Abstract
Purpose
To identify individual-/neighborhood-level correlates of membership within high HIV prevalence drug networks.
Methods
378 New York City drug users were recruited via respondent-driven sampling (2006–2009). Individual-level characteristics and recruiter-recruit relationships were ascertained and merged with 2000 tract-level US Census data. Descriptive statistics and population average models were used to identify correlates of membership in high HIV prevalence drug networks (>10.54% HIV vs. <10.54% HIV).
Results
Individuals in high HIV prevalence drug networks were more likely to be recruited in neighborhoods with greater inequality (Adjusted Odds Ratio [AOR]=5.85; 95%CI:1.40–24.42), higher valued owner-occupied housing (AOR=1.48;95%CI:1.14–1.92), and a higher proportion of Latinos (AOR=1.83; 95%CI:1.19–2.80). They reported more crack use (AOR=7.23; 95%CI:2.43–21.55), exchange sex (AOR=1.82; 95%CI:1.03–3.23), and recent drug treatment enrollment (AOR=1.62; 95%CI:1.05–2.50) and were less likely to report cocaine use (AOR=0.40; 95%CI:0.20–0.79) and recent homelessness (AOR=0.32; 95%CI:0.17–0.57).
Conclusions
The relationship between exchange sex, crack use and membership within high HIV prevalence drug networks may suggest an ideal HIV risk target population for intervention. Coupling network-based interventions with those adding risk-reduction and HIV testing/care/adherence counseling services to the standard of care in drug treatment programs should be explored in neighborhoods with increased inequality, higher valued owner-occupied housing, and a greater proportion of Latinos.
Keywords: HIV, drug use, networks, inequality, neighborhoods
The “HIV risk environment” has been characterized as a dynamic interplay between structural and network factors (1). Although both structural (1) and network (2–4) factors influence individual-level risk/health behaviors, information flow, and HIV transmission, most HIV prevention research among drug using populations examines the HIV risk environment from only one of these perspectives.
From a structural perspective, many studies have shown that HIV is disproportionately concentrated in lower income and underserved communities (5, 6). For example the 2006 CDC HIV prevalence estimates (which did not account for neighborhood characteristics) revealed significant racial/ethnic disparities (1.7% among blacks, 0.6% among Hispanics, and 0.2% among whites) (7). While the 2010 CDC HIV prevalence estimates (which stratified by neighborhood-level poverty) reported no significant racial/ethnic differences in HIV, HIV was significantly more prevalent in low-income areas (8). Racial and socioeconomic disparities in HIV are argued to be due, in part, to racial residential segregation (6, 9), which disproportionately influences poor neighborhoods and results in fewer resources in minority neighborhoods (1, 10, 11).
Other studies have identified geospatial clusters of HIV and related risk behaviors. For example, one study in the United States identified a cluster of 157 census tracts with a higher prevalence of HIV than the surrounding census tracts. This geo-spatial cluster was characterized by increased poverty, a lower density of multi-racial individuals, and a higher prevalence of HIV-related risk behaviors (12). In another example, a study among injection drug users (IDUs) in Russia reported that HIV and injection risk behaviors were geo-spatially clustered and that clusters frequently overlapped (13). Another study among IDUs reported that high-risk injection behaviors were more common in census tracts with higher unemployment rates (14). Other studies among IDUs have reported ecological associations between greater income inequality at the standard metropolitan statistical area-level and increased HIV prevalence (15, 16).
Studies using social network-based approaches have demonstrated that network norms are associated with drug-related (17–22) and sex-related HIV-risk behaviors. Specifically, among individuals from a drug-using community, self-reported condom use with main partners was associated with communication, proscriptive/injunctive, and descriptive condom use norms (23). Another study found that female IDUs who reported having peers who exchanged sex were twice as likely to report exchanging sex (24).
Few studies have assessed the combined influence of structural/neighborhood and network risk factors on HIV. Because individuals who are socially connected may also be in close geographic proximity to one another (25), risk behaviors may cluster among individuals within networks because of their shared structural environment, network norms/relationships, or both. Similarly, geo-spatial/neighborhood-level clustering of risk behaviors may be partially attributed to network relationships and social norms. For these reasons, it is important to consider network and neighborhood-level factors together to not only account for correlated data, but because there may be different mechanisms through which network-level and neighborhood-level characteristics influence individual-level behaviors.
Other motivations for examining network and structural/neighborhood factors together are 1) those with the greatest disease burden are often more difficult to reach through traditional sampling approaches and 2) poverty and inequality (which structural approaches have identified as leading causes of health disparities) are difficult to modify. Thus new strategies are needed to recruit sub-groups of hidden populations and target them with interventions that more effectively reduce health disparities. Network-based recruitment approaches could target individuals from high-risk structural environments and network-based interventions could be used to disseminate information and build supportive relationships that buffer the influence of structural factors which facilitate HIV transmission. A better understanding of how network and structural factors act independently and/or jointly may better explain racial and socioeconomic disparities in HIV and, in turn, better inform effective strategies to reduce health disparities.
As infectious disease transmission relies on the presence of both disease and behaviors that facilitate disease transmission, this analysis aims to visualize HIV clustering among a sample of drug users recruited through respondent-driven sampling (RDS) and to identify individual and neighborhood characteristics associated with membership in drug using networks with a higher HIV prevalence (referred to as high HIV prevalence networks, hereafter). The findings from this analysis are relevant for future research or interventions which aim to target these high risk drug using networks and can inform the development of multi-level intervention approaches which incorporate both network and neighborhood/structural components to reduce HIV transmission among these higher risk drug using networks.
