SUMMARY
Candida albicans causes the majority of invasive candidiasis in immunocompromised adults while Candida parapsilosis is a leading cause of neonatal candidiasis. While much work has focused on how the immune system recognizes and responds to C. albicans, less is known about host interaction with C. parapsilosis. This study investigates the human neutrophil phagocytic response to these species. Neutrophils underwent phagocytosis of C. parapsilosis yeast and C. albicans hyphae much more efficiently than C. albicans yeast. Treatment of neutrophils with a galectin-3 (gal3) blocking antibody inhibited phagocytosis of C. parapsilosis yeast and C. albicans hyphae, but not C. albicans yeast. The majority of neutrophil gal3 was expressed intracellularly and was secreted from neutrophils after treatment with C. parapsilosis mannan. When neutrophils were treated with exogenous gal3, phagocytosis of both C. albicans and C. parapsilosis yeast increased. Exposure of neutrophils to C. parapsilosis yeast increased phagocytosis of C. albicans yeast and was inhibited by gal3 blocking antibody. Taken together, these data indicate that gal3 secreted from neutrophils may act as a proinflammatory autocrine/paracrine signal in neutrophil phagocytosis and suggest that gal3 has a unique role in neutrophil response to C. parapsilosis yeast and C. albicans hyphae distinct from C. albicans yeast.
INTRODUCTION
The Candida genus includes opportunistic pathogens that cause life threatening disease in immunocompromised individuals. Systemic infections with these fungi are associated with high morbidity and mortality rates, even with antifungal treatment (Bassetti et al., 2010, Benjamin et al., 2010). Historically, C. albicans has been the leading cause of invasive nosocomial fungal infections; however the incidence of infections involving non-albicans species has dramatically increased (Blyth et al., 2009, Bassetti et al., 2010, Falagas et al., 2010). Although C. albicans remains the most common cause of invasive candidiasis in immunocompromised adults (Pfaller et al., 2010), C. parapsilosis causes 15.5–67% of invasive candidiasis in premature newborn infants, often outranking C. albicans in neonates (Spiliopoulou et al., 2012). This species-dependent predilection to cause disease in specific patient groups may be due in part to differences in the patients’ immune recognition and response to these specific pathogens. Understanding how an intact immune system recognizes and responds to these separate species will facilitate our understanding of unique patient susceptibilities.
Traditionally, studies focusing on host-Candida interactions have used C. albicans as a model organism. Although substantially advancing our understanding of fungal host defense, the assumption that the immune system recognizes and responds to other Candida species similarly to C. albicans has recently been challenged. For example, we have shown that neutrophils phagocytose C. parapsilosis much more efficiently than C. albicans yeast (Linden et al., 2010) while others have shown that dendritic cells produce more fungipods when exposed to C. parapsilosis than when exposed to C. albicans or C. tropicalis (Neumann et al., 2010). In addition, macrophages preferentially phagocytose C. glabrata over C. albicans (Keppler-Ross et al., 2010) and phagocytose C. glabrata and C. lusitaniae more efficiently than C. albicans (Dementhon et al., 2012). These differences in cellular responses to different species taken together with the unique susceptibility of specific patient groups highlight the need to study fungal pathogenesis in a species dependent manner.
The host recognizes and responds to invading pathogens by recognizing specific pathogen associated molecular patterns (PAMPs). Fungal PAMPs are generally components of the carbohydrate rich cell wall and include chitin, β-glucan, and mannan structures. PAMPs are recognized by pathogen recognition receptors (PRRs) which are found on a wide array of effector cells and include C-type lectin receptors such as dectin-1, toll-like receptors, and integrins such as complement receptor 3 (CR3) (Netea et al., 2008, Netea et al., 2010). Recently, galectin-3 (gal3), an S-type (soluble) lectin receptor which recognizes specific β-(1-2) oligomannans (Fradin et al., 2000, Kohatsu et al., 2006), has emerged as an important co-receptor in distinguishing non-pathogenic from pathogenic fungi in macrophages (Jouault et al., 2006, Esteban et al., 2011). Gal3 plays a critical role in influencing immunity to many different types of microbial infections (Bernardes et al., 2006, Farnworth et al., 2008, Ferraz et al., 2008, Nieminen et al., 2008, Ruas et al., 2009). Found in numerous cell types, gal3 influences the function of innate immune cells including neutrophils, monocytes, macrophages, endothelial cells and epithelial cells (Henderson et al., 2009, Sato et al., 2009). This lectin contains a carbohydrate recognition domain (CRD) at the C-terminus (Seetharaman et al., 1998) while the N-terminus, also known as the regulatory domain, contains multiple repeats capable of oligomerization after CRD binding (Hsu et al., 1992, Ahmad et al., 2004).
In this study, we examine the role of gal3 in neutrophil phagocytosis of C. parapsilosis and C. albicans. We show that gal3 is involved in neutrophil phagocytosis of C. parapsilosis yeast and C. albicans hyphae, but not C. albicans yeast. In addition, we demonstrate that gal3 is secreted from neutrophils and present data that indicate this secreted gal3 acts as a proinflammatory autocrine/paracrine signal in phagocytosis. Taken together, these data suggest that gal3 has a unique role in the neutrophil response to C. parapsilosis yeast and C. albicans hyphae distinct from C. albicans yeast.
RESULTS
Neutrophils have unique phagocytic responses to C. albicans yeast compared to C. parapsilosis yeast
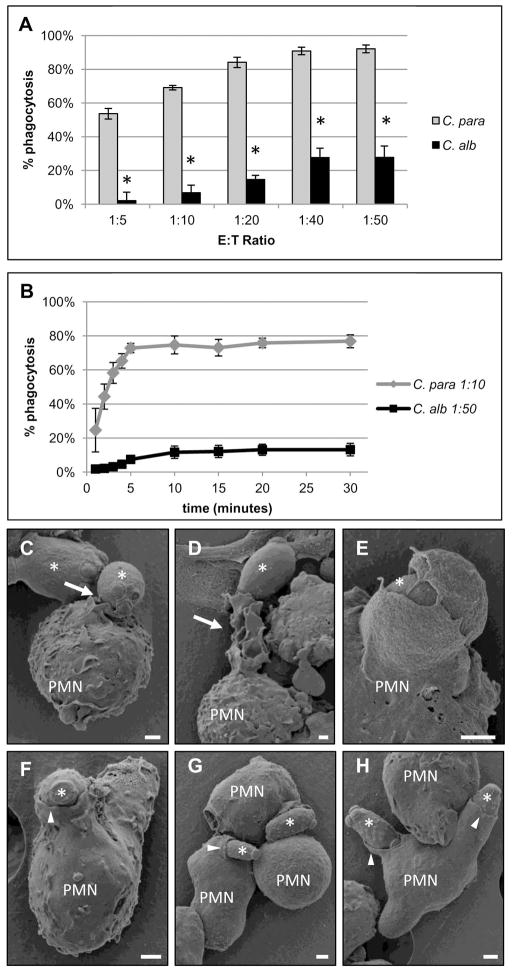
We have previously shown that neutrophils phagocytose C. parapsilosis yeast much more efficiently than C. albicans yeast (Linden et al., 2010). These results were confirmed by comparing phagocytosis rates of C. parapsilosis yeast to C. albicans yeast across a range of effector to target (E:T) ratios. Neutrophils underwent phagocytosis of C. parapsilosis yeast much more efficiently than C. albicans yeast at all E:T ratios (Figure 1A). To evaluate the kinetics, the time course of phagocytosis was investigated. Neutrophils were incubated with C. parapsilosis at an effector to target ratio (E:T) of one neutrophil to ten C. parapsilosis yeast (1:10). Neutrophils were incubated with C. albicans yeast at an E:T ratio of 1:50. Neutrophils were incubated with more C. albicans yeast than C. parapsilosis yeast in an effort to augment phagocytosis rates of the former so that conditions for kinetic comparisons would be as similar as possible. Even when neutrophils were incubated with five times more C. albicans yeast, neutrophils underwent phagocytosis of C. parapsilosis yeast much faster and more efficiently than C. albicans yeast (Figure 1B). The phagocytic process was also evaluated using scanning electron microscopy (SEM). Neutrophils had distinctly different morphological responses to the two species. When incubated with C. albicans for ten min, neutrophils exhibited membrane ruffling (Figure 1C), extrusion of large, arm-like protrusions towards single yeast (Figure 1D) and extension of finger-like projections around engulfed C. albicans yeast (Figure 1E). In response to incubation with C. parapsilosis for 2.5 min, neutrophils exhibited much less membrane ruffling and underwent phagocytosis of multiple yeast at the same time (Figure 1F–H). When incubation time was extended to 10 min with C. parapsilosis yeast, very few ongoing phagocytosis events were seen as would be predicted by the kinetics (Figure 1B), however, no membrane ruffling was observed (data not shown). C. parapsilosis yeast were more elongated and somewhat smaller than C. albicans yeast, (approximately 1×4 microns versus 4×6 microns respectively), which may also influence neutrophil phagocytic efficiency. Taken together however, these data demonstrate that neutrophils respond quite differently to these two Candida species.
