Abstract
Objectives
Inferring the pain level of a critically ill infant is complex. The ability to accurately extract the appropriate pain cues from observations is often jeopardized when heavy sedation and muscular blocking agents are administered. Near-infrared spectroscopy (NIRS) is a noninvasive method that may provide the bridge between behavioral observational indicators and cortical pain processing. We aimed to describe regional cerebral and systemic hemodynamic changes, as well as behavioral reactions in critically ill infants with congenital heart defects (CHD) during chest drain removal following cardiac surgery.
Methods
Our sample included 20 critically ill infants with CHD less than 12 months of age admitted to the cardiac intensive care unit following surgery.
Results
Cerebral deoxygenated hemoglobin concentrations significantly differed across the epochs (i.e. Baseline, Tactile stimulus, Noxious stimulus) (p = .01). Physiological systemic responses and FLACC pain scores differed significantly across the events (p < .01). The three outcome measures were not found to be associated with each other. Mean FLACC pain scores during the painful procedure was 7/10 despite administration of morphine. Midazolam administration accounted for 36% of the variance in pain scores.
Discussion
We demonstrated with a multidimensional pain assessment approach that significant cerebral, physiological and behavioral activity was present in response to a noxious procedure in critically ill infants despite the administration of analgesic treatment. Considering that the sedating agent significantly dampened pain behaviors, assessment of cerebral hemodynamic in the context of pain seems to be an important addition.
1. Introduction
To appropriately respond and treat pain in the non-communicative child, one must be able to accurately extract the appropriate pain cues from observations. Although a wide variety of pain measures exist for assessing pain in non-communicative infants and children, no one measure has been uniformly accepted. Although a considerable body of knowledge now exists, an observational tool that evaluates pain with sound validity, reliability, specificity, and sensitivity remains elusive (1). More recently, researchers have directed their efforts towards improving the psychometric properties of existing pain assessment instruments within various populations, types of pain and contextual factors (2). Clinicians still do not know if the behaviors they observe are specific to pain or if they are manifestations of other experiences such as agitation, distress, fear, stress, or sadness (1;3). This issue is even more problematic when dealing with critically ill infants, in which the expressive capacity is often jeopardized by the administration of heavy sedation and muscular blocking agents.
Most of the available paediatric pain assessment instruments are multidimensional, incorporating both behavioral (facial action, body movement, cry) and physiological (heart rate, respiratory rate, blood pressure, arterial oxygen saturation) indicators. Dissociation between these two classes of indicators has been reported with an average correlation of 0.3 (4–6). Behavioral measures, especially facial actions, are more likely to respond selectively to pain (7), while physiological indicators change in response to painful stimuli, but also for numerous reasons not specific to pain (5;8;9).
Hemodynamic and electrical brain studies into pain processing show potential for exploring these issues (10–12;14–16). One such approach employs continuous near-infrared spectroscopy (NIRS), which may provide the bridge between behavioral observational indicators and cortical pain processing, thus serving as an alternative and perhaps more specific instrument in the measure of pain in critically ill infants. In fact, recent studies in premature and term infants indicated that painful stimuli cause hemodynamic changes in specific cortical regions of the brain (11;14;16).
In this study we report on regional cerebral and systemic hemodynamic changes, as well as behavioral reactions in critically ill infants with congenital heart disease (CHD) during a routine painful procedure. We assessed the cerebrovascular and cardiovascular responses to postoperative chest-drain removal by means of continuous NIRS as well as usual monitoring of heart rate, systemic blood oxygenation, and mean arterial blood pressure. Behavioral reactions were also captured through video recordings and rated for pain retrospectively with the Face Leg Activity Cry Consolability (FLACC) pain scale. Specifically, we examined differences within subjects across phases of the procedure, factors associated with the response (sex, age, weight, and medication), as well as associations between cerebral hemodynamic changes, systemic physiological changes, FLACC pain scores and specific clinical variables.
1.1. Research questions
The following research questions guided this study:
Is a noxious stimulation associated with regional cerebral hemodynamic changes of critically ill infants as measured with NIRS?
Do regional cerebral hemodynamic (as measured with NIRS), physiological (vital signs) and behavioural (as measured with FLACC scale) reactions significantly correlate in response to a noxious stimulation in critically ill infants?
2. Materials and Methods
2.1. Study population
Forty infants less than 12 months who required a surgical procedure for repair of a CHD and who required a chest drain post-operatively were prospectively recruited between April 2009 and May 2010. We excluded infants who were diagnosed with a spinal or peripheral nervous system illness. The research protocol was approved by the Institutional Review Board at Children’s Hospital Boston, USA. We obtained parental written informed consent in all cases, and 89% of the approached parents gave their consent.
2.2. Instrumentation
The infants’ cerebral hemodynamic and associated physiological signals, as well as video recordings were continuously monitored and recorded for approximately 30 minutes surrounding the chest drain removal.
A non-invasive NIRS-based tissue oxygenation monitor (NIRO 300, Hamamastu Photonics, Japan) was used to measure changes in cerebral tissue oxygenation. This NIRS device has two channels, each consisting of an emitting and a receiving optode. When possible, the emitter optode was positioned approximately 2 cm beneath and slightly posterior to the C3/C4 position (according to the international EEG 10–20 system (14;17)); the receiver optode was positioned 4 cm apart from the emitter optode allowing illumination of the primary somatosensory cortex and possibly the underlying structures. When this optode configuration was not possible due to hair coverage, which causes interference with the laser signal, or other obstacles (i.e. intravenous indwelling catheter), optodes were placed in the frontoparietal or temporoparietal regions. Optodes were placed in a flexible rubber holder that prevented interference from external light sources and kept the distance between the emitter and receiver optodes constant at 4 cm.
