Abstract
Background
Femoroacetabular impingement (FAI) is a condition that has become increasingly identified as abnormal, repetitive abutment of the proximal femur and acetabular rim. Safe surgical dislocation of the hip has been popularized as a technique that allows surgeons to not only improve joint preservation procedures but also understand disease patterns more clearly.
Questions/Purposes
We describe the technique of surgical dislocation as well as review the indications, results, and complications that are associated with the procedure. We also present various case examples to highlight this technique.
Search Strategies
We performed a systematic review of the literature to define the indications, clinical outcomes, and complications associated with surgical dislocation of the hip for the treatment of FAI.
Results
Clinical success rates vary in the literature between 64% and 96% of patients with good results, and conversion to total hip arthroplasty ranging between 0% and 30% in patients who underwent FAI treatment with surgical dislocation. Reported major complication rates have ranged from 3.3% to 6%, most commonly in the form of trochanteric nonunion, neurapraxia, or heterotopic ossification.
Conclusions
FAI deformities encompass a wide spectrum of disease patterns. Surgical dislocation allows full access to the hip in addition to observing its pathomechanics. Strict adherence to proper technique allows the surgeon to minimize complication rates while treating the deformity at hand.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-012-9323-7) contains supplementary material, which is available to authorized users.
Keywords: hip dislocation, femoroacetabular impingement, FAI, perthes, SCFE
Introduction
Femoroacetabular impingement (FAI) disease has recently become a popular topic in the hip preservation and sports literature. This condition occurs due to an abnormally shaped proximal femur and/or acetabulum resulting in abnormal, repetitive contact between the femoral head–neck junction and the acetabular rim [6, 17]. These pathologic hip biomechanics have been proposed as precursors to acetabular labral tears, articular cartilage injury, and secondary osteoarthritis [7, 17, 21, 24, 35, 43, 44].
Cam-type FAI is caused by an insufficient concavity of the femoral head–neck junction, which creates an increased radius of curvature that is mismatched for the more congruent acetabulum. Repetitive contact between the proximal femur and the acetabular rim creates a shearing force of the labrum and the adjacent acetabular cartilage, leading to labral and chondral damage [4, 7]. These pathomechanics can be described as an “inclusion” injury due to the aspheric femoral head being forced into the joint. The vast majority of patients with cam-type FAI have no history of pediatric hip disorders, however Legg–Calve–Perthes disease [48] or slipped capital femoral epiphysis (SCFE) [25, 39] can result in this type of pathomorphology and are often more difficult to treat given the resulting complex deformity. Pincer-type FAI results from acetabular overcoverage of the femoral head, resulting in abnormal contact between the labrum and acetabular rim against the femoral neck during normal physiological hip motion [40, 43]. This can occur due to several distinct structural abnormalities, including acetabular retroversion, focal anterosuperior overcoverage, or global acetabular overcoverage. The pathomechanics of pincer FAI can be classified as an “impaction” of the prominent acetabular rim on the femoral neck. These two forms of FAI (cam and pincer) may also coexist in a combined or mixed disease pattern [6].
Treatment of FAI can be performed in a variety of methods including surgical dislocation of the hip, hip arthroscopy with or without a limited anterior approach, limited anterior approach alone, and finally a periacetabular osteotomy (PAO) when indicated. Various types of FAI are difficult to treat with arthroscopy alone due to the deformity location, severity, and complexity. We describe the technique of surgical dislocation as well as the indications, results, and complications that are associated with the procedure. We also present an Appendix (online version only) illustrating additional case examples to highlight the expanding role and versatility of this technique in treating the wide spectrum of diseases that encompass FAI.
Search Strategies
We searched PubMed on December 12, 2011 using the search terms “surgical dislocation” with “impingement” and “trochanteric flip osteotomy.” Collectively, our searches identified 56 articles, whose abstracts were reviewed by one of the authors (JRR). Studies were included if they were peer-reviewed, published in English, and not descriptions of previous reported findings. All study designs from level I to IV were eligible that described indications, outcomes, and complications of surgical dislocation of the hip. Studies were excluded if they did not concern the open surgical treatment of femoroacetabular impingement. Of the 56 articles, 16 met our criteria and are the focus of this review.
Results
Surgical Approach
Vascular Anatomy
Precise knowledge of the vascular anatomy of the hip is of paramount importance in preserving the blood supply to the femoral head during surgical dislocation. The primary source for the blood supply to the femoral head is the medial femoral circumflex artery (MFCA) [10, 18, 34, 49]. Knowledge of the course of the MFCA as it travels posteriorly is of utmost importance, as this forms the basis for the ability to perform a surgical hip dislocation from a posterior approach. The MFCA may arise from either the profunda femoral artery or the common femoral artery. From its origin, the MFCA travels posteriorly and gives off five consistent branches including the superficial, ascending, acetabular, descending, and deep branches. Preservation of the deep branch of the MFCA is the most important in preventing avascular necrosis of the femoral head [18]. The deep branch travels along the inferior border of the obturator externus, between the pectineus medially and the iliopsoas tendon laterally, toward the intertrochanteric crest. When viewed posteriorly, the deep branch can be located in the space between the quadratus femoris and the inferior gemellus. The main division of the deep branch continues its course superiorly, by crossing posterior to the obturator externus tendon and then anterior to the tendons of the superior gemellus, obturator internus, and inferior gemellus. The deep branch then perforates the hip capsule cranial to the insertion of the superior gemellus tendon and distal to the piriformis tendon. After perforating the capsule, the deep branches divide into two to four terminal branches, which course beneath the reflected capsule at the posterosuperior aspect of the femoral neck. The terminal branches perforate into bone approximately 2 to 4 mm distal to the bone–cartilage junction of the femoral head. Protection of these branches is critical to preserving the vascular integrity of the femoral head.
Operative Technique
The surgical dislocation of the hip, described by Ganz et al. [16], involves an anterior dislocation of the hip from a posterior approach with a trochanteric flip osteotomy. During dislocation of the hip, the deep branch of the MFCA is protected by the intact obturator externus muscle, thus maintaining blood supply to the femoral head [18]. The patient is positioned lateral decubitus, with the operative hip upward. A Kocher–Langenbeck or Gibson-type incision is made, through the skin and subcutaneous tissue, and the fascia lata is exposed. The fascia lata is incised longitudinally, centered between the anterior and posterior borders of the greater trochanter and carried distally along the femoral shaft. Proximally, this split is carried slightly posterior to the interval between the tensor and the gluteus maximus, in line with the direction of the gluteus maximus fibers. The trochanteric bursa, with its adipose tissue, is released from the posterior greater trochanter, as necessary, to improve visualization of the external rotators and posterior border of the gluteus medius. Electrocautery is used to mark the level of the trochanteric osteotomy, extending from the posterosuperior edge of the greater trochanter extending distally to the posterior border of the vastus lateralis ridge. The osteotomy leaves a small (5-mm) cuff of the most posteromedial gluteus medius tendon insertion and at least a portion of the piriformis tendon insertion attached to the trochanteric bed. In doing this, the deep perforating branch of the MFCA is preserved. An oscillating saw is used to create the trochanteric osteotomy along this line with a maximal thickness of approximately 1.5 cm. Alternatively, a step-cut osteotomy can be made [2] if the trochanteric fragment is not to be advanced. Once the osteotomy is complete, the posterosuperior edge of the trochanteric osteotomy commonly requires release from the most posterior fibers of the gluteus medius insertion.
