Abstract
Objective
Opioids and clonidine, used in for sedation, analgesia and control of opioid withdrawal in neonates, directly or indirectly activate opioid receptors expressed in immune cells. Therefore, our objective is to study how clinically relevant concentrations of different opioids and clonidine change cytokine levels in cultured whole blood from preterm and full-term infants.
Study design
Using blood from preterm (≤ 30 weeks gestational age, n=7) and full-term (≥37 weeks GA, n=19) infants, we investigated the changes in cytokine profile (IL-1β, IL-6, IL-8, IL-10, IL-12p70, and TNF-α), cyclic adenosine monophosphate (cAMP) levels and μ-, δ-, and κ- opioid receptor (OPR) gene and protein expression following in-vitro exposure to morphine, methadone, fentanyl, or clonidine at increasing concentrations ranging from 0 to 1 mM.
Results
Following LPS activation, IL-10 levels were 146-fold greater in cultured blood from full-term than from preterm infants. Morphine and methadone, but not fentanyl, at >10-5M decreased all tested cytokines except IL-8. In contrast, clonidine at <10-9M increased IL-6, while at >10-5M increased IL-1β and decreased TNF-α levels. All cytokine changes followed the same patterns in preterm and full-term infant cultured blood and matched increases in cAMP levels. All three μ-, δ- and κ-OPR genes were expressed in mononuclear cells from preterm and full-term infants. Morphine, methadone and clonidine, but not fentanyl, at >10-5M decreased the expression of μ-OPR, but not δ- or κ-OPRs.
Conclusion
Generalized cytokine suppression along with downregulation of μ-OPR expression observed in neonatal mononuclear cells exposed to morphine and methadone at clinically relevant concentrations contrast with the modest effects observed with fentanyl and clonidine. Therefore, we speculate that fentanyl and clonidine may be safer therapeutic choices for sedation and control of opioid withdrawal and pain in neonates.
Keywords: morphine, methadone, fentanyl, clonidine, inflammation, preterm infant, full-term infant
INTRODUCTION
Sick preterm and full-term infants are exposed to high doses of opioids in the neonatal intensive care unit (NICU) to provide sedation, analgesia and to treat neonatal abstinence syndrome (NAS). Clonidine, an α2-adrenergic receptor agonist is broadly used to provide sedation and analgesia during anesthesia induction in children 1, 2, 3, and is also safe and effective in the treatment of NAS 4. The similarities between the therapeutic effects of opioids and clonidine are suggestive of functional cross-talk between their receptors, opioid (OPR) and α2-adrenergic, respectively. Evidence of this cross-talk includes that morphine also binds to α2-adrenergic receptors 5, while clonidine indirectly activates OPRs via the induction of endogenous opiates 6. This may explain the prevention of the cardiovascular effects of clonidine by both yohimbine (α2-adrenergic receptor antagonist) and naloxone (non-specific OPR antagonist)6, 7, 8. Therefore, it is likely that opioids and clonidine share many other effects because the expression of classical OPRs (μ-, δ- and κ-) is not limited to neurons but they are also expressed in many adult immune cells from different species 9, 10, 11, 12, 13, 14. Many components of the immune response are modulated by opioids15 increasing the risk for sepsis in patients chronically exposed to these drugs 16. We speculate that opioids, and perhaps clonidine, may also change immune responses in immune cells from neonates, a population highly susceptible to infections.
Compared to adults, the acquired immune response of preterm and full-term infants is deficient due to lower T-cell proliferation, greater CD4+/CD8+ ratio and lower natural killer cell activity 17. Thus, the non-specific innate immune response becomes the primary mechanism to control infections in neonates. However, innate immunity in neonates is adapted to in-utero life, as shown by the significantly lower production of cytokines in response to Toll-like receptor (TLR)4 18 and TLR2 activation 19, hypogammaglobulinemia, and decreased complement levels and opsonization 20, all of which are required to prevent excessive inflammation in-utero. Most of these adaptations disappear by full-term gestational age 21, 22, 23, 24, a time when neonatal innate response becomes similar to that of adults.
In many adult animal models and humans, opioid exposure down-regulates cytokine production 11, 25, 26, 27, 28, 29 and, in turn, modulates the expression of OPRs as demonstrated by decreased expression of μ-OPRs in IL-6 knockout mice 30, and by enhanced OPR expression in response to increased levels of IL-6, TNF-α, and IL-1β 31, 32, 33, 34. Many similar effects have also been reported for clonidine 35, 36, 37, 38. Because cytokine production is a crucial component of the innate immune response which is essential in neonates, the potential down-regulation of cytokine production by neonatal immune cells is of clinical significance in the NICU setting. Therefore, we hypothesized that opioids (morphine, methadone, and fentanyl) and clonidine, at clinically relevant concentrations, down-regulate cytokine production and decreases OPRs expression in immune cells of cultured blood from neonates. Here, we show for the first time the comparative detailed profile of cytokine modulation that follows the exposure to opioids and clonidine in immune cells from preterm and full-term infants and the respective changes in OPRs expression.
MATERIALS AND METHODS
Patients and specimens
This study complied with the Guidelines for Human Experimentation from the U.S. Department of Health and Human Services and received approval from The Johns Hopkins Institutional Review Board. Parents provided informed consent prior to inclusion in study.
Peripheral blood (0.2 to 0.4 mL) was collected from preterm infants (≤ 30 weeks gestational age), and cord blood (1 mL) was collected from full-term infants (≥ 37 weeks gestational age). Since peripheral blood sampling was consider an invasive procedure in healthy full-term neonates, we were required to use cord blood for our experiments, a source of blood that was for the most part not available in the preterm group due to size of the umbilical vessels and the acuity of the cases. We excluded infants with known genetic disorders, major respiratory or cardiovascular anomalies, intrauterine growth restriction or small for gestational age, suspected prenatal viral infections, clinical or histological (placental pathology) chorioamnionitis, confirmed sepsis (positive cerebral spinal fluid, urine or blood culture) or suspected sepsis (extension of initial antibiotic course to more than 48h) and confirmed prenatal drug exposure to cocaine, opioids or benzodiazepines by history or urine toxicological screens at any time during pregnancy and/or at delivery. All mothers of preterm infants included in the study received at least one dose of betamethasone prior to delivery which may have buffered the cytokine response observed in our experiments.
