Abstract
Life history theory posits that organisms face a trade-off between current and future reproductive attempts. The physiological mechanisms mediating such trade-offs are still largely unknown, but glucocorticoid hormones are likely candidates as elevated, post-stress glucocorticoid levels have been shown to suppress both reproductive physiology and reproductive behavior. Aged individuals have a decreasing window in which to reproduce, and are thus predicted to invest more heavily in current as opposed to future reproduction. Therefore, if glucocorticoids are important in mediating the trade-off between current and future reproduction, aged animals are expected to show decreased hypothalamic-pituitary-adrenal (HPA) axis responses to stressors and to stimulation by corticotropin-releasing hormone (CRH), and enhanced responses to glucocorticoid negative feedback, as compared to younger animals. We tested this hypothesis in the monogamous, biparental California mouse by comparing baseline and post-stress corticosterone levels, as well as corticosterone responses to dexamethasone (DEX) and CRH injections, between old (~18–20 months) and young (~4 months) virgin adults of both sexes. We also measured gonadal and uterine masses as a proxy for investment in potential current reproductive effort. Adrenal glands were weighed to determine if older animal had decreased adrenal mass. Old male mice had lower plasma corticosterone levels 8 h after DEX injection than did young male mice, suggesting that the anterior pituitary of older males is more sensitive to DEX-induced negative feedback. Old female mice had higher body-mass-corrected uterine mass than did young females. No other differences in corticosterone levels or organ masses were found between age groups within either sex. In conclusion, we did not find strong evidence for age-related change in HPA activity or reactivity in virgin adult male or female California mice; however, future studies investigating HPA activity and reproductive outcomes in young and old breeding adults would be illuminating.
Keywords: age, trade-off, glucocorticoids, corticosterone, California mouse, stress, reproduction
1. INTRODUCTION
Life history theory posits that organisms face a trade-off between current and future reproductive attempts (56,57,68). The physiological mechanisms mediating such trade-offs are largely unknown; however, hormones have been viewed as probable candidates (33, 67, 69, 77). Specifically, the glucocorticoids, end products of the hypothalamic-pituitary-adrenal (HPA) axis, are likely to play a role (45,55). Glucocorticoids are best known for their role in the stress response, as plasma concentrations rise ~3–5 minutes following the onset of a stressor (11,58); however, basal levels are also important for organismal functioning, and basal glucocorticoid release displays a predictable diurnal pattern (61). Glucocorticoids, at both basal and post-stress concentrations are important for the response to and recovery from stressors and are critical for maintenance of homeostasis (34,61,76). These hormones influence glucose regulation (glucocorticoids can suppress insulin secretion as well as stimulate gluconeogenesis, lipolysis, glycogenolysis, and proteolysis), energy partitioning, and reproduction (2,12,16, 51,60,61, 75), as well as myriad other physiological and behavioral functions.
Across vertebrate taxa, the HPA axis plays a role in regulating reproduction in the face of stress (18,55,76): while basal levels of glucocorticoids can facilitate physiological and behavioral aspects of reproduction, elevated glucocorticoid hormones have been shown to suppress both reproductive physiology and reproductive behavior (35,75). Reproductive suppression by stress (and glucocorticoids) is thought to be adaptive in the short term, as it promotes individual survival (61), but may be detrimental when the value of current reproduction is high compared to future reproductive prospects (75). For example, aged individuals have a decreasing window in which to reproduce, and are thus predicted to invest more heavily in current as opposed to future reproduction (40,75). Consequently, it has been hypothesized that older individuals should display decreased glucocorticoid responses to stressors, as compared to young conspecifics, to buffer short-term reproductive efforts from the detrimental effects of elevated glucocorticoids (55,75).
Evidence supporting this prediction has been documented in a handful of species. For example, older terns (Sterna hirundo; 24,25) and Leach’s storm-petrels (Oceanodroma leucorhoa; 47) of both sexes showed a smaller CORT (and in terns, adrenocorticotropic hormone (ACTH)) response to handling or capture stress during the breeding season than did young adult conspecifics. In addition, old, non-breeding green sea turtles (Chelonia mydas) of both sexes exhibited lower concentrations of CORT following capture stress than did juvenile turtles (29; adult turtles were reproductively capable, but adults breed less than annually so non-breeding animals of that year were used). Aged male Sprague-Dawley rats produced less corticosterone in response to an injection of corticotropin-releasing hormone (CRH) than did young adult rats (28,62), suggesting that pituitary responsiveness to CRH and/or adrenocortical responsiveness to ACTH is blunted in old individuals. Additionally, old male F344/BN hybrid rats showed greater suppression of ACTH and CORT in response to the synthetic glucocorticoid dexamethasone (DEX) than did younger adult rats, suggesting that sensitivity to glucocorticoid negative feedback increases with advancing age (31).
In the present study we compared HPA activity and reactivity, as well as adrenal and reproductive organ masses, in young adult and aged California mice (Peromyscus californicus) of both sexes to test the hypothesis that aged animals display dampened HPA reactivity as compared to young animals, possibly as a means to protect current reproductive potential. California mice are monogamous and biparental (52–54), and both males and females invest heavily in each reproductive bout (7,8,20,21). Thus, we expected both sexes to exhibit changes in HPA reactivity (measured by circulating concentrations of corticosterone, CORT) with age. This species resides in chaparral and areas with coastal sage scrub, and ranges from Northern California down to Baja California (42). California mice have an average lifespan of 9 to 18 months in the wild (41), but can live up to 4 years in the lab (C.A. Marler, pers. comm.). Females become reproductively mature around 40 days of age (19), and males begin to breed successfully at 60–90 days of age (J. Crossland, pers. comm.; unpub. obs.). Males and females have similar home-range sizes and, once a mate has been found, remain paired for life (42, 54). A pair can produce several litters per year under favorable environmental conditions, and estimates (lab and wild) of young produced per pair per year range from 6 to 15 pups (7,8,42,54). The main breeding season is from October to May (54), but breeding does not appear to depend on photoperiod or season (42). In captivity, both males and females breed successfully until 3 years of age or later, with no age-related decrease in number of pups born per litter (unpub. obs.).
We used virgin animals to avoid potential confounds of reproductive behavior, reproductive hormones (and in females, lactation), parental experience, and any reproduction-related effects on organ mass. We measured plasma CORT concentrations under basal conditions, in response to an acute stressor (bobcat urine), and in response to pharmacological suppression (DEX) and stimulation (CRH) in young and old adult mice of both sexes. We also compared gonadal and adrenal gland masses between the age classes. In line with the hypothesis that glucocorticoids mediate the trade-off between current and future reproduction, we predicted that aged individuals of both sexes would show a decreased CORT response to the predator-odor stressor, enhanced CORT suppression in response to simulated glucocorticoid negative feedback (DEX injection), and a diminished CORT response to CRH injection, when compared to young animals. Baseline levels of CORT were not predicted to differ between the age groups, as post-stress CORT concentrations are implicated in disruption of reproductive physiology and behavior (75), whereas comparatively small changes in circulating basal CORT would not be predicted to alter reproductive investment. In terms of organ mass, we predicted that older animals would have heavier gonads and, in the case of females, heavier uteri, as an index of increased preparedness for investment in current reproduction when future reproductive potential is low. Additionally, we predicted that older individuals might have lower adrenal mass than young individuals (if CORT modulation is a function of adrenal capacity), or that adrenal mass might not differ between age groups (if CORT modulation occurs via adrenal or pituitary sensitivity).
