Abstract
Objective
To describe the effects of age at ART initiation on growth outcomes among children infected with HIV followed for 48 months after treatment initiation.
Study design
This secondary analysis describes anthropometric changes in children infected with HIV in Johannesburg, South Africa who initiated ritonavir-boosted lopinavir (LPV/r)-based ART before 24 months of age and were randomized to continue LPV/r or to receive nevirapine after achieving and maintaining virologic suppression. Weight, height, and head circumference were measured at visits over 48 months post-ART initiation. Growth patterns including z-scores for weight for age (WAZ), height for age (HAZ), BMI for age, and head circumference for age (HCAZ) were compared between children initiating ART <6 months, 6–12 months, and 12–24 months of age.
Results
195 children (mean±SD age 10.7±5.9 months), including 54(27.7%) <6 months, 69(35.4%) 6–12 months, and 72(36.9%) 12–24 months of age at ART initiation, were evaluated. In the first 12 months on treatment, children <6 months of age at ART initiation experienced more rapid improvement in WAZ (1.98 vs. 1.44, p=0.084) and HCAZ (1.24 vs. 0.45, p=0.004) than children who initiated ART between 12–24 months of age. By 48 months on ART, growth outcomes were similar, regardless of age at ART initiation. WAZ approached population norms by 12 months on ART. Although improving, HAZ remained on average 1.0 z-score below population norms at 48 months of therapy.
Conclusions
Initiation of ART before 6 months of age results in more rapid growth recovery in children infected with HIV. These data provide further evidence for the importance of prompt diagnosis and early initiation of ART for infants infected with HIV.
Keywords: height, weight, BMI, head circumference
Perinatally-acquired HIV infection is associated with poor growth, including compromised weight, height, and head circumference.1–4 As poor growth is both a sensitive indicator of HIV disease progression and an independent risk factor for mortality,4 developing interventions to optimize growth during infancy and early childhood is particularly important.4
In developed countries, the availability of antiretroviral therapy (ART) has dramatically improved both growth and survival of children infected with HIV12.5–9 ART-related improvements in weight-for-age z-scores (WAZ) occur before improvements in height-for-age z-scores (HAZ).6 In the US, children infected with HIV have been reported to achieve normal weight (WAZ=0) within a year and near normal height (HAZ=0) within 2 years after initiation of ART.7, 8 The age of the child at the time of ART initiation appears to be important. Initiating ART at ages younger than 3 years has been reported to result in more rapid early weight gain and more pronounced trajectories towards growth normalization.8, 9
Improvements in growth also have been demonstrated in studies in sub-Saharan Africa, where more than 90% of the 2.5 million children living with HIV currently reside and where childhood malnutrition is highly prevalent.10 Follow-up periods for these studies range from 4 months to 24 months after ART initiation; less attention has been given to periods beyond 24 months.11–16 Several African studies also have indicated that earlier age at initiation of treatment has a positive effect on growth.12–14 Further studies focusing on infants and young children infected with HIV, with longer follow-up periods, are necessary to investigate when ART should be initiated in order to support a more rapid catch-up to population growth norms, and to optimize long-term growth outcomes.
We describe the effects of age at ART initiation (before 6 months, 6–12 months, and 12–24 months) on growth outcomes, including weight, height, body mass index (BMI), and head circumference, among children infected with HIV followed for over 48 months after initiation of treatment in the context of a clinical trial.
METHODS
We performed a secondary data analysis of anthropometric changes in children infected with HIV followed prospectively through 48 months of therapy as part of a clinical trial (ClinicalTrials.gov: NCT00117728) from ART initiation. The analysis evaluates the effect of age at ART initiation on growth outcomes. The data were collected as a part of a randomized trial that assessed the reuse of nevirapine in children who previously were exposed to single-dose nevirapine prophylaxis at birth and who were younger than 24 months of age at ART initiation.17 Between April 8, 2005 and July 10, 2007, 323 children were recruited at Rahima Moosa Mother and Child Hospital in Johannesburg, South Africa; 195 of these children who achieved and sustained plasma HIV-1 RNA <400 copies/mL for at least 3 months within the first 12 months of treatment were randomized and included in our analysis. Signed informed consent was obtained from the child’s parent or guardian. The study was approved by the Institutional Review Boards of Columbia University (New York, NY) and the University of the Witwatersrand (Johannesburg, South Africa).
At pre-treatment, sociodemographic information was collected, a medical history was obtained, weight (kg), height (cm), and head circumference (mm) were measured, and blood samples (for CD4 T-cell determination and HIV-1 RNA quantity) were collected. Subsequent visits were scheduled at 0.5, 1, 2, 3, and every 3 months thereafter to detect viral suppression up to 12 months post-ART initiation until randomization, and at 0.5, 1, 2, 4, 6, 9, 13, 16, and 19 months after randomization. Blood samples for plasma HIV-1 RNA quantification were collected at 1, 4, 6, 9, 13, 16, and 19 months and for CD4 cell determination at 4, 6, 9, 13, 6, and 19 months and both every 3 months thereafter until study completion in June 2010. At all visits, weight, height, and head circumference were measured by trained clinicians using a standardized protocol. A digital scale was used to weigh the children, recumbent height was measured using an infantometer for children ≤24 months of age, and standing height was measured with a wall-mounted stadiometer for those >24 months of age. Head circumference was measured using a flexible anthropometric tape measure. BMI was calculated as weight (kg) divided by height (m2). Weight-for-age (WAZ), height-for-age (HAZ), BMI-for-age (BAZ), and head circumference-for-age (HCAZ) z-scores were calculated using World Health Organization standards.18 Underweight was defined as WAZ less than −2, stunting as HAZ less than −2, and wasting as BAZ less than −2. HCAZ were not available for children beyond 5 years of age.