Whole blood was diluted at 1:10 (v:v) with 4X RPMI 1640 media containing 8% (v/v) ΔH human AB serum, penicillin/streptomycin (400 IU/ml/400 ug/mL), and 8 mM L-glutamine (Sigma-Aldrich, St. Louis, MO), and plated in 200 μL aliquots on a 48-well plate. Cultured whole blood was used to mimic the response of the mixture of immune cells observed in-vivo as described previously 39. In the same plate, separate diluted whole blood aliquots were treated with morphine sulfate (Baxter, Deerfield, IL), fentanyl citrate (Hospira, Inc., Lake Forest, IL), methadone hydrochloride (Xanodyne Pharmaceuticals, Inc., Newport, KY; racemic mixture of R and S enantiomers), or clonidine hydrochloride (Sigma-Aldrich) at concentrations ranging from 0 (baseline) to 0.01 mM (fentanyl) or 1 mM (morphine, methadone, and clonidine) for 2h at 37°C and 5% CO2, followed by blood activation with lipopolysaccharide (LPS; 100 ng/mL; Sigma-Aldrich) for 18h in culture (modified from 25). Opioids and clonidine concentrations chosen for in-vitro testing were calculated based on clinically relevant blood concentrations reported in the literature 40, 41, 42, 43. Concentrations of 10-5 to 10-3M were clinically relevant for morphine and methadone, while concentrations of 10-8 to 10-7M were for fentanyl and clonidine. These calculated relevant molar concentrations may be underestimating actual in-vivo concentrations due to factors such as fluid restriction, hyperbilirubinemia, hypoalbuminemia, liver failure and drug interactions affecting sick neonates in the NICU. This is particularly true with respect to morphine and methadone, increasing the likelihood of achieving concentrations as high as 10-3M 44, 45. The concentration of LPS used for our experiments (100 ng/mL) was previously proven to produce significant TLR4 activation without decreasing cell survival 46 and has been used by others in similar models 47. Collected supernatants were stored at -20°C for cytokine analysis performed at the University of Cincinnati, Laboratory of Immunobiology. Following supernatant collection, mononuclear cells (MNC) were isolated by adherence to plastic wells as previously described 47. Ammonium-Chloride-Potassium lysis buffer (Quality Biological, Inc, Gaithersburg, MD) and Trypan Blue Exclusion (Gibco, Grand Island, NY) methods were used for cell count and to determine viability of MNC. MNC were then used to determine total cAMP concentrations and OPR expression profile.
ELISA for determination of cytokine and total cAMP concentrations
Pro-inflammatory IL-1β, IL-6, IL-8, IL-12p70 and TNF-α, and anti-inflammatory, IL-10, cytokine concentrations were measured (ELISA) using LINCOplex™ Multiplex kits (Millipore, Billerica, MA), following manufacturer's protocol, and concentrations were calculated using Luminex™ detection system (Millipore). The lowest dilution on the standard curve (0.64 pg/mL) was considered the lower limit of detection. Values below the lower limit of detection were calculated by maximum-likelihood estimation only if they represented <50% of all the measurements. In contrast, if >50% of all the measurements were below lower limit of detection, those cytokines were considered non-detectable.
Total cAMP concentrations were measured by non-acetylation procedure using the Amersham cAMP EIA System (GE Healthcare, Little Chalfont, Buckinghamshire, UK), as directed by the manufacturer. Results were reported as percentage change from baseline (no treatment, 0 mM) and standardized by mg of protein measured by Bradford Assay 48.
Real Time qRT-PCR for determination of OPR gene expression
To determine changes in μ-, δ-, and κ-OPR gene expression, total RNA was extracted from MNC. PureLink™ Micro-to-midi Total RNA Purification System (Invitrogen, Carlsbad, CA) was used according to manufacturer's specifications. RNA was utilized to generate cDNA, using iScript cDNA synthesis kit (BioRad, Hercules, CA). Reverse transcription protocol included 5 min at 25°C, 30 min at 42°C, and 5 min at 85°C. cDNA was then used to amplify the target genes by real time qRT-PCR using primers at 300 nM (Table 1). SYBR Green Supermix (BioRad) was used for signal detection by MyIQ PCR Thermocycler (BioRad). Two different amplification protocols were used for: 1) μ-OPR, 40 cycles of 1 min at 95°C, 1 min at 60°C, and 1.5 min at 72°C with 3 min for extension; and 2) δ- and κ-OPRs, 45 cycles of 1 min at 95°C, 1 min at 62°C, and 1.5 min at 72°C with 10 min for extension. The fold difference in gene expression was corrected to the reference gene, GAPDH, using the Pfaffl method 49. Melting curves were used to ascertain single PCR products.
TABLE 1.
PRIMERS FOR REAL TIME qRT-PCR
| Gene | Direction | Sequence | Base pair |
|---|---|---|---|
| OPRM1 | S | 5′- AGTTCTGTATCCCAACCTCTTCC -3′ | 60-bp |
| AS | 5′ - TCTGACGAATTCGAGTGGAG -3′ | ||
| OPRK1 | S | 5′ -GGAGAGAATAGTAGCTGTATGT-3′ | 120-bp |
| AS | 5′ -AGCAGTACCCTAAAATGATATT-3′ | ||
| OPRD1 | S | 5′ - TGCACCTGTGCATCGCGCTGA -3′ | 141-bp |
| AS | 5′ - GGCTGAAGCTGCTGGGGTC -3′ | ||
| GAPDH | S | 5′ - AACAGCGACACCCACTCCTC -3′ | 258-bp |
| AS | 5′ - GGAGGGGAGATTCAGTGTGGT -3′ | ||
AS, anti-sense; OPRD1, δ-opioid receptor; OPRK1, κ- opioid receptor; OPRM1, μ-opioid receptor; S, sense.
Western blot analysis for determination of OPR protein expression
Protein homogenates were obtained from MNC using ice-cold ethanol precipitation method, then reconstituted in 0.01M PBS (pH 7.4; Quality Biological, Inc., Gaithersburg, MD) and protein concentration was determined using the Bradford method 48. Protein (20 μg) was diluted 2:1 (v/v) with loading buffer, containing 20% (w/v) glycerol, and loaded to 15% SDS-PAGE. Proteins were transferred to nitrocellulose membrane, stained with Ponceau S, blocked with 5% nonfat dry milk with 0.1% Tween 20 in Tris-buffered saline (pH 7.4, 50 mM), and incubated overnight at 4°C with rabbit monoclonal anti μ-OPR antibody (Abcam, Cambridge, MA; 1:3,000), rabbit polyclonal anti κ-OPR antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:500) or mouse monoclonal anti β-actin antibody (Sigma-Aldrich; 1:10,000). The membrane was then washed with milk, exposed to goat anti-rabbit or anti-mouse antibodies (Bio-Rad) at 1:10,000 for 1h and then developed with enhanced chemiluminescence using SuperSignal kit (Thermo Scientific, Rockford, IL). Films were scanned using Adobe Photoshop (Adobe Systems Inc., San Jose, CA), and optical density (OD) was determined with IP Lab Gel H software (Signal Analytics, Vienna, VA) adjusting for background. β-actin was used for protein loading correction. Protein levels are expressed as arbitrary density units (ADU).
Statistical analysis
Because of the non-normal distribution of the data, nonparametric statistics using Wilcoxon and Mann-Whitney U tests was applied. Results are reported as median with interquartile range (IQR, 25th to 75th percentile) and represented as box-and-whisker plots with outliers (boxes symbolize IQR). Significance was assigned by p < 0.05 (vs. baseline) with multiple comparison correction using Bonferroni correction. All data presented as percentages do not require adjustments by white blood cell count (WBC) because ratios were calculated based on responses in the same blood, and since the correction factor (WBC) was in both the numerator and the denominator, it was cancelled from the equation. SPSS 18.0 software (SPSS Inc., Chicago, IL) was used for analysis and graphs.