The cerebral oxygenation signal obtained with the NIRS technique is based on the absorption of near-infrared light by hemoglobin, which in turn depends on the oxygenation state of hemoglobin circulating through the tissues. The NIRS technology employed in this study does not measure the absolute cerebral concentrations in oxygenated hemoglobin ([HbO2]) and deoxygenated hemoglobin ([HbH]), but rather absolute change in concentration from an unknown reference value (17–19). This method has been described in a recent review paper (20). The NIRS device allows for data sampling at 6 Hz.
The systemic arterial oxygen saturation (SaO2), the electrocardiogram (ECG), and arterial blood pressure were recorded continuously from the patient’s bedside monitor (Philips Intellivue MP70, Andover, MA). An indwelling arterial line placed either in the femoral or radial arteries provided continuous monitoring of the arterial blood pressure waveform, from which mean arterial blood pressure (MAP) was computed on a beat-by-beat basis.
2.2.1. Face Leg Activity Cry Consolability (FLACC) scale
The FLACC scale is a unidimensional behavioral pain assessment instrument to measure pain in young children in the post-operative period (21). It includes five items (Face, Leg, Activity, Cry, and Consolability) and has good inter-rater reliability (Kappa 0.52–0.82), as well as good content and convergent validity (21). The FLACC has been shown to be reliable in critically ill young children (22;23).
2.3. Data collection
The analogue signals were passed through a band-pass filter and a notch filter at 60 Hz. The band-pass filter was used for anti-aliasing, and the notch filter to exclude potential 60 Hz artifact common in the intensive care unit (ICU) setting. During measurement recording, cerebral, systemic physiological data, and video were simultaneously transferred to a portable user interface monitor (Component Neuromonitoring System, Day One Medical, LLC, Ambler PA, USA). This device is a computer-based system that continuously records, displays, stores, and analyzes in real-time physiological data from multiple monitoring sources in real-time, allowing data synchronization, display, and analysis.
Equipment set-up and data acquisition were completed by the same study investigator (MR), except on one occasion. The investigator was continuously present during the data-acquisition process to document in the patient’s study file the precise beginning and end of the chest-drain removal procedure and steps leading to the actual noxious stimulation, as well as the nature of all other events (e.g. study events, administration of medication, handling by care provider or parent, etc.) that occurred throughout the study.
2.4. Chest-drain removal procedure
Data collection was carried out in the cardiac intensive care unit (CICU) at the bedside of the patient, and the nurses performing the chest-drain removal were asked to carry out the procedure as usual. No interruption on the part of the study investigator took place. Once the equipment was set up, baseline data were gathered prior to the noxious procedure to allow calibration of NIRS device and adjustments for artefacts. Each procedure was performed according to a standardized care plan. The chest-drain removal procedure comprised a sequence of steps and a variety of sensory stimuli, including: (1) administration of analgesics and/or sedatives (e.g. morphine and/or midazolam) in the majority of the cases, (2) removal of dressing, (3) untying of purse-string sutures around the exit site, (4) removal of the drain at the height of expiration, and (5) pulling tightly on the purse string to allow closure of the exit site, immediately followed by application of a pressure bandage.
Data analysis was divided into three 30-second intervals (epochs); these were precisely marked during recordings by the study investigator on an Excel event log sheet. Once the procedure was completed, all recordings were stopped and the equipment was removed.
2.5. Data selection
Three 30-second epochs that represent quiet baseline (T0), tactile stimulation (T1) (removal of dressing), and removal of chest drain (T2), respectively were documented. The baseline (T0) period during which the infant was calm and not disturbed was captured at approximately 20 to 25 minutes prior to the drain removal. About 5 minutes prior to the removal of the chest drain, a period of tactile stimulation (T1) which was defined by removal of the dressing and preparation of the procedure took place. The epoch corresponding to the noxious stimulus response (T2) was signaled by the unit nurse as soon as the tube began to be pulled on peak expiration by the patient.
2.5.1. Video
Filmed behavioral displays were rated according to the recommended procedure for clinical use of the FLACC scale by a clinical nurse specialist (CNS) in paediatric pain experienced with the use of the instrument and blinded to the purpose of the study. The footages were played back in real time on a personal computer; each of the 30-second segments previously identified by study investigator (MR) was coded for pain scores by the CNS. Thus, the blinded coder generated three pain FLACC scores related to the study epochs, and this for each participant (Cronbach alpha of 0.8). This same procedure was repeated ten weeks after the first ratings were done to test intrarater reliability of 0.9 intraclass correlation coefficient (ICC). A second blinded CNS in paediatric pain coded half of the sample to establish interrater reliability of 0.86 (0.71–0.96) (ICC).
2.6. Data pre-processing and statistical analysis
For ease of data handling, the physiological signals were subsequently down-sampled to 20 Hz prior to further analysis. From the raw data we computed average [HbO2], [HbH], HR, MAP, and SaO2 values for each 30-second epoch. For ease of naming, each variable will be referred to by its name without mentioning “mean”. We also computed average changes for each signal (Δ[HbO2], Δ[HbH], ΔHR, ΔMAP, and ΔSaO2) between the different event epochs: Tactile vs. Baseline (T1–T0), Noxious vs. Baseline (T2-T0), and Noxious vs. Tactile (T2-T1).