Figure 1. Neutrophils have different phagocytic responses to C. parapsilosis compared to C. albicans yeast.
Results are mean ± SEM of at least three different neutrophil donors. (A) Neutrophil phagocytosis rates of C. parapsilosis (C. para) and C. albicans (C. alb) yeast at various effector to target (E:T) ratios. * p ≤ 0.05 comparing C. para and C. alb at that E:T ratio. % phagocytosis was calculated by dividing the number of neutrophils with internalized yeast by the total number of neutrophils. (B) Neutrophil phagocytosis rates of C. parapsilosis (C. para) at an E:T ratio of 1:10 or C. albicans (C. alb) at an E:T ratio of 1:50 at indicated time points. (C–H) Scanning electron microscopy (SEM) photomicrographs of fixed neutrophils after 10 min of incubation with C. albicans (C–E) or 2.5 min of incubation with C. parapsilosis (F–H). Selected neutrophils are labeled (PMN) and yeast are indicated by asterisks. White arrows indicate neutrophil membrane ruffling and pseudopodia extending towards C. albicans yeast. White arrow heads indicate neutrophil membranes smoothly advancing over C. parapsilosis yeast. Bar = 1 micron.
Endogenous galectin-3 plays an important role in neutrophil phagocytosis of C. parapsilosis yeast and C. albicans hyphae, but not C. albicans yeast
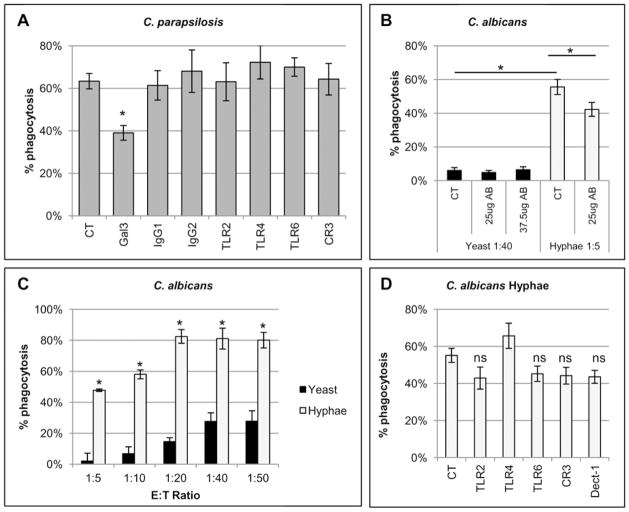
To identify the neutrophil receptors involved in efficient phagocytosis of C. parapsilosis, blocking antibodies against neutrophils PRRs known to recognize different fungal cell wall components were screened for their ability to inhibit phagocytosis. Dectin-1 was excluded from the screen because dectin-1 blocking antibody was previously shown to have no effect (Linden et al., 2010). Significant inhibition of C. parapsilosis phagocytosis was only seen with antibody directed against gal3 at an E:T ratio of 1:10 (Figure 2A). Treatment with the gal3 antibody did not inhibit the low frequency phagocytosis of C. albicans yeast at an E:T ratio of 1:40 (Figure 2B). Neutrophils were incubated with C. albicans yeast at a higher E:T ratio to increase the likelihood that differences in phagocytosis rates after antibody treatment could be observed.
Figure 2. Endogenous gal3 plays a role in neutrophil phagocytosis of C. parapsilosis yeast and C. albicans hyphae.
Results are mean ± SEM of at least three separate neutrophil donors. *p ≤ 0.05 compared to untreated controls (CT) unless otherwise indicated. (A) Phagocytosis rates of C. parapsilosis after neutrophil treatment with 25 μg blocking antibodies at an E:T ratio of 1:10. Blocking antibodies were specific for gal3, toll-like receptor 2 (TLR2), TLR4, and TLR6. Three antibodies were used to block complement receptor 3 (CR3) including antibodies against CD11b domains and CD18. IgG1 and IgG2 were isotype controls. (B) Phagocytosis rates of C. albicans yeast or hyphal forms after neutrophil treatment with a blocking antibody against gal3 at indicated doses at an E:T ratio of 1:40 or 1:5, respectively. (C) Phagocytosis rates of C. albicans yeast or hyphae at various E:T ratios. * p ≤ 0.05 comparing yeast and hyphae at that E:T ratio. (D) Phagocytosis rates of C. albicans hyphae after neutrophil treatment with 25 μg blocking antibodies at an E:T ratio of 1:5. Blocking antibodies were specific for TLR2, TLR4, TLR6 and dectin-1 (Dect-1). Three antibodies were used to block complement receptor 3 (CR3) including antibodies against CD11b domains and CD18. ns=not significant. n=9 independent experiments for CT, TLR2, CR3 and Dect-1 and n=4 for TLR2.
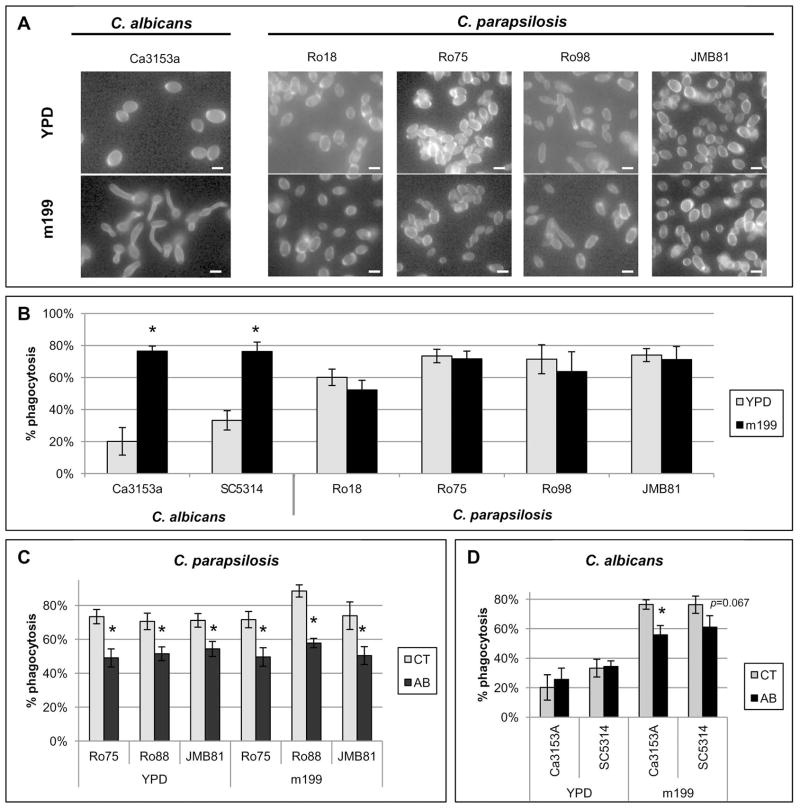
Unlike C. parapsilosis, C. albicans has the capacity to produce true hyphae, a trait necessary for virulence (Lo et al., 1997). Because macrophages have been shown to undergo phagocytosis of C. albicans yeast preferentially over C. albicans hyphae (Keppler-Ross et al., 2010), we also compared neutrophil phagocytosis rates of C. albicans yeast versus C. albicans hyphae. To produce hyphal morphology, C. albicans was grown in medium 199 to promote germ tube formation (Figure 3A). C. albicans germ tubes were phagocytosed much more efficiently than C. albicans yeast, even when neutrophils were incubated with eight times more yeast than hyphal forms (Figure 2B). Neutrophils also underwent phagocytosis of C. albicans hyphae much more efficiently than C. albicans yeast across a range of E:T ratios (Figure 2C). Because of this difference, the role of gal3 in neutrophil phagocytosis of C. albicans hyphal forms was also evaluated. Treatment with the gal3 antibody significantly inhibited neutrophil phagocytosis of C. albicans germ tubes at an E:T ratio of 1:5 (Figure 2B). Again, neutrophils were incubated with more C. albicans yeast than hyphae to ensure that differences in phagocytosis rates after treatment could be observed. Neutrophil treatment with other blocking antibodies against TLR2, TLR4, TLR6, CR3 and dectin-1 also did not significantly affect phagocytosis of C. albicans hyphae. (Figure 2D). To exclude an effect of medium 199 itself on phagocytosis rates, C. parapsilosis strains were grown under the same conditions and evaluated for morphological changes (Figure 3A). C. parapsilosis strains grown under conditions used to induce C. albicans germ tubes did not exhibit morphological changes nor were phagocytosis rates significantly affected for the strains tested (Figure 3B). Phagocytosis of C. parapsilosis strains grown under germ tube inducing conditions was also still inhibited by treatment with the gal3 blocking antibody (Figure 3C). Similar results were seen among all strains tested (Figures 3C and 3D).