2. METHODS
2.1 Animals
We used California mice that were bred and housed at the University of California, Riverside (UCR), and that were descended from mice purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC). The colony at the Stock Center was founded between 1979 and 1987 from about 60 individuals collected in the Santa Monica Mountains, CA. Our colony at UCR was established in 2007 and has since received several shipments of new animals to maintain genetic diversity. Sibling-sibling or parent-sibling matings are never performed in our colony, and first-cousin matings are avoided when possible. All mice had ad libitum access to food (Purina rodent chow 5001) and water, and were housed in polycarbonate cages (44 × 24 × 20 cm) lined with aspen shavings; cotton wool was provided for nesting material. Lights were on from 0500–1700h (14:10 L:D cycle), and ambient temperature was maintained at approximately 23 °C with humidity around 65%. Animals were weaned from their birth cage at 27–32 days of age (prior to the birth of any younger siblings), ear punched for identification and housed in same-sex groups of two or four until just before the experiment began. From the time of weaning, animals were never housed with an individual of the opposite sex and were thus virgins at the time of testing.
We used a total of 19 virgin females, 10 young (123 ± 1 days at the beginning of data collection; range: 120–124 days; ~ 4 months) and 9 old (546 ± 4 days; range: 525–566 days; ~18 months), and a total of 43 virgin males, 22 young (135 ± 2 days at the beginning of data collection; range: 120–146 days; ~4.5 months) and 21 old (624 ± 10 days; range: 497–688 days; ~20 months). The ages chosen (range: 4–20 months) are within the age range of California mice found in the wild (41) and are thus ecologically relevant. In addition, the ages chosen are in line with ages used in several other rodent aging studies (e.g., rats: 3–30 months, 28,31,32,44; mice: 3–16 months, 13). Due to limited availability of aged animals, females were tested before males (females in two cohorts during the fall of 2009 and males in three cohorts during November, 2009, January and July, 2010). UCR has full AAALAC accreditation, and all procedures were approved by the UCR IACUC and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2 Experimental Design
At least seven days prior to the start of data collection, groups of four mice were split into same-sex pairs. Hormonal data collection occurred over a period of 14–15 days (females) or 21–22 days (males). Males and females underwent data collection on slightly different timelines due to unforeseen logistical constraints. During the first week of data collection two baseline blood samples were collected from each mouse, one at 0800h and another at 2000h, to capture both the nadir and peak, respectively, of CORT levels across the diurnal cycle (23). Consecutive samples were separated by two days, and all animals were weighed 1 day prior to initial blood collection. One week (females) or two weeks (males) after collection of the first baseline blood sample, mice were exposed to bobcat urine (see section 2.5) for 5 min and a blood sample was collected immediately following exposure. Seven to eight days after predator-urine exposure, mice were injected with dexamethasone (DEX; see section 2.6). Eight hours after the DEX injection, a blood sample was collected, and mice were then immediately injected with CRH. Two additional blood samples were collected, one at 45 min and one at 90 min post-CRH. All mice were euthanized after the 90-min blood sample. For females, a vaginal lavage was performed (see section 2.2.1), and then right- and left-side adrenals and ovaries, as well as the uterus, were removed, placed in physiological saline, blotted dry 3x, and weighted to the nearest 0.00001g. For males right-side adrenal glands and testes were dissected out (left-side organs were frozen for possible future analysis), placed in physiological saline, blotted dry 3x, and weighed to the nearest 0.0001 g.
2.2.1 Vaginal Lavage
In addition to the procedures described above, we performed vaginal lavage on each female mouse 6 times over the course of the experiment in an attempt to assess estrous cycles. Cycle stage can influence baseline CORT levels in female rats and house mice (females in proestrus have higher CORT concentrations; 1, 36,46); however, previous data from California mice show that the estrous cycle does not affect CORT concentration (14,30), and male and female California mice do not differ in basal or post-stress CORT concentrations (23). We refrained from lavaging animals every day as lavage itself can be stressful to rodents (63) and can alter experimental outcomes (see 74).
The first lavage occurred two days prior to the first baseline blood sample. Then, starting two days after predator-odor exposure (11 days from first the lavage), lavages were conducted on 4 consecutive days (ending 2 days prior to the final day of the experiment). A final lavage was conducted post-mortem on the last day of data collection. Lavages were performed by gently holding the female by the scruff of the neck and inserting a glass Pasteur pipette filled with approximately 100–150μl of sterile saline into the vagina. The saline was squirted into the vaginal canal and then removed, placed onto a slide, stained with methyl blue, and immediately placed under a compound microscope for analysis. Cell types and proportions were determined, and mice were classified in one of five categories: diestrus, proestrus, estrus, metestrus, or no sample (vagina closed; 6, 19, 43).
2.3 Blood Sample Collection
Care was taken to collect blood from both individuals in a cage as quickly as possible. Additionally, order of cages sampled was random and balanced across age groups. Cages were always removed from and replaced in the colony room as quietly as possible.
Mice were anesthetized with isoflurane, and blood samples (70–210 μl) were collected from the retro-orbital sinus using heparinized glass microhematocrit tubes. Time from disturbance of the cage or end of the test to collection of the blood sample was less than 3 minutes for all but 6 out of 352 blood samples (males: 78.9 ± 2.0 s, range 40–275 s; females: 73.8 ± 4.2 s, range: 39–235 s). Blood was centrifuged for 12 min (13,300 rpm, 4°C), and plasma was collected and stored at −80°C until assay.
Final blood collection from females was performed using cardiac puncture. After euthanasia via CO2 inhalation, blood was collected from the heart with a 1ml heparinized syringe fitted with a 27G sterile needle. Average time from initial administration of CO2 to collection of blood was 284.8 ± 20.1 s (range: 189–500 s; collection time was not significantly correlated with plasma CORT, Pearson’s r=−0.064, n=19, P=0.795). Blood was processed and stored as described above.
2.4 Corticosterone Assay
Plasma was assayed in duplicate for corticosterone using an 125I double-antibody radioimmunoassay kit (#07-120102, MP Biomedicals, Costa Mesa, CA) that has been validated for this species (9). Inter- and intra-assay coefficients of variation (CVs) were 10.7% and 4.1%, respectively (N = 45 assays). Plasma samples from each age group were balanced across assays, and samples from males and females were run in separate assays. Therefore, hormone data from males and females were not directly compared to each other.
2.5 Predator-urine Exposure
Same-sex pairmates were exposed to predator urine together. Between 0800 and 0845h, animals were placed in a fresh cage that contained clean bedding but no food, water or cotton, and taken to a testing chamber. A cotton ball wetted with 1ml of bobcat urine (Maine Outdoor Solutions, Hermon, ME) was immediately placed in a corner of the test cage for 5 min. As soon as exposure ended, a blood sample was collected from each mouse and mice were returned to their home cage. We have previously found that exposure to predator urine, including bobcat urine, produces a pronounced CORT response in California mice at this time of day (9, 23).
2.6 Dexamethasone and Corticotropin-Releasing Hormone Injections
Mice were weighed one day prior to injection to permit calculation of body-mass-corrected hormone doses. Dexamethasone sodium phosphate (DEX; 4mg/ml, American Regent, Shirley, NY) was diluted with sterile saline to a concentration of 10 mg/kg and injected i.p. This dose was selected on the basis of a previous dose-response study in male and female California mice, which demonstrated that 10 mg/kg DEX effectively suppresses plasma CORT levels in both sexes 8 h following injection at approximately the same time of day as in the present experiment (23). Corticotropin-releasing hormone (CRH; C3042, Sigma Aldrich, St. Louis, MO) was diluted in sterile water to a 1 μg/ml solution, and mice were injected i.p. with 2 μg/kg CRH; this dose has been shown to increase DEX-suppressed CORT levels in this species (unpub. data).