A custom Matlab script was used to process the raw recordings and analyze the data (Matlab Student version 7.0, The Mathworks, Natick, MA). To determine whether chest-drain removal procedures induced statistically significant cerebral and systemic hemodynamic changes compared to the other epochs, a repeated measure ANOVA was conducted to compare [HbO2], [HbH], HR, MAP, and SaO2 responses, as well as FLACC pain scores between the epochs. Sex was added as a between-subject factor. Univariate linear regression analyses were used to verify associations between changes in NIRS signal values (Δ[HbO2] and Δ[HbH]), systemic physiological responses (ΔHR, ΔMAP, ΔSaO2), FLACC pain score changes, analgesic medication doses, age, weight, pediatric risk of mortality score (PRISM III) (24), and elapsed time since surgery of chest- drain removal.
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 15.0 (IBM, Somers, NY); p-values of less than 0.05 were considered significant, and Bonferroni correction was used to adjust for multiple comparison. Mauchly’s test was used to verify the assumption of sphericity, and if violated, degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity.
3. Results
3.1. Population
The families of 45 infants were approached in the pre-operative cardiac clinic to participate in the study. Forty infants were enrolled, and 32 (18 males; 14 females) participated in the immediate post-operative period. Eight recruited participants were not recorded because chest drain removal was missed (n = 6) or the patient was transferred to another unit prior to procedure (n = 2). Although all 32 infants were filmed, we were able to capture good quality video recordings of the procedure to enable behavioral pain coding in only 20 infants (12 males; 8 females). These participants comprised the sample for the present study, though not all 20 recorded data sets could be used in the analysis due to equipment issues, loss of signal, or excessive noise in the recordings. Twenty NIRS data sets for left hemisphere and 19 for right hemisphere were analyzed, 20 for HR, and 18 for SaO2. Fourteen infants had an indwelling arterial catheter allowing for continuous MAP monitoring. Infants’ characteristics are presented in Table 1.
Table 1.
Demographic and clinical characteristics of our cohort (N= 20)
| Mean age (months) | 3.7 ±1.4; range 1.5–7 |
| Mean weight (Kg) | 5.5 ±1.6; range 2.8–10 |
| Mean PRISM III scores | 8.5 ± 3.9; range 3–18 |
| Diagnosis: TOF (+ pulmonary stenosis) VSD &VSD/PFO ASD/VSD CAVC Combination of 2 or more Other |
n = 7 n = 5 n = 3 n = 1 n = 2 n = 2 |
| Mean morphine dose mg/kg/dose | 0.05; range 0.0–0.2 (median 0.05) |
| Mean midazolam dose mg/kg/dose | 0.04; range 0.0–0.1 (median 0.05) |
| Mean time from surgery at noxious procedure | 32.9 hours; range 16–96 (median 24.5) |
| Location of chest drain | Midline (n= 16) Right (n= 4) |
| Type of chest drain | Chest tube (n = 2); Blake drain (n = 18) |
| Mean maximum pain score in last 24h (n = 18) | 4.5/10; range 0–7 |
PRISM III: Pediatric RISk of Mortality; TOF: Tetralogy of Fallot; VSD: Ventricular septal defect; ASD: Atrial septal defect; PFO: Patent foramen ovale; PDA: Patent ductus arteriosis; CAVC: Complete Atrioventricular Canal; Combination: VSD/ASD/PDA, CAVC/TOF; Other: Aortic root aneurysm, hypoplastic left heart syndrome, Transitional AV canal; pain score was taken from patients’ charts.
The 20 infants underwent cardiopulmonary bypass for various CHD. Seven children had a co-morbid condition with the most common being Trisomy 21 in five patients (3 females; 2 males), one had a connective tissue disorder and one had a syndrome associated with digital abnormality not well characterized otherwise. During chest-drain removal, 35% of the participants were supported by a conventional ventilator (n = 6) or continuous positive airway pressure (CPAP) (n = 1). Cortical somatosensory placement of optodes was not achieved in six participants because of hair or access issues. In these 6 cases, optodes were placed in the frontotemporal and temporoparietal regions.
3.2. Cerebral NIRS changes
In response to chest drain removal, significant increases in regional cerebral deoxygenated hemoglobin concentrations [HbH] were found. Specifically, the right hemisphere [HbH] differed significantly between the different 30-second epochs F(2, 36) = 5.07 (p = .01). Within-subject pairwise comparisons showed significant differences between Baseline (T0) and Noxious (T2) [HbH] (p = .04) in the right hemisphere (Figure 1). We found no significant changes in the left hemisphere [HbH] or bilateral cerebral regional oxygenated hemoglobin [HbO2]. Optode placement or chest tube location were not determining factors in cerebral hemodynamic responses.
Figure 1.