The trochanteric osteotomy is mobilized anteriorly by releasing the vastus lateralis from the lateral femur to about the level of the mid-portion of the gluteus maximus tendon insertion. Remaining fibers of the posterior gluteus medius are also released and the piriformis tendon becomes visible as the trochanteric fragment is mobilized. At times, a portion of the piriformis tendon must be released from the trochanteric fragment; however, the vast majority should remain intact on the proximal femur. The leg is flexed and externally rotated, allowing further mobilization of the trochanteric fragment by elevating vastus lateralis and intermedius fibers from the anterior proximal femur. The capsule is then exposed by elevating the gluteus minimus fibers away from the piriformis and the underlying capsule. The sciatic nerve is then examined to define the patient’s individual anatomy, given that approximately 17% of patients may have variations in the course of the sciatic nerve [47]. If the sciatic nerve is exposed, care should be taken to maintain the integrity of the anastomotic branch from the inferior gluteal artery than runs on the inferior aspect of the piriformis and can contribute to femoral head vascularity [52]. If the entire nerve is found to exit through the piriformis (0.5%), a portion of the nerve exits through the piriformis (13.7%), or if the nerve is double branched (1.3%; the piriformis is sandwiched between sciatic nerve branches), the piriformis can be released (10 to 15 mm away from the insertion) to avoid stretching of the branches of the sciatic nerve during dislocation.
The capsule is incised in a z-shaped configuration, with the first limb of the incision along the anterolateral axis of the femoral neck. The second limb of the incision is made starting at the anterior border of the piriformis tendon and coursing anteroinferior. This incision must remain anterior to the lesser trochanter to avoid the main branch of the MFCA. The first branch of the MFCA, providing vascular supply to the medial femoral head, must also be preserved by not extending the incision too inferior. These two incisions create an anteroinferior flap that is elevated in order to visualize the labrum prior to the creation of the final capsular incision. A curved knife is then used in an inside-out manner to create the final limb by incising posteriorly from the first incision, parallel to the labrum and the acetabular rim.
The leg is flexed, adducted, and externally rotated to enable dislocation of the hip. The leg is brought over the front of the table and is placed to a sterile bag. Full dislocation is achieved once the ligamentum teres is surgical transected, which is followed by excision of the remaining stump of the ligamentum teres from the femoral head. Combinations of three retractors are placed to enable exposure. The first retractor (a pointed Hohman) is impacted into the supra-acetabular pelvis, which assists with retraction of the trochanteric fragment and abductor muscles. A second intracapsular retractor (pointed Cobra-like) is hooked around the anterior acetabular rim and facilitates retraction of the vastus muscles, abductor muscles, trochanteric fragment, and capsule. Finally, a third retractor (thin Cobra-like) is positioned under the transverse acetabular ligament retracting the medial calcar of the femoral neck. Positioning of the leg in 90° of flexion, slight adduction by lowering the knee, and axial pressure by an assistant allows the surgeon 360° access to the acetabulum, acetabular rim/labrum, anterior inferior iliac spine, femoral head, and proximal femur.
Postoperative Rehabilitation
Patients are 30-lb. partial weight bearing with crutches for 2–4 weeks. Continuous passive motion is used 4–6 h a day for 4 weeks. Passive motion and active-assisted exercises are performed the first 4 weeks with stationary bicycle as tolerated. At 4 weeks, patients are full weight bearing with progressive active strengthening. At 8 weeks, resistance strengthening, swimming, and elliptical machine are introduced. Patients are released to full activity 4 months after surgery.
Indications
Surgical dislocation of the hip is a technique that allows full access to the acetabular labrum, and the chondral surfaces of the acetabulum and the femoral head, mainly for joint preservation techniques. In our practice, this technique is reserved for physically young patients with relatively severe deformities that are less suitable for arthroscopic treatment. Extensive, non-focal, or circumferential deformities are appropriate for treatment with surgical dislocation, as opposed to focal disease that can be addressed with hip arthroscopy alone. Global acetabular overcoverage represents an example of circumferential disease that is difficult to treat with arthroscopy alone. Complex deformities associated with residual Perthes, SCFE, and post-traumatic disorders are also common indications for surgical dislocation. Hips with femoral deformities extending posterior to the perforating vessels are also candidates for surgical dislocation as this technique is associated with improved deformity correction [8]. Less common conditions including extra-articular impingement (femoral neck, greater and lesser trochanters) can be both diagnosed and surgically corrected with the dislocation approach. In complex deformities with associated acetabular dysplasia or proximal femoral malrotation, the dislocation can be combined with acetabular reorientation or proximal femoral osteotomy [41]. Generally, patients 35 years of age or younger are potential candidates for joint preservation procedures with the diagnosis of FAI [12]. However, carefully selected patients up to 55 years of age may also benefit from non-arthroplasty surgery, provided their joint surfaces are free of moderate to severe osteoarthritis.
The prognosis of joint preservation procedures for the treatment of FAI is likely multifactorial and is dependent not only on patient age but also activity level and underlying biological factors. Patients who present with symptomatic FAI tend to be highly active with over half of patients participating in regular sporting activities [12]. It has been postulated that hyperflexion activities may place abnormal forces on the acetabular rim as well as microtrauma to the labrochondal complex [20, 27, 29, 30]. Patients who participate in high-demand activities may not only be prone to the development of FAI but are also likely to have activity limitations that drive them to seek evaluation by a physician [12].
Patients who are relatively healthy and conditioned are candidates for joint preservation not only to assist with return to active lifestyles but also due to the technical demands of the surgery itself. Patients with elevated body-mass indices (BMI) make the surgical exposure much more difficult and sometimes visualization of the entire articular surfaces of the acetabulum and femoral head not possible. In a previous study, Nepple et al. [33] noted that male gender, increased age, and insidious onset of pain were all factors that were noted to be at higher risk for moderate to severe chondromalacia. Thus, these factors should be considered when contemplating joint preservation surgery.