RESULTS
Baseline cytokine concentrations
Blood samples were obtained from 7 preterm and 19 full-term infants of whom demographics and hematological parameters are detailed in Table 2. Diluted blood was plated, cultured for 2h (no opioids or clonidine), activated with LPS (100 ng/mL), and then incubated for 18 additional hours after which time cytokines levels were measured in supernatants to provide baseline levels. While IL-1β, IL-6, IL-10, and TNF-α concentrations were significantly greater in supernatants recovered from full-term rather than preterm cultured blood; following correction with white blood cell (WBC) count (x 1000 / mm3), only IL-10 levels remained significantly greater in supernatants recovered for full-term blood (146-fold greater than preterm blood, p<0.001, Table 3).
TABLE 2.
CHARACTERIZATION OF STUDY GROUPS
| Preterm group | Full-term group | ||
|---|---|---|---|
| Demographic and perinatal variables | n = 7 | n = 19 | |
| Gestational age (wk) | mean ± SD | 26 5/7 ± 1.8 | 39 2/7 ± 1.0 |
| Birth weight (g) | mean ± SD | 865 ± 252 | 3165 ± 374 |
| Gender | % male | (4) 57 % | (13) 68.4% |
| Race | % African American | (6) 86% | (12) 63.2% |
| 1 minute APGAR score | median, IQR | 5 (5 - 7) | 8 (8 - 9) |
| 5 minutes APGAR score | median, IQR | 7 (7 - 8) | 9 ( 9 - 9) |
| Delivery method | % cesarean section | (4) 57 % | (7) 36.8 % |
| Clinical choriamnionitis | (5)71 % | (0) 0 % | |
| Pathological chorioamnionitis | (3) 43 % | (0) 0 % | |
| Confirmed infection | (0) 0 % | (0) 0 % | |
| Hematological parameters | |||
| Hematocrit | mean ± SD | 43 ± 6 % | 49 ± 5 % |
| White blood cell count (per mm3) | mean ± SD | 8953 ± 3461 | 14233 ± 4168 |
| Neutrophils | mean ± SD | 52 ± 19 % | 49 ± 13 % |
| Lymphocytes | mean ± SD | 32 ± 17 % | 41 ± 16 % |
| Monocytes | mean ± SD | 9 ± 5 % | 8 ± 0.5 % |
| Platelet count (× 1000 per mm3) | mean ± SD | 165 ± 72 | 265 ± 30 |
TABLE 3.
BASELINE CYTOKINE CONCENTRATIONS POST- LPS ACTIVATION
| Cytokines (pg/ml) | Preterm group n = 7 | Full-term group n = 19 | p-value | ||||
|---|---|---|---|---|---|---|---|
| Pro-inflammatory | Absolute | WBC adjusted† | Absolute | WBC adjusted† | Absolute | WBC adjusted† | |
| IL-1β | Mean | 40.5 1 | 2.89 | 138.7 | 8.88 | 0.03* | 0.11 |
| 25tile | 17.13 | 1.72 | 116.5 | 6.84 | |||
| 75tile | 53.04 | 13.7 | 188.0 | 17.57 | |||
| IL-6 | Mean | 317.15 | 24.56 | 2409.2 | 166.2 | 0.001* | 0.07 |
| 25tile | 179.7 | 11.24 | 2197.2 | 118.3 | |||
| 75tile | 941.2 | 204.2 | 3999.7 | 373.3 | |||
| IL-8 | Mean | 3000 . 5 | 433.1 | 5894.9 | 453.0 | 0.41 | 0.96 |
| 25tile | 1995.4 | 192.4 | 4775.4 | 404.2 | |||
| 75tile | 9354.6 | 3697.5 | 7183.7 | 556.0 | |||
| IL-12p70 | Mean | ND | ND | 1.03 | 0.09 | -- | -- |
| 25tile | -- | -- | 0.49 | 0.03 | |||
| 75tile | -- | -- | 1.81 | 0.12 | |||
| TNF-α | Mean | 63 . 1 | 5.55 | 310.6 | 17.42 | 0.03* | 0.25 |
| 25tile | 45.0 | 3.65 | 180.6 | 9.72 | |||
| 75tile | 156.5 | 28.80 | 452.5 | 28.43 | |||
| Anti-inflammatory | |||||||
| IL-10 | Mean | 7 . 7 1 | 0.55 | 647.8 | 80.57 | <0.001* | <0.001* |
| 25tile | 4.31 | 0.38 | 557.8 | 48.90 | |||
| 75tile | 12.03 | 4.52 | 1158.0 | 149.5 | |||
p < 0.05, preterm vs. fullterm group (Mann Whitney U Test)
Cytokine concentrations adjusted by WBC = [Absolute cytokine concentration (pg/ml) / WBC (× 1000 per mm3)]
Changes in cytokine and cAMP concentrations
Full-term group
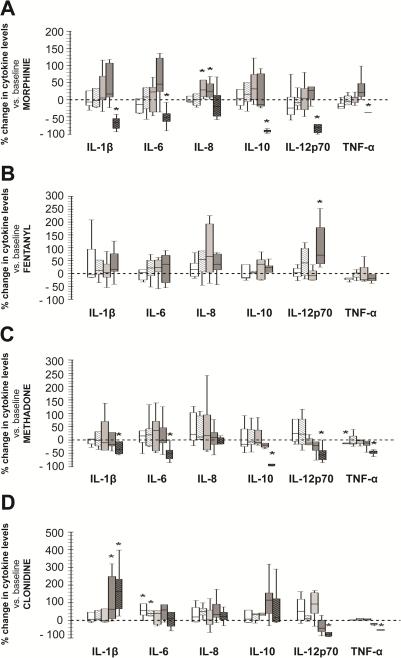
Cytokine concentrations did not change following exposure to opioid concentrations from 10-11 to 10-7M, although a ~25% increase in IL-8 levels was observed in response to morphine at 10-7 (median, p<0.05 vs. baseline). At greater concentrations, morphine and methadone (10-3M) significantly decreased all measured cytokines except for IL-8 (Fig.1A and 1C). Interestingly, fentanyl did not decrease cytokine levels at any concentration but it did increase IL-12p70 levels by a median of 70% (p=0.01 vs. baseline) at 10-5M concentration (Fig. 1B). Clonidine, on the other hand, increased IL-6 concentrations by a median of 55% (IQR 22.7 to 131.4%, p=0.01 vs. baseline) at 10-11M and 38% (IQR 25.6 to 101.2%, p=0.02 vs. baseline) at 10-9M. The highest tested concentration of clonidine (10-3M) increased IL-1β by 199% (IQR 55.7 to 480.2%, p=0.02 vs. baseline), and decreased TNF-α by 53% (32.6-63.3%, p=0.01 vs. baseline) and IL-12p70 by 92% (IQR 96.2 to 67.2%, p =0.01 vs. baseline) without modulating IL-6 or IL-8 levels (Fig.1D). The biological significance of changes reported for IL-12p70 is unknown due to the overall low baseline levels (shown in Table 3).