Children older than 6 months were initiated on treatment with LPV/r (230 mg/m2), stavudine (1 mg/kg), and lamivudine (4 mg/kg) every 12 hours. Children younger than 6 months or those receiving tuberculosis treatment were initiated using ritonavir (400–450 mg/m2), stavudine, and lamivudine every 12 hours and changed from ritonavir to LPV/r once they were older than 6 months or completed tuberculosis treatment. Eligible (ie, HIV-1 RNA <400 copies/mL for 3 months) children were randomly assigned to either continue LPV/r regimen or receive nevirapine (120 mg/m2 every 24 hours for the first 2 weeks and 200 mg/m2 every 12 hours thereafter). Children with HIV-1 RNA >1000 copies/mL were recalled and retested within 4 weeks if possible. If adherence difficulties were noted, additional counseling was provided. If persisting HIV-1 RNA levels >1000 copies/mL were noted despite adherence counseling, children in the nevirapine group were returned to the LPV/r-based regimen.
Statistical Analyses
For this analysis, only scheduled visits were included and data were truncated to the 48 month visit. At the time of this analysis, 94 (48.2%) children reached 48 months after initiation of ART or beyond. Children were divided into three groups based on their age at ART initiation: <6 months, 6–12 months, and 12–24 months. Pre-treatment characteristics for these groups were compared using chi-squared tests for categorical variables, ANOVA and pairwise t-tests for normally-distributed continuous variables, and Kruskal-Wallis tests for non-normally distributed continuous variables.
Locally weighted scatterplot smoothing (LOESS) was used to generate curves of WAZ, HAZ, BAZ, and HCAZ by time on ART stratified by age at ART initiation. Stratified plots were also generated for other potential risk factors of poor growth including sex, low birthweight (<2500 grams), high pre-treatment HIV-1 RNA (>750,000 copies/mL), randomization group, being underweight pre-treatment (WAZ <-2), and being stunted pre-treatment (HAZ <-2).
To examine the effects of age at ART initiation and other risk factors on WAZ, HAZ, BAZ, and HCAZ, generalized estimating equations (GEE) were used. A first order autoregressive correlation structure was used under the assumption that the standard errors for correlations between measurements for each subject diminished as the time interval between the measurements increased. Other correlation structures produced similar results. Separate GEE models were fit for specific time periods if inspection of the plots indicated likely different effects over different time periods. Interaction terms were used to evaluate effect modification. GEE models were also used to generate estimates of the magnitude of change in growth per year on treatment. Interaction between age group and time on ART was assessed.
To describe mean growth outcomes (WAZ, HAZ, BAZ, and HCAZ) by age at ART initiation, 3-month post-ART initiation time points were created. A window period of 45 days was allowed for each time point. Proportions of children who were underweight and stunted at each of these 3-month time points were also described.
Kaplan-Meier methods were used to describe the proportion of children failing to achieve target growth milestones. These analyses were restricted to those underweight and stunted pre-treatment. Because more than 90% of these children failed to achieve HAZ≥0 by 24 months post-ART initiation, we could not use the population norm of HAZ=0 for this analysis and instead defined the target height milestone as HAZ ≥−1. Target weight milestone was defined as WAZ ≥0. Groups were compared using log-rank tests. Hazard ratios were calculated using Cox Proportional Hazards models using <6 months as a reference group. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS Version 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
Pre-treatment Characteristics
Of the 195 children infected with HIV included in the analysis, 99 were randomized to continue on LPV/r and 96 to begin nevirapine. By the end of the study, 156 children (including 85 continued on LPV/r and 71 switched to nevirapine) completed extended follow-up, 6 children died, 28 were lost to follow up, and 5 transferred out of the study. Total observed time on ART ranged from 6.4 months to 68.3 months, with a median of 47.6 months and mean of 46.1±12.7 months.
Pre-treatment characteristics of the 195 children included in this analysis by age at ART initiation are presented in Table I. Of the 195 children, 104 (53.3%) were male. Mean age at ART initiation was 10.7±5.9 months, with 54 (27.7%) children <6 months old, 69 (35.4%) 6–12 months old, and 72 (36.9%) 12–24 months old. There were no differences in sex, randomization group, or proportions of children with low birthweight (<2500 grams) or high pre-treatment HIV-1 RNA (>750,000 copies/mL) between the age groups. Children 12–24 months at ART initiation had a lower percentage of CD4 cells prior to treatment than children 6–12 months and children <6 months at ART initiation. More children were stunted (76.3%) than underweight (51.1%) or wasted (21.4%) prior to starting therapy. Pre-treatment WAZ, HAZ, BAZ and HCAZ and proportions of children underweight, stunted, or wasted were not different between the three age groups.
Table 1.