Patient selection is of prime importance in obtaining successful clinical results. It is important to clearly identify the patient who is indeed symptomatic from the structural abnormality that is present in FAI. Patients who experience predominately groin pain that is exacerbated by hip flexion activities including sitting are likely to be of benefit from joint preservation surgery [12]. Patients with a history of childhood disorders, such as Perthes [42] or slipped capital femoral epiphysis (SCFE), are known to have residual FAI that becomes symptomatic in adulthood and are thus indications for surgical hip dislocation. A positive anterior impingement test (flexion, adduction, and internal rotation), positive Patrick’s FABER test (flexion, abduction, and external rotation), and restricted internal rotation at 90° of flexion (IRF) are all very sensitive tests for intra-articular disease [12, 26]. Finally, a radiographic confirmation of cam- and/or pincer-type lesions is critical for establishment of FAI. Optimization of patient selection, surgical technique, and preoperative planning requires a radiographic assessment in which both the acetabular and femoral osseous anatomy are detailed accurately. We obtain a comprehensive hip series including a standing anteroposterior (AP) pelvis, false profile, frog lateral, cross table lateral, and Dunn view of the hip [11]. A patient who demonstrates moderate to severe osteoarthritis, represented by 2 or more millimeters of joint space narrowing is not likely to have a “viable joint” and thus not a candidate for joint preservation given a trend for poorer results [13].
Clinical Outcomes
Our institution previously performed a systematic review of FAI surgical outcome studies prior to 2009 and found an overall clinical success rate varying between 68% and 96% with a minimum 2-year follow-up period [13]. An average of 86% of these patients had excellent or good outcomes with pain relief and return to function. The conversion to a total hip arthroplasty ranged from 0% to 26%, anywhere from 3 months to 75 months after the index joint preservation procedure. Some of the factors that were noted to be associated with a good outcome and increased satisfaction included no or mild arthritis, labral refixation as the method of treatment of labral pathology, young age, and limited chondral disease. Poor prognostic factors included more advanced preoperative osteoarthritis, advanced articular cartilage disease, older age, and more severe preoperative pain. These findings truly emphasize the negative impact of osteoarthritis on the long-term results of surgical intervention. Joint preservation surgery must therefore be recommended for patients with early disease and cautioned in those with more advanced arthritis.
A similar systematic review was performed, with the inclusion of an addition year of investigations and found similar results [28]. A clinical success rate was noted in 65% to 94% of patients with a mean follow-up of 2.5 years, and conversion to total hip arthroplasty ranging between 0% and 30% in patients who underwent FAI treatment with surgical dislocation [3, 6, 15, 31, 36, 50]. The higher percentages of conversion to total hip arthroplasty in some of the earlier studies [6, 31] are very likely due to the inclusion of a greater proportion of patients with advanced radiographic arthritis (Tonnis 2, 3) and/or concurrent acetabular dysplasia, in addition to a shift in the treatment of labral disease from debridement to repair. This false impression of higher “failures” are not similarly seen in the later studies that developed stricter inclusion criteria and had THA conversion rates of 0–5% [1, 9, 22, 36, 50].
Espinosa et al. [15] also demonstrated that labral treatment during surgical hip dislocation may impact final outcomes. Comparison of the clinical scores between labral resection and labral repair revealed significantly better outcomes at both 1 year and at 2 years after treatment with labral repair. Radiographic signs of osteoarthritis were also significantly more prevalent in the labral resection group. Similar results have also been noted during arthroscopic labral treatment of FAI hips [23].
Studies of professional athletes also provide a unique view of surgical outcomes since these outcomes represent the ability to return to the highest possible level of competition and activity. Some may argue that improvements in pain and range of motion after operative treatment are the result of activity modification rather than the effect of surgery itself. Nevertheless, investigations in a population of high-level athletes and determining their satisfaction and return to play can assist with outcome determination. In a recent study by Naal et al., 22 elite, professional male athletes with the diagnosis of FAI were treated with surgical hip dislocation [32]. Labral pathology was noted in 93% of the hips, while adjacent full-thickness chondral lesions were noted in 77%. At an average follow-up of 45 months (minimum 1-year follow-up), 96% of the athletes were still competing professionally, 19 at their previous level while two athletes competing in the minor leagues. The mean activity levels were 9.8 per the UCLA scale and 7.6 per the Hip Sports Activity Scale. Eighty-six percent of the athletes were satisfied with their sports ability. Interestingly, eight of the 22 had open procedures on bilateral hips and seven of these patients were completely satisfied with their hip surgeries and their sports ability. These favorable outcomes are comparable with arthroscopic treatment of athletes with FAI [38]. Values for the Hip Outcome Score—Activities of Daily Living and Sport subscales of 95 and 89 points were found in the open surgical dislocation group [32] and 94 and 89 points in the arthroscopic group [38]. In a separate study, Philippon speculated that arthroscopic rather than open treatment of FAI would lead to a better chance of full return to professional athletics [37]. They reported that 93% initially returned to professional competition, but this percentage fell to 78% at an average of 1.6 years after surgery, while in the study by Naal et al. [32], 96% remained at the professional level an average of 3.8 years after surgical dislocation. These data suggest that open surgical dislocation procedures are at least comparable to arthroscopic interventions when comparing return to high-level athletics.
Complications
Despite precise knowledge of the anatomy of the hip and the surgical technique, surgical dislocation has inherent risks. In their initial experience of over 200 cases, Ganz et al. [16] reported a “major” complication rate of 3.3%. In a systematic review performed at our institution [13], major complications were defined as avascular necrosis, femoral head–neck fracture, loss of fixation resulting in reoperation, trochanteric nonunion, failure of labral refixation, inadequate osteochondroplasty requiring revision, deep infection, and symptomatic heterotopic ossification. The major complications reported in this initial series of patients by Ganz et al. [16] included two cases of partial sciatic neurapraxia (0.9%), three cases of trochanteric nonunion (1.4%), and two cases of Brooker grade III heterotopic ossification (0.9%) that was along the acetabular rim and required excision due to motion loss. The two patients with sciatic neurapraxia had complete resolution without residual deficits 6 months postoperatively, which was attributed to previous surgery and scarring around the nerve. The overall incidence of heterotopic ossification was 37%; however, 86% of these patients were classified as Brooker grade I.
In review of previous literature regarding surgical dislocation of the hip, complications were difficult to collectively evaluate due to reporting inconsistency [13]. We therefore graded the complications into major, moderate, and minor categories. Major complications are stated above. Moderate complications include symptomatic hardware (with or without removal) and minor complications consisted of asymptomatic or minimal heterotopic ossification and a miscellaneous category (urinary tract infections, postoperative fevers, etc.). The major complication rate varied from 0% to 6% among the six studies. There were no minor complications that were noted. A recent study investigating pain at the lateral hip noted 46% of patients with pain at the greater trochanter after surgical dislocation [5]. The presence of greater trochanteric pain, however, had no influence on the overall clinical outcome scores. Some studies have demonstrated improvement of trochanteric pain with subsequent removal of hardware [4, 19, 22]. Trochanteric nonunion has been sporadically reported with wide ranges (1.9–20%) [22, 36, 50]. The higher rate of nonunion has not been previously reported and is likely due to surgeon inexperience, improper fixation technique, or insufficient protection postoperatively. [50] We have not only gone to the use of a z-shaped osteotomy cut to provide improved stability of the trochanteric fragment after reduction and refixation but have also limited the application of bone wax to the osteotomy site.