Figure 3. Evaluation of additional C. albicans and C. parapsilosis stains.
Results are mean ± SEM of at least two separate neutrophil donors in three separate experiments. Between-group comparisons were made by the Fisher LSD test. * p ≤ 0.05. (A) Photomicrographs of C. albicans or C. parapsilosis strains grown as yeast in YPD broth or grown in medium 199 (m199) to induce germ tube formation. Cells were stained with Calcofluor White to facilitate imaging. C. albicans exhibited germ tube growth while C. parapsilosis strains exhibited no detectable morphological change. Bar = 4 microns. (B) Neutrophil phagocytosis rates of C. albicans and C. parapsilosis grown in YPD or m199 at an E:T of 1:10. (C–D) Neutrophil phagocytosis rates of selected C. parapsilosis strains (C) or C. albicans strains (D) grown in YPD or m199 at an E:T of 1:10. Phagocytosis rates of untreated neutrophils (CT) were compared to phagocytosis rates of neutrophils treated with gal3 blocking antibody (AB).
Neutrophils express galectin-3 intracellularly and secrete galectin-3 when stimulated
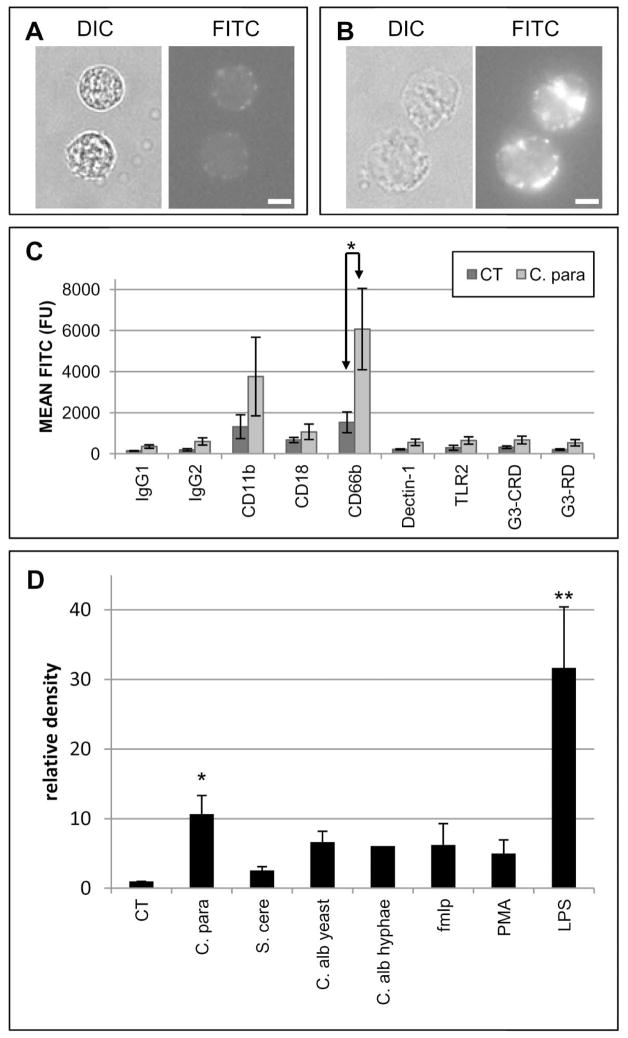
To determine the cellular location of gal3 in neutrophils, indirect immunofluorescence assays were performed with intact and permeabilized cells. A limited amount of gal3 was detected on the cell surface (Figure 4A) while the majority was detected intracellularly (Figure 4B). Treatment with C. parapsilosis yeast did not increase cell surface expression of gal3 (Figure 4C). Treatment did significantly increase CD66b expression, which is consistent with neutrophil activation and degranulation (Stocks et al., 1995, Fernandez et al., 2005, Hajkova et al., 2009). Increased cell surface expression of CD66b suggests that gal3 could be secreted during degranulation.
Figure 4. Neutrophils express galectin-3 intracellularly and secrete galectin-3 after stimulation.
All results are representative of at least three different donors ± SEM. *p≤0.05 and **p≤ 0.005 compared to control. Surface (A) or intracellular (B) expression of gal3 in neutrophils by immunofluorescence assay. Bar = 5 microns. (C) Surface receptor expression of untreated neutrophils (CT) or neutrophils treated with whole C. parapsilosis yeast (C. para) by FACS analysis. Surface expression of CD11b, CD18, CD66b, dectin-1, TLR2, the gal3 carbohydrate recognition domain (G3-CRD), and the gal3 regulatory domain (G3-RD) were evaluated. Isotype control antibodies (IgG1 and IgG2) were used as controls. Expression was measured as arbitrary fluorescent units (FU) and expressed as mean FITC. (D) Gal3 secretion from neutrophils by western blot analysis and densitometric measurements. Densitometric measurements are expressed as mean relative density compared to untreated controls of at least three separate donors, except C. albicans hyphae (two donors). Neutrophils were treated with mannan isolated from C. parapsilosis (C. para), S. cerevisiae (S. cere), C. albicans yeast (C. alb yeast) or C. albicans hyphae (C. alb hyphae); or fMLP, PMA and LPS.
Supernatants of treated neutrophils were examined for the presence of secreted gal3 by western blot (Figure 4D). Because the structure and composition of fungal mannans differs among species and each induces unique immunological responses (Netea et al., 2006, McKenzie et al., 2010, Rizzetto et al., 2010), neutrophils were treated with mannan isolated from C. albicans, C. parapsilosis and Saccharomyces cerevisiae. Because the structure of mannan is also known to differ between the different morphological forms on C. albicans (Shibata et al., 2007), mannan was extracted from both C. albicans yeast and C. albicans hyphae. Neutrophils were treated with mannan instead of whole yeast because gal3 is known to adhere to Candida species expressing specific β-(1-2) oligomannan (Fradin et al., 2000, Kohatsu et al., 2006). Mannan treatment had no effect on neutrophil viability as determined by Trypan Blue exclusion (data not shown). Neutrophils were also treated with LPS, fMLP, and PMA to determine if gal3 secretion was a specific response to fungal stimuli. For densitometer measurements, secretion was expressed as relative density compared to the untreated control supernatants. Though there was some variation from donor to donor, treatment with C. parapsilosis mannan, C. albicans yeast mannan, C. albicans hyphae mannan, PMA and LPS all induced gal3 secretion relative to control as determined by western blot (data not shown). As determined by densitometry measurements, however, only C. parapsilosis mannan and LPS treatment induced a significant amount of gal3 secretion compared to the untreated control, and secretion induced by C. parapsilosis mannan was nearly double that of all other species tested. No significant difference was observed between C. parapsilosis and C. albicans mannan. Because LPS induced the greatest amount of gal3 secretion, we confirmed that all of our mannan preparations were LPS free (data not shown). These data indicate that C. parapsilosis mannan may have unique stimulatory properties capable of inducing gal3 secretion. However gal3 secretion is not unique to fungal stimuli, as treatment with LPS also induced gal3 secretion.