Pre-treatment characteristics of 195 children infected with HIV by age at initiation of antiretroviral therapy (ART)
| Age at ART Initiation | |||||
|---|---|---|---|---|---|
| Characteristic | All Children a (n=195) |
<6 months (n=54) |
6–12 months (n=69) |
12–24 months (n=72) |
p-valueb |
| Sex, n (%) | |||||
| Male | 104 (53.3) | 23 (42.6) | 41 (59.4) | 40 (55.6) | 0.159 |
| Female | 91 (46.7) | 31 (57.4) | 28 (40.6) | 32 (44.4) | |
| Age at ART Initiation (months), Mean ± SD | 10.7 ± 5.9 | 4.1 ± 1.0 | 9.1 ± 1.7 | 17.2 ± 3.6 | <0.0001 |
| Randomization Group, n (%) | |||||
| Remain on LPV/r | 99 (50.8) | 28 (51.9) | 29 (42.0) | 42 (58.3) | 0.151 |
| Switch to NVP | 96 (49.2) | 26 (48.1) | 40 (58.0) | 30 (41.7) | |
| HIV-1 RNA (copies/mL), n (%) | |||||
| <100000 | 19 (11.0) | 3 (6.5) | 8 (12.3) | 8 (12.9) | |
| 100000–750000 | 58 (33.5) | 10 (21.7) | 21 (32.3) | 27 (43.6) | 0.069 |
| ≥750000 | 96 (55.5) | 33 (71.7) | 36 (55.4) | 27 (43.6) | |
| CD4 Percentage, n (%) | |||||
| <10 | 26 (14.0) | 1 (1.9) | 10 (15.2) | 15 (22.7) | |
| 10–14.9 | 37 (19.9) | 6 (11.5) | 15 (22.1) | 16 (23.5) | 0.003 |
| ≥15 | 123 (66.1) | 45 (86.5) | 41 (62.1) | 37 (54.4) | |
| Median (IQR) | 18.5 (13.4, 24.0) | 23.6 (18.4, 29.9) | 19.3 (13.4, 22.6) | 15.6 (10.9, 19.7) | <0.0001 |
| Birthweight, grams | |||||
| Low <2500, n (%) | 24 (15.7) | 8 (17.4) | 9 (17.7) | 7 (12.5) | 0.712 |
| Weight-for-age z-score (WAZ) | |||||
| Mean ± SD | −2.18 ± 1.67 | −2.20 ± 1.50 | −2.15 ± 1.66 | −2.20 ± 1.80 | 0.980 |
| Score <2 SD below mean, n (%) | 91 (51.7) | 22 (47.8) | 36 (55.4) | 33 (50.8) | 0.722 |
| Height-for-age z-score (HAZ) | |||||
| Mean ± SD | −3.12 ± 1.68 | −2.94 ± 1.71 | −3.02 ± 1.62 | −3.36 ± 1.72 | 0.363 |
| Score <2 SD below mean, n (%) | 132 (76.3) | 34 (73.9) | 50 (76.9) | 48 (77.4) | 0.904 |
| BMI for-age z-score (BAZ) | |||||
| Mean ± SD | −0.43 ± 2.11 | −0.67 ± 2.17 | −0.54 ± 2.01 | −0.14 ± 2.17 | 0.383 |
| Score <2 SD below mean, n (%) | 37 (21.4) | 12 (26.1) | 15 (23.1) | 10 (16.1) | 0.420 |
| Head circumference-for-age z-score (HCAZ) | |||||
| Mean ± SD | −0.58 ± 1.42 | −0.74 ± 1.58 | −0.66 ± 1.35 | −0.38 ± 1.35 | 0.418 |
| Score <2 SD below mean, n (%) | 24 (15.9) | 9 (22.0) | 8 (13.8) | 7 (13.5) | 0.461 |
Data were available for all 195 children except CD4 (186), VL (173), WAZ (176), HAZ (173), BAZ (173), HCAZ (151). Denominators are as shown.
Categorical variables were compared across groups using chi-squared tests; when medians are shown, the Kruskal-Wallis test was used; when means are shown, variables were compared using ANOVA.
Abbreviations: SD, standard deviation; IQR, interquartile range displaying 25th and 75th percentile
Growth by Age at ART Initiation
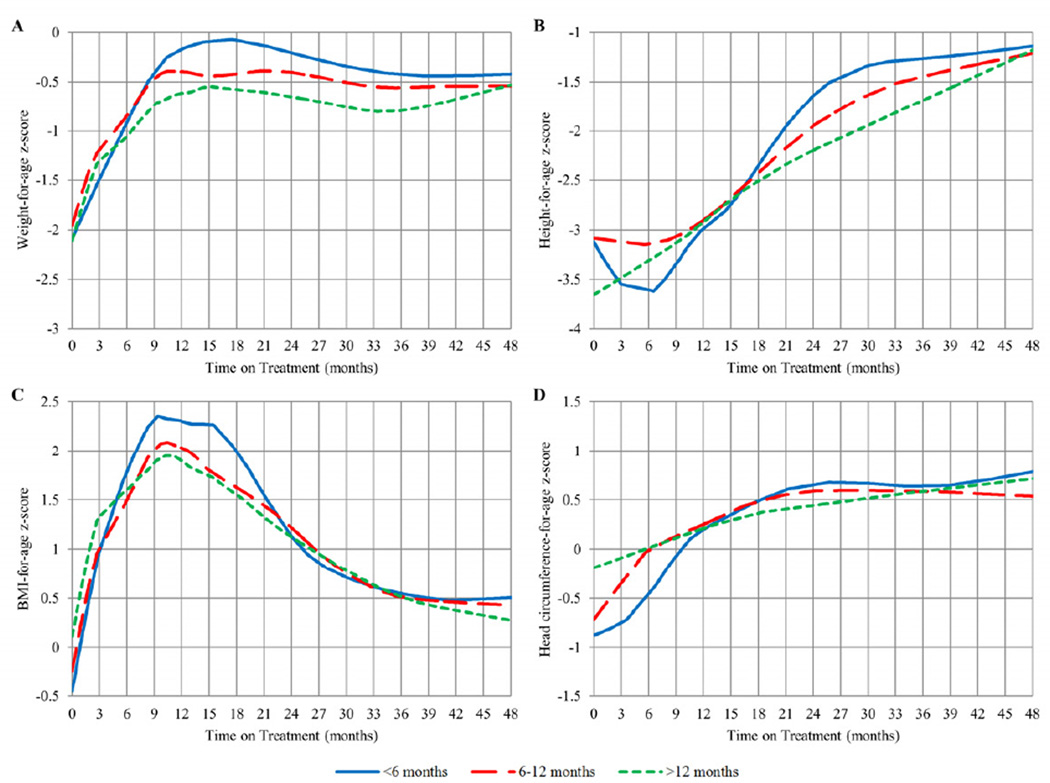
Children contributed 4160 weight, 4156 height, and 4066 head circumference measurements through 48 months to the curves of WAZ, HAZ, BAZ, and HCAZ by time on ART stratified by age at ART initiation (Figure 1). Mean WAZ, HAZ, BAZ, and HCAZ by age group at 3-month post-ART initiation time points are presented in Table II (available at www.jpeds.com).
Figure 1.
LOESS plots of WAZ, HAZ, BAZ, and HCAZ over time from ART initiation (in months) stratified by age at ART initiation
Table II.