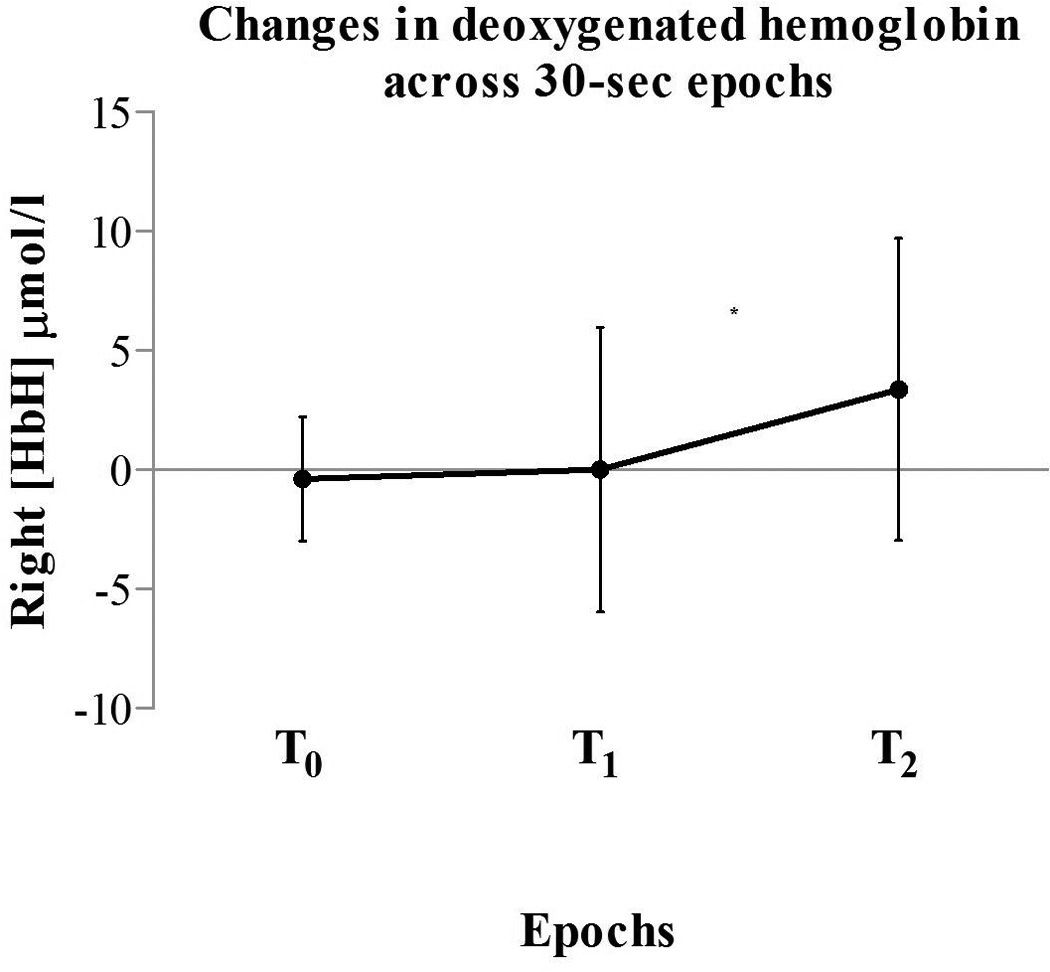
Changes in right hemisphere [HbH] values in all subjects (n = 19). The figure shows significant [HbH] increases from baseline (T0) to noxious stimulation (T2) *p < 0.05. Vertical bars denote the standard deviations and the middle points denote the mean values.
T0: baseline; T1: tactile stimulation; T2: noxious stimulation.
3.3. Systemic physiological changes
In response to chest-drain removal, we found significant increases in systemic mean arterial blood pressure (MAP). MAP differed significantly between the different 30-second epochs F(1.38, 16.52) = 19.18 (p < .001). Within-subject pairwise comparisons showed significant differences between the three 30-second epochs: T2 and T0 MAP (15.75 CI [6.62, 24.89]; p = .001); T2 and T1 MAP (p = .005); T0 and T1 MAP (p = .012).
Heart rate (HR) differed significantly between the different 30-second epochs F(1.28, 24.27) = 6.87 (p = .01). Within-subject pairwise comparisons showed significant differences between T2 and T0 HR (11.35 CI [1.27, 21.42]; p = .02). No other significant differences were found; systemic arterial blood oxygenation (SaO2) level was not found to differ significantly across the 30-second epochs.
3.4. FLACC pain scores
Mean FLACC pain scores at the three epochs were 0.25 (SD 0.12); CI [0.01, .51] at baseline, at tactile 3.25 (SD 0.56); CI [2.08, 4.23], and at noxious 6.7 (0.66); CI [5.32, 8.08]. Overall, FLACC pain scores differed significantly between the different 30-second epochs F(1, 19) = 102.64 (p < .001) (Table 2; Figure 2). Female infants had significantly higher FLACC scores than males F(1, 18) = 9.65 (p = .006) across all events. In addition to the standard pharmacological treatment, two participants received sucrose prior to chest-drain removal and both had 9/10 FLACC pain scores in response to the noxious procedure.
Table 2.
FLACC pain scores regression analysis (n = 20)
| Epochs | Mean difference | 95% CI | p-value |
|---|---|---|---|
| T1-T01 | 3.00 | [1.49, 4.51] | < .001 |
| T2-T02 | 6.45 | [4.65, 8.25] | < .001 |
| T2-T13 | 3.45 | [1.65, 5.25] | < .001 |
For Tactile stimulation - Baseline
For Chest-drain removal - Baseline
For Chest-drain removal - Tactile stimulation
T0 baseline; T1 tactile stimulus; T2 noxious stimulation (chest-drain removal).
Figure 2.