Exogenous galectin-3 increases phagocytosis of C. parapsilosis and C. albicans yeast
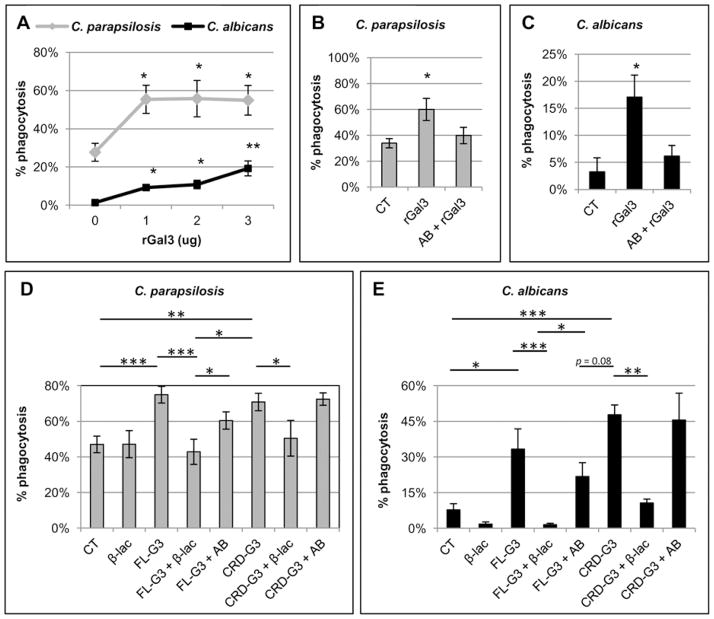
Because neutrophils secrete gal3 when stimulated with C. parapsilosis mannan and binding of gal3 to the neutrophil cell surface has been shown to increase phagocytosis (Fernandez et al., 2005, Farnworth et al., 2008), we determined whether the addition of exogenous gal3 could increase phagocytosis of both C. parapsilosis and C. albicans yeast. Neutrophils were treated with recombinant gal3 (rGal3) prior to the addition of yeast. Neutrophils were combined with C. parapsilosis yeast an E:T ratio of 1:5 and C. albicans yeast at an E:T ratio of 1:20 to ensure that differences in phagocytosis rates after treatment could be observed if present. Treatment with rGal3 increased phagocytosis rates of both C. parapsilosis and C. albicans yeast (Figure 5A), whereas pretreatment of rGal3 with the gal3 blocking antibody inhibited the increase in phagocytosis rates of both species (Figure 5B and 5C, respectively).
Figure 5. Exogenous galectin-3 augments phagocytosis of C. parapsilosis and C. albicans.
Results are mean ± SEM of at least three different neutrophil donors. * p ≤ 0.05, **p≤ 0.005, ***p≤ 0.0005 compared to untreated controls. (A) Phagocytosis rates of C. albicans and C. parapsilosis at an E:T ratio of 1:20 and 1:5, respectively, after neutrophil treatment with rGal3 at the indicated doses. Untreated neutrophils (0 μg) were used as a control. Phagocytosis of C. parapsilosis at an E:T ratio of 1:5 (B) and C. albicans at an E:T ratio of 1:20 (C) after treatment with 2 μg of rGal3 alone or after pretreatment with 25 μg of gal3 blocking antibody before adding neutrophils (AB + rGal3). Untreated neutrophils (CT) were used as control. Phagocytosis of C. parapsilosis yeast at an E:T ratio of 1:5 (D) and C. albicans yeast at an E:T ratio of 1:20 (E) was measured after treatment with 3 μg full length recombinant gal3 (FL-G3) or a truncated form of recombinant gal3 containing only the CRD domain (CRD-G3). FL-G3 and CRD-G3 were pretreated with either β-lactose (β-lac) to inhibit CRD binding or a gal3 antibody (AB) that inhibits regulatory domain oligomerization prior to neutrophil treatment.
Galectin-3 dependent increase in neutrophil phagocytosis requires recognition of the neutrophil cell surface via the carbohydrate recognition domain with a lesser role for the regulatory domain
In some biological systems, the ability of gal3 to augment the inflammatory response is dependent on oligomerization of the regulatory domain (Rabinovich et al., 2002, Sato et al., 2009). To determine if the CRD or the regulatory domain played a role in the increased phagocytosis of C. parapsilosis and C. albicans yeast, a series of experiments were performed using full length rGal3 (FL-G3), a truncated form of rGal3 (CRD-G3), and inhibitors of CRD binding (β-lactose, β-lac) and regulatory domain oligomerization (antibody, AB) (Figures 5D and 5E). Phagocytosis of C. parapsilosis at an E:T ratio of 1:5 (Figure 5D) and C. albicans at an E:T ratio of 1:20 (Figure 5E) was measured to ensure that differences in phagocytosis rates after treatment could be observed. For C. parapsilosis, both the FL-G3 and CRD-G3 increased phagocytosis relative to the untreated control. To further delineate the mechanism, both FL-G3 and CRD-G3 were pretreated with either β-lactose (β-lac) to inhibit CRD binding or a gal3 antibody (AB) that inhibits regulatory domain oligomerization prior to neutrophil treatment. When FL-G3 was pretreated with β-lactose, phagocytosis rates were similar to the untreated control, whereas pretreatment with blocking antibody had a less pronounced inhibitory effect. When CRD-G3 was pretreated with β-lactose, phagocytosis was inhibited compared to the CRD-G3 treated neutrophils. CRD-G3 pretreatment with the blocking antibody had no effect as expected given its lack of the regulatory domain. For phagocytosis of C. albicans, similar trends were observed. Treatment with both the FL-G3 and CRD-G3 increased neutrophil phagocytosis compared to the untreated control. When FL-G3 was pretreated with β-lactose, phagocytosis rates were similar to the untreated control, whereas pretreatment with the blocking antibody led to much less inhibition. When CRD-G3 was pretreated with β-lactose, phagocytosis was inhibited compared to the CRD-G3 treated neutrophils while pretreatment with the blocking antibody led to no inhibition. Taken together, these data indicate that gal3 mediated phagocytosis of C. parapsilosis and C. albicans is regulated by CRD binding to the neutrophil cell surface, and to a lesser extent, regulatory domain oligomerization.
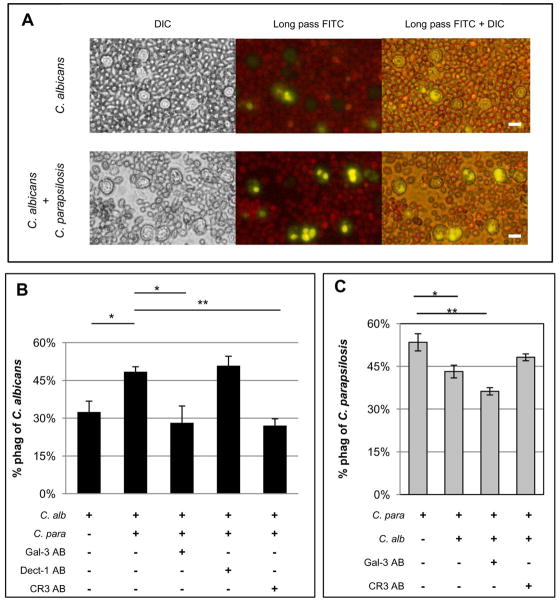
Coincubation of C. albicans with C. parapsilosis increases neutrophil phagocytosis of C. albicans yeast
C. parapsilosis mannan induces a significant amount of gal3 secretion compared to untreated controls. This finding together with the observation that exogenous gal3 increases neutrophil phagocytosis of C. albicans led to the hypothesis that C. parapsilosis induced gal3 secretion will increase phagocytosis of C. albicans yeast. To test this hypothesis, labeled C. albicans were incubated either with neutrophils alone or in combination with unlabeled C. parapsilosis (Figure 6A). The total number of yeast was kept constant for each condition. When C. albicans was incubated in the presence of C. parapsilosis, phagocytosis of C. albicans significantly increased (Figure 6B). Pretreatment of neutrophils with blocking antibodies against gal3 or CR3 inhibited the increase in phagocytosis, but dectin-1 blocking antibody did not, suggesting that the presence of C. parapsilosis increases phagocytosis of C. albicans and is dependent on both gal3 and CR3. Unexpectedly, the presence of C. albicans inhibited phagocytosis of C. parapsilosis (Figure 6C), but the possibility that this result was due to steric interference by the large number of C. albicans vs. C. parapsilosis yeast cannot be excluded. Pretreatment with the gal3 blocking antibody inhibited phagocytosis of C. parapsilosis even further, but pretreatment with the CR3 blocking antibodies did not. Again, these data suggest that gal3 plays an important role in phagocytosis of C. parapsilosis, even in the presence of C. albicans.
Figure 6. Exposure of neutrophils to C. parapsilosis increases phagocytosis of C. albicans.
All images are representative fields of at least three different donors and results are mean ± SEM of at least three different donors. *p≤0.05, **p≤ 0.005. Bar = 10 microns. (A) Images of neutrophil phagocytosis of C. albicans labeled yellow when incubated alone (top panel) or in combination with unlabeled C. parapsilosis (bottom panel). (B) Neutrophil phagocytosis rates of C. albicans (C. alb) when incubated alone, coincubated with C. parapsilosis (C. para), or coincubated with C. parapsilosis after neutrophils were pretreated with blocking antibodies against gal3 (Gal-3 AB), dectin-1 (Dect-1 AB), or CR3 (CR3 AB). (C) Neutrophil phagocytosis rates of C. parapsilosis (C. para) when incubated alone, coincubated with C. albicans (C. alb), or coincubated with C. albicans after neutrophil pretreatment with blocking antibodies against gal3 (Gal-3 AB) or CR3 (CR3 AB).