Mean weight-for-age z-score (WAZ), height-for-age z-score (HAZ), BMI-for-age z-score (BAZ), and head circumference-for-age z-score (HCAZ) of 195 children with perinatal HIV infection over time from antiretroviral therapy (ART) initiation to 48 months stratified by age at initiation of ART (<6, 6–12, 12–24 months)
| Time on Treatment (Months)a | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Outcome |
Age at ART Initiation (months) |
0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 | 39 | 42 | 45 | 48 |
| WAZ | <6 | −2.20 (46) | −1.38 (47) | −0.79 (47) | −0.34 (50) | −0.21 (51) | −0.08 (50) | −0.04 (46) | −0.15 (43) | −0.25 (45) | −0.30 (47) | −0.38 (48) | −0.35 (45) | −0.45 (47) | −0.54 (40) | −0.43 (40) | −0.47 (39) | −0.47 (27) |
| 6–12 | −2.15 (65) | −1.17 (64) | −0.89 (67) | −0.58 (65) | −0.33 (68) | −0.33 (66) | −0.33 (66) | −0.35 (62) | −0.33 (61) | −0.45 (62) | −0.55 (57) | −0.52 (56) | −0.62 (55) | −0.56 (57) | −0.50 (47) | −0.48 (41) | −0.45 (32) | |
| 12–24 | −2.20 (65) | −1.33 (65) | −0.94 (68) | −0.76 (71) | −0.64 (70) | −0.49 (69) | −0.63 (67) | −0.58 (63) | −0.63 (65) | −0.71 (65) | −0.78 (64) | −0.84 (65) | −0.82 (63) | −0.81 (59) | −0.69 (53) | −0.54 (41) | −0.56 (40) | |
| All | −2.18 (176) | −1.29 (176) | −0.88 (182) | −0.58 (186) | −0.41 (189) | −0.32 (185) | −0.36 (179) | −0.38 (168) | −0.42 (171) | −0.51 (174) | −0.59 (169) | −0.60 (166) | −0.65 (165) | −0.65 (156) | −0.55 (140) | −0.50 (121) | −0.50 (99) | |
| HAZ | <6 | −2.94 (46) | −3.57 (47) | −3.46 (47) | −3.33 (50) | −2.84 (51) | −2.79 (50) | −2.58 (46) | −1.78 (43) | −1.50 (45) | −1.38 (47) | −1.32 (48) | −1.30 (45) | −1.28 (47) | −1.26 (40) | −1.25 (40) | −1.09 (39) | −1.30 (27) |
| 6–12 | −3.02 (65) | −3.17 (64) | −3.11 (67) | −3.23 (65) | −2.92 (68) | −2.65 (66) | −2.23 (66) | −2.15 (62) | −1.83 (61) | −1.70 (62) | −1.50 (57) | −1.41 (56) | −1.52 (55) | −1.32 (57) | −1.24 (47) | −1.27 (41) | −1.18 (32) | |
| 12–24 | −3.36 (62) | −3.61 (65) | −3.47 (68) | −3.16 (71) | −2.86 (70) | −2.66 (69) | −2.51 (67) | −2.29 (63) | −1.99 (65) | −2.04 (65) | −1.99 (64) | −1.84 (64) | −1.80 (63) | −1.69 (59) | −1.39 (53) | −1.19 (41) | −1.17 (40) | |
| All | −3.12 (173) | −3.44 (176) | −3.33 (182) | −3.23 (186) | −2.88 (189) | −2.69 (185) | −2.42 (179) | −2.11 (168) | −1.80 (171) | −1.74 (174) | −1.64 (169) | −1.54 (165) | −1.56 (165) | −1.44 (156) | −1.30 (140) | −1.19 (121) | −1.21 (99) | |
| BAZ | <6 | −0.67 (46) | 1.04 (47) | 1.80 (47) | 2.30 (50) | 2.10 (51) | 2.27 (50) | 2.21 (46) | 1.38 (43) | 0.99 (45) | 0.82 (47) | 0.68 (48) | 0.69 (45) | 0.54 (47) | 0.40 (40) | 0.55 (40) | 0.34 (39) | 0.55 (27) |
| 6–12 | −0.54 (65) | 1.02 (64) | 1.38 (67) | 1.98 (65) | 2.12 (68) | 1.90 (66) | 1.58 (66) | 1.51 (62) | 1.24 (61) | 0.95 (62) | 0.61 (57) | 0.57 (56) | 0.54 (66) | 0.44 (57) | 0.44 (47) | 0.50 (41) | 0.44 (32) | |
| 12–24 | −0.14 (62) | 1.36 (65) | 1.85 (68) | 1.85 (71) | 1.78 (70) | 1.84 (69) | 1.53 (67) | 1.40 (63) | 1.02 (65) | 0.95 (65) | 0.81 (64) | 0.56 (64) | 0.53 (63) | 0.44 (59) | 0.32 (53) | 0.34 (41) | 0.30 (40) | |
| All | −0.43 (173) | 1.14 (176) | 1.66 (182) | 2.02 (186) | 1.99 (189) | 1.98 (185) | 1.72 (179) | 1.43 (168) | 1.09 (171) | 0.92 (174) | 0.70 (169) | 0.60 (165) | 0.53 (165) | 0.43 (156) | 0.43 (140) | 0.39 (121) | 0.41 (99) | |
| HCAZ | <6 | −0.74 (41) | −0.71 (47) | −0.42 (47) | 0.01 (50) | 0.12 (51) | 0.33 (50) | 0.52 (46) | 0.60 (43) | 0.75 (45) | 0.71 (47) | 0.69 (48) | 0.70 (45) | 0.56 (47) | 0.63 (40) | 0.60 (40) | 0.68 (39) | 0.89 (27) |
| 6–12 | −0.66 (58) | −0.29 (64) | −0.03 (67) | −0.07 (65) | 0.31 (68) | 0.49 (66) | 0.62 (66) | 0.62 (62) | 0.64 (61) | 0.63 (62) | 0.60 (57) | 0.68 (56) | 0.47 (55) | 0.53 (57) | 0.70 (47) | 0.70 (41) | 0.40 (31) | |
| 12–24 | −0.38 (52) | −0.16 (65) | −0.01 (68) | 0.16 (71) | 0.24 (70) | 0.30 (69) | 0.44 (67) | 0.45 (63) | 0.56 (65) | 0.53 (65) | 0.52 (64) | 0.58 (65) | 0.53 (64) | 0.41 (54) | 0.59 (38) | 0.77 (16c) | 1.53 (4c) | |
| All | −0.58 (151) | −0.35 (176) | −0.12 (182) | 0.04 (186) | 0.24 (189) | 0.38 (185) | 0.52 (178) | 0.55 (168) | 0.64 (171) | 0.62 (173) | 0.59 (169) | 0.65 (166) | 0.52 (165) | 0.51 (151) | 0.63 (125) | 0.70 (96c) | 0.69 (62c) | |
| Max nb | 195 | 176 | 182 | 186 | 189 | 185 | 179 | 168 | 171 | 175 | 169 | 170 | 165 | 157 | 140 | 122 | 99 | |
Data shown are means (n) in each 3 month time on treatment interval using the closest unique visit ± 45 days (1.5 months) of the time point
n presented is the maximum number of subjects at each 3 month period. Subgroups may not add to the maximum n due to unattained measurements or missing data.