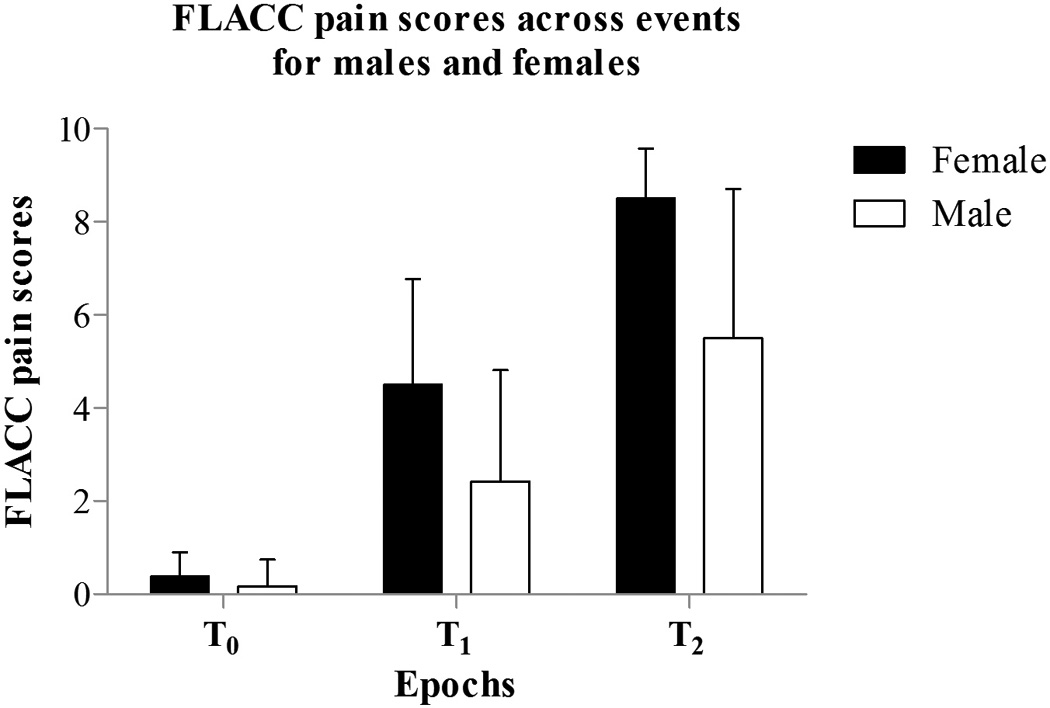
Mean FLACC pain scores for baseline (T0), tactile (T1), and noxious (T2) events between males (n = 12) and females (n = 8). The figure shows significant differences in overall FLACC scores across the three events (p < 0.001) and between males and females (p < 0.01).
3.5. Changes in NIRS, physiological signals and pain scores across 30-second epochs
We performed univariate analyses to explore the association among study parameters related to changes (Δ) observed during the different 30-second epochs, as well as exploring the relationship between these and clinical factors. Table 3 shows those relationships that achieved statistical significance on univariate analysis. Only left hemisphere Δ[HbO2] was associated with ΔHR, explaining 32% of the change. No other NIRS signal change across the three 30-second epochs was found to be significantly correlated with physiological signals. Total FLACC pain scores and its five indicators were not significantly associated with the NIRS and physiological signal changes across the different epochs.
Table 3.
Relationship (p value) between cerebral and physiological measures, and clinical factors (univariate analysis) during chest-drain removal.
| Parameter | Δ[HbO2] | Δ[HbH] | ΔMAP | ΔHR | ΔSaO2 |
|---|---|---|---|---|---|
| T1–T0 | |||||
| ΔMAP | |||||
| ΔHR | 0.19 (.035)1 | ||||
| ΔSaO2 | |||||
| Morphine | −137.78 (.005)1 | ||||
| Midazolam | −114.87 (.017)1 | −71.27 (.012) | |||
| Age | |||||
| Weight | 4.6 (.032) | ||||
| T2–T0 | |||||
| ΔMAP | |||||
| ΔHR | |||||
| ΔSaO2 | |||||
| Morphine | |||||
| Midazolam | |||||
| Age | −1.94 (.044)1 | ||||
| Weight | 3.93 (.029) | −1.46 (.002) | |||
| T2–T1 | |||||
| ΔMAP | |||||
| ΔHR | |||||
| ΔSaO2 | |||||
| Morphine | −82.54 (.002)1 | −90.24 (.022) | |||
| Midazolam | 105.97 (.029)1 | −63.7 (.015)1 −137.49 (.008)2 |
−146.63 (.005) | ||
| Age | 2.9 (.044) | ||||
| Weight | −1.11 (.017) | ||||
Left hemisphere;
Right hemisphere;
[HbO2]: oxygenated hemoglobin concentration; [HbH]: deoxygenated hemoglobin concentration; MAP: mean arterial blood pressure; HR: heart rate; SaO2 arterial blood oxygen saturation.
T0 baseline; T1 tactile stimulus; T2 noxious stimulation (chest-drain removal).
In response to chest-drain removal, infants with the comorbid condition Trisomy 21 did not differ significantly in their cerebral and physiological hemodynamic response or FLACC pain scores when compared to the rest of the sample. Infants’ age and weight significantly correlated with some of the measured variables. Results from the univariate analyses are found in Table 3.
3.5.1. Effects of medication
The administration of analgesic medication (morphine) was significantly associated with less changes in [HbO2] (β −137.78, p = .005) and [HbH] (β −82.54, p = .002). Morphine doses also decreased the response in HR (β −90.24, p = .022). The administration of analgesic was not significantly associated with FLACC pain scores changes.
Sedation medication administration (midazolam) was associated with significantly less changes in left hemisphere [HbO2] and bilateral [HbH] (β −114.87, p = .017; β −63.7, p = .015; β −137.49, p = .008). Midazolam doses also significantly decrease the responses of the HR and SaO2 (β −146.63, p = .005; β −71.27, p = .012). Administration of a sedative was significantly correlated with less FLACC pain scores changes between T0 and T2 (−65.76; p = .005) and 19% of the changes between T0 and T1 (−40.53; p = .05). These data are presented in Table 3.
3.5.2. Individual changes between T2-T0
For comparison purposes, we divided the sample in two groups: (1) the moderate to severe pain group (FLACC pain scores ≥ 5) and (2) the no pain to mild pain group (FLACC pain scores ≤ 4). For the first group, mean FLACC scores were 8/10, mean cerebral hemodynamic (i.e. [HbO2] and [HbH]) varying from −0.6 to 4.7 µmol/l with large standard deviations, and smaller mean doses of morphine/midazolam. The second group received higher mean doses of these same drugs with lower mean FLACC scores 2/10, and mean cerebral hemodynamic changes between −0.33 to 3.3 µmol/l. These results can be found in the supplemental Tables S1 and S2 which show the results of the descriptive analyses of individual cerebral hemodynamic and FLACC score changes from baseline to chest-drain removal.