Animals were injected with DEX at 0730–0830h on the last day of testing, and then placed back in their home cage. Eight h following DEX injection, at 1530–1630h, each animal was blood sampled, injected with CRH, and then returned to its home cage. A second blood sample was collected via the retro-orbital sinus from each animal 45 min following CRH injection, and the animal was again returned to its home cage. Finally, 90 min after CRH injection, a third blood sample was collected from either the heart via cardiac puncture following CO2 inhalation (females) or from the retro-orbital sinus (males).
This study was conducted primarily to elucidate the effect of age and not injection per se; therefore, a vehicle-injection group was not included. For the present study we had measures of basal hormone level from all animals, and the post-injection data generated allowed us to adequately compare age groups to one another. Additionally, we have previously characterized the response to both pharmacological and sham injection in this species; thus, a vehicle-injection group was not included here (see 23).
2.7 Analysis
Data were checked for normality using the Shapiro-Wilk test and transformed if necessary. All CORT values were log10-transformed prior to analysis, but non-transformed values are presented for ease of interpretation. Each mouse’s plasma CORT concentration from the 0800h sample was used as a baseline for within-subjects analyses of the response to bobcat urine. CORT data were analyzed via repeated-measures ANOVA. Additionally, area under the curve (AUC) was calculated on post-injection CORT concentrations using two formulas (see 73) to quantify total CORT release over time following CRH treatment. AUCg corresponds to the integrated amount of hormone produced over time with respect to a starting value of zero (not taking post-DEX CORT concentration into account). AUCi is calculated using a baseline value (here, post-DEX CORT levels) and measures CORT increase over time from each individual animal’s starting value. One young male’s 2000h plasma sample was lost during processing, leaving 21 samples for young males at that time point. Additionally, due to problems with sample processing, three post-CRH-injection plasma samples from old males were lost, leaving 20, 45 min post-CRH and 19, 90 min post-CRH samples from old males for analysis. Hormone concentrations were correlated using Pearson’s correlation.
Body mass was analyzed by ANOVA. Organ masses (gonads, uteri, and adrenal glands) were analyzed using an ANCOVA with final body mass as a covariate, following the methods of Tomkins and Simmons (73), except that organ mass was not subtracted from body mass due to differences in the number of significant figures (body mass was measured to the nearest 0.01 g and organs to the 0.0001 or 0.00001 g). For females, associations between right and left organ masses were evaluated using Pearson’s correlation. The left ovary of one old female was damaged during dissection and thus that animal was not included in analysis of ovarian mass.
As described above, females were tested several months before males, and male and female blood samples were run in different assays. Additionally, final blood samples were collected via cardiac puncture in females but via retro-orbital puncture in males. Therefore, we chose to analyze male and female data separately in the initial analysis. However, since previous studies have noted sex differences in post-stress CORT levels in this species (70,71), an additional analysis was conducted on combined data from the sexes to explore possible sex differences in CORT concentrations in a preliminary manner.
3. RESULTS
3.1 Females
3.1.1 Basal CORT
Plasma CORT concentrations in female California mice were markedly higher at 2000h than at 0800h (1466.44 ± 169.27 vs. 42.48 ± 5.67 ng/ml; n=19; F1,17= 353.72, P <0.001; Table 1). However, CORT levels did not differ between young and old adult females (F1,17= 0.46, P=0.505), nor was there a time*group interaction (F1,17=0.01, P=0.914). Across all females, plasma CORT level at 0800h was not correlated with plasma CORT level at 2000h (r=0.090, n=19, P=0.715).
Table 1.
Plasma CORT concentrations (ng/ml) in young and old virgin California mice by sex. All concentrations are reported as mean ± SEM.
| Condition | Female | Male | |||||
|---|---|---|---|---|---|---|---|
| Time | Young | Old | P | Young | Old | P | |
|
|
|
||||||
| Basal | 0800h | 45.06 ± 7.03 | 39.62 ± 9.44 | 0.505a | 54.76 ± 10.48 | 52.78 ± 10.78 | 0.999 |
| Basal | 2000h | 1522.85 ± 229.17 | 1403.75 ± 263.65 | --a | 1682.94 ± 128.64 | 1839.60 ± 167.59 | --a |
| Post-stress | 0800–0900h | 966.85 ± 170.34 | 780.85 ± 182.74 | 0.356b | 654.14 ± 82.54 | 709.87 ± 98.31 | 0.978b |
| Post-DEX | 1530–1630h | 81.80 ± 13.28 | 79.30 ± 15.35 | 0.665c | 104.55 ± 10.80 | 73.75 ± 7.97 | 0.582c,d |
| 45 min post-CRH | 1615–1715h | 87.00 ± 7.96 | 165.14 ± 82.53 | --c | 357.94 ± 101.72 | 309.85 ± 140.53 | --c |
| 90 min post-CRH | 1700–1800h | 637.65 ± 184.76 | 1025.61 ± 334.53 | --c | 899.96 ± 184.72 | 1199.05 ± 261.93 | --c |
P-values correspond to main effect of age group in a repeated-measures analysis on basal CORT (0800h and 2000h).
P-values correspond to main effect of age group in a repeated-measures analysis (0800h basal vs. post-stress).
P-values correspond to main effect of age group in a repeated-measures analysis of post-injection CORT (post-DEX, 45 min post-CRH, 90 min post-CRH).
Group*time interaction, P=0.016; old males had lower post-DEX CORT concentration than young males P=0.009.
3.1.2 CORT Response to Predator-urine Exposure
Exposure to predator urine increased plasma CORT above baseline levels measured at the same time of day (878.74 ± 123.06 vs. 42.48 ± 5.67 ng/ml; n=19; F1,17= 175.77, P <0.001; Table 1), but there was no effect of age group (F1,17= 0.90, P=0.356), nor was there a time*group interaction (F1,17 =0.14, P=0.714). For both age groups combined, post-urine-exposure CORT levels were not correlated with time-matched baseline concentrations (r=0.182, n=19, P=0.445).
3.1.3 CORT Response to DEX and CRH Injection
Injection of DEX followed by CRH elicited an increase in plasma CORT (8 h post-DEX vs. 45 min post-CRH vs. 90 min post-CRH: 80.62 ± 9.80 vs. 124.01 ± 39.18 vs. 821.42 ± 186.08 ng/ml; main effect of time: F2,34= 61.78, P <0.001; Fig. 1), but CORT response did not differ between age groups (F1,17= 0.20, P=0.665), nor was there a time*group interaction (F2,34 =0.73, P=0.494; Table 1). CORT levels increased at each time point measured, as CORT values were higher 90 min post-CRH than 45 min post-CRH (t=8.00, P<0.001; Fisher’s LSD), and as CORT values both 45 and 90 min post-CRH were higher than levels 8 h after DEX injection and immediately before CRH treatment (t=2.42, P=0.029; t=8.27, P<0.001, respectively; Fisher’s LSD).
Figure 1.
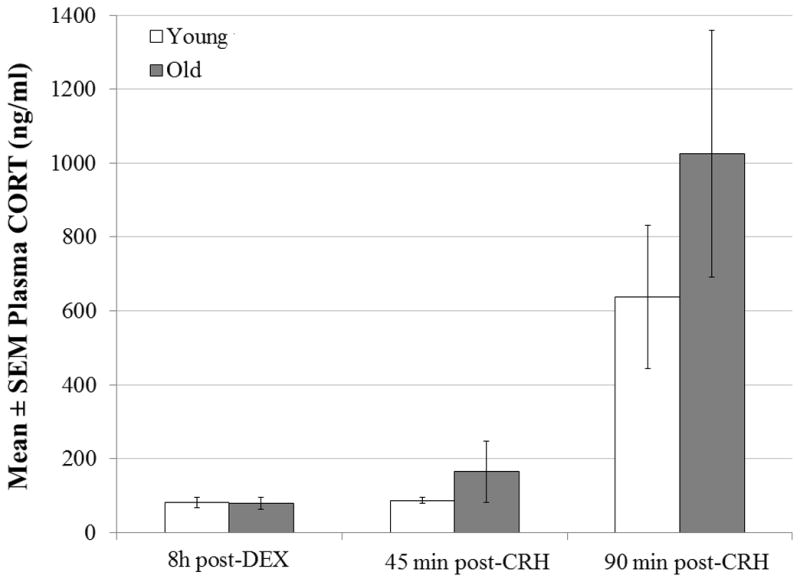
Plasma CORT concentrations in young (n=10) and old (n=9) adult, virgin female California mice following an injection of DEX (10mg/kg, i.p.; 0730–0830 h) and a subsequent injection of CRH (2 μg/kg, i.p.; 1530–1630 h). Age group did not affect the response to injection at any time point measured, but CORT levels increased across time (P <0.001) and all time points differed from one another (post-DEX vs. 45 min post-CRH: P=0.029; post-DEX vs. 90 min post-CRH: P<0.001; 45 min post-CRH vs. 90 min post-CRH: P<0.001).