Decreased n is due to HCAZ estimates not being available for children beyond 5 years of age
Weight
For all age groups, WAZ increased rapidly during the first 12 months on treatment and stabilized thereafter at approximately 0.5 z-scores below population norms through 48 months. In the first 12 months on treatment, children <6 months and 6–12 months of age at ART initiation experienced increases of 1.98 and 1.96 z-scores, respectively, and children 12–24 months at ART initiation increased 1.4 z-scores; this difference was not significant (p=0.084). Between 18–36 months on ART, children who initiated ART 12–24 months had a significantly lower WAZ than children who initiated ART <6 months (b= −0.469, p=0.016). Between 36–48 months, children 12–24 months at ART initiation increased 0.106 z-scores whereas children <6 months dropped 0.1 z-scores (p=0.014) (Figure 1, A).
Height
HAZ increased steadily through 48 months but all three groups failed to reach population norms (HAZ=0), remaining on average 1.0 z-score below population norms. Between 12–24 months on treatment, children <6 months at ART initiation experienced a greater increase in height (1.56 z-scores), and children 12–24 months at ART initiation increased 0.755 z-scores (p=0.004). Between 24–36 months on ART, children who initiated ART 12–24 months had a significantly lower HAZ than children who initiated ART <6 months (b=−0.545, p=0.009). However, between 36–48 months on treatment, height growth for children <6 months at ART initiation slowed down, with a negligible change of 0.04 z-scores, and those who initiated ART 12–24 months of age experienced a 0.286 increase in z-score; this difference was significant (p=0.029) (Figure 1, B).
BMI
For all age groups, BAZ increased dramatically in the first 12 months of treatment as WAZ increased, and then declined between 12 and 48 months on treatment as HAZ began to increase with no further changes in WAZ. No significant differences in BAZ were seen between age groups after 21 months on ART (Figure 1, C).
Head Circumference
HCAZ steadily rose after ART initiation for all groups from a sub-normal z-score to a z-score above 0. Children 12–24 months at ART initiation had a significantly higher HCAZ than children <6 months at ART initiation in the first 6 months on treatment (b=0.580, p=0.026). In the first 12 months on treatment, children <6 months at ART initiation increased 1.24 z-scores compared with children 12–24 months at ART initiation, who increased 0.452 z-scores (p=0.004). After 12 months on ART, there were no significant differences in HCAZ between groups (Figure 1, D).
Growth by Other Risk Factors
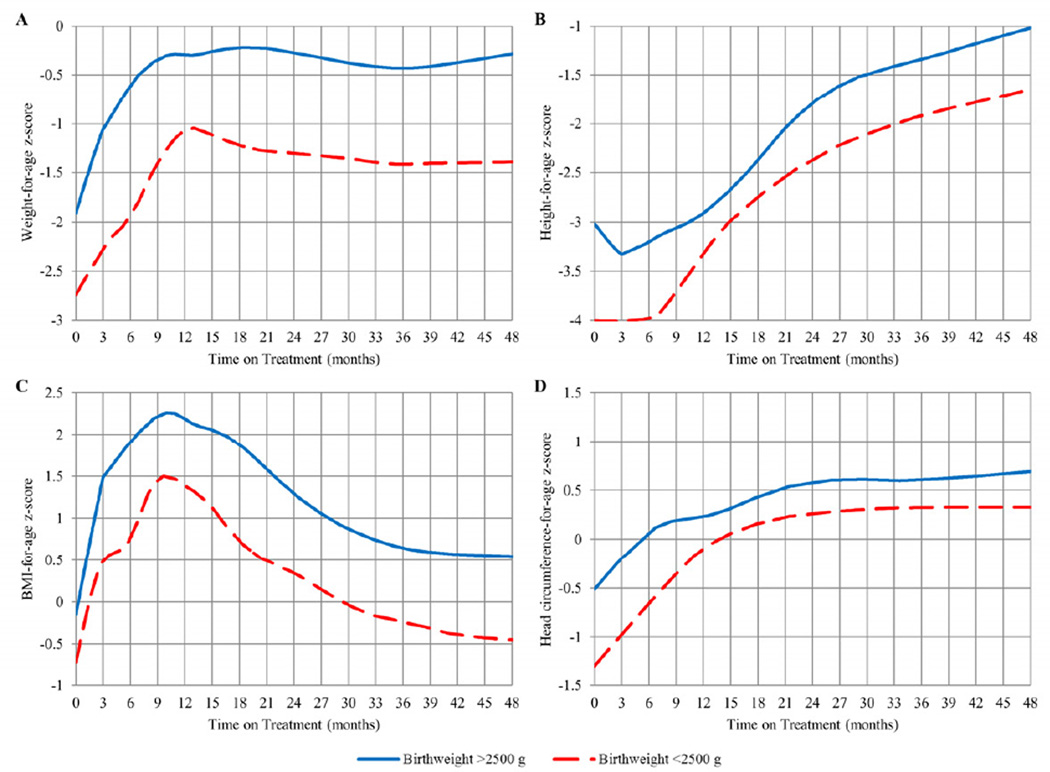
Although females had a significantly higher HAZ than males (b=0.311, p =0.049) for all 48 months on treatment, no significant differences in WAZ, BAZ, or HCAZ were detected (data not presented). No significant interactions between age at ART initiation and sex were detected. The 24 children with low birthweight (<2500 grams) consistently had a significantly lower WAZ (b=−0.974, p<0.0001), HAZ (b=−0.630, p=0.003), BAZ (b=−0.845, p<0.0001), and HCAZ (b=−0.523, p=0.015) for all 48 months on ART compared with children with higher birthweight (Figure 2). No differences in growth outcomes relative to time on ART were detected between children with pre-treatment VL >750,000 copies/mL and VL <750,000 copies/mL.