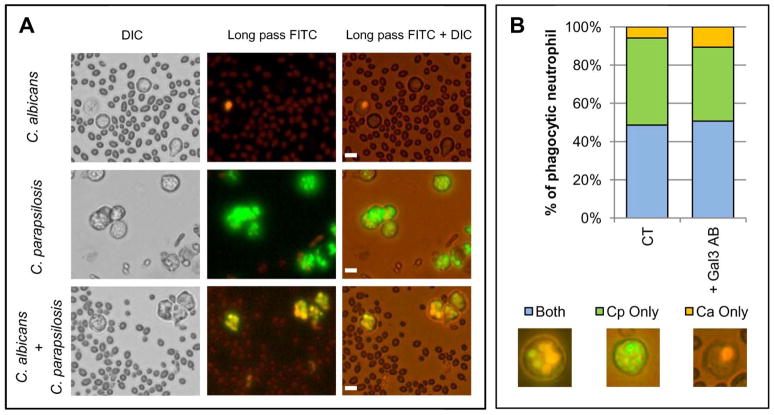
Because the presence of C. parapsilosis increased phagocytosis of C. albicans, we tested whether neutrophils selectively underwent phagocytosis of one species over the other and if this was dependant on gal3. This experiment was designed to determine if gal3 increased phagocytosis overall or if it increased phagocytosis of a specific species. C. parapsilosis was labeled green, C. albicans was labeled orange, and both were combined with neutrophils (Figure 7A). Compared to neutrophils that contained only C. albicans, a significantly larger number of phagocytic neutrophils contained either C. parapsilosis alone or both C. parapsilosis and C. albicans (Figure 7B). Pretreatment with the gal3 antibody did not significantly change the distribution of the phagocytic contents. Taken together, these data suggest that neutrophils undergo phagocytosis of C. parapsilosis more efficiently than C. albicans, but the presence of C. parapsilosis activates phagocytic pathways that are not selective to C. parapsilosis alone.
Figure 7. Neutrophils do not selectively phagocytose C. parapsilosis yeast over C. albicans yeast during coincubation.
All images are representative fields of at least three different donors. Bar = 10 microns. (A) Images of neutrophil phagocytosis of C. albicans dyed orange with ethidium bromide alone (top panel), C. parapsilosis dyed green with FITC alone (middle panel), or both orange C. albicans and green C. parapsilosis together (bottom panel). (B) Percent of total phagocytic neutrophils containing only C. albicans (Ca) or C. parapsilosis (Cp) or both species together. Images under the graph represent neutrophils containing both C. albicans and C. parapsilosis (left panel), green C. parapsilosis only (middle panel), or orange C. albicans only (right panel). Phagocytic contents of untreated neutrophils (CT) and neutrophils pretreated with gal3 blocking antibody (+Gal3 AB) were evaluated.
DISCUSSION
Several recent studies have demonstrated that the immune system responds differently to individual Candida species, suggesting that fungal pathogenesis must be approached in a species specific manner. This study adds to this collection of work by demonstrating that neutrophils have distinctly different phagocytic responses to C. parapsilosis versus C. albicans yeast. Neutrophils undergo phagocytosis of C. parapsilosis yeast much more efficiently and faster than C. albicans yeast and also have different morphological responses. Neutrophils exhibit extensive membrane ruffling and produce long, arm like projections towards single C. albicans yeast reminiscent of dectin-1 mediated protrusions generated when neutrophils are exposed to unopsonized zymosan particles (Lee et al., 2003, Kennedy et al., 2007). In comparison, when neutrophils were confronted with C. parapsilosis yeast, no membrane ruffling was observed. Neutrophil membranes were, however, observed advancing over the surface of C. parapsilosis yeast in a process morphologically similar to phagocytosis of serum opsonized zymosan particles (Kennedy et al., 2007). In addition, neutrophils were observed engulfing multiple C. parapsilosis yeast at the same time. These differences in phagocytic efficiency and appearance may be due, in part, to gal3. For the first time, we demonstrate that neutrophils secrete gal3, and this secreted gal3 may act as a proinflammatory autocrine/paracrine signal responsible for augmenting neutrophil phagocytosis.
By performing a screen with blocking antibodies against neutrophil receptors known to recognize fungal PAMPs, gal3 was identified to be involved in efficient phagocytosis of C. parapsilosis yeast but not C. albicans yeast. Although dectin-1, CR3, and possibly TLR2 have been identified as receptors involved in neutrophil phagocytosis of C. albicans (Kennedy et al., 2007, van Bruggen et al., 2007, van Bruggen et al., 2009, Linden et al., 2010, Tessarolli et al., 2010, Li et al., 2011), treatment with blocking antibodies against these PRRs did not inhibit phagocytosis of C. parapsilosis yeast. Interestingly, gal3 also appears to play a role in phagocytosis of C. albicans hyphae which are also phagocytosed much more efficiently than C. albicans yeast. Although important in neutrophil phagocytosis, gal3 does not appear to function as a traditional membrane receptor; instead, it appears to augment phagocytosis by acting as a proinflammatory autocrine/paracrine signal. We propose that the exposure of neutrophils to C. parapsilosis yeast and C. albicans hyphae, but not C. albicans yeast, induces gal3 secretion which binds to the neutrophil cell surface, increasing neutrophil phagocytic efficiency. Because treatment with the gal3 blocking antibody only partially inhibited phagocytosis of both C. parapsilosis yeast and C. albicans hyphae, it is likely that multiple neutrophil receptors may have a role in these processes. Experiments using different combinations of blocking antibodies in conjunction with the gal3 blocking antibody may reduce phagocytosis rates even more and may elucidate other receptors and pathways involved in these phagocytic pathways.
The effects of exogenous gal3 on neutrophil function have been well documented (Kuwabara et al., 1996, Karlsson et al., 1998, Sato et al., 2002, Nieminen et al., 2008); however, this study is the first to show that neutrophils secrete gal3 and this secretion augments phagocytosis. Several lines of evidence support this conclusion: 1) the majority of neutrophil gal3 is expressed intracellularly but can be secreted when stimulated, 2) C. parapsilosis mannan induces the most gal3 secretion from neutrophils among fungal stimuli tested, 3) exogenous gal3 increases phagocytosis of both C. parapsilosis and C. albicans yeast, 4) neutrophil exposure to C. parapsilosis increases phagocytosis of C. albicans and this increase is inhibited by a gal3 blocking antibody.
To the best of our knowledge, there is no molecular difference between endogenous and exogenous gal3. However, for endogenous gal3 to augment phagocytosis, it must first be secreted from stimulated neutrophils. Once secreted, this gal3 can further activate neutrophil effector functions, augmenting and increasing phagocytosis. In our system, exposure of neutrophils to C. parapsilosis yeast induces more gal3 secretion than exposure to C. albicans yeast. This secreted gal3 can then augment neutrophil phagocytosis of either target, which is consistent with the finding that exogenous gal3 increases phagocytosis of both species. Because C. albicans yeast did not induce gal3 secretion, gal3 was unavailable to augment phagocytosis of C. albicans yeast. However, secreted gal3 is apparently nonselective in its action, as exposure of neutrophils to C. parapsilosis increased phagocytosis of C. albicans yeast, and this increase was inhibited by gal3 antibody. We propose that the role of gal3 in neutrophil phagocytosis is determined by the ability of the target to induce gal3 secretion.
The mechanism of gal3 secretion from neutrophils remains unclear. Gal3 lacks a signal sequence and is secreted into the microenvironment by activated or damaged cells through a non-classical secretory pathway (Hughes, 1999). It has been proposed that gal3 can be secreted from activated or damaged cells by translocation, exocytosis, or vesicular transport. As neutrophil degranulation is a form of vesicular transport, gal3 secretion could be a result of neutrophil degranulation; however data supporting a specific mechanism of neutrophil gal3 secretion are inconclusive.