3.6. Sex disparity
Sex of the patient was found to be a determining factor in the cerebral and behavioral responses (supplemental Figures S1 and S2). Females responded more strongly than males across the events in the right hemisphere [HbO2] F(1, 17) = 4.3 (p = .05). As stated previously, female infants had significantly higher pain scores across the events as well (Figure 2). We found that the change in FLACC scores of males from T0 to T2 was significantly dampened by the midazolam administered doses (β = −70.43, CI [−134.43, −6.43], p = .03; r(11) = −0.61). This was not the case in female infants.
4. Discussion
We demonstrated varying cerebrovascular and systemic hemodynamic changes, as well as pain behavioral manifestations in response to chest-drain removal in critically ill infants with CHD following open heart surgery. We demonstrated the gender differences: females showed significantly stronger cerebral hemodynamic response and higher pain scores across the events when compared to males. Finally, we failed to find significant associations between these three multidimensional “pain” parameters.
Previous studies that have reported on cerebral hemodynamic responses to noxious stimuli have focused on preterm and term born neonates (11;14;16). These studies showed significant bilateral mean increases in [HbO2] (14) or in the maximum change in the contralateral total hemoglobin concentrations ([HbT] = [HbO2] + [HbH]) (11;16) when compared to baseline. None of these studies reported on the measured changes in the [HbH] or incorporated pharmacological treatment for pain into their analyses, therefore making comparison with our findings difficult. Regional cerebral activation typically results in regional increases in both [HbO2] and [HbT] with a decrease in [HbH] (25). However, contrasting results have been reported, including no change or increases in [HbH] with increases in both [HbO2] and [HbT] (26–28).
To date, there is only one study reporting on associations between pain scores and cerebral hemodynamic responses in infants. In twelve preterm and term neonates (measured on 33 occasions), Slater et al. (11) found moderate correlations (r = .57; p = .001) between scores of a multidimensional pain scale (Premature Infant Pain Profile- PIPP (29)) and maximum change in the contralateral [HbT] in response to heel lance. Moreover, they showed that the cortical response had a stronger association with the behavioral components of the PIPP (i.e. facial expression) compared with the physiological components (i.e. HR and SaO2 changes from baseline) (r = .4; p = .04). Although some correlations between the behavioral FLACC pain scores and cerebral hemodynamic responses were present in our study, they did not attain statistical significance. However, this is most certainly due to lack of power since in order to attain a statistically significant correlation between any of these signals, a minimal association of 0.55 was needed. Similar to our finding, Gelinas et al. (30) did not find significant associations between behavioral pain scores, adult patient’s self-report, and regional cerebral oxygenation (rSO2) during nociceptive procedures, although taken individually, these parameters did show significant changes from their baseline measure.
Recently Frank et al. (31), recommended that the COMFORT (32) pain scale be used as the main pain assessment instrument for recognizing post-operative and procedural pain in the critically ill neonate when compared to three other tools. The COMFORT scale was best at identifying analgesic effectiveness. This composite pain assessment instrument may be more suitable for critical care contexts where significant clinical factors (e.g. inotropes, ventilator support, etc) can impact pain measures (31). The FLACC scale, not designed specifically for this population, is not sensitive enough to these and other factors.
Experimental findings have consistently demonstrated that women experience pain more intensely than men do (33–35). Nonetheless, the lower pain scores found in male infants in our study population could be explained by the significant impact (i.e. r = −.61) that midazolam had on their behavioral manifestations when compared to females; thus dampening males’ capacity to express their pain experience. The exact mechanism underlying this gender difference and the role of midazolam calls for further investigations with a larger sample to insure reproducibility of these findings. As many physiological, structural, hormonal, psychological, and environmental factors influence how the human brain responds to noxious stimulation, whether and how these explain the gender differences in brain activation that we found would require a much larger sample size than was available in our study (36).
Although we expected some level of pain in response to chest-drain removal, we did not anticipate the mean FLACC pain scores to be severe (7/10) since nearly all infants (n = 19) received an analgesic. Additionally, the sedating agent’s (midazolam) ability to blunt/dampen the behavioral response to pain should be taken into account when conducting pain evaluations solely on the basis of behavioral manifestations. Although cerebral hemodynamics responses were decreased by pharmacological treatments, changes in [HbO2] and [HbH] irrespective of hemisphere were still present in the infants with lower FLACC pain scores (e.g. FLACC 1/10 with Δ[HbO2] Left, Right = 6.32, 1.12 and Δ[HbH] Left, Right = 7.29, 3.79 µmol/l). Thus, adding cerebral measurements to a behavioral assessment may complement the pain evaluation when there is a risk of dampened behaviors, e.g., highly sedated or paralyzed patients. These data underscore the need for further investigations of pain responses in non-communicative patients which combine simultaneous cerebral and behavioral assessment.
A recent study by Slater et al. (10) demonstrated that neonates were still showing somatosensory neuronal activity after receiving sucrose, a treatment known to be effective for reducing procedural pain response (37), even though their behavioral pain scores were significantly lowered. The effectiveness of this widely used non-pharmacological pain treatment was questioned, but the validity of the behavioral pain measure was not questioned. This discrepancy stresses the importance of assessing the two dimensions of pain (38–40): the sensory dimension as reflected by somatosensory hemodynamic activity and the emotional dimension as reflected by behavioral manifestations. Each dimension may be more differentially sensitive to non-pharmacological and pharmacological treatments. This again emphasizes the necessity of using a multidimensional approach to completely capture the pain experience, especially in non-verbal critically ill populations.