In addition to repeated-measures ANOVA, we analyzed time-integrated CORT responses to CRH using two calculations for area under the curve (AUC; 50). Female age did not influence either AUCg (F1,17=0.34, P=0.565) or AUCi (F1,17=2.63, P=0.151).
For both age groups combined, post-DEX CORT concentrations were significantly and positively correlated with CORT levels 45 min post-CRH (r=0.852, n=19, P<0.001), but not 90 min post-CRH (r=0.362, n=19, P=0.128). Additionally, 45 min post-CRH CORT levels were significantly and positively correlated with 90 min post-CRH levels (r=0.537, n=19, P=0.018).
3.1.4 Body Mass
Body mass of female California mice was influenced by a main effect of day (F1,17 =19.21, P<0.001) and a day*group interaction (F1,17=6.33, P=0.022), but not a main effect of age group (F1,17= 1.79, P=0.199). Old females lost body mass from the start to the end of the experiment (approximately 2 weeks; t=4.75, P<0.001) whereas young females did not (t=1.36, P=0.193; Sidak-corrected post-hoc tests following repeated-measures ANOVA). Females did not differ in body mass at the start of the experiment (P=0.117) or on the day prior to dissection (P=0.323; Table 2).
Table 2.
Body mass and organ masses in young and old virgin California mice by sex. P values are for main effect of age group from one-way ANOVA (body mass) or ANCOVA (organ masses). Values are presented as mean ± SEM or mean with lower and upper confidence interval (for log-transformed data) and are reported in grams; bolded P-values are <0.05.
| Sex | Variable | Young | Old | P | Covariates |
|---|---|---|---|---|---|
| Females | (n=10) | (n=9) | |||
| body mass - start | 43.24 ± 2.02 | 49.69 ± 3.46 | 0.117 | -- | |
| body mass - end | 42.36 ± 2.10 | 46.47 ± 3.56 | 0.323 | -- | |
| total ovarya | 0.0111 ± 0.0012 | 0.0138 ± 0.0013 | 0.156 | body mass - end | |
| total adrenalb | 0.0171 ± 0.0013 | 0.0185 ± 0.0014 | 0.499 | body mass - end | |
| uterusc | 0.0421 (−1.47, −1.28) | 0.0635 (−1.30, −1.10) | 0.017 | body mass - end | |
| Males | (n=22) | (n=21) | |||
| body mass - start | 39.66 ± 1.54 | 53.39 ± 1.44 | <0.001 | -- | |
| body mass - end | 41.53 ± 1.68 | 53.67 ± 1.74 | <0.001 | -- | |
| r. testisd | 0.1977 ± 0.0210 | 0.1428 ± 0.0202 | 0.133 | body mass - end, group*body mass | |
| r. adrenale | 0.0097 (−2.10, −2.01) | 0.0087 (−2.06, −1.97) | 0.229 | body mass - end |
body-mass-corrected averages at body mass of 44.61 g
body-mass-corrected averages at body mass of 44.31 g
back-transformed body-mass-corrected averages at body mass of 44.31 g
body-mass-corrected averages at body mass of 47.46 g
back-transformed body-mass-corrected averages at body mass of 47.46 g
3.1.5 Organ Masses
Body mass on the day prior to dissection was used as a covariate for organ-mass analyses. Uterine mass was log10-transformed prior to analysis to meet normality assumptions. Across all females, right and left adrenal gland masses were highly correlated (r=0.892, n=19, P<0.001), as were right and left ovary masses (r=0.615, n=18, P=0.007). Therefore, only total (left + right) organ masses were used in the remaining analyses.
Initially, ANCOVAs were computed using group, body mass, and the group*body mass interaction. For all organs, the interaction term was not significant and was dropped from the model. Body mass remained in the model as a covariate and was significant for log10-transformed uterine mass (F1,16=8.27, P=0.011; Table 1), total ovarian mass (F1,15=11.32, P=0.004), and total adrenal gland mass (F1,16=5.56, P=0.031). After accounting for body mass, old females had significantly higher log10-transformed uterine mass (F1,16 = 7.10, P=0.017), but neither total adrenal gland mass (F1,16=0.479, P=0.499) nor total ovarian mass (F1,15=2.23, P=0.156) differed by group.
3.1.6 Vaginal Lavage
Lavages were carried out in an attempt to characterize cycle lengths of females. We were not able to discern a reliable pattern of cyclicity in vaginal smears in either age group. However, we did observe that 9 females (5 young and 4 old) had vaginal cytology typical of estrus in at least one lavage, suggesting that these animals were undergoing estrous cycles.
3.2 Males
3.2.1 Basal CORT
Plasma CORT levels were dramatically higher at 2000h when compared to 0800h levels, regardless of age group (1761.27 ± 105.05 vs. 53.79 ± 7.42 ng/ml; n=42; F1,40=881.27, P<0.001). However, there was neither a main effect of age group (F1,40 <0.001, P=0.999; Table 1) nor a time*group interaction (F1,40=0.221, P=0.641). Across both age groups, basal CORT concentrations at 0800h and 2000h were not correlated with one another (r=−0.021, n=42, P=0.897).
3.2.2 CORT Response to Predator-urine Exposure
Exposure to bobcat urine significantly increased plasma CORT concentrations in male California mice when compared to time-matched baseline values (F1,41 =283.79, P<0.001; Table 1), but there was no effect of age group (F1,41= 0.01, P = 0.978), nor was there a group*time interaction (F1,41=0.40, P=0.531). Post-urine-exposure CORT levels were not correlated with 0800h basal CORT concentrations (r=0.082, n=43, P=0.602).
3.2.3 CORT Response to DEX and CRH Injection
DEX and CRH treatment affected CORT levels over the three time points measured, as there was a main effect of time (8 h post-DEX vs. 45 min post-CRH vs. 90 min post-CRH: 89.51 ± 7.09 vs. 335.04 ± 84.54 vs. 1038. 56 ± 156.45 ng/ml; F2,78 = 82.90, P<0.001; Fig. 2). Irrespective of age group, CORT levels 90 min post-CRH were higher than those post-DEX (t=11.39, P<0.001; Table 1) and 45 min post-CRH (t=9.78, P<0.001; Fisher’s LSD). Additionally, CORT levels 45 min post-CRH were higher than those obtained 8 h post-DEX injection (t=3.94, P=0.001; Fisher’s LSD). We did not find a significant main effect of age group on CORT levels (F1,39=0.31, P=0.582), but there was a time*group interaction (F2,78 =4.33, P=0.016). Specifically, young males had higher CORT levels than did old males 8 h after DEX injection (t=2.74, P=0.009; Sidak-corrected post-hoc test; Fig. 2), but CORT concentrations did not differ between the two age groups either 45 min (P=0.212) or 90 min (P=0.155) following CRH injection. Neither AUCg (F1,39= 0.76, P=0.785) nor AUCi (F1,36=0.12, P=0.734) differed between young and old males.