The neutrophil receptors involved in induction of gal3 secretion also remain to be defined. Although gal3 recognizes specific β-(1-2) oligomannans (Fradin et al., 2000, Kohatsu et al., 2006), it is unlikely that this recognition is responsible for initiating gal3 secretion for several reasons. Previous studies have demonstrated that C. albicans expresses β-(1-2) oligomannans (Fradin et al., 2000) while C. parapsilosis does not (Suzuki, 1997). Additionally, because C. parapsilosis mannan induced more gal3 secretion than C. albicans mannan it seems improbable that β-(1-2) oligomannan expression on yeast plays a role in initiating gal3 secretion. It is also unlikely that dectin-1, TLR2, TLR4, TLR6 or CR3 play a role in secretion, as treatment with these blocking antibodies did not inhibit phagocytosis of C. parapsilosis yeast. If these receptors were necessary for gal3 secretion, treatment with the blocking antibodies should have inhibited gal3 secretion, therefore reducing phagocytosis. It seems probable, however, that several receptors may play a role in neutrophil gal3 secretion as LPS, a known TLR4 ligand, induced the most gal3 secretion. The involvement of additional receptors is also suggested by the observation that treatment with the gal3 blocking antibody inhibited phagocytosis of both C. parapsilosis yeast and C. albicans hyphae, yet only mannan extracted from C. parapsilosis yeast induced a significant amount of gal3 compared to the untreated control. Since the mannan from C. albicans hyphae was less stimulatory of galectin-3 secretion, the fungal ligand inducing galectin-3 secretion in response to C. albicans hyphae may be a cell wall protein. Indeed, many aspects of host immunity respond quite differently to C. albicans yeast versus hyphal morphologies (Gow et al., 2012). Experiments investigating gal3 secretion specifically in conjunction with specific targets and specific blocking antibodies would be informative. More studies are needed to determine the mechanisms and receptors involved in neutrophil gal3 secretion.
The main neutrophil receptors for gal3 have been reported to be CD66a and CD66b (Feuk-Lagerstedt et al., 1999). Interestingly, co-ligation of CD66a and CD66b with monoclonal antibodies increased neutrophil receptor clustering and integrin-mediated adhesion, suggesting that gal3 mediated activation of neutrophils may be mediated by gal3 ligation of these receptors (Stocks et al., 1995, Ruchaud-Sparagano et al., 1997). In addition, previous studies have shown that primed neutrophils treated with exogenous gal3 internalize the lectin at 37°C within 5 min (Nieminen et al., 2005), indicating that internalization of gal3 may play a role in neutrophil activation. This internalization of gal3 may explain why we did not see an increase in gal3 surface expression after neutrophil treatment with whole C. parapsilosis yeast at 37°C for 15 min. Alternatively, because we did not cross-link gal3 to the cell surface prior to FACS analysis, bound gal3 may have been washed off and resulted in absence of gal3 staining. Gal3 may also exert its proinflammatory effect on neutrophil function by activating the p38 mitogen activated protein kinase (MAPK) pathway, an important signaling pathway in neutrophils (Fernandez et al., 2005). Gal3 mediated activation of the p38 MAPK pathway or other important signaling pathways may be activating CR3, a receptor that requires activation for proper functioning. Gal3 mediated CR3 activation may explain the increase in neutrophil phagocytosis of C. albicans yeast when coincubated with C. parapsilosis yeast. This is supported by the observation that blocking antibodies against both gal3 and CR3 inhibited increased phagocytosis. The observation that CR3 blocking antibodies also inhibited the increase in C. albicans phagocytosis suggests that neutrophil contact with C. parapsilosis leads to CR3 activation, a necessary step in efficient functioning of this receptor (Hynes, 2002). Although a recent study has suggested that neutrophil recognition of C. albicans yeast requires CR3 activation via dectin-1 signaling (Li et al., 2011), dectin-1 does not appear to play a role when C. parapsilosis is present. Such interactions between gal3 and CR3 are the focus of ongoing investigation.
The proinflammatory activity of gal3 has been shown to be dependent on oligomerization of the regulatory domain after ligand recognition by the carbohydrate recognition domain (CRD) (Liu et al., 1995, Kuwabara et al., 1996, Nieminen et al., 2007, Karlsson et al., 2009). Oligomerization can result in ligand cross-linking on the same cell surface, resulting in augmentation of many inflammatory responses. To determine the mechanistic role of the CRD and regulatory domain (RD) of gal3 in phagocytosis of Candida species, we treated neutrophils with both full length and truncated gal3. Full length gal3 contains both the CRD and RD of gal3, while the truncated form only contains the CRD. In addition, we also treated gal3 with β-lactose, a competitive inhibitor of the CRD. Data from these experiments indicated CRD binding to the neutrophil cell surface was sufficient to augment neutrophil phagocytosis. The RD also appears to play a role, as treatment of the FL rGal3 with a blocking antibody that inhibits RD oligomerization partially inhibited the increase in neutrophil phagocytosis. The RD of gal3 could influence phagocytosis through several different mechanisms including establishing cell surface receptor lattices, causing receptor clustering, and by bridging or cross-linking microbes to the neutrophil cell surface (Sato et al., 2004). Further investigation into the exact mechanisms involved in gal3 dependant phagocytosis is still needed.
In addition to augmenting neutrophil phagocytosis, gal3 secreted from activated neutrophils may play an important role in host defense against invasive fungal disease by activating additional neutrophil effector functions, having direct antifungal activity, and playing a role in additional immune recruitment. Gal3 has been shown to play a role in neutrophil effector functions including ROS production, degranulation and increased phagocytosis and inhibition of apoptosis (Farnworth et al., 2008, Forsman et al., 2008, Karlsson et al., 2009, Alves et al., 2010, Fermino et al., 2011). Gal3 has also been shown to kill Candida species expressing specific β(1,2) linked oligomannans, including C. albicans (Kohatsu et al., 2006). Gal3 has also been implicated in integrin independent neutrophil recruitment to Streptococcus pneumonia infected lungs (Sato et al., 2002, Farnworth et al., 2008, Nieminen et al., 2008) by acting as a bridging molecule between neutrophils and endothelial cells (Sato et al., 2002, Gil et al., 2006, Nieminen et al., 2007). Understanding how neutrophil secreted gal3 influences host defense will further our understanding of fungal pathogenesis and may lead to the development of novel therapeutics.
EXPERIMENTAL PROCEDURES
Organisms
C. albicans strains used in this study were SC5314 and Ca3153A. C. parapsilosis strains included the clinical isolates Ro75-R1, Ro18-G3, Ro88-R2, and Ro98-R1 from colonized infants (Bliss et al., 2008) and the clinically invasive isolate 15-72391-101, referred to here as JMB81 (Benjamin et al., 2010). Strains were maintained on YPD plates (1% yeast extract, 2% peptone, 2% dextrose, 2% agar). Overnight (ON) cultures were grown for 16 h at 37°C with vigorous agitation in YPD broth. C. albicans strain Ca3153A and C. parapsilosis strain Ro75-R1 were used for phagocytosis assays unless otherwise noted. To induce germ tube formation, yeast from washed ON cultures were incubated in medium 199 at 37°C. C. albicans strains were incubated for 60 min while C. parapsilosis strains were incubated for 90 min.
Calcofluor White staining
Strains grown in YPD or medium 199 were washed with HBSS, stained with 20 μg/ml Calcofluor White for 15 min at room temperature, washed again, and examined by fluorescence microscopy.
Neutrophil isolation
Human neutrophils were isolated from healthy adult peripheral blood by density gradient centrifugation as previously described (Linden et al., 2010). Briefly, leukocytes were separated from whole blood using Histopaque-1077 (Sigma), dextran sedimentation and hypotonic lysis of contaminating erythrocytes. Cells were adjusted to the appropriate concentration in Hank’s Balanced Salt Solution (HBSS) with Ca/Mg. All experiments involving human subjects were approved by the Institutional Review Board.
Phagocytosis assay with a single Candida species
Yeast from ON cultures were heat killed at 65°C for 90 min and labeled with fluorescein isothiocyanate (FITC). To induce germ tube formation, C. albicans yeast was incubated in medium 199 for 1 h at 37°C and then heat killed. Phagocytosis assays were performed as previously described (Linden et al., 2010). Live organisms were also tested in phagocytosis assays with similar results (Linden et al., 2010). Briefly, 5×105 neutrophils were combined with the number of yeast appropriate to achieve the desired effector to target ratio (E:T). Cells were pelleted and incubated at 37°C for 30 min unless otherwise noted. For select experiments, cell pellets were resuspended in ice cold HBSS to stop phagocytosis at indicated time points. To visualize phagocytosis, resuspended cells were combined with ethidium bromide (100 μg/ml) and examined by fluorescence microscopy. Intracellular yeast appeared green while external yeast appeared orange. To calculate percent phagocytosis, the number of neutrophils with internalized green yeast was divided by the total number of neutrophils. A minimum of 100 neutrophils were counted for each condition. Where indicated, neutrophils were treated with 25 μg of blocking antibodies against TLR2 (Clone TL2.1, Abcam), TLR4 (Clone HTA125, Abcam), TLR6 (Clone C585, Invitrogen) and gal3 (Clone B2C10, Genway) on ice for 30 min prior to the addition of yeast. To block the CR3 receptors, neutrophils were treated with a cocktail of antibodies against the lectin (Clone ICR44, eBioscience) and I domain of CD11b (Clone vim12, Invitrogen) as well as CD18 (Clone CBL158, Millipore) at 25 μg each. IgG1 and IgG2 antibodies were used as isotype controls. Neutrophils were also treated with 1–5 μg of human rGal3 (Biovision, Milpitas, CA) for 30 min on ice prior to addition of yeast. To block the effects of rGal3, 2 μg of rGal3 was pretreated with 25 μg gal3 blocking antibody before addition of neutrophils.