Figure 1.
Percent change in cytokine levels in supernatants of cultured blood from full-term infants following exposure to morphine (A), fentanyl (B), methadone (C), and clonidine (D). Concentrations were: 10-11M (white), 10-9M (hashed-white), 10-7M (light grey), 10-5M (dark grey), and 10-3M (hashed-black). Changes represented as box-and-whisker plot, boxes represent IQR and median (solid line inside the boxes). Reference line at 0 represents baseline (0 M). *, p≤0.01 (vs. baseline, Wilcoxon test); n=7.
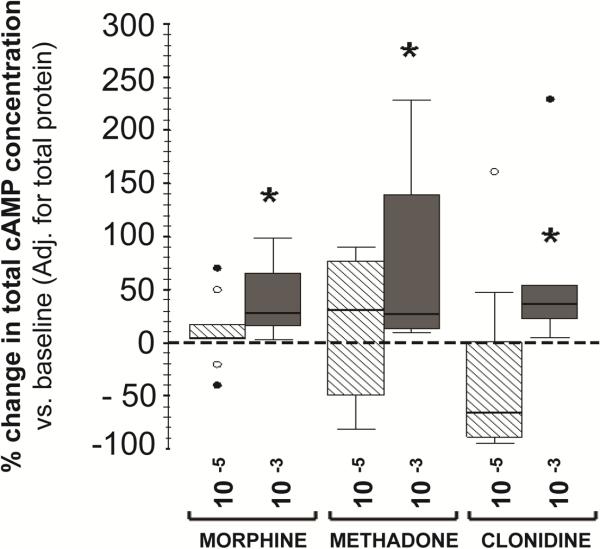
Morphine, methadone and clonidine treatment at 10-3M modulated the cytokine profile and increased total cAMP levels in MNC isolated by adherence from full-term infants by 38.9% (17.5-214.6%, p=0.005), 55.8% (13.5-327.1%, p=0.005) and 39.5% (21.8–252.2%, p=0.005), respectively by treatment group (Fig.2). No changes in cAMP were observed following in-vitro exposure to fentanyl at 10-5M (data not shown).
Figure 2.
Percent change in protein-adjusted total cAMP concentrations following treatment with morphine, methadone, and clonidine at concentrations of 10-5M (hashed-white) and 10-3M (dark grey). Changes presented as box-and-whisker plot. Reference line at 0 represents baseline condition (0 M). *, p < 0.01 (vs. baseline, Wilcoxon rank test). ○, outliers; ●, extremes; n=10 (full-term infants).
Preterm group
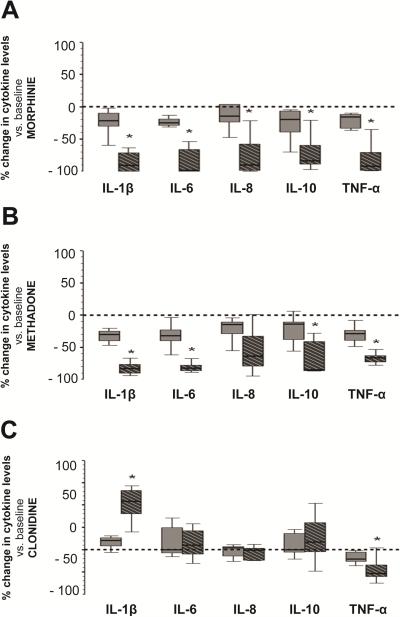
Only two concentrations, 10-5 and 10-3M, were tested for morphine, methadone and clonidine due to the small blood sample volume permitted from preterm infants. Similarly, fentanyl was not tested since the pattern of cytokine change was very limited in our previous experiments using blood from full-term infants (described above). Cytokine concentrations in supernatants of cultured diluted peripheral blood from preterm infants showed a similar pattern of changes compared to those observed experiments using cord blood from full term infants: morphine and methadone at 10-3M decreased all measured cytokines (Fig.3A and 3B), while clonidine at 10-3M, increased IL-1β by median of 108% (IQR 80.2 to 132.6%, p=0.01), and decreased TNF-α by 53% (33.7-59.8%, p=0.01; Fig.3C). As shown in Table 3, IL-12p70 expression was not detected.
Figure 3.
Percent change in cytokine levels in supernatants of cultured blood cells from preterm infants following exposure to morphine (A), methadone (B), and clonidine (C). Concentrations included 10-5 M (dark grey) and 10-3 M (hashed-black). Changes represented as box-and-whisker plot, with boxes representing IQR and median (solid line in boxes). Reference line at 0 represents baseline condition (0 M). *, p≤0.01 (vs. baseline, Wilcoxon rank test); n=7.
MNC viability following 18 h exposure to opioids or clonidine at 10-3M was evaluated in both study groups and no difference was found between groups or treatments with an average viability of 95 ± 3%.
Changes in OPR expression
Gene expression
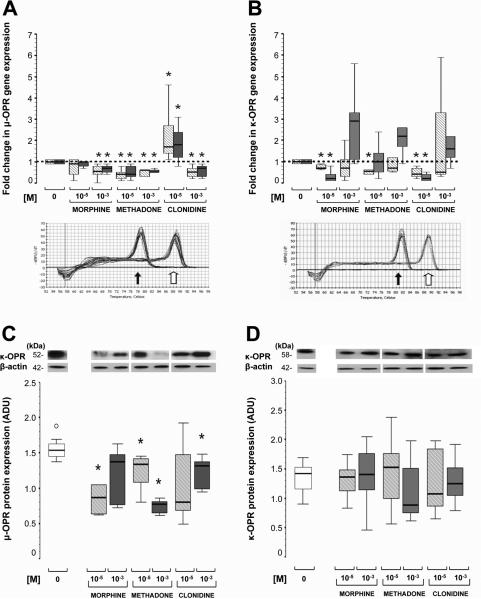
All of the three major classical OPR genes (μ-OPR1, δ-OPR1 and κ-OPR1) were expressed in MNC from preterm and full-term infants isolated by adherence. While morphine (10-5M) did not modulate μ-OPR1 gene expression, higher concentrations (10-3M) decreased gene expression by a median of 35% (IQR 16-53%, p=0.01 vs. baseline) and 20% (10-60%, p=0.02 vs. baseline) in MNC from both preterm and full-term infants, respectively. μ-OPR1 gene expression, in MNC from preterm and full-term infants, was also inhibited by methadone at 10-5M by a median of 57% (IQR 37-71%, p=0.04 vs. baseline) and 20% (10-60%, p=0.02 vs. baseline), respectively; and at 10-3M by 44% (38-76%, p=0.04) and 40% (36-50%, p=0.02), respectively. In contrast, clonidine (10-5M) significantly increased μ-OPR1 gene expression by over 80% (median), while higher concentrations (10-3M) inhibited gene expression by over 30% in both preterm and full-term MNC (p≤0.02 for both groups vs. baseline; Fig. 4A). Fentanyl, only tested in MNC from full-term infants, did not modify μ-OPR1 expression (data not shown).