METHODS
The data used were from the Social Ties Associated with Risk of Transition into injection drug use (START), a longitudinal study aiming to identify social risk factors for transitioning from non-injection to injection drug use among young adult heroin, crack and cocaine users in New York City (NYC). START methods have been previously described (26). In brief, participants were eligible if they were 18–40 years old and were active injection or non-injection drug users. While participants were recruited through targeted street outreach and RDS, this analysis was restricted to the respondent-driven sample where social network data were available. RDS participants were given three coupons to recruit drug using peers. Participants could bring in more than three peers but were only compensated for the first three. Recruiter-recruit relationships were determined as part of the RDS eligibility screening process. Most participants described their recruiter as a friend or acquaintance (83%); 6% as a relative, 7% as a stranger, and 4% as other. While peer recruits overlapped with risk network members, RDS recruits tended to be higher risk than the drug using networks reported in the social/risk network inventory.(26)
Between July 2006 and June 2009, 621 participants were screened to participate in the respondent-driven sample and 439 were eligible. Of those eligible, 32 were removed from the analysis because they were recruited on the mobile research van which had changing site locations that made RDS less optimal. Five others (and their six peer recruits) were dropped due to inconsistencies in self-reported drug use at follow-up visits. Finally, 18 seeds were dropped from this analysis because they did not recruit peers (N=378). All study materials were approved by the institutional review boards at Columbia University and the New York Academy of Medicine.
Individual-level variables
After providing informed consent, individuals completed a 90-minute interviewer-administered questionnaire, which ascertained demographics, network characteristics and relationships, drug use and sex behaviors, and health service use. Individual-level variables included in the analysis were selected because of the strong association between these characteristics and HIV in the prior literature.
Neighborhood-level variables
Baseline data were merged with tract-level data from the 2000 United States Census via the census tract where he/she was recruited for START. Participants most often described their recruitment neighborhood as the location where they hung out, purchased drugs and spent most of their time. Previous research comparing street recruitment and neighborhood residence found that the majority of illicit drug users in urban settings (85%) lived in the neighborhoods where they were recruited.(27)
Neighborhood covariates selected for inclusion in this analysis were selected based on findings from prior literature on health disparities in HIV prevalence and incidence. The following categories of tract-level neighborhood attributes were used for this analysis: minority composition, educational attainment, unemployment, income/poverty, inequality, and crowding. Inequality was measured using the index of concentration at the extremes (ICE), which conceptualizes the concentration of affluence and poverty as falling along a continuum ranging from −1.0 to +1.0 (ICE=−1.0 when all families are poor and +1.0 when all families are affluent) (28).
Network analysis
Because standard statistical models cannot capture the interconnectedness of network data, simulations were used to derive a meaningful measure of HIV clustering within networks. RDS recruit-recruiter ties were displayed visually using NetDraw (29) (Figure 1). To determine whether clustering by HIV status could be explained by chance, the observed network was compared with a null distribution (1,000 randomly generated networks with the same network topology and overall prevalence of HIV, but with HIV status distributed randomly) (30). If HIV clusters more than what would be expected by chance, the probability that an ego is HIV positive given that his/her alter is HIV positive would be higher in the observed network than in the null distribution and would not be included within the 95% confidence interval for the null distribution (P<0.05) (Table 1).
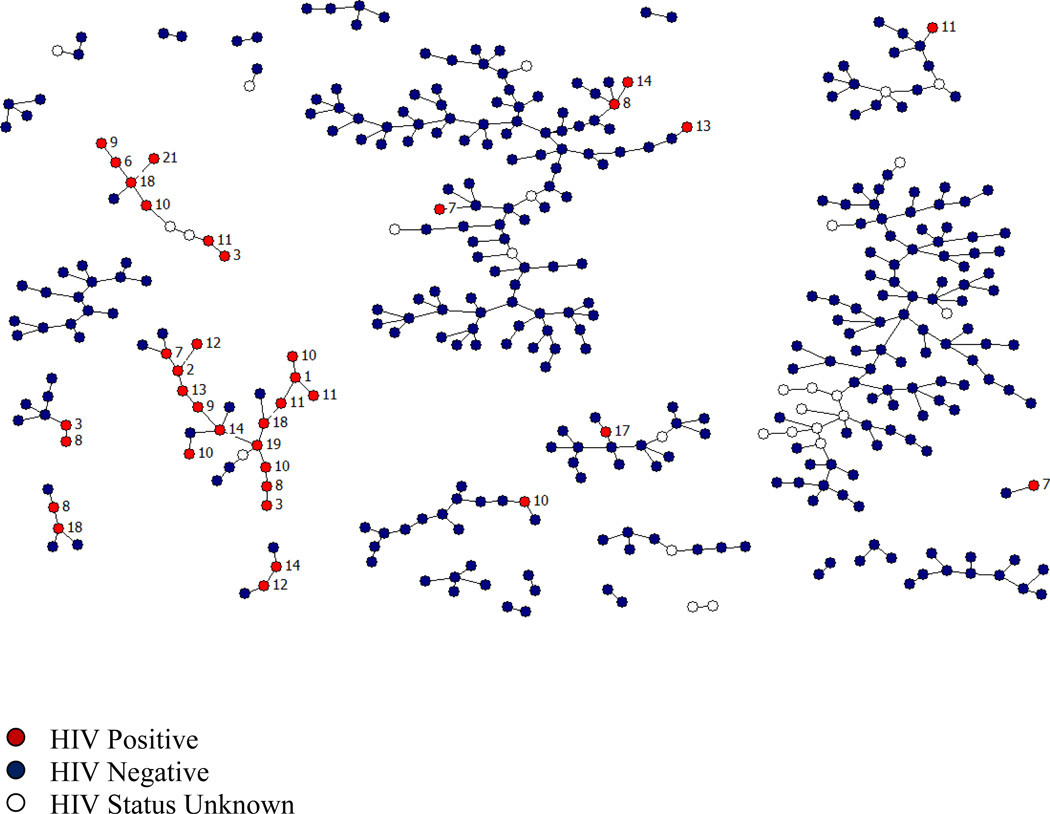
Figure 1.
HIV status among 378 New York City drug users recruited via respondent-driven sampling (2006–2009). Each circle represents an individual (red=HIV positive, blue=HIV negative, and white=missing HIV status). Lines between individuals indicate RDS recruiter-recruit relationships. Labels indicate the number of years since his/her first HIV positive diagnosis.
Table 1.