Several limitations of our study are apparent. First or foremost, our sample size limited the extent of our analyses and conceivably our ability to obtain statistically significant associations between cerebral hemodynamic changes and behavioral pain scores. Additionally, we could not perform multivariate statistical tests which would have allowed us to describe more precisely interactions between our variables and possibly important clinical indicators. Second, optode placement and consistent interrogation of the same brain regions is a major concern with NIRS. Currently multichannel NIRS devices that cover multiple regions of the scalp are not readily available but their use in adult and older children functional NIRS research is increasing in popularity (25;41–44).
Controlling for movement artefact and possible confounding environmental factors in this clinical research, although very difficult, represents an additional limitation to the study. However, since the purpose of this research was to explore, through the use of NIRS technology, cerebral hemodynamic changes to a frequently performed noxious procedure in critically ill patients, we wanted to capture changes in a routine clinical setting. For this reason, we elected not to interfere with standard care and apply a more strict control over possible confounding factors.
Although understanding of the multidimensional experience of pain has advanced over the last century, avenues remain unexplored, particularly in vulnerable populations such as the non-communicative patients. Near-infrared spectroscopy has potential as a technique for assessing pain evoked cerebral activation in critically ill infants. Given the complexity of NIRS technology and known limitations (20), at his stage it may be best to consider this neurodiagnostic technique solely as a research tool. Through further research, we will improve our understanding of pain perception, increase the psychometric features of currently available pain assessment instruments, and perhaps assess the efficacy of pharmacological and non-pharmacological treatments. Determining what constitutes a clinically significant change in the measured parameters (i.e. [HbO2], [HbH], and [HbT]) when compared with normal fluctuations that occur in the brain tissue by sampling both healthy and critically ill infants of differing developmental ages is also needed. Therefore, setting standards for specific use in pain measurement could facilitate the generalization of findings.
A major question that is raised by clinicians caring for non-communicative patients is whether this technique will move beyond research to become a bedside monitoring technique for pain assessment. Moreover, can this technology help us monitor the cerebral hemodynamic changes due to prolonged pain versus acute procedural pain, and can it make this distinction. When looking at the research findings and current available devices, this may seem improbable. Nevertheless, this approach should not be abandoned since its usefulness as a portable means to functional brain mapping is evolving well and setbacks are being resolved (41–42).
Using a multidimensional pain assessment approach, we demonstrated that significant cerebral, physiological and behavioral activity was present in response to a noxious procedure in critically ill infants despite the administration of analgesic treatment. The fact that the average FLACC scores were high and that the sedative agent, rather than analgesic treatment, reduced the pain ratings significantly may urge health-care professionals to question current pain management protocols during chest-drain removal in critically ill infant. Despite low pain scores in some infants, this group still showed relatively high cerebral hemodynamic activity in response to chest-drain removal. Hence, there is additional information from brain activity in these circumstances. Dampened behaviors by a sedating agent during a procedure known to be painful may jeopardize the discriminate validity of certain behavioral cues. This is especially relevant when heavy sedation and/or blocking agents are administered. Further research using an approach that combines cerebral and behavioral evaluation is warranted.
Acknowledgements
This work was supported in part by the United States National Institutes of Health through grants R01EB001659, K24NS057568, and R21HD056009 by the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, and the National Institute of Child Health and Human Development, respectively. Further support was provided by the Lifebridge Fund. M. Ranger was supported by doctoral and international Fellowships from the Quebec Health Research Fund and the Quebec Interuniversity Nursing Intervention Research Group. We are very thankful to the nurses of the pre-operative clinic and CICU at Children’s Hospital Boston and families of patients, whose generous help and participation made this work possible. We also thank our colleagues Dr. Mustafa Sulemanji and Ms. Heather O’Leary for significant contributions and guidance in the performance of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Figure S1. Mean changes in left hemisphere [HbO2] across events in males (n = 12) and females (n = 8). Vertical bars denote the standard deviations and the middle points denote the mean values. T0: baseline; T1: tactile stimulation; T2: noxious stimulation.
Figure S2. Mean changes in right hemisphere [HbO2] across events in males (n = 11) and females (n = 8). Vertical bars denote the standard deviations and the middle points denote the mean values. T0: baseline; T1: tactile stimulation; T2: noxious stimulation.