A retrospective, multicenter analysis of complications was performed by the Academic Network for Conservational Hip Outcomes Research (ANCHOR) Study Group [45]. Excluding grade I and II heterotopic ossification (18 hips; 5.4%), the complication rate was 4.8% in 334 hips. Trochanteric nonunion was noted in 1.8% of hips, which were all treated successfully with revision open reduction and internal fixation. There were two cases of deep vein thrombosis in the calf and one case of deep infection that was treated with irrigation and debridement. One patient suffered complete sciatic nerve paralysis that partially resolved, however with residual numbness and pain at final follow-up.
Recently, intra-articular adhesions between the femoral neck and joint capsule have been identified as a cause of persistent postoperative pain. These adhesions form between the joint capsule and the area of prior resection from the femoral head–neck junction, which may lead to intra-articular impingement. Three percent of 750 patients who underwent a previous open osteochondroplasty were thought to have residual pain secondary to adhesions, which were also confirmed on MR arthrogram [14]. Adhesions were noted during arthroscopic treatment in all suspected patients at the anterior and anterolateral position of the femoral head–neck junction and the femoral neck, on average 19 months after the index procedure. Lysis of these adhesions resulted in resolution of hip pain in 86% of patients.
Case Presentations
Various disorders of the hip joint can result in abnormal pathomechanics and thus subject the native hip joint to repetitive microtrauma, which is thought to predispose the patient to the development of osteoarthritis over time. Appropriate indications and sound surgical technique can help restore the homeostasis of the hip and thus prevent progression of hip disease. We present the following cases to demonstrate the utility of the surgical dislocation of the hip as a technique for the treatment of various forms of “complex FAI.”
Acute SCFE open reduction and internal fixation
Acetabular protrusio
Residual Perthes deformity
Residual Perthes deformity with acetabular dysplasia (online only)
Post-traumatic avascular necrosis (online only)
Residual deformity after SCFE treated with proximal femoral osteotomy (online only)
I. Acute SCFE Open Reduction and Internal Fixation
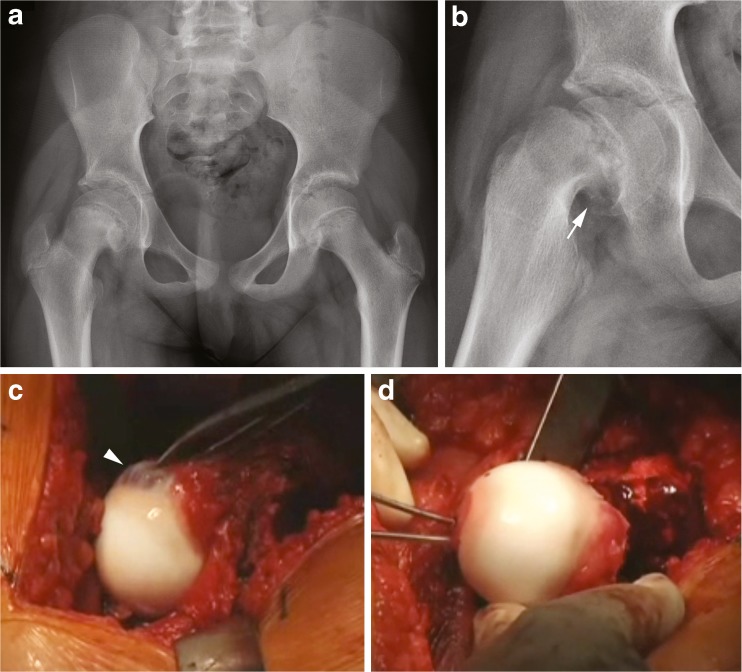
A 10-year-old female patient developed worsened hip pain and inability to weight-bear 1 week after a fall. She previously had an episode of similar pain 4 months prior, but returned to activities without pain. Hip range of motion was 0° to 45° with obligate external rotation. Internal rotation and external rotation at maximum flexion was −20° and 70°, respectively. Radiographs demonstrated a slipped capital femoral epiphysis with posteromedial callus, suggestive of an acute on chronic SCFE (Fig. 1a, b).
Fig. 1.
Acute on chronic slipped capital femoral epiphysis. a A decreased epiphyseal height and a break in Klein’s line is noted in the right hip on the AP pelvic radiograph. b Frog-leg lateral reveals callus formation at the posterior femoral neck with a severe displacement of the epiphysis (arrow). c Intraoperative image of remodeling of the anterolateral femoral head–neck junction (arrowhead). d Reduction and antegrade fixation of the epiphyseal fragment through the fovea centralis.
A modified Dunn procedure was performed with surgical hip dislocation and reduction of the femoral epiphysis as previously described [46, 51]. Prior to dislocation of the hip, the epiphyseal fragment was pinned in situ with two threaded Kirschner wires. Extended retinacular flap were created, which began with trimming of the posterosuperior portion of the stable greater trochanter down to the level of the femoral neck. The periosteum of the neck was then incised along the anterior neck, which was then followed by release of the periosteum and retinaculum from the femoral neck with the use of a scalpel and periosteal elevator. Posteriorly, this was continued down to the level of the lesser trochanter. The medial flap contains a branch of the MFCA, which supplies the inferomedial portion of the epiphysis. Once the retinacular flaps were developed, the femoral head was dislocated. The provisional Kirschner wires were removed and the epiphyseal fragment was separated from the metaphysis with the use of the 10-mm chisel. In this case, a fair amount of callus was resected from the posteromedial neck with a chisel and rongeur and the remaining physis was also removed from the epiphyseal fragment with the use of a curette. The epiphysis was then manually reduced onto the femoral neck while visually checking the tension of the medial retinaculum (Fig. 1d). Once reduction was performed and accepted, the femoral epiphysis was stabilized with two antegrade, threaded Kirschner wires placed through the fovea and exiting the lateral femoral cortex, distal to the trochanteric osteotomy site. A single 6.5-mm cannulated, partially threaded screw was also inserted retrograde to improve the construct stability (Fig. 2). The hip was reduced, range of motion evaluated, and the head–neck offset at the anterolateral femur corrected with a round bur, improving hip flexion and internal rotation.