Figure 2.
LOESS plots of WAZ, HAZ, BAZ, and HCAZ over time from ART initiation (in months) stratified by birthweight
We have previously reported significantly higher changes in WAZ post-randomization in the group that switched to NPV compared with those continuing on LPV/r.17, 19 The differences were most consistent in the first year after randomization. In these prior papers, we also reported no differences between the groups in WAZ and HAZ over time. In this analysis, we found no evidence of interaction between age at ART initiation and randomization group.19,22
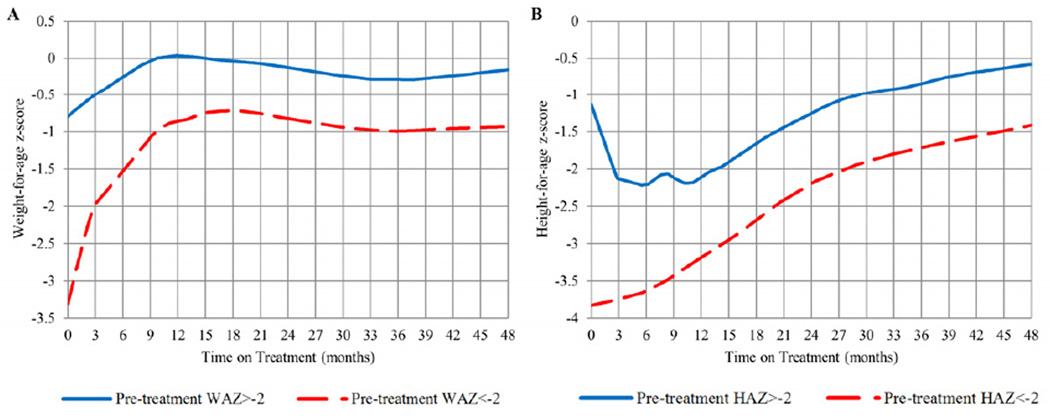
Children Underweight or Stunted Pre-treatment
Plots of WAZ and HAZ over time on treatment by pre-treatment categories are presented in Figure 3 (available at www.jpeds.com). The 91 children underweight pre-treatment had a significantly lower WAZ for all 48 months on treatment compared with children who were not underweight (b=−1.336, p<0.0001). In the first 12 months on treatment, children underweight pre-treatment increased 2.49 WAZ compared with children not underweight, who increased 0.85 WAZ (p<0.0001). The 132 children stunted pre-treatment had a significantly lower HAZ for all 48 months on treatment compared with non-stunted children (b=−0.76, p<0.0001). In the first 12 months on treatment, children stunted pre-treatment declined 1.21 z-scores in HAZ compared with non-stunted children, who increased 0.674 z-scores in HAZ (p<0.0001). Children underweight and stunted pre-treatment also had a significantly lower BAZ and HCAZ for all 48 months on treatment (data not presented).
Figure 3.
LOESS plots of WAZ over time from ART initiation (in months) stratified by pre-treatment WAZ category (<-2 vs. >-2) and HAZ over time stratified by pre-treatment HAZ category (<-2 vs. >-2)
Hazard ratios of failing to achieve target population growth norms of those underweight and stunted pre-treatment are presented in Table III. Underweight children age 12–24 months at ART initiation were 2.4 times more likely to fail to achieve the target weight milestone (WAZ=0) compared with underweight children started at <6 months (p=0.039). Stunted children age 12–24 months at ART initiation were 3.0 times more likely to fail to achieve the target height milestone (HAZ=−1) compared with stunted children started at <6 months (p=0.013).
Table III.
Effect of age at initiation of antiretroviral therapy (ART) on failing to achieve target growth milestonesa by 24 months post-ART initiation among 91 children infected with HIV who were underweight (WAZ less than -2) and 132 children stunted (HAZ less than-2) pre-treatment
| Growth Outcome Age at ART Initiation |
n Total Pre-treatment |
n Failing to Achieve Target Growth Milestonea |
Probability of Failing to Achieve Target Growth Milestonea |
Hazard Ratio (95% CI) |
p-valueb |
|---|---|---|---|---|---|
| Weight-for-age Z-Score | WAZ less than -2 | ||||
| <6 | 22 | 10 | 0.412 | - | - |
| 6–12 | 36 | 26 | 0.717 | 2.54 (1.10, 5.89) | 0.030 |
| 12–24 | 33 | 23 | 0.691 | 2.42 (1.05, 5.61) | 0.039 |
| Height-for-age Z-Score | HAZ less than-2 | ||||
| <6 | 34 | 20 | 0.556 | - | - |
| 6–12 | 50 | 31 | 0.613 | 1.10 (0.55, 2.20) | 0.786 |
| 12–24 | 48 | 40 | 0.830 | 3.02 (1.27, 7.20) | 0.013 |
Target growth milestone was defined as WAZ ≥0 or HAZ ≥-1 using WHO Growth Standards
Hazard ratios and were calculated using Cox Proportional Hazards models using <6 months at ART initiation as the reference group
DISCUSSION
In this study of South African children infected with HIV initiated on ART before 24 months of age and maintained on therapy with adequate virologic and immunologic response, WAZ, HAZ, BAZ, and HCAZ all improved considerably following treatment initiation. Early rapid weight gain was most pronounced among children started on ART during the first 6 months of life. A similar pattern in statural growth also was seen following some inconsistencies in the initial 6 months on ART. Growth benefits with earlier age of ART initiation have been reported in some studies of African children.12–14 In contrast to our study, however, these reports did not distinguish between children started on ART before or after 6 months of life. Although we did not specifically investigate reasons for the more rapid catch-up growth in children who initiate ART less than 6 months of age, a number of HIV-related neuroendocrine and gastrointestinal abnormalities such as growth hormone insensitivity and nutrient malabsorption, which adversely affect growth, may be less prevalent in these younger age groups.20
Children <6 months of age were initiated on ritonavir for an average of 2 months before changing to LPV/r therapy, as dosing guidelines for LPV/r were not yet established for this age group at the time of this study. In light of ritonavir’s known adverse gastrointestinal effects, it is possible that better growth could have been achieved had these children started on LPV/r at earlier ages.