Measures of Clustering by HIV Status (Observed and Expected Risk Ratios) Among RDS-recruited Illicit Drug Users in New York City, 2006–2009 (N=378)
| N degrees of separation |
Number of N-degree paths |
Number of individuals separated by N degrees |
Observed Risk Ratio (HIV+ ego | HIV+ alter) / (HIV+ ego | HIV− alter) |
95% Confidence Interval for the expected Risk Ratio (HIV+ ego | HIV+ alter) / (HIV+ ego | HIV− alter) from 1000 random samplesa |
|---|---|---|---|---|
| 1 | 700 | 378 | 13.63 | −1.00, 1.25 |
| 2 | 1406 | 356 | 9.29 | −0.79, 0.98 |
| 3 | 1330 | 336 | 10.84 | −0.76, 0.91 |
| 4 | 1500 | 321 | 15.67 | −0.66, 0.84 |
| 5 | 1672 | 306 | 13.82 | −0.66, 0.76 |
| 6 | 1788 | 283 | 16.81 | −0.70, 0.76 |
95% confidence intervals for the null distribution reflect the range of risk ratios that were produced by the middle 950 randomly generated networks when all 1,000 estimates were ranked numerically. Observed risk ratios not included in the 95% confidence interval for the expected risk ratio are significantly different from what we would expect by chance (P<0.05).
Degree of separation is defined as the social distance, or the smallest number of intermediates, between alter-ego pairs. The association between an ego’s HIV status and his/her alter’s HIV status (for 1–6 degrees of separation) was also examined (Risk Ratios in Table 1; Risk Differences in Figure 2).
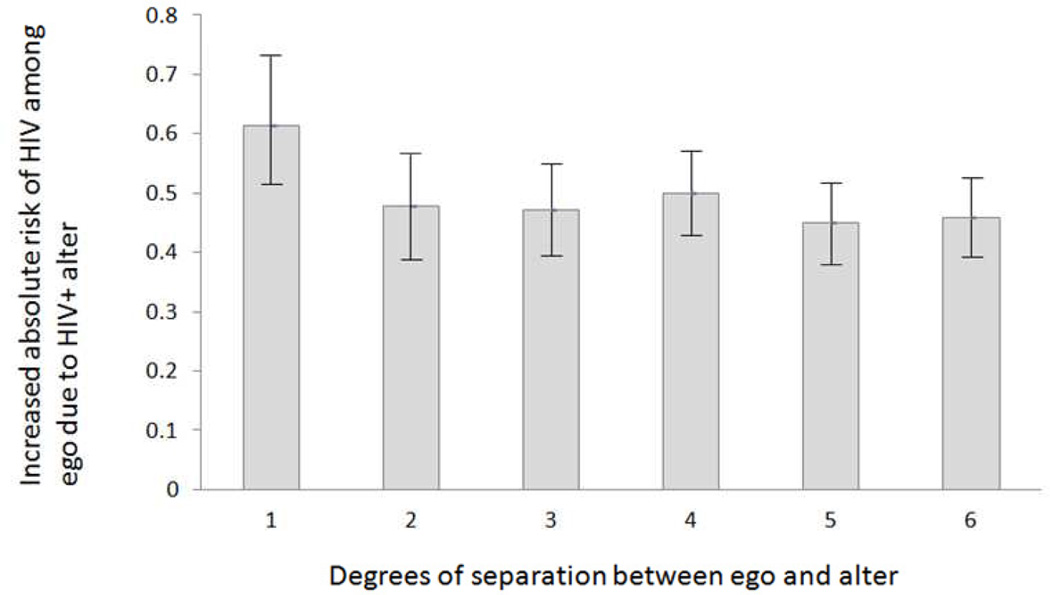
Figure 2.
Absoulte Increased Risk of HIV Among Egos Associated With Having an HIV Positive Alter Separated by 1–6 Degrees. Absolute risk is calculated as the difference between the observed risk and the risk from the null distribution (1,000 random samples with HIV status randomly distributed). Error bars denote 95% confidence intervals for the observed risk of HIV for alter-ego pairs separated by 1–6 degrees.
Statistical analysis
As mentioned above, network simulations were used to derive a meaningful measure of HIV clustering. In the Framingham Heart Study, the association between an ego’s attribute and his/her alter’s attribute was no different from that expected by chance after three degrees of separation (31–33). Because 1) the increased risk of HIV observed for egos with an HIV positive alter was significantly different from that expected by chance for alter-ego pairs separated by 1–6 degrees (P<0.05) and 2) the increased risk of HIV among those with HIV positive alters did not vary in strength or significance for alter-ego pairs separated by 1–6 degrees (Table 1 and Figure 2), individuals were classified based on their membership in an RDS recruitment network with greater than expected HIV (>10.54%) vs. less than expected HIV (<10.54%). The expected prevalence assuming random assignment of HIV status across the sample was 10.54%. Of note, the highest HIV prevalence among the low-prevalence networks was 7.14% and the lowest HIV prevalence among the high-prevalence networks was 28.57%.
Because network clusters included individuals from multiple neighborhoods and individuals within networks were not confined to specific neighborhoods, a multi-level model with two levels of clustering was not possible. We also considered a hierarchical model with two random intercepts (one for network clusters and a second for neighborhood clusters), however the models did not converge. Therefore, we selected the clustering level most relevant to this analysis (networks) and clustered individuals belonging to the same RDS recruitment network. To account for correlation between observations from individuals within the same RDS recruitment network, we clustered on RDS recruitment network membership using a generalized estimating equations approach and calculated Huber-White robust standard errors using STATA 10 (34). Neighborhood variables (except ICE) were standardized by z-score in the bivariate and multivariable regressions. Descriptive statistics and logistic regression (with GEE and Huber-White robust standard errors) were used to identify individual and neighborhood-level factors associated with membership in RDS recruitment networks with a higher HIV prevalence.
RESULTS
Our sample includes 378 individuals, 28 RDS recruitment networks and 350 one-degree ties (Table 1). The median number of individuals per RDS recruitment network was 4.5 (min=2; max=103; IQR:2–11.75). Sample characteristics are presented in Table 2. The absolute increased risk of HIV associated with having an HIV positive alter ranged from 45%–61%, was significant for alter-ego pairs separated by 1–6 degrees, and did not significantly vary by the number of degrees of separation (Figure 2; P<0.05). The relative increased risk of HIV associated with having an HIV positive alter ranged from RR=9.29 to RR=16.81 (Table 1; P<0.05).
Table 2.