Figure 2.
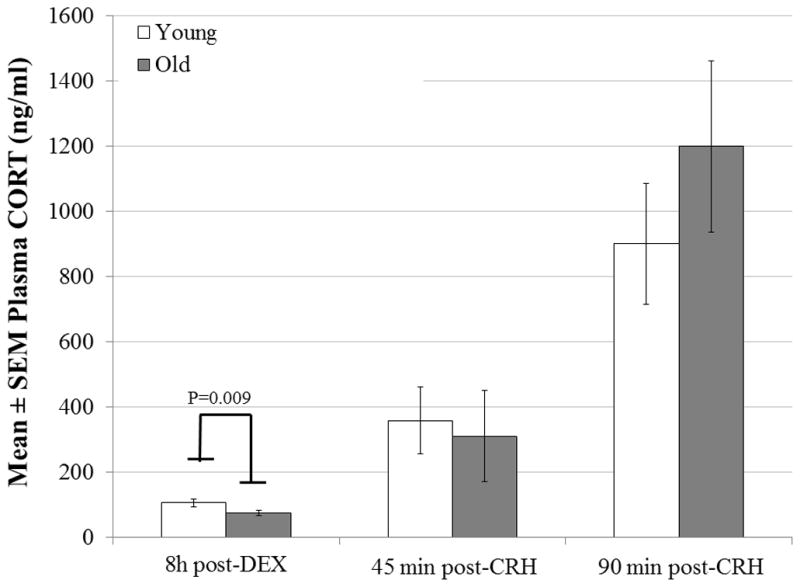
Plasma CORT levels in young (n=22) and old (n=21) adult, virgin male California mice following an injection of DEX (10mg/kg, i.p.; 0730–0830 h) and a subsequent injection of CRH (2 μg/kg, i.p.; 1530–1630 h). Regardless of age group, CORT increased over time (P<0.001; post-DEX vs. 45 min post-CRH: P=0.001; post-DEX vs. 90 min post-CRH: P<0.001; 45 min post-CRH vs. 90 min post-CRH: P<0.001). Age group significantly affected CORT responses over time (group*time interaction, P=0.016). CORT levels were higher in young males than in old males at 8h post-DEX (P=0.009) but not at the remaining time points.
Across all males, as in females, post-DEX CORT levels were significantly and positively correlated with 45 min post-CRH CORT levels (r=0.412, n=42, P=0.007) but not with 90 min post-CRH CORT levels (r=0.059, n=41, P=0.713). Plasma CORT concentrations at 45 min post-CRH were significantly and positively correlated with concentrations at 90-min post-CRH (r=0.606, n=41, P<0.001), again mirroring results found in females.
3.2.4 Body Mass
Body mass of male California mice increased across the 3-week period of data collection (main effect of day: F1,41 =5.41, P=0.025; Table 2). Overall, old males were heavier than young males (F1,41 =33.80, P<0.001), but the change in mass over time did not differ between the two age groups (day*group interaction: F1,41 =2.940, P=0.094).
3.2.5 Organ Masses
Ending body mass differed between young and old males (t41=5.02; P<0.001; Table 2) and was used as a covariate for analyses of organ masses. Adrenal gland mass was log10-transformed to fit normality assumptions. Initially the interaction term of group*body mass was included in the model. The term was nearly significant (F1,39=3.85, P=0.057) for analysis of right testis mass and therefore was retained in this ANCOVA, but it was not significant for right adrenal gland mass (F1,39=0.13, P=0.720) and was thus removed. The effect of body mass was significant for right adrenal (F1,40=5.50, P=0.024) and right testis (F1,39=4.51, P=0.040). Age did not affect body-mass-corrected right adrenal (F1,40=1.50, P=0.229; Table 2) or right testis (F1,39=2.35, P=0.133; Table 2) mass.
3.3 Comparisons Between the Sexes
As described above, CORT data from males and females were not strictly comparable, due to minor differences in methodology and to the fact that blood samples from the two sexes were assayed separately. Therefore, the results of analyses comparing CORT levels between the sexes should be interpreted cautiously.
3.3.1 Basal CORT
When we analyzed data from both sexes together, results mirrored those from female-only and male-only analyses. Basal CORT was affected by time of sample, with 2000h CORT levels being higher than 0800h levels (1669.44 ± 90.44 vs. 49.42 ± 5.46 ng/ml; F1,57=1041.67, P<0.001); however, there was no time*sex (P=0.521), time*age (P=0.725), or time*sex*age (P=0.867) interaction. Additionally, there was no main effect of sex (P=0.106) or age (P=0.544), nor a sex*age interaction (P=0.545).
3.3.2 CORT Response to Predator-urine Exposure
As with the basal CORT results, combined post-stress analysis mirrored results from single-sex analyses. Predator-urine exposure resulted in a significant increase in plasma CORT concentration (741.85 ± 58.48 vs. 49.42 ± 5.46 ng/ml; F1,58=406.48, P<0.001), but there was no time*sex (P=0.194), time*age (P=0.959) or time*sex*age (P=0.514) interaction. Additionally, there was no main effect of sex (P=0.776) or age (P=0.417), nor was there a sex*age interaction (P=0.434).
3.3.3 CORT Response to DEX and CRH Injection
For the sexes combined, the DEX/CRH injection paradigm significantly altered plasma CORT levels, as there was a main effect of time (F2,112=117.88, P<0.001). This effect depended on age (time*age interaction: F2,112=3.20, P=0.045; main effect of age: P=0.991; see Supplemental Fig. A) but not sex (main effect of sex: P=0.165; time*sex interaction: P=0.259; time*sex*age interaction: P=0.552; sex*age interaction: P=0.514). Sex did not affect AUCg or AUCi results (AUCg: sex P=0.206, age P=0.547, sex*age P=0.777; AUCi: sex P=0.475, age P=0.201, sex*age P=0.383).
4. DISCUSSION
We used virgin male and female California mice to test the hypothesis that aged animals show reduced glucocorticoid responses to stress and CRH injection, and enhanced responses to negative feedback, when compared to young adults, possibly as a means to maximize current reproductive potential. We predicted that old animals would have lower circulating CORT levels in response to predator-odor exposure, following DEX injection, and at two time points following CRH injection. Sustained elevation of glucocorticoids can be detrimental to reproductive efforts (35,64,65,72); therefore, enhanced negative feedback or reduced CORT output should be beneficial in preserving reproductive function by minimizing the duration or magnitude of CORT elevation. Additionally, we predicted that older animals would have larger reproductive organs and might have smaller adrenal glands as compared to young animals. We found that old male mice were more sensitive to DEX negative feedback than were young male mice, thus providing partial support for the hypothesis; however, we did not find any evidence that HPA activity changes with age in adult females. No other differences in HPA function between old and young adult mice were noted in either sex. Regardless of age group, all mice experienced an increase in CORT concentration above basal levels after exposure to predator urine and in response to CRH injection. Additionally, all animals displayed a pronounced diurnal rhythm in CORT profiles. Old female mice had heavier body-mass-corrected uteri than did young females, but neither adrenal nor gonadal mass differed between age groups in either sex. When the sexes were analyzed together, the same general pattern emerged and there was no main effect of sex or sex-by-age interaction on basal CORT, post urine-exposure CORT or post-injection (DEX or CRH) CORT levels.