SEM Analysis of phagocytic neutrophils
Neutrophils (1×107) were combined with C. albicans strain Ca3153A or C. parapsilosis strain Ro98-R1 at an E:T ratio of 1:50 or 1:10 for 10 or 2.5 min, respectively. Cells were fixed in 2% v/v glutaraldehyde or 3% v/v paraformaldehyde for 30 min and transferred to 13mm isopore filters. Samples were rinsed with 0.1M sodium cacodylate, post fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 30 min, and rinsed again. Rinsed samples were dehydrated in an ascending ethanol series from 10% to 100% ethanol. Samples were critical point dried using liquid CO2 (Tousimis CPD), mounted and metal coated with 60/50 gold-palladium, and examined using a Hitachi S-5800 field emission scanning electron microscope (FESEM).
Phagocytosis assay with multiple Candida species
For phagocytosis experiments involving coincubation of C. albicans and C. parapsilosis, yeast were differentially labeled. When evaluating phagocytosis of C. albicans when coincubated with C. parapsilosis, C. albicans was labeled with cell tracker orange (Invitrogen) and then heat killed. Heat killed C. parapsilosis was left unlabeled. When evaluating phagocytosis of C. parapsilosis when coincubated with C. albicans, C. parapsilosis was labeled with cell tracker orange and C. albicans was left unlabeled. When incubated alone, neutrophils were combined at an E:T of 1:30 or 1:10 for C. albicans or C. parapsilosis, respectively. When coincubated, neutrophils were combined with C. albicans at an E:T ratio of 1:20 plus C. parapsilosis at an E:T ratio of 1:10. Phagocytosis assays were performed as described above, and external fluorescence of yeast was quenched with Trypan Blue. Where indicated, neutrophils were treated with 25 μg blocking antibodies against gal3 (Clone B2C10, Genway) and dectin-1 (Clone GE2, Abcam) on ice for 30 min prior to addition of yeast. To block the CR3 receptors, neutrophils were treated with a cocktail of antibodies against the lectin domain (Clone ICR44, eBioscience) and I domain of CD11b (Clone vim12, Invitrogen) as well as CD18 (Clone CBL158, Millipore) at 25 μg each.
For selective phagocytosis experiments, heat killed C. albicans were stained with ethidum bromide (100 mg/ml) for 10 min at room temperature and C. parapsilosis were stained with FITC as described above and extensively washed in 3% BSA/PBS. When incubated alone, neutrophils were combined at an E:T of 1:30 or 1:10 for C. albicans or C. parapsilosis, respectively. When coincubated, neutrophils were combined with C. albicans at an E:T ratio of 1:20 plus C. parapsilosis at an E:T ratio of 1:10. Phagocytosis assays were performed as described above and external fluorescence of yeast was quenched with Trypan Blue. To determine the percent of phagocytic neutrophils, neutrophils were scored as containing orange C. albicans only, green C. parapsilosis only, or both; divided by the total number of neutrophils with internalized yeast. Where indicated, neutrophils were treated with the gal3 blocking antibody.
Immunofluorescence Assay
To determine cell surface and intracellular expression of gal3, neutrophils were fixed in 3.3% formalin or 3.3% formalin + 0.1% tween-20, respectively, for 30 min at room temperature. Fixed cells were blocked in 3% BSA/PBS and probed with anti-gal3 antibody (Clone B2C10, Genway), followed by the appropriate secondary antibody. Cells were examined by fluorescence microscopy.
FACS analysis
Neutrophils were treated with unlabeled, heat killed C. parapsilosis yeast at an E:T ratio of 1:10. Cells were pelleted and incubated at 37°C for 30 min. Untreated pelleted neutrophils incubated at 37°C for 30 min were used as controls. Cell suspensions were fixed in 3.3% formalin for 30 min, blocked in 3% BSA/PBS and probed with antibodies against TLR2 (Clone TL2.1, Abcam), TLR4 (Clone HTA125, Abcam), TLR6 (Clone C585, Invitrogen) and gal3 (Clone B2C10, Genway), CD18 (Clone CBL158, Millipore), CD11b (Clone ICR44, eBioscience), and CD66b (Clone 80H3, Gentex). Two separate antibodies against gal3 were used; one that recognized the CRD domain (Clone Gal397, Biolegend) and another that recognized the regulatory domain (Clone B2C10, Genway). IgG1 and IgG2 isotypes were used as controls. Appropriate secondary antibodies were applied, and cells were examined with a BD Biosciences FACSCanto instrument.
Western blot analysis and Densitometry Measurements
Neutrophils (1×107) were treated with 15 mg/ml mannan, 0.1 mg/ml fMLP, 2.5 μg/ml PMA, or 125 mg/ml LPS for 15 min at 37°C. Untreated neutrophils incubated for 15 min at 37°C were used as control. Whole cell neutrophil lysates were collected by lysing neutrophils in 5.8% Octyl β-D-glucopyranoside in PBS plus protease inhibitors on ice for 30 min. Mannan was extracted from C. albicans strain Ca3153A and C. parapsilosis strain Ro75-R1 grown in YPD at 37°C for 48 h and S. cerevisiae strain By5751a grown at 30°C for 48 h as previously described (van de Veerdonk et al., 2009). Mannan extracts were tested for endotoxin using the Limulus Amebocyte Lysate Chromogenic Endotoxin Quantitation Kit (Pierce) per manufacturer’s instructions. To extract mannan from C. albicans hyphae, strain Ca3153A was grown in m199 at 37°C for at least 48 h. Cells were spun at 16.1 × g for 10 min and supernatants were separated by SDS-PAGE, analyzed by western blot with gal3 antibody (Galectin-397) and an appropriate secondary antibody. Bands were visualized by chemiluminescence, and densitometry measurements were made using ImageJ software.
Statistics
For parametric variables, comparisons were made by ANOVA. Between-group comparisons were made by the Holm-Sidak test unless otherwise indicated. P values ≤0.05 were considered significant. Tests appropriate for nonparametric variables were used as indicated.
Acknowledgments
Many thanks to Drs. Daniel Hsu and Fu-Tong Liu at UC Davis for the generous gift of full length and truncated forms of rGal3, Richard Tucker for assistance with statistical analysis, Matthew Hirakawa for assistance with scanning electron microscopy, and the Eunice Kennedy Shriver NICHD Neonatal Research Network for supplying clinical isolate 14-72391-101. This project was supported by grants from the National Center for Research Resources (5P20RR018728-10) and the National Institute of General Medical Sciences (8P20GM103537-10) from the National Institutes of Health.
Footnotes
The authors have no conflict of interest to declare except that Dennis Kunkel is owner and founder of Dennis Kunkel Microscopy, Inc.