Individual and Neighborhood Correlates of Membership in a Drug Network With Greater Than Expected HIV vs. Less Than Expected HIV in New York City 2006–2009.
| Greater Than Expected HIV N=52 (13.76%) |
Less Than Expected HIV N=326 (86.24%) |
All N=378 |
|||||
|---|---|---|---|---|---|---|---|
| INDIVIDUAL-LEVEL VARIABLES | N | (%) | N | (%) | N | (%) | 2 sided P value |
| HIV positive | 30 | (61.22) | 7 | (2.32) | 37 | (10.54) | 0.000 |
| HIV status unknown | 3 | (5.80) | 24 | (7.40) | 27 | (7.14) | 0.679 |
| Total income >10,000 | 7 | (15.22) | 47 | (15.11) | 54 | (15.13) | 0.985 |
| Homeless in past 6 months | 24 | (46.15) | 235 | (72.09) | 259 | (68.52) | 0.000 |
| Race/Ethnicity | 0.003 | ||||||
| Hispanic | 11 | (21.15) | 119 | (36.5) | 130 | (34.39) | |
| Black | 40 | (76.92) | 171 | (52.45) | 211 | (55.82) | |
| Other | 1 | (1.92) | 36 | (11.04) | 37 | (9.79) | |
| HIV test in the past 6 months | 14 | (66.67) | 213 | (70.07) | 227 | (69.85) | 0.743 |
| Ever tested for an STD (Herpes, Gonorrhea, Syphilis, Chlamydia) | 46 | (88.46) | 221 | (70.83) | 267 | (73.15) | 0.008 |
| Drug treatment in the past 6 months | 26 | (50) | 116 | (35.58) | 142 | (37.57) | 0.046 |
| Inject | 2 | (3.85) | 30 | (9.2) | 32 | (8.47) | 0.198 |
| Any cocaine past 6 months | 31 | (59.62) | 257 | (79.32) | 288 | (76.6) | 0.002 |
| Any crack past 6 months | 49 | (94.23) | 273 | (84) | 322 | (85.41) | 0.052 |
| Any heroin past 6 months | 22 | (42.31) | 156 | (48.6) | 178 | (47.72) | 0.400 |
| Exchange sex in the past year | 26 | (50.98) | 95 | (30.06) | 121 | (32.97) | 0.003 |
| Male | 41 | (78.85) | 241 | (74.8) | 282 | (74.8) | 0.469 |
| High school education or more | 32 | (61.54) | 165 | (50.61) | 197 | (52.12) | 0.143 |
| Always use condoms | 16 | (31.17) | 98 | (30.82) | 114 | (30.89) | 0.937 |
| Ever done city time or gone to jail (short stay of ≤ 1 year) | 34 | (69.39) | 234 | (69.64) | 268 | (79.76) | 0.051 |
| Ever done time upstate or gone to a state or federal prison | 17 | (34.69) | 125 | (43.55) | 142 | (42.26) | 0.246 |
| Age, Median (IQR) | 35.50 | (32.00, 38.50) | 34.00 | (29.00, 38.00) | 34.50 | (29.00, 38.00) | 0.034 |
| Number of drug using networks, Median (IQR) | 1 | (0, 2) | 1 | (0, 2) | 1 | (0, 2) | 0.429 |
| Number of people he/she uses, Median (IQR) drugs with | 1 | (0, 1) | 1 | (0, 1) | 1 | (0, 1) | 0.452 |
| Years HIV positive, Median (IQR) | 9.80 | (7.43, 12.86) | 11.31 | (7.89, 14.25) | 10.02 | (7.61, 13.12) | 0.4983 |
| NEIGHBORHOOD-LEVEL VARIABLES | Median | IQR | Median | IQR | Median | IQR | 2 sided P value |
| Minority Composition | |||||||
| Percent Latino | 20.09 | (13.68, 67.27) | 27.73 | (13.81, 49.77) | 23.64 | (13.68, 49.77) | 0.183 |
| Percent Black | 44.76 | (17.56, 76.93) | 55.92 | (44.44, 75.78) | 55.92 | (44.44, 75.78) | 0.040 |
| Percent White | 2.50 | (1.11, 3.52) | 2.34 | (1.13, 11.69) | 2.34 | (1.13, 11.67) | 0.362 |
| Percent foreign born | 19.85 | (16.58, 30.86) | 16.58 | (14.51, 22.57) | 16.58 | (14.51, 22.87) | 0.339 |
| Educational attainment | |||||||
| Percent with bachelor's degrees and beyond (25+) | 9.04 | (6.01, 17.21) | 6.29 | (3.03, 9.87) | 6.70 | (3.74, 10.77) | 0.003 |
| Unemployment | |||||||
| Percent unemployed | 18.32 | (5.56, 19.37) | 19.28 | (14.98, 22.55) | 19.25 | (14.19, 21.10) | 0.012 |
| Income / Poverty | |||||||
| Median household income | 19,434 | (16148, 24091) | 15,900 | (9829, 19434) | 15,900 | (9829, 20816) | 0.000 |
| Percent poverty- individuals | 38.54 | (29.39, 47.31) | 43.91 | (31.19, 49.12) | 43.91 | (30.92, 49.12) | 0.039 |
| Percent housing units vacant | 10.25 | (4.56, 21.06) | 9.82 | (5.36, 13.74) | 9.86 | (4.91, 13.74) | 0.414 |
| Percent owner-occupied housing | 7.95 | (2.86, 12.99) | 4.20 | (1.40, 12.99) | 5.38 | (1.40, 12.99) | 0.003 |
| Median value of owner-occupied housing | 350,000 | (176250, 410400) | 177,750 | (17500, 363200) | 186,200 | (17500, 363200) | <0.001 |
| Inequality | |||||||
| ICE (Index of concentration at the extremes)a | −0.12 | (−0.28, 0.28) | −0.21 | (−0.31, −0.08) | −0.21 | (−0.31, −0.02) | 0.019 |
| Crowding | |||||||
| Percent crowding (>1 res/room)b | 15.35 | (12.82, 20.62) | 14.41 | (12.82, 16.25) | 14.41 | (12.82, 17.62) | 0.030 |
(51)
Occupied housing units with >1 person/room are considered crowded
Members of high HIV prevalence networks were more likely to report 1) a prior sexually transmitted disease test (Herpes, Gonorrhea, Syphilis, Chlamydia), 2) recent drug treatment enrollment, 3) crack use, 4) exchanging sex for money/drugs, and 5) ≥ high school diploma/GED. They were less likely to report homelessness, injection drug use, and cocaine use (Table 3).
