Abstract
VEGF-stimulated angiogenesis depends on a cross-talk mechanism involving VEGF receptor 2 (VEGFR2), vascular endothelial (VE)-cadherin and the αVβ3 integrin. Since we have shown that αVβ3 integrin activation is dependent on its incorporation, along with the insulin-like growth factor-1 receptor (IGF1R) kinase, into a ternary receptor complex organized by the matrix receptor syndecan-1 (Sdc1), we questioned the role of this core complex in VEGF-stimulated angiogenesis. We find that the Sdc1-coupled ternary receptor complex is required for VEGF signaling and for stimulation of vascular endothelial cell migration by VE-cadherin engagement. VE-cadherin binding to Fc/VE-cadherin chimeras activates Sdc1-coupled IGF1R and αVβ3 integrin; this depends on VEGFR2 and c-Src activated by the cadherin. Blocking homotypic VE-cadherin engagement disrupts VEGF-stimulated cell migration, which is restored by clustering the cadherin in the absence of cell-cell adhesion. This cadherin-dependent stimulation requires VEGFR2 and IGF1R and is blocked by synstatin (SSTN92-119), a peptide that competitively disrupts the Sdc1-coupled ternary complex and prevents αVβ3 integrin activation that is required for VEGFR2 activation. VEGFR2-stimulated angiogenesis in the mouse aortic ring explant assay is disrupted by SSTN, but only early in the process, suggesting that IGF1R coupling to Sdc1 and αVβ3 integrin comprises a core activation mechanism activated by VE-cadherin that is necessary for VEGFR2 and integrin activation during the initial stages of endothelial cell dissemination during angiogenesis.
Keywords: Aortic ring, synstatin, blocking antibodies, scratch wound
INTRODUCTION
Angiogenesis, the process by which new blood vessels arise from pre-existing vessels, relies on the activation and signaling of several classes of receptors, notably VEGF receptor 2 (VEGFR2; also known as Flk1 or KDR)) and integrins. The process also depends on coupling the signaling from these receptors to the breakdown of adherens junctions (AJ) that maintain the impermeable blood vessel wall. It is known that VEGF-mediated activation of VEGFR2 in quiescent endothelial cells targets multiple proteins in the VE-cadherin-rich AJ, most notably the cadherin-catenin complex itself, and leads to the loss of stable VE-cadherin-mediated adhesion [1]. VEGFR2 also activates c-Src, a tyrosine kinase that associates directly with VE-cadherin and is believed to be required for VEGF-induced phosphorylation of VE-cadherin and other targets in the junctional complex [2]. Despite the importance of VEGF stimulation in disrupting VE-cadherin-rich junctions, however, homotypic VE-cadherin interactions appear necessary during the VEGF-stimulated outgrowth phase as well, as VE-cadherin blocking antibodies are known to block angiogenesis [3–5].
A functional interaction between VEGFR2 and the αVβ3 integrin is also central to angiogenesis and is especially important in pathological angiogenesis (reviewed in [5, 6]). Blockade of αVβ3 integrin activity using blocking antibodies and chemical inhibitors is known to disrupt angiogenesis in in vitro and in vivo models [7–13]. This is supported by recent studies showing that angiogenesis is disrupted in diYF knock-in mice that express β3 integrin subunit with Y747F and Y759F mutations [14, 15]. These mutations disrupt c-Src-dependent integrin activation and phosphorylation downstream of VEGFR2. This work also extends prior studies [16] that revealed a role for αVβ3 integrin in the activation of VEGFR2 by VEGF. These findings point to a complicated cross-talk mechanism that governs the angiogenesis process and remains poorly understood despite intensive study.
Our prior work shows that activation of the αVβ3 integrin in many, and perhaps all, cell types requires the cell surface proteoglycan syndecan-1 (Sdc1) and the insulin-like growth factor-1 receptor (IGF1R) [17–20]. This mechanism relies on capture of either αVβ3 (or αVβ5) integrin by Sdc1, utilizing an interaction site that spans amino acids 92-119 in the Sdc1 extracellular domain [18, 20]. The Sdc1 and integrin pair provide a docking face that captures the IGF1R, which, when activated, leads to activation of the integrin. Although capture of IGF1R as a member of the ternary receptor complex does not cause activation of either it or the integrin directly, the receptor tyrosine kinase and subsequently the integrin are activated either by IGF1, or by clustering of the ternary complex when Sdc1 engages the extracellular matrix [20]. We have derived a peptide, called synstatin (SSTN92-119) that mimics the interaction site in Sdc1, competitively displaces the integrin and IGF1R from the complex and in this manner blocks integrin activation [18]. Thus, this peptide serves as a highly specific probe for integrin activation that depends on Sdc1-coupled IGF1R.
