 7/1 | 11 | Yes | None |
| 13 | 19/F | 25.7 | T12 burst fracture/kyphosis and myelopathy | T12 corpectomy/Synex | None | DBM, rib | 250 | 7/1 | 12 | Yes | None |
| 14 | 72/M | 31.3 | T11 pathologic fracture with spondylolisthesis | T10-12 discectomy/Nuvasive PEEK | PSFI T8-L2 | ICBG, DBM, rib | 350 | 52/5 | 2 | No | Death from metastatic cancer |
| 15 | 36/F | 22.0 | T7-8 HNP/myelopathy | T7-8 discectomy/Nuvasive PEEK | None | Rib | 1250 | 39/3 | 6 | Yes | Incidental durotomy requiring reoperation and closure with subarachnoid drain |
| 16 | 88/M | 35.4 | T8-9 proximal junctional kyphosis | T8-9 discectomy/Nuvasive PEEK | PSFI T6-11 | ICBG, DBM cancellous allograft | 350 | 14/4 | 12 | Yes | Surgical site infection requiring reoperation |
| 17 | 50/F | 21.1 | T9-10 chronic Chance fracture/painful kyphosis | T10 corpectomy/Synex and TSLP | None | DBM, rib | 500 | 12/5 | 12 | Yes | None |
| 18 | 55/F | 25.4 | T12 burst fracture/painful kyphosis | T11-L1 discectomy/Nuvasive PEEK | None | DBM, rib | 300 | 19/2 | 12 | Yes | None |
Synex Synthes expandable cage (Westchester, PA), Nuvasive Nuvasive PEEK cage (San Diego, CA), PSFI posterolateral instrumented fusion with Depuy Expedium Instrumentation (Raynham, MA), DBM Grafton Putty (Osteotech, Shrewsbury, NJ), ICBG iliac crest bone graft
Fig. 1.
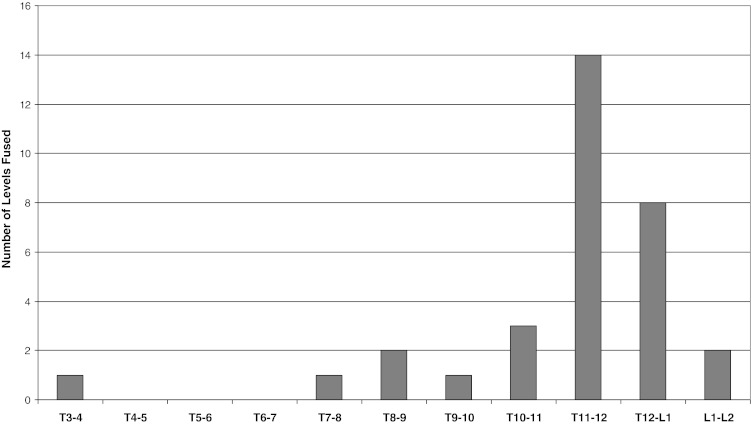
Distribution of fused spinal levels.
The surgical procedure has several important highlights. Following standard single-lumen intubation, the patient is positioned in the lateral decubitus position on a radiolucent table. Care needs to be taken during positioning to ensure that the operative disc space is orthogonal to the floor. The position of the incision is marked using fluoroscopy directly above the operative disc space. Following a skin incision of 4–5 cm, blunt dissection is used to separate muscle layers overlying the rib. A 6-cm section of the rib can be resected to allow for access to two interspaces for corpectomy or adjacent-level discectomies. For one-level discectomy and fusion, rib resection is not usually needed (Fig. 2). The chest cavity is then entered and the lung is swept anteriorly using the surgeon’s finger to allow for passage of the MaXcess retractor (Nuvasive, San Diego, CA). At T12-L1 and L1-2, it is often necessary to cross the diaphragm (T12-L1 from above, L1-2 from below) to access the intervertebral disc. In these cases, care is taken to open the diaphragm at its junction with the chest wall to allow for repair.
Fig. 2.