Figure 4.
Fold change in μ- (A), and κ- (B) OPR gene expression in MNC from preterm (hashed-white) and full-term (grey) infants in response to morphine, methadone, and clonidine at 10-5M and 10-3M. Protein expression of μ- (C) and κ- (D) OPR in MNC from full-term infants to similar treatments at 10-5M (hashed-grey) and 10-3M (dark grey). Changes represented as box-and-whisker plot, with boxes representing IQR and median (solid line in boxes). For gene expression (A and B), reference line at 1 represents gene expression at baseline condition (0 M) and melting curves identified a single PCR product for each OPR (block arrow) along with GAPDH (housekeeping gene, white arrow). For protein expression (C and D), representative 15% SDS-PAGE immunoblots for respective OPRs with β-actin loading controls are also presented. *, p< 0.05 (vs. baseline, Wilcoxon rank test). ○, outliers, n=7/group.
High concentrations of morphine, methadone or clonidine did not modulate the gene expression of δ-OPR1 (data not shown). In contrast, morphine and clonidine at 10-5M inhibited κ-OPR1 gene expression by a median of 32% (IQR 15-35%, p=0.01vs. baseline) and 48% (25-70%, p=0.02), respectively in MNC from preterm infants; and by 80% (64-88%, p=0.02) and 82% (59-92%, p=0.02), respectively in those from full-term infants (Fig 4B).
Protein expression
For those OPRs with modified gene expression following opioid or clonidine exposure, we determined the effect on protein expression using Western blot. Due to sample volume limitations with preterm infants, studies for OPR protein expression were only performed in MNC from full-term infants. Generally, the effect of the drugs at the different concentrations altered gene and protein expression to the same degree. For example, in response to concentrations of 10-5M of morphine and methadone, μ-OPR1 protein expression was decreased by a median of 44.2% (IQR 32.2-56.3%, p=0.02 vs. baseline) and 15.1% (3-35.4%, p=0.02), respectively. At higher doses (10-3M), only methadone significantly decreased μ-OPR1 protein expression by a median of 52.1% (IQR 43.6-57.7%, p=0.03 vs. baseline). While clonidine at 10-5M increased μ-OPR1 gene expression, protein levels for μ-OPR1 did not differ from control levels. However, at 10-3M clonidine did decrease μ-OPR1 protein expression by a median of 14.5% (IQR 7.9-43.8%, p=0.02 vs. baseline; Fig. 4C), as it did for gene expression. Fentanyl did not change μ-OPR1 protein expression Unlike the results for gene expression, κ-OPR1 protein levels were unchanged following treatment with either opioids or clonidine at any tested concentrations (Fig. 4D).
DISCUSSION
Opioids, such as morphine, methadone, and fentanyl, are widely used in neonates for the treatment of NAS, pain, and agitation. Clonidine, used for anesthesia induction in children 1, 2, 3, has been recently proposed as a new adjunct therapy for NAS 4. While opioid exposure inhibits cytokine production by adult immune cells 25, 26, 27, ours is the first systematic evaluation of the effects of opioids and clonidine on neonatal immune cells. Using cultured blood from preterm and full-term infants to mimic the in-vivo response generated by the mixture of different immune cells, we show that: i) all OPRs receptors are expressed in neonatal MNC; ii) the kind and concentration of analgesic/sedative agent can differentially modulate the level of gene and protein expression of OPRs, the level of total cAMP, and the production of cytokines; and iii) these effects were for the most part similar between cells from preterm and full-term infants. Specifically, we find that clinically relevant concentrations (10-3M) of morphine and methadone decrease μ-OPR1 gene and protein expression, while increasing cAMP accumulation and broadly decreasing overall cytokine production. Conversely, fentanyl does not modulate cytokine production at any clinically relevant concentration and clonidine has less of an effect on OPR expression and cytokine production than methadone or morphine. These data suggest that concentrations of morphine and methadone that are translatable to the clinical setting in the treatment of sick infants in the NICU may be decreasing properties of the innate immune response in neonates, such as cytokine production.
OPRs are coupled to protein Gi/Go and their activation by low concentrations of opiates decreases the accumulation of cAMP, a critical second messenger that when inhibited leads to up-regulation of several cytokines 50. Morphine at low concentrations enhances, while at high concentrations reduces, LPS-induced Nuclear Factor-kappa B (NF-κB) in adult immune cells, suggesting that opioids affect cytokine production at the transcriptional level and its direction is concentration dependent 27, 51. Chronic administration or high concentrations of opioids is associated with increased cAMP (i.e., adenylyl cyclase superactivation), which acts to inhibit NF-κB and cytokine production 52. Neither clonidine nor opioids have been explored with respect to their modulation of cAMP and their resulting effects on cytokine production in-vivo or in-vitro in neonates until now. Here, we suggest that the same principles for OPR activation in MNC from adults may apply to those from preterm and full-term infants. We demonstrate that, in newborns, cAMP production is increased in MNC exposed to morphine, methadone or clonidine at 10-3M, explaining the significant down-regulation of pro-inflammatory (TNF-α, IL-1β, and IL-6) and anti-inflammatory (IL-10) cytokine production. This generalized cytokine suppression has unknown repercussions in the neonatal innate immune response and the risk for sepsis; however, since in-utero adaptations persist in preterm infants and resolve by full-term gestational age 21, 22, 23, 24, we can speculate that these effects may have a greater relevance in the sick preterm infant in the NICU. Furthermore, the cytokine responses observed in culture blood from preterm infants may be buffered by the use of perinatal steroids, suggesting that the real effects could be even greater. Our data also show that μ-OPR1 expression is down-regulated following exposure to 10-3M concentrations of morphine and methadone, but not fentanyl, which was also reported in the central nervous system linked to high doses and development of opioid tolerance 53, 54. We speculate that down-regulation of μ-OPR1 expression occurs in response to the decrease in cytokine production as suggested by the attenuated μ-OPR expression reported in IL-6 knockout mice 30 and the enhanced expression in response to increase IL-6, TNF-α, and IL-1β 31, 32, 33, 34.