Old male California mice displayed lower CORT concentrations 8 h following injection of a standardized dose of DEX than did young males. This finding suggests that the anterior pituitary of older males is more sensitive to DEX-induced negative feedback; it is unclear whether central sensitivity to glucocorticoids also differs between young and old males, as DEX does not readily cross the blood-brain barrier (10). This increased pituitary sensitivity with old age supports the hypothesis that decreased future opportunities for breeding should favor a more easily suppressed HPA axis. However, we did not find any differences in CRH-stimulated CORT concentrations between the age groups within either sex, even when we analyzed total CORT output with post-DEX values taken into consideration (AUCi values). This suggests that HPA axis sensitivity to CRH is not dampened in older animals. Additionally, age did not affect CORT levels following 5-minute exposure to predator urine, suggesting that HPA axis sensitivity to an acute stressor is not altered by age.
Our equivocal findings on effects of age on CORT are consistent with previous research, as several other studies have also noted mixed findings on effects of age on HPA activity and responsiveness to DEX. In hybrid (F344/BN) male rats, 30-month-old males had lower post-DEX CORT concentrations than 3-month-old males, but age groups (3 vs. 15 vs. 30 months) did not differ in their CORT response to restraint stress (31), similar to our findings in male California mice. Additionally, 24-month-old F344/BN hybrid males had lower post-DEX CORT concentrations as compared to 3-month-old males when the DEX injection was given systemically (44). However, when DEX was administered directly into the brain (prefrontal cortex, hippocampus, or hypothalamus), the suppressive effect of DEX on plasma CORT was abolished in old rats whereas young rats still experienced a decrease (44), suggesting that age differentially affects responsiveness to glucocorticoid negative feedback at both the brain and the pituitary. In contrast, old (15–27 yrs) female rhesus monkeys (Macaca mulatta) displayed an earlier release from DEX-induced cortisol suppression than did younger adult (7–8 yrs) females; however, the two age groups did not differ in glucocorticoid concentrations following CRH injection (22). Additionally, post-DEX and post-CRH cortisol levels were higher in older men and women compared to their younger counterparts (27). In sum, these findings suggest that pituitary sensitivity to DEX and/or glucocorticoids increases with old age in male rodents but decreases with old age in primates.
Effects of aging on HPA responses to stress also differ among studies. For example, old (24 months) female Fisher 344 rats had lower CORT levels following 15-min exposure to a novel environment and to a bout of anesthesia with blood collection, as compared to young female rats (3 months; 4). However, young and old females did not differ in CORT levels following a 3-min novel-environment exposure. Moreover, young and old male Fisher 344 rats did not differ in CORT concentration following any of the three stress paradigms (4), suggesting that the effects of age on CORT responses can be modulated by both sex and stressor type. Old (22–24 months) and young (3 months) male Wistar rats did not differ in CORT response following 15 min in a novel environment, but old males displayed higher baseline CORT concentrations than did young males (32). Moreover, 33-month-old male F344/BN hybrid rats had higher CORT levels following a 30-min novel environment exposure compared to 15- and 3-month-old males, but the three age groups did not differ in the CORT response to restraint stress or in morning basal CORT levels (26), contrary to findings on basal levels in Wistar rats and again suggesting that strain, stressor type, and possibly age categories can alter results. In humans, a meta-analysis indicated that older adults have more active HPA axes (higher post-stress and post-DEX cortisol levels) than do young adults, and that this difference is more pronounced in women compared to men (48), again pointing to a sex difference. The occurrence of mixed results across several species suggests that age effects on HPA function are complex, and that numerous organismal and experimental variables, including sex, strain, stressor type, test paradigm, age classification, and injection route, can influence the interactions between age and HPA responsiveness.
In contrast to our hormonal results, we found no support for our predictions regarding age effects on organ masses in males, but found partial support in females. Despite being virgins, old females had higher body-mass-corrected uterine mass than did young females, possibly suggesting that older females were better prepared for pregnancy as uterine mass has been noted to increase prior to the breeding season (P. maniculatus, 15) or in response to gonadal steroids present around the time of estrus (Rattus norvegicus, 38). In contrast, age (onset of adulthood vs. young adult) did not affect body-mass-corrected uterine mass in Mongolian gerbils (Meriones unguiculatus; 59), nor did uterine mass differ between juvenile and mature female Pine voles (Microtus pinetorum; 66).
Despite differences in uterine mass, old and young P. californicus females did not differ in total ovarian mass in this study. Unfortunately, we were not able to use estrous-cycle stage as a covariate because we could not accurately monitor estrous cyclicity. However, similar numbers of old (4/9) and young (5/10) females expressed vaginal cytology typical of estrus at least once during the study; therefore, we believe that differences in uterine mass were not due to age group differences in the number of animals undergoing estrous cycles. Previous reports on California mice show that estrous cycles in this species are longer and more variable in length (range: 5–20 days, median: 9 days; 19) than in rats and house mice (mice: 4–5 days; 6; rats: 4–5 days; 17; 37), but are consistent with cycle lengths in other Peromyscus species (3). Although studies on Rattus and Mus found higher CORT levels during proestrus, estrous cycle stage does not affect CORT levels of female California mice following a 3 h social interaction (30) or 40 min following a resident-intruder test (14); therefore, estrous cycling should not have confounded our CORT results.
In conclusion, we did not find consistent evidence for age-related changes in HPA activity or reactivity in virgin male or female California mice. This suggests that aged and young adult California mice are not differentially modulating the perception of potential stressors (i.e. stressful or not), nor are they undergoing age-related alterations in HPA responsiveness to stress or exogenous HPA hormones. Nonetheless, it remains possible that the HPA axis plays a role in the trade-off between current and future reproductive investment and in age-related modulation of this trade-off. For instance, aged animals might increase production of corticosteroid-binding globulin (CBG), thus decreasing the amount of free vs. bound CORT and mitigating the effects of elevated glucocorticoids (5,39,75). Unfortunately, we were not able to determine concentrations of CBG at this time, but development of a CBG assay or a glucocorticoid binding affinity assay (see 49) for California mice would be beneficial for future studies. Moreover, aged individuals might exhibit reduced responsiveness of the reproductive system or hypothalamic-pituitary-gonadal (HPG) axis to elevated glucocorticoids (75). In semelparous species such as salmon (Oncorhynchus spp.) and certain dasyurid marsupials (Antechinus spp.), individuals are able to maintain reproductive function despite near-lethal levels of circulating glucocorticoids (see 75 for review). While the mechanism for this ability is unknown, reduced HPG sensitivity has been proposed (75). Future studies in California mice and other species should evaluate the interactions of age and stress effects on CORT concentrations and HPG-level measures, and should investigate the relationship between HPA reactivity and reproductive outcomes in actively breeding, young and old adults.
Supplementary Material
Plasma CORT levels in young (n=32) and old (n=28) adult, virgin, male and female California mice following an injection of DEX (10mg/kg; 0730–0830 h) and a subsequent injection of CRH (2 μg/kg; 1530–1630 h). CORT increased over time (P<0.001; post-DEX vs. 45 min post-CRH: P=0.003; post-DEX vs. 90 min post-CRH: P<0.001; 45 min post-CRH vs. 90 min post-CRH: P<0.001), but pattern of change over time differed with age (time*age interaction, P=0.045). Age groups did not differ from each other at any of the three time points, but a subtle difference existed across time young animals compared to old animals. In young animals, post-DEX CORT levels did not differ from 45 min post-CRH (P=0.064), but these two time points did differ in old animals (P=0.046); CORT levels 90 min post-CRH were different from all other time points for each age group (P<0.001). Sex did not significantly affect CORT level (P=0.165) and is thus sexes are not shown separately on the graph. * P<0.05 within age group.