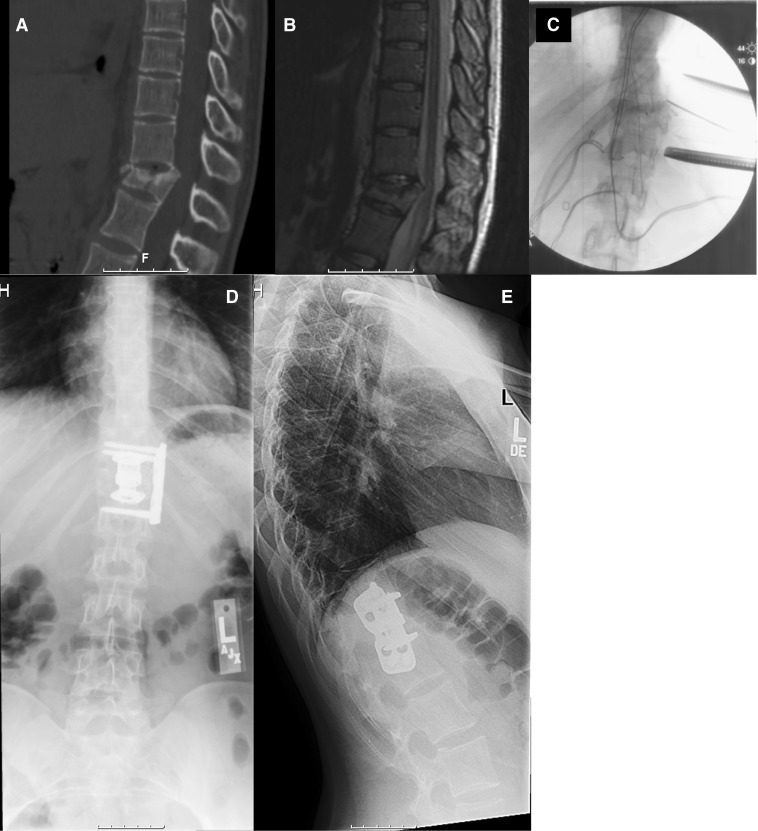
Twenty-four-year-old male with myelopathy. a Sagittal T2 MRI demonstrating disc herniations at T11-12 and T12-L1 causing cord compression. b Intraoperative fluoroscopic images demonstrating retractor positioning and localization of the interspace. c, d AP and lateral radiographs demonstrating Nuvasive PEEK cages with a lateral thoracic spine locking plate. e Sagittal CT at 8 months after the index surgery shows completed fusion of the instrumented levels.
Lateral radiographs are used to confirm that the retractor is centered over the correct interspace. This provides excellent exposure for discectomy of up to two adjacent levels or a single level corpectomy. Our preference is to use Nuvasive PEEK cages for discectomy and Synthes expandable cages (Synex, Westchester, PA) for corpectomy reconstruction (Fig. 3). Synthes thoracic spine locking plates can be used to augment anterior-only constructs through the same exposure. The wound is then closed in layers over a closed suction chest tube. Multiple non-adjacent levels are generally approached through separate small incisions.
Fig. 3.
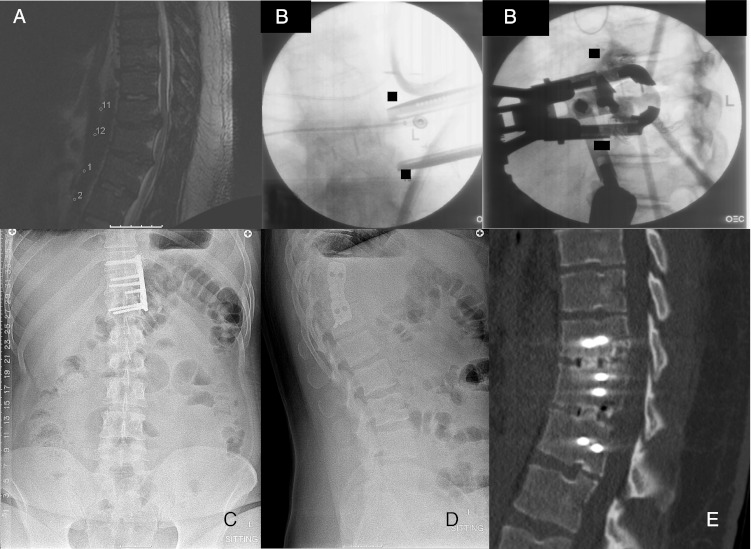
Nineteen-year-old female with chronic T12 burst fracture and painful progressive local kyphosis. a Preoperative sagittal CT scan demonstrating collapse with local kyphosis and retropulsion of fragments into the canal. b Preoperative sagittal fat saturation inversion recovery MRI demonstrating a chronic fracture and cord compression. c Intraoperative fluoroscopic image demonstrating retractor placement and localization of the affected spinal level. d, e Postoperative AP and lateral radiographs demonstrating Synex expandable cage and thoracic spine locking plates in place with restoration of normal alignment.