Similar to other studies, height improvements lagged behind weight.7, 8, 11, 12 Although the general trajectories of growth were similar to a study of children in the United States, the children in the United States normalized weight (WAZ=0) within 6 months on ART and nearly normalized height (HAZ=0) after 2 years on ART,8 whereas children in our study, on average, required 12 months to reach normal weight and continued to be almost one standard deviation below normal height after 48 months on ART.9 Other African studies have also demonstrated increases in WAZ and HAZ without reaching normal values.15, 16,20 It is unknown if height increases beyond 48 months will eventually catch-up to the population norm (HAZ=0) with continued viral suppression on ART. This lower attained height may reflect high rates of stunting in the underlying population, irrespective of HIV.21
ART showed a clear benefit for children with low birthweight, who experienced a growth pattern similar to children with normal birthweight. However, growth outcomes of children with low birthweight remain significantly lower through 48 months on treatment, and these children did not catch-up to those with normal birthweight. This growth restriction is similar to that observed in uninfected low birthweight babies who also attain a weight for age below that of their normal birthweight peers.22
Of note, the proportions of children in our cohort underweight (51.7%) and stunted (76.3%) pre-treatment were similar to other African studies.12, 14, 16 In the first 12 months on treatment, children underweight pre-treatment experienced a significant catch-up in weight and children stunted pre-treatment experienced a significant catch-up in height towards normalization compared with children not underweight or stunted. However, their attained weight and height remained below those children who were not underweight or stunted pre-treatment. Our results are consistent with other observations in African cohorts.14, 16 A study in Malawi reported that children with low pre-treatment z-scores demonstrated the steepest increase after starting ART.16 Underweight and stunted children in our study who initiated treatment before 6 months of age achieved target growth milestones more rapidly than children who initiated treatment 12–24 months of age.16
We have previously reported that, relative to the time of randomization, there are short-term weight increases in the “switch group.”17, 19 This is consistent with the P1060 trial of LPV/r vs. nevirapine-based primary therapy for exposed infants, which saw improved WAZ and HAZ for children on nevirapine, albeit not significant, at all study visits up to 24 months.23
By 48 months of treatment, all growth measurements had attained similar levels regardless of age at treatment initiation. We did not evaluate if there are additional beneficial health outcomes conferred to those with early rapid growth improvement. In general, suboptimal postnatal weight gain is associated with an increased risk for diarrhea, pneumonia, malaria, measles, and death,24 and a more rapid growth recovery may reduce the overall risk of morbidity and mortality from infectious diseases. Rapid height catch-up also reduces risk for irreversible stunting, which can be stigmatizing and lead to poor pregnancy and birth outcomes and decreased work capacity in later life.25 Data from otherwise healthy children suggests that optimal postnatal growth in early infancy may also improve later intellectual development.26,27 Although the interpretation of head circumference in our study was complicated by pre-treatment differences between the age groups, the observed overall improvement in head circumference is encouraging.
Although our study extends the length of follow-up reported in most studies of HIV-specific childhood growth in Africa and incorporates more frequent measurements, there are a number of limitations. This was a randomized clinical trial that monitored children closely and included only those who attained viral suppression in the first 12 months of therapy. Thus, growth trajectories are unlikely to be representative of cohorts with poorer adherence, or of children with poorer initial virologic response to treatment. It should be noted that children were retained in the analysis even if they had virologic failure at a later time point. However, numbers of later failure were too few for meaningful analysis of the effect of viral failure on growth. Additionally, most children in our cohort were formula fed and did not receive the benefits of breastfeeding. It is unknown what additional benefits this may provide. Furthermore, our study did not include measurement of other known or potential pathways or mechanisms for growth differences, such as immune activation, neuroendocrine or gastrointestinal abnormalities, or dietary practices. However, it is unlikely that diet or other nutritional factors would differ between children initiating therapy at different ages and is unlikely to influence the results. Finally, an estimated 9% of children in Gauteng province are underweight according to the most recent National Food Consumption Survey.21 Thus, this study may not be as generalizable to other populations in sub-Saharan Africa with higher background rates of underweight children.28 The study also lacks a control (uninfected) group but age and sex adjusted population z-scores are utilized in order to make standardized comparisons.
Our study demonstrates that excellent growth outcomes can be achieved in virally suppressed children with good adherence on ART, and presents strong evidence that early ART plays an important role in the rate of growth recovery in young children infected with HIV. Our findings provide further support for the importance of prompt identification and immediate initiation of ART for infants infected with HIV in order to optimize growth outcomes. Even though implementing early infant diagnosis services in low-resource settings has proved challenging, our results, as well as those from the CHER study in which earlier ART led to a 76% reduction in mortality and 75% reduction in HIV disease progression,29 reinforce the importance of these services. Whether this rapid growth will be beneficial for subsequent health outcomes across the lifecourse requires further study. As children infected with HIV commonly now survive to adolescence and adulthood on lifelong ART, optimizing early growth may play an important role in improving their long-term clinical outcomes and quality of life.