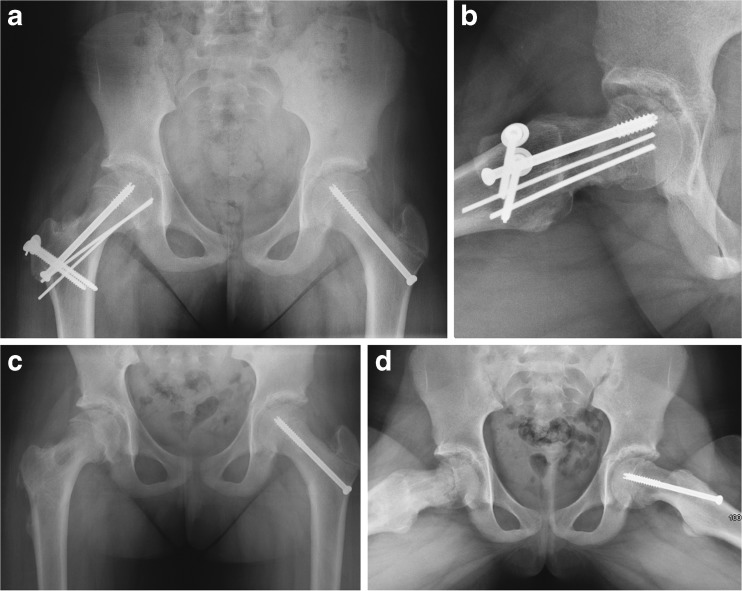
Fig. 2.
SCFE treated with Modified Dunn. a Pelvic radiograph shows reconstitution of a more normal femoral head and neck relationship after reduction of the epiphyseal fragment. b Frog-leg lateral also demonstrates improved reduction in addition to an anterior osteochondroplasty. c, d Final radiographs after removal of hardware on the right hip approximately 1 year from her index operation.
The patient underwent removal of hardware at 7 months postoperatively (Fig. 2c, d) and has no pain and return to full activities including basketball and cheerleading. Hip range of motion improved with 95° of flexion, IRF to 25°, and external rotation in flexion (ERF) to 30°. Her modified Harris hip score improved from 18 to 100.
II. Acetabular Protrusio
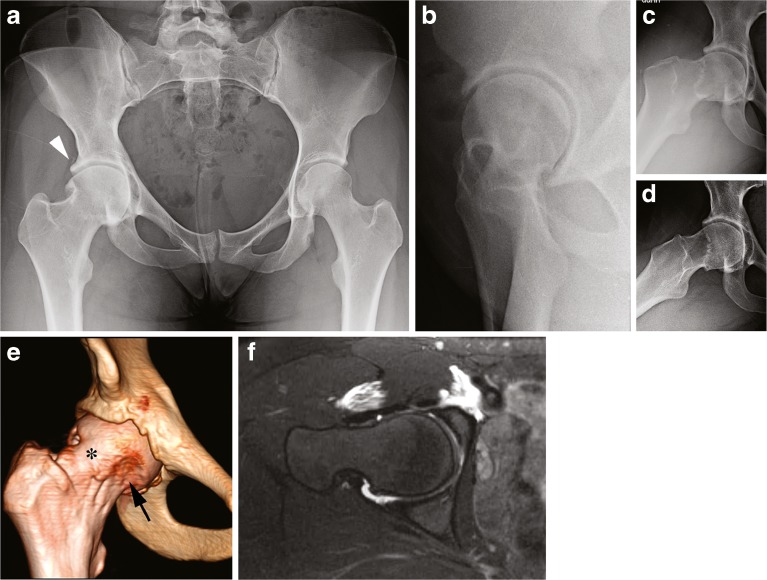
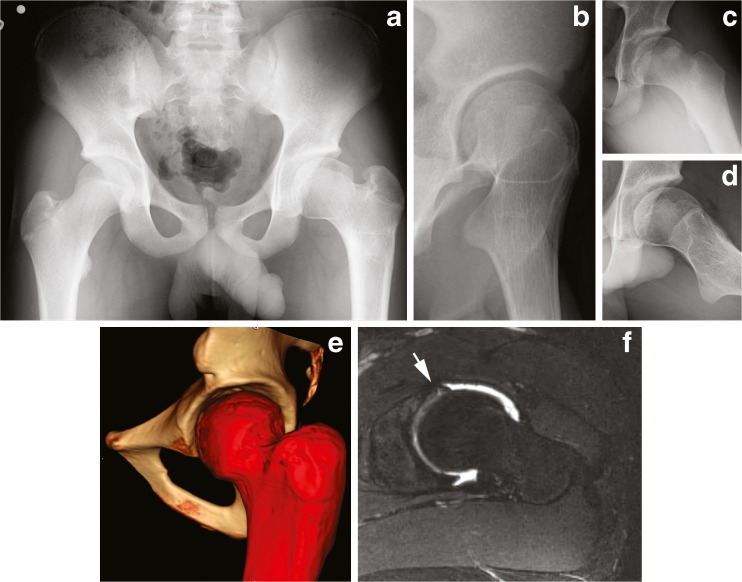
A 19-year-old female dancer presented with right hip pain and restricted motion after undergoing arthroscopic labral repair and osteochondroplasty at another institution 1 year prior without improvement. Her right hip range of motion was 0° to 90°, with IRF of 0°, and external rotation in flexion (ERF) of 15°. Anterior and lateral impingement testing and Patrick’s FABER test were positive. Radiographs demonstrated acetabular protrusio with global overcoverage, a femoral head–neck deformity, ossified labrum, and circumferential insufficient head–neck offset (Fig. 3).
Fig. 3.
Acetabular protrusio. a AP pelvic radiograph demonstrates protrusio, elevated lateral center edge angle, negative acetabular inclination, and ossified labrum (arrowhead). b False profile radiograph reveals elevated anterior center edge angle. c, d Dunn view and frog-leg lateral shows circumferential insufficient head–neck offset. e, f Three-dimensional CT scan and an axial slice of a T2-weighted MR arthrogram demonstrates the inadequate arthroscopic femoral osteochondroplasty (arrow) with continued anterolateral prominence (asterisk).
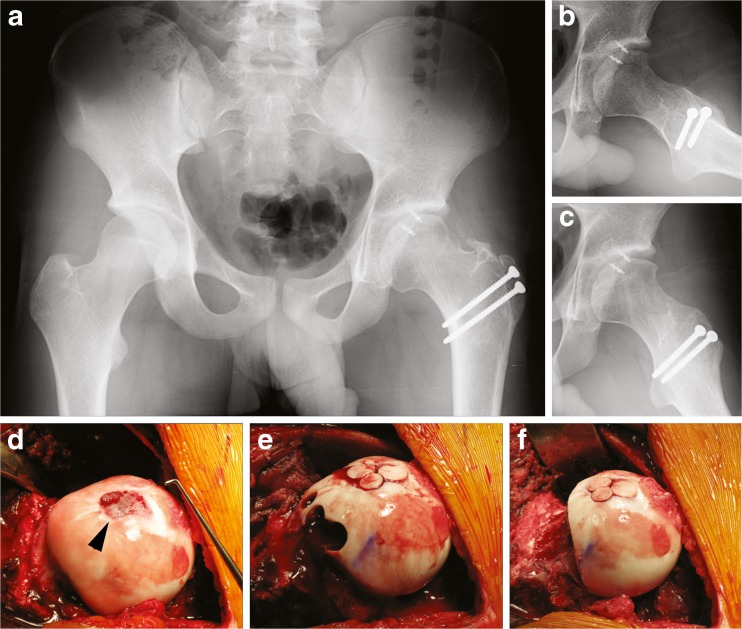
Surgical hip dislocation revealed a circumferentially deep acetabulum, chondromalacia of the acetabulum and femoral head, and an acetabular labral tear (Fig. 4). The previous arthroscopically performed head–neck osteochondroplasty was primarily anterior and anterolateral, with remaining insufficient offset laterally and posterolaterally. A near-circumferential femoral head/neck junction contour was performed with a combination of osteotomes and round bur, with care to protect and preserve the deep branches of the MFCA and the medial synovial fold. A majority of the labrum was taken down and an acetabular rim trimming performed with a round bur. The most anterior and posterior aspects of the labrum were repaired; however, a fascia lata autograft was used to reconstruct the labral tissue over approximately 25 mm.