Bone morphogenetic protein (Infuse, Medtronic, Minneapolis, MN) was utilized to enhance fusion in 9 of 18 patients. The remaining cases utilized a combination of rib autograft (when it was necessary as part of the surgical approach), demineralized bone matrix (Grafton Putty, Osteotech, Shrewsbury, NJ), or cancellous allograft.
Early pulmonary complications were identified based on clinical notes and radiographic studies such as chest radiographs and CT scans. Non-pulmonary complications were similarly identified based on a review of both inpatient and outpatient clinical records combined with the available radiographic studies. Fusion was assessed using unenhanced CT of the thoracic or thoracolumbar spine with a 16-MDCT scanner (MX8000 Philips Healthcare). The determination of final fusion of the operative level was made by the senior author (RCH). Resolution of patient symptoms was documented in the senior authors’ outpatient medical records. Mean follow-up was 14 months (range, 2–36 months).
Results
Pulmonary effusion was a common early postoperative complication. The mean estimated blood loss was 577 ml (range, 100–1500 ml), which includes the posterior portion of the procedure when relevant. Eleven of the patients were extubated in the OR. All patients were extubated by the first postoperative day. Mean stay in a monitored care setting was 3 days (range, 1–7 days). Chest tubes were removed at a mean of 2 days (range, 1–5 days). Eight patients developed a pulmonary effusion following removal of the chest tube. Six were minimally symptomatic. In one patient, the effusion was significant enough to require reinsertion of a chest tube on postoperative day 2. In a second patient, respiratory symptoms related to the effusion were deemed significant enough to warrant transfer from the inpatient floor to a monitored care setting.
Six patients had experienced perioperative complications unrelated to pulmonary effusion. Two patients developed new cardiac arrythmias in the immediate postoperative period and were successfully treated with medical management. One patient had an incidental durotomy during the posterior portion of the procedure that was primarily repaired without incident. A second patient had an incidental durotomy during the anterior portion of the procedure in which the repair failed and CSF began to collect in the pulmonary cavity. This was addressed successfully by revision repair and placement of a subarachnoid drain. One patient presented with a surgical site infection 7 months after his index procedure requiring surgical debridement and antibiotic therapy.
Seventeen of the 18 patients went on to develop radiographic evidence of solid fusion. The patient who did not have evidence of radiographic fusion died from widely metastatic cancer 2 months following her surgery to address a pathological fracture causing spinal cord compression. Twelve patients experienced excellent pain relief and were satisfied with the result of the surgical procedure. One patient had a fracture with pullout of the distal pedicle screws requiring revision fusion at 1 month following the index surgery. One patient had a fracture of the sacrum at the level of the distal instrumentation which required extension of the fusion construct to the pelvis. One patient developed adjacent segment degeneration proximal to the fusion construct requiring further surgery. Two patients continued to have moderate axial back pain after the surgery despite evidence of adequate decompression and solid fusion on repeat imaging.