Table 3.
Individual and Neighborhood Correlates of Membership in a Drug Network with Greater Than Expected HIV vs. Less Than Expected HIV in New York City 2006–2009.
| Odds Ratio |
95% Confidence Interval |
|
|---|---|---|
| INDIVIDUAL-LEVEL VARIABLES | ||
| Total income >10,000 | 1.01 | 0.48, 2.11 |
| Homeless in past 6 months | 0.33 | 0.20, 0.55 |
| Race/Ethnicity | ||
| Hispanic | 3.33 | 0.32, 35.14 |
| Black | 8.42 | 0.96, 73.85 |
| Other | Ref | Ref |
| HIV test in the past 6 months | 0.85 | 0.44, 1.65 |
| Ever tested for an STD (Herpes, Gonorrhea, Syphilis, Chlamydia) | 3.16 | 1.67, 5.98 |
| Drug treatment in the past 6 months | 1.81 | 1.18, 2.79 |
| Inject | 0.39 | 0.16, 0.99 |
| Any cocaine past 6 months | 0.38 | 0.17, 0.85 |
| Any crack past 6 months | 3.11 | 1.23, 7.84 |
| Any heroin past 6 months | 0.78 | 0.35, 1.73 |
| Exchange sex in the past year | 2.42 | 1.42, 4.11 |
| Male | 1.30 | 0.50, 3.38 |
| High school education or more | 1.56 | 1.04, 2.35 |
| Always use condoms | 1.03 | 0.56, 1.89 |
| Age | 1.07 | 0.99, 1.15 |
| NEIGHBORHOOD-LEVEL VARIABLES | ||
| Minority Composition | ||
| Percent Latinoa | 1.29 | 0.98, 1.69 |
| Percent Blacka | 0.60 | 0.30, 1.19 |
| Percent Whitea | 1.35 | 0.64, 2.83 |
| Percent foreign borna | 0.95 | 0.72, 1.25 |
| Educational attainment | ||
| Percent with bachelor's degrees and beyond (25+)a | 1.85 | 1.31, 2.63 |
| Unemployment | ||
| Percent unemployeda | 0.80 | 0.58, 1.10 |
| Income / Poverty | ||
| Median household incomea | 2.36 | 1.48, 3.77 |
| Percent poverty- individualsa | 0.81 | 0.57, 1.14 |
| Percent housing units vacanta | 1.07 | 0.84, 1.36 |
| Percent owner-occupied housinga | 0.75 | 0.58, 0.97 |
| Median value of owner-occupied housinga | 1.40 | 1.13, 1.76 |
| Inequality | ||
| ICE (Index of concentration at the extremes)b | 3.68 | 1.33, 10.20 |
| Crowding | ||
| Percent crowding (>1 res/room)a,c | 1.49 | 1.02, 2.19 |
Those in high HIV prevalence networks were more likely to have been recruited in neighborhoods characterized by: greater inequality; a higher percentage of residents with ≥bachelor’s degree; higher median household incomes; higher valued owner-occupied housing (a higher median value for owner-occupied housing in that census tract); and greater residential crowding.
The final multivariable model assessed individual and census tract-level correlates of membership in high HIV prevalence networks. Members of high HIV prevalence networks were more likely to be recruited in neighborhoods characterized by greater inequality (Adjusted Odds Ratio [AOR]:5.85), higher valued owner-occupied housing (AOR=1.48), and a higher proportion of Latinos (AOR:1.83). Individuals in high HIV prevalence networks were more likely to have exchanged sex for money/drugs in the past year (AOR:1.82), to have used crack (past 6 months) (AOR:7.23), and to have been enrolled in drug treatment (past 6 months) (AOR:1.62). They were less likely to have used cocaine (AOR:0.40) and to report homelessness in the past 6 months (AOR:0.32).
DISCUSSION
In this sample, membership in high HIV prevalence networks was not random; individuals who recruited (or were recruited by) HIV positive individuals were much more likely to be HIV positive and the strength and significance of this association did not diminish with increasing degrees of separation. Our data highlight an association between exchanging sex, crack use, and increased HIV prevalence in these drug using networks. Additionally, high HIV prevalence network members were more likely to be recruited in neighborhoods characterized by increased inequality, higher-valued owner-occupied housing, and a greater proportion of Latinos.
Prior studies examining the “reach” of attributes (e.g., smoking, obesity, happiness, and loneliness) have consistently reported a threshold of three degrees of influence. There are several possible explanations for the difference in the degree to which networks exhibit clustered behavior. First, because HIV is infectious, it’s “reach” is likely greater, particularly among networks connected by drug use and in most instances drug/sex behaviors that directly facilitate HIV transmission. In this sample, these data suggest HIV was predominately acquired through sexual transmission which is consistent with literature that suggests the HIV epidemic among drug users is primarily sustained through sexual transmission (35). Members of high HIV prevalence networks were more likely to report exchanging sex and crack use which has been well established in the literature (36, 37).
Second, because the data were cross-sectional, we cannot determine whether high HIV prevalence network members knew each other prior to seroconversion or whether they met after being diagnosed. However, the number of years since first HIV diagnosis (median=10) did not differ by group. In either case, HIV negative individuals and others with unknown HIV status within these high HIV prevalence networks are at increased risk for HIV through high-risk sex behaviors (i.e., 94% and 51% of individuals in high HIV prevalence networks reported crack use and exchanging sex, respectively). While possible that HIV positive individuals in high HIV prevalence networks acquired HIV from high-risk behaviors with others not in these networks, their current HIV status and risk behaviors still pose a risk of transmission to their current sex and/or injecting network members (some of whom are in this dataset). The diffusion of a network-driven intervention would likely reach additional at-risk network members (including those not captured here).