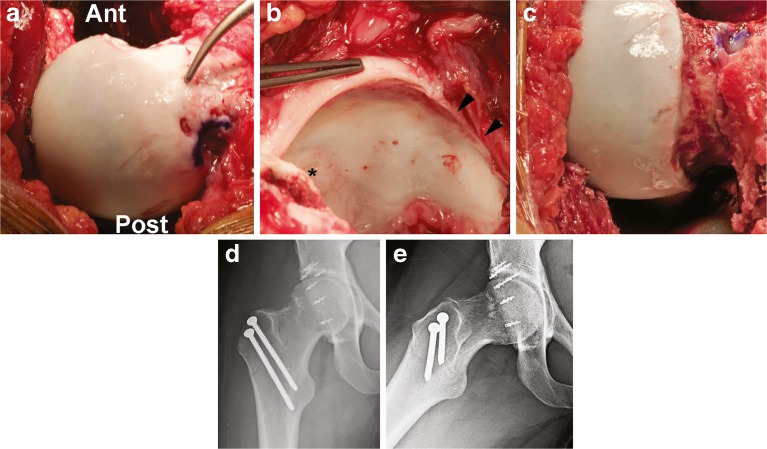
Fig. 4.
Acetabular protrusio. a, b Surgical hip dislocation revealed a circumferentially deep acetabulum, chondromalacia of the superolateral and superior acetabulum (asterisk) and anterior–superior and posterior–superior femoral head, and a 15-mm labral tear, in addition to labral deficiency along the anterior and superolateral rim (arrowhead). c After circumferential osteochondroplasty. d, e Radiographs showing less acetabular coverage and improvement of the head–neck offset. Ant anterior, Post posterior.
The patient elected to have her trochanteric screws removed 1 year from surgical dislocation and had marked improvement with return to dance with minimal pain. She had improvement of IRF to 10° and external rotation in flexion (ERF) to 20°. Her modified Harris hip score improved from 70 to 96.
III. Residual Perthes deformity
A 15-year-old male patient, diagnosed with Perthes disease at 9 years of age, presented with left hip pain and restricted motion. He was previously treated with a varus- and flexion-producing proximal femoral osteotomy (PFO) as a child and thereafter removal of hardware. He presented with mechanical symptoms of “popping” and “catching” in addition to pain. Physical examination revealed a positive Trendelenberg sign and a limb length discrepancy of 1.5 cm with the left being short. His left hip range of motion was 0° to 95°, with an IRF of 10°, external rotation in flexion (ERF) of 10°, and abduction of 20°. Anterior and lateral impingement testing and Patrick’s FABER test were positive. Radiographs demonstrated coxa magna, coxa breva, coxa vara, and a prominent greater trochanter (Fig. 5).
Fig. 5.
a, b AP pelvis and false profile radiographs demonstrate coxa magna, coxa breva, coxa vara, and a prominent greater trochanter. c, d Dunn view and frog-leg lateral demonstrate insufficient head–neck offset. Three-dimensional CT (e) also shows this deformity and a T2-weighted MR arthrogram (f) is suggestive of a labral tear (arrow).
Surgical hip dislocation was performed revealing an aspherical femoral head with an anterolateral prominence, and a full-thickness central defect approximately 20 × 15 mm in size. A 20-mm labral tear extended from superolateral to anterior, which was taken down to allow a mild acetabular rim trimming, followed by repair with two suture anchors. A mosaicplasty of the femoral head defect was then performed with three donor plugs harvested from the anterolateral head that was planned to be resected (Fig. 6). The remainder of the femoral head–neck junction was recontoured. The patient also had a relatively high trochanter when compared to the femoral head center, and thus a trochanteric advancement as well as a relative femoral neck lengthening was performed.
Fig. 6.
a, b, c Radiographs demonstrate correction of the head neck offset after osteochondroplasty in addition to an improvement in the trochanteric height. d Aspherical femoral head with an anterolateral prominence, and a full-thickness central defect (arrowhead) of approximately 20 × 15 mm. e Mosaicplasty of the femoral head defect was performed with three donor plugs harvested from anterolateral head that was later resected (f) and recontoured.
The patient elected to have his trochanteric screws removed at approximately 1 year from the surgical dislocation, and at 2 years postoperatively he has major improvement in symptoms and activity, however with mild residual abductor weakness. He has improvement of the IRF to 10°, external rotation in flexion (ERF) to 70°, and abduction to 40°.
Conclusions
Femoroacetabular impingement deformities are varied and may encompass a wide spectrum of disease patterns. Some of these FAI deformities are non-focal and complex, and are best treated with surgical dislocation of the hip. This technique allows the surgeon the ability to have full access to the acetabular labrum, and the chondral surfaces of the acetabulum and the femoral head in addition to observing the pathomechanics of motion in these FAI patients. This technique provides comprehensive deformity correction, with access to both the intra-articular and extra-articular structures, and without the limitations and difficulties that are encountered during hip arthroscopy. Strict adherence to proper technique, in addition to a sound knowledge of the anatomy of the hip, allows the surgeon to minimize complication rates while treating the deformity at hand.