All tested opioids, morphine, methadone and fentanyl, bind to all classical OPRs (μ-, δ-, κ-) but their potency at each receptor site depends on their biochemical composition. For instance, fentanyl binds more avidly than morphine to μ-OPRs (subtype 1 and 2), while morphine binds more avidly than fentanyl to κ- and δ-OPRs 55, which may explain the lack of cytokine changes seen with fentanyl exposure in our experiments. Furthermore, while μ-OPR may be the primary site for the effects seen in the central nervous system with morphine treatment, it may not be the sole site inducing changes in cytokine expression by immune cells in neonates reported above 56, 57, 58, 59 and perhaps the activation of κ- and δ- OPRs have a greater influence in those changes. In that sense, based on our results, we may argue both ways, that: i) κ- and δ-OPR overstimulation with high concentrations of morphine and methadone may inhibit overall cytokine production by neonatal immune cells by adenylyl cyclase superactivation (as described above), secondarily decreasing μ-OPR expression in MNC and inducing further predominance of κ- and δ-OPRs (decreased competitive binding to μ-OPR), or ii) high morphine and methadone concentrations, may produce primarily a decline in μ-OPR expression at the transcriptional level, inducing a decrease in cytokine expression by a greater predominance of κ- and δ-OPR activation. Because, in our experiments, fentanyl, a more potent μ-OPR agonist, does not modulate cytokine production or OPR expression, we speculate that the effect of morphine and methadone in neonatal immune cells is linked to κ- and δ-OPRs via the potential mechanisms explained above. Although fentanyl exposure does inhibit IL-1β and TNF-α in rodent models 60 and in adult human whole blood experiments 61, 62, this effect is diminished after 3 days of exposure 63, perhaps related to a decline in μ-OPR expression. Therefore, we speculate that lower μ-OPR expression in immune cells from neonates versus those from adults may explain the difference in response to fentanyl observed in our experiments, a speculation that we cannot confirm with these experiments since an adult group was not included.
Clonidine, an α2-adrenergic receptor agonist, prevents the immunomodulatory effects reported with morphine withdrawal in animals 8, 64. In human adult whole blood and in cord blood MNC, clonidine also stimulates production of the anti-inflammatory cytokine, IL-10, while decreasing the production of the pro-inflammatory cytokine, TNF-α 7, 38. It appears from our data that in MNC from newborns (full-term cord blood or preterm peripheral blood), clonidine does not modify the production of IL-10 but it does decrease TNF-α, confirming previous studies 7, 65. In neonatal immune cells, the mechanism underlying these changes is still unknown; however, since cross-talk between α2-adrenergic receptors and OPRs is evident 6, 7, 8, we postulate that the changes in cytokine expression is, at least in part, indirectly linked to activation of OPRs, as suggested by the increase cAMP accumulation which was also observed with clonidine treatment (Fig 2).
The translation of the concentrations of opioids and clonidine that induce decreases in cytokine production and μ-OPR1 expression in-vitro to those relevant in the NICU setting is cumbersome; however, there are a few previous studies that may put these results in perspective. With respect to morphine, when used for post-operative analgesia in full-term infants, serum concentrations of 10 and 32 ng/dl, are achieved with doses as small as 5 and 10 μg/k/h, respectively 40, 44, suggesting that changes in dosages can produce exponential increases in serum concentrations in neonates. Since morphine is adjusted to response in the NICU, doses may be higher than 200 μg/k every 2-4h, suggesting that serum concentrations of 10-5 and 10-3M are not unlikely. Furthermore, lower volume of distribution (fluid restriction or increased interstitial edema), hyperbilirubinemia, hypoalbuminemia, liver failure, lower clearance of the drug and drug interactions in sick neonates may further increase the risk of greater serum morphine concentrations 44. These factors also affect methadone blood concentrations which may vary up to 40- fold despite the same dosage schedule, explaining the equally variable therapeutic responses 45, 66, 67. In neonates exposed in-utero to methadone, withdrawal symptoms appear when blood concentrations fall below 88 ng/dl 41, perhaps representing the threshold for NAS treatment (10-5M); however, based on the many factors described above levels of 10-3M may still be clinically relevant. Fentanyl potency is 100-fold greater than that of morphine, suggesting that concentrations of 10-5M represent concentrations as high as 10-3M of morphine. In infants undergoing cardiac surgery, plasma fentanyl levels are 15 ng/ml (10-7M) during IV infusion at 18 μg/k/h 42; however, this rate is significantly greater than that used for sedation in the NICU. Lastly, the pharmacokinetics of clonidine during different therapeutic paradigms for NAS treatment in neonates demonstrate that the clearance of the drug improves rapidly during the second week of life with levels that can be as high as ~1.5 ng/mL (10-8 M) at doses of 2 μg/ k/ dose q4h 43. Therefore, clinically relevant concentrations for morphine and methadone are in the range of 10-5 to 10-3M, while for fentanyl and clonidine are in the range of 10-8 to 10-7M, suggesting that fentanyl and clonidine may have a safer immunomodulatory profile compared to morphine and methadone.
Opioids are titrated to effect for many clinical uses in neonates including treatment of pain, control of NAS, and induction of anesthesia. Clonidine is used as an effective adjunct therapy for NAS and significantly decreases the duration of opioid exposure without negative impact on cardiovascular outcomes 4. While further studies must be conducted to examine the immunomodulatory effects of opioids in-vivo, our study using neonatal cultured blood shows significant inhibition of cytokine release at clinically relevant concentrations of morphine and methadone. Our results also suggest that fentanyl and clonidine may be safer alternatives in neonates, since their modulation of cytokine production and OPRs is limited. Sick neonates have significant deficiencies in acquired immunity and adaptations of innate immunity, which increase their baseline risk for developing infections in the NICU setting. Therefore, a greater understanding of the potential immunosuppressant effects of perinatal opioid exposure is imperative for judicious use of these drugs in the NICU.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr. Frances Northington and Dr. Robert Bennett for their support; Mrs. Debra Flock and Ms. Ariel Mason for their administrative assistance and the obstetric team at Johns Hopkins Hospital for their kind support with patient enrollment. We are also indebted to our patients and their families for their willingness to participate in this study.
FINANCIAL SUPPORT: National Institute for Drug Abuse (NIDA) R25DA021630 (E.B.G).