Also of note, the reach of HIV >3 degrees of separation in this sample may reflect limitations in our network data. Rather than sociometric data, our data reflect RDS recruitment ties. Because individuals could only be recruited by one other person, it is possible that the number of one-degree ties is under-estimated and the number of several-degree links is overestimated. Thus, individuals separated by >1 degree may be directly connected to one another, but because of the way in which network links were generated, we were unable to discern these ties. The impact of this limitation on our subsequent findings is reduced because we analyzed individuals in the same recruitment network together, rather than focusing on the relationship between social distance and HIV status. Additionally, it is possible that individuals in distinct networks knew one another, but that this was not captured in our data. This could result in miss-specification of the clustering variable and consequently artificially narrow confidence estimates.
Despite the limitations of our network data, the networks in this study represent actual recruitment linkages, similar to those that would be generated with a network-driven intervention used in the field. Consequently, our findings demonstrate the feasibility of reaching HIV positive NIDUs and HIV-at-risk NIDUs using a network-driven approach (such as RDS) and could potentially increase uptake of 1) HIV care and treatment adherence among HIV positive NIDUs, and 2) HIV testing services among at-risk HIV negative NIDUs, particularly in neighborhoods that are most vulnerable.
We also observed an association between homelessness and membership in high HIV prevalence networks, however, it was not in the expected direction. In prior studies among IDUs, homelessness was associated with increased HIV prevalence and HIV-related risk behaviors; this relationship is thought to result from fewer socioeconomic resources, inadequate access to medical care, and reduced treatment adherence (38, 39). The fact that individuals with HIV/AIDS in New York are provided with housing services by the HIV/AIDS Services Administration may in part explain the observed inverse association between homelessness and HIV prevalence. (40) The HIV/AIDS Services Administration (HASA) within the New York City Human Resource Association is among the most comprehensive government programs serving people living with HIV/AIDS in the world. However, in November 2011, HASA started to enforce mandatory drug treatment for drug users applying for and living in HASA housing. Thus, data collected after 2011 should be explored with respect to homelessness.
Members of high HIV prevalence networks were also more likely to have been recruited in neighborhoods with a greater proportion of Latino residents. To better understand these findings, we mapped the data using ArcGIS and the association between being recruited in a neighborhood with a higher percent Latino population and membership in a drug using network with a higher prevalence of HIV seemed to be driven by those individuals who were recruited in the Bronx, which has a greater concentration of Latino residents than other New York City boroughs where participants were recruited. While there were fewer participants recruited in the Bronx than in other New York City boroughs, most were members of high HIV prevalence drug using networks and were recruited in census tracts with a greater concentration of Latino residents.. We therefore hypothesized that this association could be partially by explained by the New York-Puerto Rico “airbridge” in the Bronx, which is characterized by high-risk illicit drug users from Puerto Rico who migrate to New York and typically reside in Latino neighborhoods. Recent migrants from Puerto Rico typically represent a higher risk group with an increased burden of HIV compared with Latinos and other groups born in NYC and those engaging in high risk drug and sex behaviors with these individuals are also at increased risk for HIV acquisition (41–44).
Individuals in drug using networks with a higher prevalence of HIV were also more likely to be recruited in neighborhoods with greater inequality which is consistent with prior research findings (15, 16). Our data also revealed that members of drug using networks with a higher prevalence of HIV were more likely to be recruited from neighborhoods with higher-valued owner-occupied property. The association between high HIV prevalence networks and both 1) income inequality and 2) higher-valued owner-occupied property may at first seem paradoxical. However, this is consistent with the impact of the New York-Puerto Rico airbridge and expanding gentrification in low-income, Black and Latino neighborhoods in NYC (45). Researchers have suggested that in the presence of heightened income inequality during periods of gentrification and migration of marginalized Puerto Rican communities into NYC neighborhoods, relatively high rates of property ownership and income inequality could coexist (46, 47). More detailed investigation of HIV network clusters in Latino neighborhoods that focus on aspects of migration are needed to support this explanation and/or elucidate other possible explanations.
Other drawbacks also require some discussion. Because HIV status was measured via self-report, those reporting unknown or negative status may have been HIV positive but unaware of or unwilling to disclose their HIV status. However, self-reported unknown HIV status was not significantly different by group. Additionally, of the 27 individuals who reported unknown HIV status, 12 reported that he/she had never been tested for HIV, 5 had been tested for HIV (but not in the past 6 months), and 11 refused to answer any questions about their HIV testing history. Prior studies suggest that the growing prevalence of HIV among NIDUs may be partially due to overlapping sexual partnerships between NIDUs and IDUs (48–50). Due to our eligibility criteria, 1) our findings are generalizable only to the target population and 2) information on all relevant network relationships may not be captured. For example, study participants may engage in high-risk sex with HIV positive individuals not recruited/eligible for START; however, this is also likely to be the case in other network-based studies. Finally, individuals may have selectively engaged in high-risk drug and sex practices with individuals of the same serostatus. However, given the high number of HIV positive individuals observed in the high HIV prevalence networks, the HIV negative individuals in these drug networks (both those enrolled and not enrolled in START) are likely at an increased risk for disease acquisition compared with those in lower prevalence drug using networks. As noted above, network-based interventions could effectively reach members of high HIV prevalence drug use networks who were and were not enrolled in the study through diffusion.
Despite the limitations discussed above, our findings highlight the interplay between network and neighborhood correlates of HIV and drug/sex risk behaviors and can be used to guide the development of more effective interventions to reduce health disparities. Interestingly, individuals in high HIV prevalence networks were more likely to have recently been enrolled in drug treatment, which suggests a venue for targeted interventions. Our data also suggest that the influence of HIV may extend beyond six degrees of separation. Thus, network-driven approaches which also take features of the social/structural environment into account may be appropriate to reduce HIV transmission and/or to support care seeking and HIV drug adherence for HIV positive individuals, but further research is needed to better characterize neighborhood factors associated with HIV-clustering among drug-using populations in NYC.
Table 4.
Final Multivariable Logistic Regression with Robust Huber-White Standard Error Estimates Clustering on RDS Recruitment Network Membership, New York City (2006–2009)
| Adjusted Odds Ratio |
95% Confidence Interval |
|
|---|---|---|
| Individual-level variables | ||
| Exchange sex in the past year | 1.82 | 1.03, 3.23 |
| Any crack past 6 months | 7.23 | 2.43, 21.55 |
| Homeless in past 6 months | 0.32 | 0.17, 0.57 |
| Any cocaine past 6 months | 0.40 | 0.20, 0.79 |
| Drug treatment in the past 6 months | 1.62 | 1.05, 2.50 |
| Neighborhood-level variables | ||
| Percent Latinoa | 1.83 | 1.19, 2.80 |
| Median value of owner-occupied housinga | 1.48 | 1.14, 1.92 |
| ICE (Index of concentration at the extremes)b | 5.85 | 1.40, 24.42 |
standardized by z-score
(51)
Acknowledgements
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grant numbers R01 DA019964, K01 DA033879-01A1, and T32 DA023356).