Electronic supplementary material
Perthes disease with acetabular dysplasia. a AP pelvic radiograph demonstrated a Perthes-type hip with coxa magna, coxa breva, coxa vara, and a prominent greater trochanter. This also demonstrates acetabular retroversion (cross-over sign) and dysplasia with a diminished lateral center edge angle and elevated acetabular index. b False profile radiograph also demonstrated diminished anterior center edge angle. c, d Dunn view and frog-leg lateral also reveals the aspherical deformity present. Axial (e) and sagittal (f, g) T2 MR arthrogram images demonstrate an anterior and superolateral degenerative labral tear (arrowhead). (JPEG 45 kb)
a AP radiograph demonstrating correction of the femoral head–neck offset, trochanteric height, and acetabular dysplasia. b After removal of hardware demonstrating full osseous union of the trochanteric and pelvic osteotomies. (JPEG 24 kb)
Post-traumatic avascular necrosis. a, b AP pelvis and false profile radiographs revealed central femoral head collapse and extrusion of an anterolateral femoral head fragment. c, d Frog-leg lateral and Dunn views demonstrate diminished head–neck offset and impingement. Three-dimensional (e) and an axial slice (f) CT scan again demonstrated the central femoral head impaction (asterisk) and lateral extrusion with a preserved posteromedial femoral head (arrowhead). (JPEG 40 kb)
a–c Postoperative radiographs demonstrating improvement of the femoral head sphericity and increased femoral head–neck offset. d AP radiograph after removal of prominent hardware demonstrating preservation of the hip joint, 2 years after the reconstruction. (JPEG 29 kb)
Residual SCFE. Radiographs demonstrate a posteriorly displaced femoral head with a prominent anterolateral head–neck junction, with an impingement trough (arrow), in addition to a high greater trochanter in relation to the center of the femoral head. (JPEG 27 kb)
a, b Restoration of the femoral head–neck offset in addition to the trochanteric height. c Removal of the prominence from the head–neck junction. d Relative femoral neck lengthening. e Labral repair. (JPEG 69 kb)
(DOCX 27 kb)
Disclosures
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article. One or more of the authors has or may receive monies from a commercial entity that may be perceived as a potential conflict of interest.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Anderson LA, Erickson JA, Severson EP, Peters CL. Sequelae of Perthes disease: treatment with surgical dislocation and relative femoral neck lengthening. JPO. 2010;30(8):758–766. doi: 10.1097/BPO.0b013e3181fcbaaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastian JD, Wolf AT, Wyss TF, Notzli HP. Stepped osteotomy of the trochanter for stable, anatomic refixation. Clin Orthop Relat Res. 2009;467:732–738. doi: 10.1007/s11999-008-0649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaule PE, Le Duff MJ, Zaragoza E. Quality of life following femoral head–neck osteochondroplasty for femoroacetabular impingement. J Bone Joint Surg. 2007;89(4):773–779. doi: 10.2106/JBJS.F.00681. [DOI] [PubMed] [Google Scholar]
- 4.Beaule PE, Zaragoza E, Copelan N. Magnetic resonance imaging with gadolinium arthrography to assess acetabular cartilage delamination. A report of four cases. J Bone Joint Surg. 2004;86:2294–2298. doi: 10.2106/00004623-200410000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Beck M, Buchler L. Prevalence and impact of pain at the greater trochanter after open surgery for the treatment of femoro-acetabular impingement. J Bone Joint Surg. 2011;93(Suppl 2):66–69. doi: 10.2106/JBJS.J.01718. [DOI] [PubMed] [Google Scholar]
- 6.Beck M, Kalhor M, Leunig Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 7.Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;418:67–73. doi: 10.1097/00003086-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. AJSM. 2011;39(Suppl):20S–28S. doi: 10.1177/0363546511412734. [DOI] [PubMed] [Google Scholar]
- 9.Botser IB, Smith TW, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270–278. doi: 10.1016/j.arthro.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Chung SM. The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg. 1976;58:961–970. [PubMed] [Google Scholar]
- 11.Clohisy JC, Carlisle JC, Beaule PE, Kim YJ, Trousdale RT, Sierra RJ, et al. A systematic approach to the plan radiographic evaluation of the young adult hip. J Bone Joint Surg. 2008;90(suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467:638–644. doi: 10.1007/s11999-008-0680-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clohisy JC, St John LC, Schutz AL. Surgical treatment of femoroacetabular impingement: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(2):555–564. doi: 10.1007/s11999-009-1138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudda M, Mamisch TC, Krueger A, Werlen S, Siebenrock KA, Beck M. Hip arthroscopy after surgical hip dislocation: is predictive imaging possible? Arthroscopy. 2011;27(4):486–492. doi: 10.1016/j.arthro.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg. 2006;88:925–935. doi: 10.2106/JBJS.E.00290. [DOI] [PubMed] [Google Scholar]
- 16.Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip. A technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119–1124. doi: 10.1302/0301-620X.83B8.11964. [DOI] [PubMed] [Google Scholar]
- 17.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 18.Gautier E, Ganz K, Krugel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br. 2000;82:679–683. doi: 10.1302/0301-620X.82B5.10426. [DOI] [PubMed] [Google Scholar]
- 19.Graves ML, Mast JW. Femoroacetabular impingement: do outcomes reliably improve with surgical dislocations? Clin Orthop Relat Res. 2009;467(3):717–723. doi: 10.1007/s11999-008-0648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt D, Clohisy JC, Prather H. Acetabular labral tears of the hip in women. Phys Med Rehabil Clin N Am. 2007;18:497–520. doi: 10.1016/j.pmr.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Ito K, Minka MA, II, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect: a MRI-based quantitative anatomical study of the femoral head–neck offset. J Bone Joint Surg. 2001;83B:171–176. doi: 10.1302/0301-620X.83B2.11092. [DOI] [PubMed] [Google Scholar]
- 22.Kempthorne JT, Armour PC, Rietveld JA, Hooper GJ. Surgical dislocation of the hip and management of femoroacetabular impingement: results of the Christchurch experience. ANZ J Surg. 2011;81(6):446–450. doi: 10.1111/j.1445-2197.2010.05489.x. [DOI] [PubMed] [Google Scholar]
- 23.Larson CM, Giveans MR. Arthroscopic debridement versis refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369–376. doi: 10.1016/j.arthro.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M. Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clin Orthop Relat Res. 2004;418:61–66. doi: 10.1097/00003086-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Leunig M, Casillas MM, Hamlet M, Hersche O, Notzli H, Slongo T, et al. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Othop Scand. 2000;71:370–375. doi: 10.1080/000164700317393367. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald S, Garbuz D, Ganz R. Clinical evaluation of the symptomatic young adult hip. Semin Arthroplasty. 1997;8:3–9. [Google Scholar]