References
- Ahmad N, Gabius HJ, Andre S, Kaltner H, Sabesan S, Roy R, et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- Alves CM, Silva DA, Azzolini AE, Marzocchi-Machado CM, Carvalho JV, Pajuaba AC, et al. Galectin-3 plays a modulatory role in the life span and activation of murine neutrophils during early Toxoplasma gondii infection. Immunobiology. 2010;215:475–485. doi: 10.1016/j.imbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Bassetti M, Mikulska M, Viscoli C. Bench-to-bedside review: therapeutic management of invasive candidiasis in the intensive care unit. Crit Care. 2010;14:244. doi: 10.1186/cc9239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin DK, Jr, Stoll BJ, Gantz MG, Walsh MC, Sanchez PJ, Das A, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;126:e865–873. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes ES, Silva NM, Ruas LP, Mineo JR, Loyola AM, Hsu DK, et al. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am J Pathol. 2006;168:1910–1920. doi: 10.2353/ajpath.2006.050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231–235. doi: 10.1097/INF.0b013e31815bb69d. [DOI] [PubMed] [Google Scholar]
- Blyth CC, Chen SC, Slavin MA, Serena C, Nguyen Q, Marriott D, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- Dementhon K, El-Kirat-Chatel S, Noel T. Development of an in vitro model for the multi-parametric quantification of the cellular interactions between Candida yeasts and phagocytes. PLoS One. 2012;7:e32621. doi: 10.1371/journal.pone.0032621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci U S A. 2011;108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954–966. doi: 10.1016/j.ijid.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Farnworth SL, Henderson NC, Mackinnon AC, Atkinson KM, Wilkinson T, Dhaliwal K, et al. Galectin-3 reduces the severity of pneumococcal pneumonia by augmenting neutrophil function. Am J Pathol. 2008;172:395–405. doi: 10.2353/ajpath.2008.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermino ML, Polli CD, Toledo KA, Liu FT, Hsu DK, Roque-Barreira MC, et al. LPS-induced galectin-3 oligomerization results in enhancement of neutrophil activation. PLoS One. 2011;6:e26004. doi: 10.1371/journal.pone.0026004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez GC, Ilarregui JM, Rubel CJ, Toscano MA, Gomez SA, Beigier Bompadre M, et al. Galectin-3 and soluble fibrinogen act in concert to modulate neutrophil activation and survival: involvement of alternative MAPK pathways. Glycobiology. 2005;15:519–527. doi: 10.1093/glycob/cwi026. [DOI] [PubMed] [Google Scholar]
- Ferraz LC, Bernardes ES, Oliveira AF, Ruas LP, Fermino ML, Soares SG, et al. Lack of galectin-3 alters the balance of innate immune cytokines and confers resistance to Rhodococcus equi infection. Eur J Immunol. 2008;38:2762–2775. doi: 10.1002/eji.200737986. [DOI] [PubMed] [Google Scholar]
- Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin-3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–5598. [PubMed] [Google Scholar]
- Forsman H, Salomonsson E, Onnheim K, Karlsson J, Bjorstad A, Leffler H, et al. The beta-galactoside binding immunomodulatory lectin galectin-3 reverses the desensitized state induced in neutrophils by the chemotactic peptide f-Met-Leu-Phe: role of reactive oxygen species generated by the NADPH-oxidase and inactivation of the agonist. Glycobiology. 2008;18:905–912. doi: 10.1093/glycob/cwn081. [DOI] [PubMed] [Google Scholar]
- Fradin C, Poulain D, Jouault T. beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun. 2000;68:4391–4398. doi: 10.1128/iai.68.8.4391-4398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil CD, La M, Perretti M, Oliani SM. Interaction of human neutrophils with endothelial cells regulates the expression of endogenous proteins annexin 1, galectin-1 and galectin-3. Cell Biol Int. 2006;30:338–344. doi: 10.1016/j.cellbi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2012;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova V, Svobodova A, Krejcova D, Ciz M, Velebny V, Lojek A, et al. Soluble glucomannan isolated from Candida utilis primes blood phagocytes. Carbohydr Res. 2009;344:2036–2041. doi: 10.1016/j.carres.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Zuberi RI, Liu FT. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992;267:14167–14174. [PubMed] [Google Scholar]
- Hughes RC. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172–185. doi: 10.1016/s0304-4165(99)00177-4. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Christenson K, Matlak M, Bjorstad A, Brown KL, Telemo E, et al. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009;19:16–20. doi: 10.1093/glycob/cwn104. [DOI] [PubMed] [Google Scholar]
- Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–3438. [PubMed] [Google Scholar]
- Kennedy AD, Willment JA, Dorward DW, Williams DL, Brown GD, DeLeo FR. Dectin-1 promotes fungicidal activity of human neutrophils. Eur J Immunol. 2007;37:467–478. doi: 10.1002/eji.200636653. [DOI] [PubMed] [Google Scholar]
- Keppler-Ross S, Douglas L, Konopka JB, Dean N. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot Cell. 2010;9:1776–1787. doi: 10.1128/EC.00156-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu L, Hsu DK, Jegalian AG, Liu FT, Baum LG. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J Immunol. 2006;177:4718–4726. doi: 10.4049/jimmunol.177.7.4718. [DOI] [PubMed] [Google Scholar]
- Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. J Immunol. 1996;156:3939–3944. [PubMed] [Google Scholar]
- Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Li X, Utomo A, Cullere X, Choi MM, Milner DA, Jr, Venkatesh D, et al. The beta-glucan receptor Dectin-1 activates the integrin Mac-1 in neutrophils via Vav protein signaling to promote Candida albicans clearance. Cell Host Microbe. 2011;10:603–615. doi: 10.1016/j.chom.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden JR, Maccani MA, Laforce-Nesbitt SS, Bliss JM. High efficiency opsonin-independent phagocytosis of Candida parapsilosis by human neutrophils. Med Mycol. 2010;48:355–364. doi: 10.1080/13693780903164566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- McKenzie CG, Koser U, Lewis LE, Bain JM, Mora-Montes HM, Barker RN, et al. Contribution of Candida albicans cell wall components to recognition by and escape from murine macrophages. Infect Immun. 2010;78:1650–1658. doi: 10.1128/IAI.00001-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–1650. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31:346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Neumann AK, Jacobson K. A novel pseudopodial component of the dendritic cell anti-fungal response: the fungipod. PLoS Pathog. 2010;6:e1000760. doi: 10.1371/journal.ppat.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen J, Kuno A, Hirabayashi J, Sato S. Visualization of galectin-3 oligomerization on the surface of neutrophils and endothelial cells using fluorescence resonance energy transfer. J Biol Chem. 2007;282:1374–1383. doi: 10.1074/jbc.M604506200. [DOI] [PubMed] [Google Scholar]
- Nieminen J, St-Pierre C, Bhaumik P, Poirier F, Sato S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by Streptococcus pneumoniae. J Immunol. 2008;180:2466–2473. doi: 10.4049/jimmunol.180.4.2466. [DOI] [PubMed] [Google Scholar]
- Nieminen J, St-Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009) Diagn Microbiol Infect Dis. 2010;68:278–283. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Baum LG, Tinari N, Paganelli R, Natoli C, Liu FT, Iacobelli S. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23:313–320. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- Rizzetto L, Kuka M, De Filippo C, Cambi A, Netea MG, Beltrame L, et al. Differential IL-17 production and mannan recognition contribute to fungal pathogenicity and commensalism. J Immunol. 2010;184:4258–4268. doi: 10.4049/jimmunol.0902972. [DOI] [PubMed] [Google Scholar]
- Ruas LP, Bernardes ES, Fermino ML, de Oliveira LL, Hsu DK, Liu FT, et al. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS One. 2009;4:e4519. doi: 10.1371/journal.pone.0004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud-Sparagano MH, Stocks SC, Turley H, Dransfield I. Activation of neutrophil function via CD66: differential effects upon beta 2 integrin mediated adhesion. Br J Haematol. 1997;98:612–620. doi: 10.1046/j.1365-2141.1997.2523070.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Nieminen J. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj J. 2004;19:583–591. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]
- Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J Immunol. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem. 1998;273:13047–13052. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- Shibata N, Suzuki A, Kobayashi H, Okawa Y. Chemical structure of the cell-wall mannan of Candida albicans serotype A and its difference in yeast and hyphal forms. Biochem J. 2007;404:365–372. doi: 10.1042/BJ20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliopoulou A, Dimitriou G, Jelastopulu E, Giannakopoulos I, Anastassiou ED, Christofidou M. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia. 2012;173:219–228. doi: 10.1007/s11046-011-9498-3. [DOI] [PubMed] [Google Scholar]
- Stocks SC, Kerr MA, Haslett C, Dransfield I. CD66-dependent neutrophil activation: a possible mechanism for vascular selectin-mediated regulation of neutrophil adhesion. J Leukoc Biol. 1995;58:40–48. doi: 10.1002/jlb.58.1.40. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr Top Med Mycol. 1997;8:57–70. [PubMed] [Google Scholar]
- Tessarolli V, Gasparoto TH, Lima HR, Figueira EA, Garlet TP, Torres SA, et al. Absence of TLR2 influences survival of neutrophils after infection with Candida albicans. Med Mycol. 2010;48:129–140. doi: 10.3109/13693780902964339. [DOI] [PubMed] [Google Scholar]
- van Bruggen R, Drewniak A, Jansen M, van Houdt M, Roos D, Chapel H, et al. Complement receptor 3, not Dectin-1, is the major receptor on human neutrophils for beta-glucan-bearing particles. Mol Immunol. 2009;47:575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- van Bruggen R, Zweers D, van Diepen A, van Dissel JT, Roos D, Verhoeven AJ, Kuijpers TW. Complement receptor 3 and Toll-like receptor 4 act sequentially in uptake and intracellular killing of unopsonized Salmonella enterica serovar Typhimurium by human neutrophils. Infect Immun. 2007;75:2655–2660. doi: 10.1128/IAI.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]