Footnotes
CONFLICT OF INTEREST: None
REFERENCES
- 1.Mikawa K, Nishina K, Maekawa N, Obara H. Oral clonidine premedication reduces postoperative pain in children. Anesth Analg. 1996;82:225–230. doi: 10.1097/00000539-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Nishina K, Mikawa K, Shiga M, Maekawa N, Obara H. Oral clonidine premedication reduces minimum alveolar concentration of sevoflurane for tracheal intubation in children. Anesthesiology. 1997;87:1324–1327. doi: 10.1097/00000542-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Sumiya K, Homma M, Watanabe M, Baba Y, Inomata S, Kihara S, et al. Sedation and plasma concentration of clonidine hydrochloride for pre-anesthetic medication in pediatric surgery. Biol Pharm Bull. 2003;26:421–423. doi: 10.1248/bpb.26.421. [DOI] [PubMed] [Google Scholar]
- 4.Agthe AG, Kim GR, Mathias KB, Hendrix CW, Chavez-Valdez R, Jansson L, et al. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: a randomized, controlled trial. Pediatrics. 2009;123:e849–856. doi: 10.1542/peds.2008-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hocker J, Bohm R, Meybohm P, Gruenewald M, Renner J, Ohnesorge H, et al. Interaction of morphine but not fentanyl with cerebral alpha2-adrenoceptors in alpha2-adrenoceptor knockout mice. J Pharm Pharmacol. 2009;61:901–910. doi: 10.1211/jpp/61.07.0009. [DOI] [PubMed] [Google Scholar]
- 6.Gyires K, Ronai AZ, Mullner K, Furst S. Intracerebroventricular injection of clonidine releases beta-endorphin to induce mucosal protection in the rat. Neuropharmacology. 2000;39:961–968. doi: 10.1016/s0028-3908(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 7.Maes M, Lin A, Kenis G, Egyed B, Bosmans E. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res. 2000;96:245–253. doi: 10.1016/s0165-1781(00)00216-x. [DOI] [PubMed] [Google Scholar]
- 8.West JP, Dykstra LA, Lysle DT. Immunomodulatory effects of morphine withdrawal in the rat are time dependent and reversible by clonidine. Psychopharmacology (Berl) 1999;146:320–327. doi: 10.1007/s002130051123. [DOI] [PubMed] [Google Scholar]
- 9.Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol. 2000;7:719–723. doi: 10.1128/cdli.7.5.719-723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SL, Wu GD, Patel NA, Vidal EL, Fiala M. The effects of interaction between morphine and interleukin-1 on the immune response. Adv Exp Med Biol. 1998;437:67–72. doi: 10.1007/978-1-4615-5347-2_8. [DOI] [PubMed] [Google Scholar]
- 11.Gaveriaux-Ruff C, Matthes HW, Peluso J, Kieffer BL. Abolition of morphine-immunosuppression in mice lacking the mu-opioid receptor gene. Proc Natl Acad Sci U S A. 1998;95:6326–6330. doi: 10.1073/pnas.95.11.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makarenkova VP, Esche C, Kost NV, Shurin GV, Rabin BS, Zozulya AA, et al. Identification of delta- and mu-type opioid receptors on human and murine dendritic cells. J Neuroimmunol. 2001;117:68–77. doi: 10.1016/s0165-5728(01)00313-7. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy L, Szabo I, Nitsche JF, Pintar JE, Rogers TJ. Expression of functional mu-opioid receptors during T cell development. J Neuroimmunol. 2001;114:173–180. doi: 10.1016/s0165-5728(01)00248-x. [DOI] [PubMed] [Google Scholar]
- 14.Sibinga NE, Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annual review of immunology. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- 15.Roy S, Ninkovic J, Banerjee S, Charboneau RG, Das S, Dutta R, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6:442–465. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Barke RA, Ma J, Charboneau R, Roy S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch Immunol Ther Exp (Warsz) 2008;56:299–309. doi: 10.1007/s00005-008-0035-0. [DOI] [PubMed] [Google Scholar]
- 17.Gasparoni A, Ciardelli L, Avanzini A, Castellazzi AM, Carini R, Rondini G, et al. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol Neonate. 2003;84:297–303. doi: 10.1159/000073638. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, et al. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis. 2007;195:296–302. doi: 10.1086/509892. [DOI] [PubMed] [Google Scholar]
- 19.Strunk T, Richmond P, Simmer K, Currie A, Levy O, Burgner D. Neonatal immune responses to coagulase-negative staphylococci. Curr Opin Infect Dis. 2007;20:370–375. doi: 10.1097/QCO.0b013e3281a7ec98. [DOI] [PubMed] [Google Scholar]
- 20.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 21.Forster-Waldl E, Sadeghi K, Tamandl D, Gerhold B, Hallwirth U, Rohrmeister K, et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr Res. 2005;58:121–124. doi: 10.1203/01.PDR.0000163397.53466.0F. [DOI] [PubMed] [Google Scholar]
- 22.Hallwirth U, Pomberger G, Pollak A, Roth E, Spittler A. Monocyte switch in neonates: high phagocytic capacity and low HLA-DR expression in VLBWI are inverted during gestational aging. Pediatr Allergy Immunol. 2004;15:513–516. doi: 10.1111/j.1399-3038.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 23.Jones CA, Holloway JA, Warner JO. Phenotype of fetal monocytes and B lymphocytes during the third trimester of pregnancy. J Reprod Immunol. 2002;56:45–60. doi: 10.1016/s0165-0378(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 24.Marodi L. Innate cellular immune responses in newborns. Clin Immunol. 2006;118:137–144. doi: 10.1016/j.clim.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Chao CC, Molitor TW, Close K, Hu S, Peterson PK. Morphine inhibits the release of tumor necrosis factor in human peripheral blood mononuclear cell cultures. Int J Immunopharmacol. 1993;15:447–453. doi: 10.1016/0192-0561(93)90057-6. [DOI] [PubMed] [Google Scholar]
- 26.Peterson PK, Sharp B, Gekker G, Brummitt C, Keane WF. Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J Clin Invest. 1987;80:824–831. doi: 10.1172/JCI113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NF kappa B activation in macrophages. Biochem Biophys Res Commun. 1998;245:392–396. doi: 10.1006/bbrc.1998.8415. [DOI] [PubMed] [Google Scholar]
- 28.Taenzer AH, Groom R, Quinn RD. Fentanyl plasma levels after modified ultrafiltration in infant heart surgery. The Journal of extra-corporeal technology. 2005;37:369–372. [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfs TG, Derikx JP, Hodin CM, Vanderlocht J, Driessen A, de Bruine AP, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010;16:68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi M, Maggi R, Pimpinelli F, Rubino T, Parolaro D, Poli V, et al. Presence of a reduced opioid response in interleukin-6 knock out mice. Eur J Neurosci. 1999;11:1501–1507. doi: 10.1046/j.1460-9568.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 31.Borner C, Kraus J, Schroder H, Ammer H, Hollt V. Transcriptional regulation of the human muopioid receptor gene by interleukin-6. Mol Pharmacol. 2004;66:1719–1726. doi: 10.1124/mol.104.003806. [DOI] [PubMed] [Google Scholar]
- 32.Kraus J, Borner C, Giannini E, Hollt V. The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol. 2003;64:876–884. doi: 10.1124/mol.64.4.876. [DOI] [PubMed] [Google Scholar]
- 33.Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K, et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815–823. doi: 10.1136/gut.2005.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vidal EL, Patel NA, Wu G, Fiala M, Chang SL. Interleukin-1 induces the expression of mu opioid receptors in endothelial cells. Immunopharmacology. 1998;38:261–266. doi: 10.1016/s0162-3109(97)00085-4. [DOI] [PubMed] [Google Scholar]