- 27.Mason JB. Acetabular labral tears in the athlete. Clin Sports Med. 2001;20:779–790. doi: 10.1016/S0278-5919(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda DK, Carlisle JC, Arthurs SC, Wierks CH, Philippon MJ. Comparative systematic review of the open dislocation, mini-open, and arthroscopic surgeries for femoroacetabular impingement. Arthroscopy. 2011;27(2):252–269. doi: 10.1016/j.arthro.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy J, Noble P, Aluisio FV, Schuck M, Wright J, Lee JA. Anatomy, pathologic features, and treatment of acetabular labral tears. Clin Orthop Relat Res. 2003;406:38–47. doi: 10.1097/00003086-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 30.McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The role of labral lesions to the development of early degenerative hip disease. Clin Orthop Relat Res. 2001;393:25–37. doi: 10.1097/00003086-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Murphy S, Tannast M, Kim YJ, Buly R, Millis MB. Of the adult hip for femoroacetabular impingment: indications and preliminary clinical results. Clin Orthop Relat Res. 2004;429:178–181. doi: 10.1097/01.blo.0000150307.75238.b9. [DOI] [PubMed] [Google Scholar]
- 32.Naal FD, Miozzari HH, Wyss TF, Notzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingment in high-level athletes. AJSM. 2011;39(3):544–550. doi: 10.1177/0363546510387263. [DOI] [PubMed] [Google Scholar]
- 33.Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. AJSM. 2011;39(2):296–303. doi: 10.1177/0363546510384787. [DOI] [PubMed] [Google Scholar]
- 34.Notzli HP, Siebenrock KA, Hempfing A, Ramseier LE, Ganz R. Perfusion of the femoral head during surgical dislocation of the hip. Monitoring by laser Doppler flowmetry. J Bone Joint Surg Br. 2002;84:300–304. doi: 10.1302/0301-620X.84B2.12146. [DOI] [PubMed] [Google Scholar]
- 35.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head–neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg. 2002;84B:556–560. doi: 10.1302/0301-620X.84B4.12014. [DOI] [PubMed] [Google Scholar]
- 36.Peters CL, Schabel K, Anderson L, Erickson J. Open treatment of femoroacetabular impingement is associated with clinical improvement and low complication rate at short-term follow up. Clin Orthop Relat Res. 2010;468:504–510. doi: 10.1007/s11999-009-1152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philippon MJ, Schenker M, Briggs Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):908–914. doi: 10.1007/s00167-007-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philippon MJ, Yen YM, Briggs KK, Kuppersmith DA, Maxwell RB. Early outcomes after hip arthroscopy for femoroacetabular impingement in the athletic adolescent patient: a preliminary report. JPO. 2008;28(7):705–710. doi: 10.1097/BPO.0b013e318186eb2e. [DOI] [PubMed] [Google Scholar]
- 39.Rab GT. The geometry of slipped capital femoral epiphysis: implications for movement, impingement, and corrective osteotomy. J Pediatr Orthop. 1999;19:419–424. doi: 10.1097/00004694-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br. 1999;81:281–288. doi: 10.1302/0301-620X.81B2.8291. [DOI] [PubMed] [Google Scholar]
- 41.Schoenecker PL, Clohisy JC, Millis MB, Wenger DR. Surgical management of the problematic hip in adolescent and young adult patients. JAAOS. 2011;19(5):275–286. doi: 10.5435/00124635-201105000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Shore BJ, Novais EN, Millis MB, Kim YJ. Low early failure rates using surgical approach in healed Legg–Calve–Perthes disease. Clin Orthop Relat Res. 2012;470:2441–2449. doi: 10.1007/s11999-011-2187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siebenrock KA, Schoeniger R, Ganz R. Anterior femoro-acetabular impingement due to acetabular retroversion. Treatment with periacetabular osteotomy. J Bone Joint Surg Am. 2003;85:278–286. doi: 10.2106/00004623-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Siebenrock KA, Wahab KH, Werlen S, Kalhor M, Leunig M, Ganz R. Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Orthop Relat Res. 2004;418:54–60. doi: 10.1097/00003086-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Sink EL, Beaule PE, Sucato D, Kim YJ, Millis MB, Dayton M, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg. 2011;93:1132–1136. doi: 10.2106/JBJS.J.00794. [DOI] [PubMed] [Google Scholar]
- 46.Slongo T, Kakaty D, Krause F, Ziebarth K. Treatment of slipped capital femoral epiphysis with a modified Dunn procedure. J Bone Joint Surg. 2010;92(19):2898–2908. doi: 10.2106/JBJS.I.01385. [DOI] [PubMed] [Google Scholar]
- 47.Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequence: a review. Clin Anat. 2010;23:8–17. doi: 10.1002/ca.20893. [DOI] [PubMed] [Google Scholar]
- 48.Snow SW, Keret D, Scarangella S, Bowen JR. Anterior impingement of the femoral head: a late phenomenon of Legg–Calve–Perthes’ disease. J Pediatr Orthop. 1993;13:286–289. doi: 10.1097/01241398-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Trueta J, Harrison MJM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953; 442–460. [DOI] [PubMed]
- 50.Yun HH, Shon W, Yun JY. Treatment of femoroacetabular impingement with surgical dislocation. Clin Orthop Surg. 2009;1:146–154. doi: 10.4055/cios.2009.1.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziebarth K, Zilkens C, Spencer S, Leunig M, Ganz R, Kim YJ. Capital realignment for moderate and severe SCFE using a modified Dunn procedure. Clin Orthop Relat Res. 2009;467(3):704–716. doi: 10.1007/s11999-008-0687-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlorowicz M, Szczodry M, Czubak J, Ciszek B. Anatomy of the medial femoral circumflex artery with respect to the vascularity of the femoral head. J Bone Joint Surg Br. 2011;93(11):1471–1474. doi: 10.1302/0301-620X.93B11.26993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perthes disease with acetabular dysplasia. a AP pelvic radiograph demonstrated a Perthes-type hip with coxa magna, coxa breva, coxa vara, and a prominent greater trochanter. This also demonstrates acetabular retroversion (cross-over sign) and dysplasia with a diminished lateral center edge angle and elevated acetabular index. b False profile radiograph also demonstrated diminished anterior center edge angle. c, d Dunn view and frog-leg lateral also reveals the aspherical deformity present. Axial (e) and sagittal (f, g) T2 MR arthrogram images demonstrate an anterior and superolateral degenerative labral tear (arrowhead). (JPEG 45 kb)
a AP radiograph demonstrating correction of the femoral head–neck offset, trochanteric height, and acetabular dysplasia. b After removal of hardware demonstrating full osseous union of the trochanteric and pelvic osteotomies. (JPEG 24 kb)
Post-traumatic avascular necrosis. a, b AP pelvis and false profile radiographs revealed central femoral head collapse and extrusion of an anterolateral femoral head fragment. c, d Frog-leg lateral and Dunn views demonstrate diminished head–neck offset and impingement. Three-dimensional (e) and an axial slice (f) CT scan again demonstrated the central femoral head impaction (asterisk) and lateral extrusion with a preserved posteromedial femoral head (arrowhead). (JPEG 40 kb)
a–c Postoperative radiographs demonstrating improvement of the femoral head sphericity and increased femoral head–neck offset. d AP radiograph after removal of prominent hardware demonstrating preservation of the hip joint, 2 years after the reconstruction. (JPEG 29 kb)
Residual SCFE. Radiographs demonstrate a posteriorly displaced femoral head with a prominent anterolateral head–neck junction, with an impingement trough (arrow), in addition to a high greater trochanter in relation to the center of the femoral head. (JPEG 27 kb)
a, b Restoration of the femoral head–neck offset in addition to the trochanteric height. c Removal of the prominence from the head–neck junction. d Relative femoral neck lengthening. e Labral repair. (JPEG 69 kb)
(DOCX 27 kb)