Acknowledgments
We would like to thank the UCR vivarium staff for their assistance with animal maintenance. We also thank Vanessa Yang, Julia Cho, Kevin Measor, Omar Aldaas, Aaron Stamp, Saif Hossain, Juan Pablo Perea-Rodriguez, Gavrielle Concepcion, and Dr. Trynke de Jong for help with various aspects of experimental preparation and data collection, and Matthew Wolak, Brian Gray, and Dr. Zach Hohman for advice on statistical procedures. We also thank Dr. Mark Chappell and two anonymous reviewers for helpful comments on a previous draft of this manuscript. This work was supported by NIH MH087806 and by funds from the University of California, Riverside.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinol. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- 2.Boonstra R. Equipped for life: The adaptive role of the stress axis in male mammals. J Mammal. 2005;86:236–247. [Google Scholar]
- 3.Bradley EL, Terman CR. Ovulation in Peromyscus maniculatus bairdi under Laboratory Conditions. J Mammal. 1979;60:543–549. [Google Scholar]
- 4.Brett LP, Chong GS, Coyle S, Levine S. The pituitary-adrenal response to novel stimulation and ether stress in young adult and aged rats. Neurobiol Aging. 1983;4:133–138. doi: 10.1016/0197-4580(83)90037-4. [DOI] [PubMed] [Google Scholar]
- 5.Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- 6.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;48:A.4I.1–A.4I.8. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantoni D, Brown RE. Male influence of interbirth interval in the monogamous California mouse when required to forage for food. Ann NY Acad Sci. 1997:486–489. doi: 10.1111/j.1749-6632.1997.tb51946.x. [DOI] [PubMed] [Google Scholar]
- 8.Cantoni D, Brown RE. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Anim Behav. 1997;54:377–386. doi: 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- 9.Chauke M, Malisch JL, Robinson C, de Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 11.Dallman MF, Bhatnagar S. Chronic stress and energy balance: role of the hypothalamo–pituitary–adrenal axis. In: McEwen BS, Goodman HM, editors. Handbook of Physiology; Section 7: The Endocrine System; Volume IV: Coping with the Environment: Neural and Endocrine Mechanisms. Oxford University Press; New York: 2001. pp. 179–210. [Google Scholar]
- 12.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: Critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 13.Dalm S, Enthoven L, Meijer OC, van der Mark MH, Karsssen AM, de Kloet ER, Oitzl MS. Age-related changes in hypothalamic-pituitary-adrenal axis activity of male C57BL/6J mice. Neuroendocrinology. 2005;81:372–380. doi: 10.1159/000089555. [DOI] [PubMed] [Google Scholar]
- 14.Davis ES, Marler CA. The progesterone challenge: Steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 2003;44:185–198. doi: 10.1016/s0018-506x(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 15.Demas GE, Nelson RJ. Social, but not photoperiodic, influences on reproductive function in male Peromyscus aztecus. Biol Reprod. 1998;58:385–389. doi: 10.1095/biolreprod58.2.385. [DOI] [PubMed] [Google Scholar]
- 16.Ferin M. Stress and the reproductive system. In: Wassarmen P, Neill JD, editors. Knobil and Neill’s Physiology of Reproduction. Elsevier Academic Press; 2006. pp. 2627–2696. [Google Scholar]
- 17.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg N, Wingfield JC. Stress and reproduction: Reciprocal relationships. In: Norris DO, Jones RE, editors. Hormones and Reproduction in Fishes, Amphibians, and Reptiles. Plenum Press; New York: 1987. pp. 461–503. [Google Scholar]
- 19.Gubernick DJ. Reproduction in the California mouse, Peromyscus californicus. J Mammal. 1988;69:857–860. [Google Scholar]
- 20.Gubernick DJ, Wright SL, Brown RE. The significance of father’s presence for offspring survival in the monogamous California mouse, Peromyscus californicus. Anim Behav. 1993;46:539–546. [Google Scholar]
- 21.Gubernick DJ, Teferi T. Adaptive significance of male paternal care in a monogamous mammal. Proc Biol Sci. 2000;267:147–150. doi: 10.1098/rspb.2000.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gust DA, Wilson ME, Stocker T, Conrad S, Plotsky PM, Gordon TP. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab. 2000;85:2556–2563. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- 23.Harris BN, Saltzman W, de Jong TR, Milnes MR. Hypothalamic-pituitary-adrenal (HPA) axis function in the California mouse (Peromyscus californicus): Changes in baseline activity, reactivity, and fecal excretion of glucocorticoids across the diurnal cycle. Gen Comp Endocrinol. 2012;179:436–450. doi: 10.1016/j.ygcen.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidinger BJ, Nisbet ICT, Ketterson ED. Changes in adrenal capacity contribute to a decline in the stress response with age in a long-lived seabird. Gen Comp Endocrinol. 2008;156:564–568. doi: 10.1016/j.ygcen.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Heidinger BJ, Nisbet ICT, Ketterson ED. Older parents are less responsive to a stressor in a long-lived seabird: a mechanism for increased reproductive performance with age? Proc Biol Sci B. 2006;273:2227–2231. doi: 10.1098/rspb.2006.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JP, Larson BR, Speert DB, Seasholtz AF. Hypothalamo-pituitary-adrenocortical dysregulation in aging F344/Brown-Norway F1 hybrid rats. Neurbiol Aging. 2001;22:323–332. doi: 10.1016/s0197-4580(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 27.Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: Importance of gender. Neurobiol Aging. 1994;15:227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 28.Hylka VW, Sonntage WE, Meites J. Reduced ability of old male rats to release ACTH and corticosterone in response to CRF administration. Proc Soc Exp Bio Med. 1984;175:1–4. doi: 10.3181/00379727-175-1-rc1. [DOI] [PubMed] [Google Scholar]
- 29.Jessop TS, Hamann M. Interplay between age class, sex and stress response in green turtles (Chelonia mydas) Aust J Zool. 2005;53:131–136. [Google Scholar]
- 30.Karelina K, Walton JC, Weil ZM, Norman GJ, Nelson RJ, DeVries AC. Estrous phase alters social behavior in a polygynous but not a monogamous Peromyscus species. Horm Behav. 2010;58:193–199. doi: 10.1016/j.yhbeh.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Kasckow JW, Segar TM, Xiao C, Furay AR, Evanson NK, Ostrander MM, Herman JP. Stability of neuroendocrine and behavioral responsiveness in aging Fischer 344/Brown-Norway hybrid rats. Endocrinology. 2005;146:3105–3112. doi: 10.1210/en.2004-1648. [DOI] [PubMed] [Google Scholar]
- 32.Keck ME, Hatzinger M, Wotjak CT, Landgraf R, Holsboer R, Neumann ID. Aging alters intrahypothalamic release patterns of vasopressin and oxytocin in rats. Eur J Neurosci. 2000;12:1487–1494. doi: 10.1046/j.1460-9568.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 33.Ketterson ED, Nolan V., Jr Adaptation, exaptation, and constraint: a hormonal perspective. Am Nat. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- 34.Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Lerman SA, Miller GK, Bohlman K, Albaladejo V, Léonard JF, Devas V, Clark RL. Effects of corticosterone on reproduction in male Sprague-Dawley rats. Reprod Toxicol. 1997;11:799–805. doi: 10.1016/s0890-6238(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 36.Lo MJ, Kau MM, Wang PS. Effect of aging on corticosterone secretion in diestrous rats. J Cell Biochem. 2006;97:351–358. doi: 10.1002/jcb.20576. [DOI] [PubMed] [Google Scholar]
- 37.Long JA, Evans HM. The oestrous cycle in the rat and it associated phenomena. Mem Univ Calif. 1922;6:1–143. [Google Scholar]
- 38.Lundeen SG, Carver JM, McKean ML, Winneker RC. Characterization of the ovariectomized rat model for the evaluation of estrogen effects on plasma cholesterol levels. Endocrionology. 1997;138:1552–1558. doi: 10.1210/endo.138.4.5083. [DOI] [PubMed] [Google Scholar]