Abbreviations
- AOR
Adjusted Odds Ratio
- CI
Confidence Interval
- HIV
Human Immunodeficiency Virus
- HASA
HIV/AIDS Services Administration
- ICE
Index of Concentration at the Extremes
- IDU
Injection Drug User
- NIDU
Non-injection Drug User
- NYC
New York City
- OR
Odds Ratio
- RDS
Respondent-Driven Sampling
- RDSAT
Respondent-Driven Sampling Analytic Tool
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rhodes T, Singer M, Bourgois P, Friedman SR, Strathdee SA. The social structural production of HIV risk among injecting drug users. Social science & medicine. 2005;61(5):1026–1044. doi: 10.1016/j.socscimed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Curtis R, Friedman SR, Neaigus A, Jose B, Goldstein M, Ildefonso G. Street-level drug markets: Network structure and HIV risk. Social networks. 1995;17(3–4):229–249. [Google Scholar]
- 3.Friedman SR, Neaigus A, Jose B, Curtis R, Goldstein M, Ildefonso G, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997 Aug;87(8):1289–1296. doi: 10.2105/ajph.87.8.1289. [Research Support, U.S. Gov't, P.H.S.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latkin C, Mandell W, Vlahov D, Knowlton A, Oziemkowska M, Celentano D. Personal network characteristics as antecedents to needle-sharing and shooting gallery attendance. Social networks. 1995;17(3–4):219–228. [Google Scholar]
- 5.Williams KM, Prather CM. Racism, Poverty and HIV/AIDS Among African Americans. African Americans and HIV/AIDS. :31–51. [Google Scholar]
- 6.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiologic reviews. 2004;26(1):22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 7.Campsmith M, Rhodes P, Hall H, Green T. HIV prevalence estimates—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–1076. [PubMed] [Google Scholar]
- 8.Denning P, DiNenno E, editors. Communities in Crisis: Is There a Generalized HIV Epidemic in Impoverished Urban Areas of the United States? 2010. [Google Scholar]
- 9.Acevedo-Garcia D. Residential segregation and the epidemiology of infectious diseases. Social Science & Medicine. 2000;51(8):1143–1161. doi: 10.1016/s0277-9536(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 10.White K, Borrell LN. Racial/ethnic residential segregation: Framing the context of health risk and health disparities. Health & Place. doi: 10.1016/j.healthplace.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landrine H, Corral I. Separate and unequal: residential segregation and black health disparities. Ethnicity & disease. 2009;19(2):179. [PubMed] [Google Scholar]
- 12.Hixson BA, Omer SB, del Rio C, Frew PM. Spatial Clustering of HIV Prevalence in Atlanta, Georgia and Population Characteristics Associated with Case Concentrations. Journal of Urban Health. 2011:1–13. doi: 10.1007/s11524-010-9510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heimer R, Barbour R, Shaboltas AV, Hoffman IF, Kozlov AP. Spatial distribution of HIV prevalence and incidence among injection drugs users in St Petersburg: implications for HIV transmission. AIDS (London, England) 2008;22(1):123. doi: 10.1097/QAD.0b013e3282f244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bluthenthal RN, Do DP, Finch B, Martinez A, Edlin BR, Kral AH. Community characteristics associated with HIV risk among injection drug users in the San Francisco Bay Area: a multilevel analysis. Journal of Urban Health. 2007;84(5):653–666. doi: 10.1007/s11524-007-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman SR, Lieb S, Tempalski B, Cooper H, Keem M, Friedman R, et al. HIV among injection drug users in large US metropolitan areas, 1998. Journal of Urban Health. 2005;82(3):434–445. doi: 10.1093/jurban/jti088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman SR, Des Jarlais DC, editors. Economic inequality, poverty, and laws against syringe access as predictors of metropolitan area rates of drug injection and of HIV. 2000. [Google Scholar]
- 17.Davey-Rothwell MA, Latkin CA. Gender differences in social network influence among injection drug users: Perceived norms and needle sharing. Journal of Urban Health-Bulletin of the New York Academy of Medicine. 2007 Sep;84(5):691–703. doi: 10.1007/s11524-007-9215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007 Nov;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 19.Latkin CA, Davey MA, Hua W. Social context of needle selling in Baltimore, Maryland. Subst Use Misuse. 2006;41(6–7):901–913. doi: 10.1080/10826080600668720. [Research Support, N.I.H., Extramural]. [DOI] [PubMed] [Google Scholar]
- 20.Shaw SY, Shah L, Jolly AM, Wylie JL. Determinants of injection drug user (IDU) syringe sharing: the relationship between availability of syringes and risk network member characteristics in Winnipeg, Canada. Addiction. 2007 Oct;102(10):1626–1635. doi: 10.1111/j.1360-0443.2007.01940.x. [Research Support, Non-U.S. Gov't]. [DOI] [PubMed] [Google Scholar]
- 21.Unger JB, Kipke MD, De Rosa CJ, Hyde J, Ritt-Olson A, Montgomery S. Needle-sharing among young IV drug users and their social network members: The influence of the injection partner's characteristics on HIV risk behavior. Addict Behav. 2006 Sep;31(9):1607–1618. doi: 10.1016/j.addbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Tobin KE, Davey-Rothwell M, Latkin CA. Social-level correlates of shooting gallery attendance: a focus on networks and norms. AIDS Behav. 2010 Oct;14(5):1142–1148. doi: 10.1007/s10461-010-9670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latkin CA, Forman V, Knowlton A, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Social Science & Medicine. 2003;56(3):465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 24.Davey-Rothwell MA, Latkin CA. An examination of perceived norms and exchanging sex for money or drugs among women injectors in Baltimore, MD, USA. International journal of STD & AIDS. 2008;19(1):47–50. doi: 10.1258/ijsa.2007.007123. [DOI] [PubMed] [Google Scholar]