Reference List
- 1.Buttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Pediatr Anesth. 2000;10:303–318. doi: 10.1046/j.1460-9592.2000.00530.x. [DOI] [PubMed] [Google Scholar]
- 2.Stevens B, McGrath P, Gibbins S, et al. Determining behavioural and physiological responses to pain in infants at risk for neurological impairment. Pain. 2007;127:94–102. doi: 10.1016/j.pain.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Ramelet AS, Abu-Saad HH, Rees N, et al. The challenges of pain measurement in critically ill young children: a comprehensive review. Aust Crit Care. 2004;17(1):33–45. doi: 10.1016/s1036-7314(05)80048-7. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG. Reflections on measuring pain in infants: dissociation in responsive systems and "honest signalling". Arch Dis Child Fetal Neonatal Ed. 1998;79:F152–F156. doi: 10.1136/fn.79.2.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens BJ, Johnston CC, Horton L. Multidimensional Pain Assessment in Premature Neonates: A Pilot Study. J Obstet Gynecol Neonatal Nurs. 1993;22:531–541. doi: 10.1111/j.1552-6909.1993.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnston CC, Stevens BJ, Yang F, et al. Differential response to pain by very premature neonates. Pain. 1995;61:471–479. doi: 10.1016/0304-3959(94)00213-X. [DOI] [PubMed] [Google Scholar]
- 7.Craig KD, Whitfield MF, Grunau RVE, et al. Pain in the preterm neonate: behavioural and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 8.Johnston CC, Stevens BJ, Franck LS, et al. Factors Explaining Lack of Response to Heel Stick in Preterm Newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 9.Sweet SD, McGrath PJ. Physiological Measures of Pain. In: Finley GA, McGrath PJ, editors. Measurement of Pain in Infants and Children. Seattle: IASP Press; 1998. pp. 59–81. [Google Scholar]
- 10.Slater R, Cornelissen L, Fabrizi L, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. 2010;376:1225–1232. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slater R, Cantarella A, Franck L, et al. How well do clinical pain assessment tools reflect pain in infants? PLoS Medicine / Public Library of Scienc. 2008;5(6):e129. doi: 10.1371/journal.pmed.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slater R, Worley A, Fabrizi L, et al. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14:321–326. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Bartocci M, Bergqvist LL, Lagercrantz H, et al. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1–2):109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Sava S, Lebel AA, Leslie DS, et al. Challenges of functional imaging research of pain in children. Mol Pain. 2009;5:30. doi: 10.1186/1744-8069-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater R, Cantarella A, Gallella S, et al. Cortical Pain Responses in Human Infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartocci M. Brain Functional Near Infrared Spectroscopy in Human Infants. Karolinska Institutet; 2006. [Google Scholar]
- 18.Soul JS, du Plessis AJ. Near-infrared spectroscopy. Seminars in Pediatric Neurology. 1999;6:101–110. doi: 10.1016/s1071-9091(99)80036-9. [DOI] [PubMed] [Google Scholar]
- 19.Wolfberg AJ, du Plessis AJ. Near-Infrared Spectroscopy in the Fetus and Neonate. Clinics in Perinatology. 2006;33:707–728. doi: 10.1016/j.clp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Ranger M, Johsnton CC, Limperopoulos C, et al. Cerebral Near-Infrared Spectroscopy as a Measure of Nociceptive Evoked Activity in Critically Ill Infants. Pain Res Manag. 2011;16(5):331–336. doi: 10.1155/2011/891548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkel SI, Voepel-Lewis T, Shayevitz JR, et al. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–297. [PubMed] [Google Scholar]
- 22.Merkel S, Voepel-Lewis T, Malviya S. Pain assessment in infants and young children: the FLACC scale. Am J Nurs. 2002;102(10):55–58. doi: 10.1097/00000446-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Voepel-Lewis T, Zanotti J, Dammeyer JA, et al. Reliability and Validity of the Face, Legs, Activity, Cry, Consolability Behavioral Tool in Assessing Acute Pain in Critically Ill Patients. Am J Crit Care. 2010;19:55–61. doi: 10.4037/ajcc2010624. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24 doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40:511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- 26.Hoshi Y, Tamura M. Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man. Neurosci Lett. 1993;150:5–8. doi: 10.1016/0304-3940(93)90094-2. [DOI] [PubMed] [Google Scholar]
- 27.Kato T, Kamei A, Takashima S, et al. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. J Cereb Blood Flow Metab. 1993;13:516–520. doi: 10.1038/jcbfm.1993.66. [DOI] [PubMed] [Google Scholar]
- 28.Kleinschmidt A, Obrig H, Requardt M, et al. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. J Cereb Blood Flow Metab. 1996;16:817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Stevens B, Johnston C, Petryshen P, et al. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12(1):13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Gelinas C, Choiniere M, Ranger M, et al. Toward a new approach for the detection of pain ináadult patients undergoing cardiac surgery: Near-infrared spectroscopy--A pilot study. Heart Lung. 2011;39:485–493. doi: 10.1016/j.hrtlng.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Franck LS, Ridout D, Howard R, et al. A comparison of pain measures in newborn infants after cardiac surgery. Pain. 2011;152:1758–1765. doi: 10.1016/j.pain.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Ambuel B, Hamlett KW, Marx CM, et al. Assessing distress in pediatric intensive care environments: the COMFORT scale. J Ped Psychol. 1992;17(1):95–109. doi: 10.1093/jpepsy/17.1.95. [DOI] [PubMed] [Google Scholar]
- 33.Riley JL, III, Robinson ME, Wise EA, et al. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 34.Fillingim RB, King CD, Ribeiro-Dasilva MC, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goffaux P, Michaud K, Gaudreau J, et al. Sex differences in perceived pain are affected by an anxious brain. Pain. 2011;152:2065–2073. doi: 10.1016/j.pain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Derbyshire SWG. Gender, Pain, and the Brain. Pain: Clinical Updates. 2008;16:1–4. [Google Scholar]
- 37.Stevens B, Yamada J, Ohlsson A. Cochrane Database of Systematic Reviews (1):CD001069, 2010;[Update of Cochrane Database Syst Rev. 2004;(3):CD001069; PMID: 15266438] Sucrose for analgesia in newborn infants undergoing painful procedures. [DOI] [PubMed] [Google Scholar]
- 38.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 39.International Association for the Study of Pain. Editorial The need of a taxonomy. Pain. 1979;6:247–252. [Google Scholar]
- 40.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becerra L, Harris W, Joseph D, et al. Diffuse optical tomography of pain and tactile stimulation: Activation in cortical sensory and emotional systems. NeuroImage. 2008;41:252–259. doi: 10.1016/j.neuroimage.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoshi Y, Chen SJ. Regional cerebral blood flow changes associated with emotions in children. Pediatr Neurol. 2002;27:275–281. doi: 10.1016/s0887-8994(02)00432-0. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63:921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci Biobehav Rev. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]