Discussion
In this series, we present 18 cases where a modification of the XLIF techniques was utilized to provide access to the anterior column of the spine either as part of a stand-alone procedure or in the context of circumferential decompression and fusion. This series included medically complex patients such as those with pathological fracture from metastatic disease as well as relatively healthy patients with isolated thoracic herniations of the nucleus pulposus. The most commonly encountered complications were early pulmonary complications such as pleural effusion. Non-pulmonary complications including infection and incidental durotomy were encountered in a small number of patients. Overall, a large majority of the patients were able to achieve symptomatic relief and solid intervertebral fusion.
There are numerous limitations inherent within our study design. The study was retrospective and lacked validated outcome measures. The patient population was heterogeneous and the surgical approach included both anterior-only and circumferential fusion. The number of patients in the series was limited. The heterogeneous nature of the patient cohort did not allow us to establish a control group for comparison.
One notable complication encountered in this series was the presence of pulmonary effusions manifesting after postoperative day (POD) 2 which occurred only in patients where bone morphogenetic protein (BMP) was used [9]. This study does not allow us to assign causality to this observation, but further investigation is warranted given the known propensity of BMP to cause inflammatory reactions and seromas [3, 20]. The series by Karikari et al. [8] did not report comparable findings despite a similar operative technique. Although they were not encountered in this series, potential complications of operating in the thoracic spine, including delayed pneumothorax, diaphragmatic hernia, major vascular injury, and rib pain, are certainly possible given the limited size of our series. Finally, studies from the adolescent idiopathic scoliosis population have shown less effect on pulmonary function when minimally invasive techniques are used rather than open thoracotomy to access the adolescent spine [14]. However, the effect of minimally invasive lateral extracavitary approaches on long-term pulmonary function has not been investigated.
The non-pulmonary complications observed in our study, including infection, incidental durotomy, pullout of instrumentation, and cardiopulmonary complications, are comparable to other reported studies including medically complex patients and revision spinal arthrodesis [4, 5, 7].
Although this is an initial report with a limited number of cases, it does suggest that this adaptation of the XLIF technique is effective with specific technical advantages as compared to other techniques for accessing the anterior thoracic and thoracolumbar spine. The benefits of more minimally invasive approaches to the thoracic spine are exemplified by the body of literature comparing thoracoscopically assisted spinal arthrodesis to open thoracotomy. Multiple studies have demonstrated decreased incision size, less blood loss, decreased length of ICU stays, decreased duration of chest tube drainage, less pain, and shorter hospital stays [1, 6, 10, 12, 16]. However, thoracoscopic techniques have distinct disadvantages including a steep technical learning curve and a lack of three-dimensional visualization of the operative field, which have limited their widespread adoption [19]. A major advantage of the thoracic XLIF technique compared to thoracoscopy is the avoidance of periods of prolonged lung deflation which contributes to atelectasis and may pose a particular risk in the patient with preexisting pulmonary compromise. A second advantage of this technique is the lack of a need for an access surgeon due to the relatively low risk of injury to nearby vascular structures compared to open thoracotomy.
Our results differ from the previous report by Karikari et al. [8] in that our series includes the application of this technique to indications including revision and circumferential arthrodesis procedures. Similar to their report, we found an acceptable rate of complications and a high rate of arthrodesis through a small incision. Overall, visualization and access to the spinal pathology using this technique was excellent. Further study with larger samples and longer follow-up are necessary to better characterize the safety and efficacy of this technique.
Disclosures
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participating in the study was obtained.
Footnotes
Level of Evidence: Therapeutic Study Level IV: See levels of evidence for a complete description.