- 25.Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sexually transmitted diseases. 2005;32(8):506. doi: 10.1097/01.olq.0000161191.12026.ca. [DOI] [PubMed] [Google Scholar]
- 26.Rudolph AE, Crawford ND, Latkin C, Heimer R, Benjamin EO, Jones KC, et al. Subpopulations of illicit drug users reached by targeted street outreach and respondent driven sampling strategies: Implications for research and public health practice. Annals of Epidemiology. 2011;21(4):280–289. doi: 10.1016/j.annepidem.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuller CM, Borrell LN, Latkin CA, Galea S, Ompad DC, Strathdee SA, et al. Effects of race, neighborhood, and social network on age at initiation of injection drug use. Am J Public Health. 2005 Apr;95(4):689–695. doi: 10.2105/AJPH.2003.02178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massey DS. The prodigal paradigm returns: Ecology comes back to sociology. Does it take a village. 2001:41–48. [Google Scholar]
- 29.Borgatti SP. Netdraw network visualization. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- 30.Szabo G, Barabasi A. Network Effects in Service Usage. [Retrieved February 29, 2012];2007 (from http://lanl.arxiv.org/abs/physics/0611177.). [Google Scholar]
- 31.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. New England journal of medicine. 2008;358(21):2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. New England journal of medicine. 2007;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 33.Fowler JH, Christakis NA. The dynamic spread of happiness in a large social network. BMJ: British medical journal. 2008;337:a2338. doi: 10.1136/bmj.a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.StataCorp. Statistical Software: Release 10. College Station, TX: Stata Corp LP; 2007. [Google Scholar]
- 35.Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. Journal of Urban Health. 2003;80 doi: 10.1093/jurban/jtg078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Booth R, Kwiatkowski C, Chitwood D. Sex related HIV risk behaviors: differential risks among injection drug users, crack smokers, and injection drug users who smoke crack. Drug and Alcohol Dependence. 2000;58(3):219–226. doi: 10.1016/s0376-8716(99)00094-0. [DOI] [PubMed] [Google Scholar]
- 37.Edlin BR, Irwin KL, Faruque S, McCoy CB, Word C, Serrano Y, et al. Intersecting epidemics--crack cocaine use and HIV infection among inner-city young adults. Multicenter Crack Cocaine and HIV Infection Study Team. N Engl J Med. 1994 Nov 24;331(21):1422–1427. doi: 10.1056/NEJM199411243312106. [Multicenter Study Research Support, U.S. Gov't, P.H.S.]. [DOI] [PubMed] [Google Scholar]
- 38.Song JY, Safaeian M, Strathdee SA, Vlahov D, Celentano DD. The prevalence of homelessness among injection drug users with and without HIV infection. Journal of Urban Health. 2000;77(4):678–687. doi: 10.1007/BF02344031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galea S, Vlahov D. Social determinants and the health of drug users: socioeconomic status, homelessness, and incarceration. Public Health Reports. 2002;117(Suppl 1):S135. [PMC free article] [PubMed] [Google Scholar]
- 40.NYCDOMH. HIV Care, Treatment, and Housing. The City of New York. [cited 2012 7/3/12];2012 Available from: http://www.nyc.gov/html/doh/html/ah/hiv-care-treatment-housing.shtml.
- 41.Deren S, Kang SY, Colón HM, Robles RR. The Puerto Rico–New York airbridge for drug users: Description and relationship to HIV risk behaviors. Journal of Urban Health. 2007;84(2):243–254. doi: 10.1007/s11524-006-9151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deren S, Oliver-Velez D, Finlinson A, Robles R, Andia J, Colón HM, et al. Integrating qualitative and quantitative methods: comparing HIV-related risk behaviors among Puerto Rican drug users in Puerto Rico and New York. Substance use & misuse. 2003;38(1):1–24. doi: 10.1081/ja-120016563. [DOI] [PubMed] [Google Scholar]
- 43.Deren S, Kang SY, Colón HM, Andia JF, Robles RR, Oliver-Velez D, et al. Migration and HIV risk behaviors: Puerto Rican drug injectors in New York City and Puerto Rico. Journal Information. 2003;93(5) doi: 10.2105/ajph.93.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robles RR, Colón HM, Matos TD, Reyes JC, Marrero CA, Lopez CM. Risk factors and HIV infection among three different cultural groups of injection drug users. Handbook on risk of AIDS: Injection drug users and sexual partners. 1993:256–274. [Google Scholar]
- 45.Rodriguez H. Household composition, employment patterns, and income inequality: Puerto Ricans in New York and other areas of the US mainland. Hispanic Journal of Behavioral Sciences. 1992;14(1):52–75. [Google Scholar]
- 46.Vigdor JL, Massey DS, Rivlin AM. Does Gentrification Harm the Poor? Brookings-Wharton Papers on Urban Affairs. 2002:133–182. [with Comments]. [Google Scholar]
- 47.Walks RA, Maaranen R. Gentrification, social mix, social polarization: testing the linkages in large Canadian cities. Urban Geography. 2008;29(4):293–326. [Google Scholar]
- 48.Strathdee SA, Sherman SG. The role of sexual transmission of HIV infection among injection and non-injection drug users. J Urban Health. 2003 Dec;80(4):7–14. doi: 10.1093/jurban/jtg078. [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Des Jarlais DC, Semaan S. HIV prevention and psychoactive drug use: a research agenda. J Epidemiol Community Health. 2009 Mar;63(3):191–196. doi: 10.1136/jech.2008.079301. [Article]. [DOI] [PubMed] [Google Scholar]
- 50.Neaigus A, Miller M, Gyarmathy VA, Friedman SR. HIV Heterosexual Sexual Risk From Injecting Drug Users Among HIV-Seronegative Noninjecting Heroin Users. Substance Use & Misuse. 2011;46(2–3):208–217. doi: 10.3109/10826084.2011.521473. [Article]. [DOI] [PubMed] [Google Scholar]
- 51.Casciano R, Massey DS. Neighborhoods, employment, and welfare use: Assessing the influence of neighborhood socioeconomic composition. Social Science Research. 2008;37(2):544–558. doi: 10.1016/j.ssresearch.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]