Acknowledgments
Supported by the National Institute of Child Health and Human Development (HD 47177, HD 61255) and Secure the Future Foundation (RES 219).
List of Abbreviations
- ART
Antiretroviral therapy
- BAZ
BMI for age z-score
- GEE
Generalized estimating equations
- HAZ
Height for age z-score
- HCAZ
Head circumference for age z-score
- HIV
Human immunodeficiency virus
- LPV/r
Ritonavir-boosted lopinavir
- WAZ
Weight for age z-score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Bobat R, Coovadia H, Moodley D, Coutsoudis A, Gouws E. Growth in early childhood in a cohort of children born to HIV-1-infected women from Durban, South Africa. Ann Trop Paediatr. 2001;21:203–210. doi: 10.1080/02724930120077772. [DOI] [PubMed] [Google Scholar]
- 2.Moye J, Jr, Rich KC, Kalish LA, Sheon AR, Diaz C, Cooper ER, et al. Natural history of somatic growth in infants born to women infected by human immunodeficiency virus. Women and Infants Transmission Study Group. J Pediatr. 1996;128:58–69. doi: 10.1016/s0022-3476(96)70428-6. [DOI] [PubMed] [Google Scholar]
- 3.Henderson R, Miotti P, Saavedra J, Dallabetta G, Chiphangwi J, Liomba G, et al. Longitudinal growth during the first 2 years of life in children born to HIV-infected mothers in Malawi, Africa. Pediatr AIDS HIV Infect. 1996;7:91. [PubMed] [Google Scholar]
- 4.Berhane R, Bagenda D, Marum L, Aceng E, Ndugwa C, Bosch RJ, et al. Growth failure as a prognostic indicator of mortality in pediatric HIV infection. Pediatrics. 1997;100:E7. doi: 10.1542/peds.100.1.e7. [DOI] [PubMed] [Google Scholar]
- 5.Buchacz K, Cervia JS, Lindsey JC, Hughes MD, Seage GR, 3rd, Dankner WM, et al. Impact of protease inhibitor-containing combination antiretroviral therapies on height and weight growth in HIV-infected children. Pediatrics. 2001;108:E72. doi: 10.1542/peds.108.4.e72. [DOI] [PubMed] [Google Scholar]
- 6.Guillen S, Ramos JT, Resino R, Bellon JM, Munoz MA. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- 7.Verweel G, van Rossum AM, Hartwig NG, Wolfs TF, Scherpbier HJ, de Groot R. Treatment with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected children is associated with a sustained effect on growth. Pediatrics. 2002;109:E25. doi: 10.1542/peds.109.2.e25. [DOI] [PubMed] [Google Scholar]
- 8.Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, Krogstad PA, et al. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24:352–357. doi: 10.1097/01.inf.0000157095.75081.43. [DOI] [PubMed] [Google Scholar]
- 9.Steiner F, Kind C, Aebi C, Wyler-Lazarevitch CA, Cheseaux JJ, Rudin C, et al. Growth in human immunodeficiency virus type 1-infected children treated with protease inhibitors. Eur J Pediatr. 2001;160:611–616. doi: 10.1007/s004310100820. [DOI] [PubMed] [Google Scholar]
- 10.WHO. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. 2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241500395_eng.pdf.
- 11.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–251. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 12.McGrath CJ, Chung MH, Richardson BA, Benki-Nugent S, Warui D, John-Stewart GC. Younger age at HAART initiation is associated with more rapid growth reconstitution. AIDS. 2011;25:345–355. doi: 10.1097/QAD.0b013e32834171db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musoke PM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D, Mubiru MM, et al. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC Pediatr. 2010;10:56. doi: 10.1186/1471-2431-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutcliffe CG, van Dijk JH, Munsanje B, Hamangaba F, Sinywimaanzi P, Thuma PE, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. doi: 10.1186/1471-2334-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45:311–317. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigel R, Phiri S, Chiputula F, Gumulira J, Brinkhof M, Gsponer T, et al. Growth response to antiretroviral treatment in HIV-infected children: a cohort study from Lilongwe, Malawi. Trop Med Int Health. 2010;15:934–944. doi: 10.1111/j.1365-3156.2010.02561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coovadia A, Abrams EJ, Stehlau R, Meyers T, Martens L, Sherman G, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. JAMA. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Child Growth Standards. Available at: http://www.who.int/childgrowth/en/
- 19.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–530. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim RJ, Rutstein RM. Impact of antiretroviral therapy on growth, body composition and metabolism in pediatric HIV patients. Paediatr Drugs. 2010;12:187–199. doi: 10.2165/11532520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Labadarios D, Steyn NP, Maunder E, MacIntryre U, Gericke G, Swart R, et al. The National Food Consumption Survey (NFCS): South Africa, 1999. Public Health Nutr. 2005;8:533–543. doi: 10.1079/phn2005816. [DOI] [PubMed] [Google Scholar]
- 22.Hack M, Weissman B, Borawski-Clark E. Catch-up growth during childhood among very low-birth-weight children. Arch Pediatr Adolesc Med. 1996;150:1122–1129. doi: 10.1001/archpedi.1996.02170360012002. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–198. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 25.Dewey KG, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7(Suppl 3):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinonen K, Raikkonen K, Pesonen AK, Kajantie E, Andersson S, Eriksson JG, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121:e1325–e1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 27.Pongcharoen T, Ramakrishnan U, DiGirolamo AM, Winichagoon P, Flores R, Singkhornard J, et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch Pediatr Adolesc Med. 2012;166:411–416. doi: 10.1001/archpediatrics.2011.1413. [DOI] [PubMed] [Google Scholar]
- 28.United Nations Children’s Fund. The State of the World’s Children 2006 - Excluded and Invisible. 2005
- 29.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]