- 35.Novak-Jankovic V, Paver E, rcirc, en V, Bovill JG, Ihan A, et al. Effect of epidural and intravenous clonidine on the neuro-endocrine and immune stress response in patients undergoing lung surgery. Eur J Anaesthesiol. 2000;17:50–56. doi: 10.1046/j.1365-2346.2000.00602.x. [DOI] [PubMed] [Google Scholar]
- 36.Persec J, Persec Z, Bukovic D, Merc V, Pavelic J, Zupic T. Preoperative clonidine or levobupivacaine--effect on systemic inflammatory stress response. Coll Antropol. 2009;33:573–577. [PubMed] [Google Scholar]
- 37.von Dossow V, Baehr N, Moshirzadeh M, von Heymann C, Braun JP, Hein OV, et al. Clonidine attenuated early proinflammatory response in T-cell subsets after cardiac surgery. Anesth Analg. 2006;103:809–814. doi: 10.1213/01.ane.0000237308.28739.d8. [DOI] [PubMed] [Google Scholar]
- 38.Xu B, Makris A, Thornton C, Ogle R, Horvath JS, Hennessy A. Antihypertensive drugs clonidine, diazoxide, hydralazine and furosemide regulate the production of cytokines by placentas and peripheral blood mononuclear cells in normal pregnancy. Journal of hypertension. 2006;24:915–922. doi: 10.1097/01.hjh.0000222762.84605.03. [DOI] [PubMed] [Google Scholar]
- 39.Connor TJ, Kelly JP, Leonard BE. An assessment of the acute effects of the serotonin releasers methylenedioxymethamphetamine, methylenedioxyamphetamine and fenfluramine on immunity in rats. Immunopharmacology. 2000;46:223–235. doi: 10.1016/s0162-3109(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 40.Geiduschek JM, Lynn AM, Bratton SL, Sanders JC, Levy FH, Haberkern CM, et al. Morphine pharmacokinetics during continuous infusion of morphine sulfate for infants receiving extracorporeal membrane oxygenation. Crit Care Med. 1997;25:360–364. doi: 10.1097/00003246-199702000-00027. [DOI] [PubMed] [Google Scholar]
- 41.Kuschel CA, Austerberry L, Cornwell M, Couch R, Rowley RS. Can methadone concentrations predict the severity of withdrawal in infants at risk of neonatal abstinence syndrome? Arch Dis Child Fetal Neonatal Ed. 2004;89:F390–393. doi: 10.1136/adc.2003.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kussman BD, Zurakowski D, Sullivan L, McGowan FX, Davis PJ, Laussen PC. Evaluation of plasma fentanyl concentrations in infants during cardiopulmonary bypass with low-volume circuits. J Cardiothorac Vasc Anesth. 2005;19:316–321. doi: 10.1053/j.jvca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Xie HG, Cao YJ, Gauda EB, Agthe AG, Hendrix CW, Lee H. Clonidine clearance matures rapidly during the early postnatal period: a population pharmacokinetic analysis in newborns with neonatal abstinence syndrome. J Clin Pharmacol. 2011;51:502–511. doi: 10.1177/0091270010370587. [DOI] [PubMed] [Google Scholar]
- 44.Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–217. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 45.Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- 46.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65:203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 47.Horrigan LA, Kelly JP, Connor TJ. Caffeine suppresses TNF-alpha production via activation of the cyclic AMP/protein kinase A pathway. Int Immunopharmacol. 2004;4:1409–1417. doi: 10.1016/j.intimp.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic acids research. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trescot AM, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain physician. 2008;11:S133–153. [PubMed] [Google Scholar]
- 51.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnet MP, Beloeil H, Benhamou D, Mazoit JX, Asehnoune K. The mu opioid receptor mediates morphine-induced tumor necrosis factor and interleukin-6 inhibition in toll-like receptor 2-stimulated monocytes. Anesth Analg. 2008;106:1142–1149. doi: 10.1213/ane.0b013e318165de89. table of contents. [DOI] [PubMed] [Google Scholar]
- 53.Meuser T, Giesecke T, Gabriel A, Horsch M, Sabatowski R, Hescheler J, et al. Mu-opioid receptor mRNA regulation during morphine tolerance in the rat peripheral nervous system. Anesth Analg. 2003;97:1458–1463. doi: 10.1213/01.ANE.0000081721.75663.87. [DOI] [PubMed] [Google Scholar]
- 54.Zhu ZP, Badisa RB, Palm DE, Goodman CB. Regulation of rat MOR-1 gene expression after chronic intracerebroventricular administration of morphine. Mol Med Report. 2012;5:513–516. doi: 10.3892/mmr.2011.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52:389–396. doi: 10.1016/0024-3205(93)90152-s. [DOI] [PubMed] [Google Scholar]
- 56.Chao CC, Gekker G, Hu S, Sheng WS, Portoghese PS, Peterson PK. Upregulation of HIV-1 expression in cocultures of chronically infected promonocytes and human brain cells by dynorphin. Biochem Pharmacol. 1995;50:715–722. doi: 10.1016/0006-2952(95)00176-z. [DOI] [PubMed] [Google Scholar]
- 57.Kaneider NC, Dunzendorfer S, Wiedermann CJ. Heparan sulfate proteoglycans are involved in opiate receptor-mediated cell migration. Biochemistry. 2004;43:237–244. doi: 10.1021/bi035295i. [DOI] [PubMed] [Google Scholar]
- 58.Parkhill AL, Bidlack JM. Reduction of lipopolysaccharide-induced interleukin-6 production by the kappa opioid U50,488 in a mouse monocyte-like cell line. Int Immunopharmacol. 2006;6:1013–1019. doi: 10.1016/j.intimp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Sharp BM, Li MD, Matta SG, McAllen K, Shahabi NA. Expression of delta opioid receptors and transcripts by splenic T cells. Ann N Y Acad Sci. 2000;917:764–770. doi: 10.1111/j.1749-6632.2000.tb05441.x. [DOI] [PubMed] [Google Scholar]
- 60.Oh WS. Effect of fentanyl on TNF-alpha and IL-1beta levels during global ischemia/reperfusion in rats. International journal of tissue reactions. 2002;24:11–21. [PubMed] [Google Scholar]
- 61.McBride WT, Armstrong MA, McMurray TJ. An investigation of the effects of heparin, low molecular weight heparin, protamine, and fentanyl on the balance of pro- and anti-inflammatory cytokines in in-vitro monocyte cultures. Anaesthesia. 1996;51:634–640. doi: 10.1111/j.1365-2044.1996.tb07844.x. [DOI] [PubMed] [Google Scholar]
- 62.Rao Y, Wang YL, Li JG, Ke JJ. Effects of morphine and fentanyl on tumor necrosis factor-alpha and interleukin-6 concentrations in human whole blood in vitro. Chinese medical journal. 2004;117:303–304. [PubMed] [Google Scholar]
- 63.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110:385–392. doi: 10.1016/j.pain.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 64.Kaye AD, Banister RE, Hoover JM, Baluch AR, Jacobs S, Shah RV. Chronic pain and ultrarapid opioid detoxification. Pain Pract. 2005;5:33–42. doi: 10.1111/j.1533-2500.2005.05105.x. [DOI] [PubMed] [Google Scholar]
- 65.Mosqueda-Garcia R, Kunos G. Opiate receptors and the endorphin-mediated cardiovascular effects of clonidine in rats: evidence for hypertension-induced mu-subtype to delta-subtype changes. Proc Natl Acad Sci U S A. 1987;84:8637–8641. doi: 10.1073/pnas.84.23.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 67.Felder C, Uehlinger C, Baumann P, Powell K, Eap CB. Oral and intravenous methadone use: some clinical and pharmacokinetic aspects. Drug Alcohol Depend. 1999;55:137–143. doi: 10.1016/s0376-8716(98)00190-2. [DOI] [PubMed] [Google Scholar]