- 39.Malisch JL, Breuner CW. Steroid-binding proteins and free steroids in birds. Mol Cell Endocrinol. 2010;316:42–52. doi: 10.1016/j.mce.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Meddle SL, Owen-Ashley NT, Richardson MI, Wingfield JC. Modulation of the hypothalamic–pituitary–adrenal axis of an Arctic-breeding polygynandrous songbird, the Smith’s longspur, Calcarius pictus. Proc Biol Sci. 2003;270:1849–1856. doi: 10.1098/rspb.2003.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merritt J. California mouse. In: Wilson DE, Ruff S, editors. The Smithsonian Book of North American Mammals. Smithsonian Institution Press; Washington, D.C: 1999. pp. 565–566. [Google Scholar]
- 42.Merritt JF. Peromyscus californicus. Mammalian Species. 1978;85:1–6. [Google Scholar]
- 43.Mettus RV, Rane SG. Characterization of the abnormal pancreatic development, reduced growth and infertility in Cdk4 mutant mice. Onocgene. 2003;22:8413–8421. doi: 10.1038/sj.onc.1206888. [DOI] [PubMed] [Google Scholar]
- 44.Mizoguchi K, Ikeda R, Shoji H, Tanaka Y, Maruyama W, Tabira T. Aging attenuates glucocorticoid negative feedback in rat brain. Neurosci. 2009;159:250–270. doi: 10.1016/j.neuroscience.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Moore IT, Hopkins WA. Interactions and trade-offs among physiological determinants of performance and reproductive success. Int Comp Biol. 2009;49:441–451. doi: 10.1093/icb/icp081. [DOI] [PubMed] [Google Scholar]
- 46.Nichols DJ, Chevins PFD. Plasma corticosterone fluctuations during the oestrous cycle of the house mouse. Experientia. 1981;37:319–320. doi: 10.1007/BF01991678. [DOI] [PubMed] [Google Scholar]
- 47.O’Reilly KM. The effects of age and gender on adrenocortical response to stress in Leach’s Storm-Petrels. Pacific Seabirds. 1999;26:42–45. [Google Scholar]
- 48.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Perogamvros I, Kayahara M, Trainer PJ, Ray DW. Serum regulates cortisol bioactivity by corticosteroid-binding globulin-dependent and independent mechanisms, as revealed by combined bioassay and physicochemical assay approaches. Clinical Endocrinology. 2011;75:31–38. doi: 10.1111/j.1365-2265.2011.04003.x. [DOI] [PubMed] [Google Scholar]
- 50.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 51.Reeder DM, Kramer KM. Stress in free-ranging mammals: Integrating physiology, ecology, and natural history. J Mammal. 2005;86:225–235. [Google Scholar]
- 52.Ribble DO. The monogamous mating system of Peromyscus californicus as revealed by DNA fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- 53.Ribble DO. Lifetime reproductive success and its correlates in the monogamous rodent, Peromyscus californicus. J Anim Ecol. 1992;61:457–468. [Google Scholar]
- 54.Ribble DO, Salvioni M. Social-organization and nest co-occupancy in Peromyscus californicus, a monogamous rodent. Behav Ecol Sociobiol. 1990;26:9–15. [Google Scholar]
- 55.Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evol. 2002;17:462–468. [Google Scholar]
- 56.Roff DA. The Evolution of Life Histories: Theory and Analysis. Chapman & Hall; New York: 1992. pp. 1–535. [Google Scholar]
- 57.Roff DA. The Evolution of Threshold Traits in Animals. Q Rev Biol. 1996;71:3–35. [Google Scholar]
- 58.Romero ML, Reed JM. Collecting baseline corticosterone samples in the field: is under 3 minutes good enough? Comp Biochem Physiol A Mol Integr Physiol. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Saltzman W, Ahmed S, Fahimi A, Wittwer DJ, Wegner FH. Social suppression of female reproductive maturation and infanticidal behavior in cooperatively breeding Mongolian gerbils. Horm Behav. 2006;49:527–537. doi: 10.1016/j.yhbeh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Sapolsky RM. Endocrinology of the Stress-Response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. Bradford Book: Massachusetts Institute of Technology; Cambridge, Massachusetts: 2002. pp. 409–450. [Google Scholar]
- 61.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endoc Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 62.Scaccianoce S, Nicolai R, Cigliana G, Angelucci L. Reduced glucocorticoid response to corticotropin secretagogues in the aged Sprague-Dawley rat. Neuroendocrinology. 1995;62:32–38. doi: 10.1159/000126985. [DOI] [PubMed] [Google Scholar]
- 63.Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. J Am Assoc Lab Anim Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- 64.Silverin B. Corticosterone-binding proteins and behavioral effects of high levels of corticosterone during the breeding period in the pied flycatcher. Gen Comp Endocrinol. 1986;64:67–74. doi: 10.1016/0016-6480(86)90029-8. [DOI] [PubMed] [Google Scholar]
- 65.Silverin B. Behavioural and hormonal responses of the pied flycatcher to environmental stressors. Anim Behav. 1998;55:1411–1420. doi: 10.1006/anbe.1997.0717. [DOI] [PubMed] [Google Scholar]
- 66.Solomon NG, Vandenbergh JG, Wekesa KS, Barghusen L. Chemical cues are necessary but insufficient for reproductive activation of female Pine voles (Microtus pinetorum) Biol Reprod. 1996;54:1038–1045. doi: 10.1095/biolreprod54.5.1038. [DOI] [PubMed] [Google Scholar]
- 67.Stearns SC. Trade-Offs in Life-History Evolution. Funct Ecol. 1989;3:259–268. [Google Scholar]
- 68.Stearns SC. The Evolution of Life Histories. Oxford University Press; 1992. [Google Scholar]
- 69.Stearns SC. Life history evolution: successes, limitations, and prospects. Naturwissenschaften. 2000;87:476–486. doi: 10.1007/s001140050763. [DOI] [PubMed] [Google Scholar]
- 70.Trainor BC, Takahashi E, Silva AL, Crean KK, Hostetler C. Photoperiod and sex differences in hormonal responses to social conflict in the monogamous California mouse. Horm Behav. 2010;58:506–512. doi: 10.1016/j.yhbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse. PLoS One. 2011;6:e174051–11. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tilbrook AJ, Turner AI, Clarke JJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Repro. 2000;5:105–113. doi: 10.1530/ror.0.0050105. [DOI] [PubMed] [Google Scholar]
- 73.Tomkins JL, Simmons LW. Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim Behav. 2002;63:1009–1016. [Google Scholar]
- 74.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 75.Wingfield JC, Sapolsky RM. Reproduction and Resistance to Stress: When and How. J Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 76.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone-behavior interactions: The “Emergency Life History Stage”. Amer Zool. 1998;38:191–206. [Google Scholar]
- 77.Zera AJ, Harshman LG. The Physiology of Life History Trade-Offs in Animals. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma CORT levels in young (n=32) and old (n=28) adult, virgin, male and female California mice following an injection of DEX (10mg/kg; 0730–0830 h) and a subsequent injection of CRH (2 μg/kg; 1530–1630 h). CORT increased over time (P<0.001; post-DEX vs. 45 min post-CRH: P=0.003; post-DEX vs. 90 min post-CRH: P<0.001; 45 min post-CRH vs. 90 min post-CRH: P<0.001), but pattern of change over time differed with age (time*age interaction, P=0.045). Age groups did not differ from each other at any of the three time points, but a subtle difference existed across time young animals compared to old animals. In young animals, post-DEX CORT levels did not differ from 45 min post-CRH (P=0.064), but these two time points did differ in old animals (P=0.046); CORT levels 90 min post-CRH were different from all other time points for each age group (P<0.001). Sex did not significantly affect CORT level (P=0.165) and is thus sexes are not shown separately on the graph. * P<0.05 within age group.