References
- 1.Beisse R, Muckley T, Schmidt MH, Hauschild M, Buhren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128–36. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 2.Beisse R, Muckley T, Schmidt MH, et al. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128–136. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- 3.Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62:ONS423-31. doi: 10.1227/01.neu.0000326030.24220.d8. [DOI] [PubMed] [Google Scholar]
- 4.Edwards CC, Bridwell KH, Patel A, Rinella AS, Berra A, Lenke LG. Long adult deformity fusions to L5 and the sacrum. A matched cohort analysis. Spine. 2004;29:1996–2005. doi: 10.1097/01.brs.0000138272.54896.33. [DOI] [PubMed] [Google Scholar]
- 5.Emami A, Deviren V, Berven S, Smith JA, Hu SS, Bradford DS. Outcome and complications of long fusions to the sacrum in adult spine deformity. Spine. 2002;27:776–86. doi: 10.1097/00007632-200204010-00017. [DOI] [PubMed] [Google Scholar]
- 6.Han PP, Kenny K, Dickman CA. Thorascopic approaches to the thoracic spine: experience with 241 surgical procedures. Neurosurgery. 2002;51:S88–S95. doi: 10.1097/00006123-200211002-00013. [DOI] [PubMed] [Google Scholar]
- 7.Huang TJ, Hsu RW, Li YY, Cheng CC. Minimal access spinal surgery (MASS) in treating thoracic spine metastasis. Spine. 2006;31:1860–1863. doi: 10.1097/01.brs.0000225995.56028.46. [DOI] [PubMed] [Google Scholar]
- 8.Karikari IO, Nimjee SM, Hardin CA, et al. Extreme lateral interbody fusion approach for isolated thoracic and thoracolumbar spine diseases: Initial clinical experience and early outcomes. J Spinal Disord Tech. 2011;24:368-75. [DOI] [PubMed]
- 9.Kepler CK, Huang RC, Meredith D, Cunningham M, Boachie-Adjei O. Delayed pleural effusion after anterior thoracic spinal fusion using bone morphogenetic protein-2. Spine. 2011;36:E365–9. doi: 10.1097/BRS.0b013e3181f55057. [DOI] [PubMed] [Google Scholar]
- 10.Khoo LT, Beisse P, Potulski M. Thorascopic-assisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurgery. 2002;51:S104–117. [PubMed] [Google Scholar]
- 11.Kossmann T, Jacobi D, Trentz O. The use of a retractor system (SynFrame) for open, minimal invasive reconstruction of the anterior column of the thoracic and lumbar spine. Eur Spine J. 2001;10:396–402. doi: 10.1007/s005860100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative painrelated morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285–9. doi: 10.1016/0003-4975(93)90667-7. [DOI] [PubMed] [Google Scholar]
- 13.Lidar Z, Lifshutz J, Bhattacharjee S, Kurpad SN, Maiman DJ. Minimally invasive, extracavitary approach for thoracic disc herniation: technical report and preliminary results. Spine J. 2006;6:157–63. doi: 10.1016/j.spinee.2005.05.377. [DOI] [PubMed] [Google Scholar]
- 14.Lonner BS, Auerbach JD, Estreicher MB, et al. Pulmonary function changes after various anterior approaches in the treatment of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2009;22:551-8. [DOI] [PubMed]
- 15.Lubelski D, Abdullah KG, Steinmetz MP, et al. Lateral extracavitary, costotransversectomy, and transthoracic thoracotomy approaches to the thoracic spine: review of techniques and complications. J Spinal Disord Tech. 2011. doi:10.1097/BSD.0b013e31823f3139. [DOI] [PubMed]
- 16.Mack MJ, Regan JJ, Bobechko WP, et al. Application of thorascopy for diseases of the spine. Ann Thorac Surg. 1993;56:736–8. doi: 10.1016/0003-4975(93)90966-L. [DOI] [PubMed] [Google Scholar]
- 17.Mayer HM. Microsurgical anterior approach to T5-T10 (Mini- TTA) In: Mayer HM, editor. Minimally Invasive Spine Surgery. 2. Berlin: Springer; 2006. pp. 129–37. [Google Scholar]
- 18.Ozgur BM. Aryan He, Pimenta L et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal D. Endoscopic approaches to the thoracic spine. Eur Spine J. 2000;9:S8–16. doi: 10.1007/PL00010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31:2813–9. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 21.Tis JE, O'Brien MF, Newton PO, et al. Adolescent idiopathic scoliosis treated with open instrumented anterior spinal fusion: five-year follow-up. Spine. 2010;35:64-70. [DOI] [PubMed]