Despite the extensive work on αVβ3 integrin in angiogenesis and its interdependence with VEGFR2, there is little work investigating the potential role of Sdc1 and IGF1R in this mechanism. Our initial work shows that the Sdc1-coupled ternary complex is present on endothelial cells and is required for αVβ3 and αVβ5 integrin activation [18, 20]. The inhibitory SSTN peptide blocks endothelial cell migration in scratch wound assays, and disrupts angiogenesis in the aortic ring assay in vitro as well as well as in the corneal pocket angiogenesis assay in vivo [18]. SSTN also blocks the growth of tumor xenografts at least partly by targeting angiogenesis, as the tumors in SSTN-treated mice display dramatically reduced levels of neovessel formation [18].
In the present work, we assess the hypothesis that the cross-talk that occurs between VEGFR2, αVβ3 integrin and VE-cadherin that is essential for angiogenesis is dependent on Sdc1-coupled IGF1R. Using the aortic ring angiogenesis assay, we find that the Sdc1-coupled IGF1R is essential for the initial onset of angiogenesis, and is then no longer required. In addition, we find that homotypic VE-cadherin engagement, which is required for endothelial cell migration to close scratch wounds, causes VEGFR2- and Src-dependent activation of the Sdc1-coupled ternary complex and that the ternary complex is necessary for VEGFR2 activation by VEGF. This suggests a model in which Sdc1-coupled IGF1R acts as a central mediator of integrin and VEGFR2 activation in endothelial cells during the onset of angiogenesis.
RESULTS
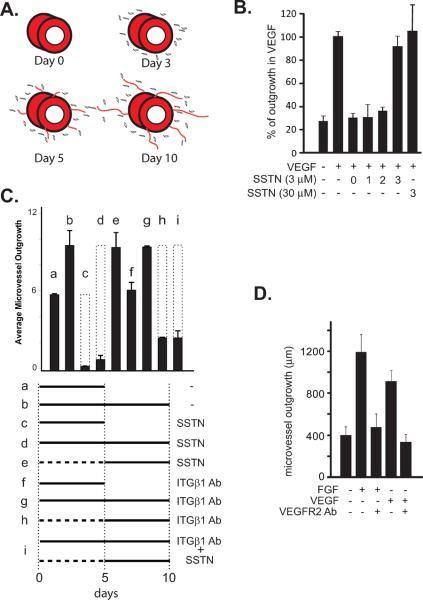
Prior work has shown that SSTN92-119 blocks angiogenesis using in vitro and in vivo assays. In mouse aortic rings explanted to type I collagen gels and stimulated with VEGF, microvessel outgrowth is blocked by SSTN92-119 present throughout the assay, displaying an IC50 of ca. 0.3 μM [18]. Specific cell staining shows that it is largely the outgrowth of endothelial cells that is disrupted by the peptide, whereas mural cells (pericytes) show unimpeded outgrowth into the collagen gel [18]. These findings are summarized in the cartoon in Fig. 1A, demonstrating that outgrowth begins with the appearance of single cells - largely desmin-positive pericyes [18] – followed by rapid microvessel formation one or two days later. Microvessels are typically not yet apparent at day 3, but are clearly present at day 5 and continue to grow in length until termination of the experiment at day 10.
Figure 1. SSTN acts early during aortic ring angiogenesis in collagen gels.
A. Cartoon of aortic ring outgrowth, showing mural cells (pericytes) and microvessel outgrowth after 3, 5 and 10 days. B. Segments of mouse aorta are explanted to collagen gels in the absence or presence of VEGF (50 ng/mL) for 10 days. Explants are treated with vehicle alone (−), or SSTN92-119 (3 μM or 30 μM) on day 0, 1, 2 or 3 as shown. C. Explants cultured in 20 ng/mL FGF for either 5 days (a, c, f) or 10 days (b, d, e, g, h, i) are treated with SSTN92-119 (3 μM), 10 μg/mL β1 blocking antibody P5D2, or combinations of P5D2 and SSTN for the times shown. Total length of microvessel outgrowth (μm) is shown. The dotted bars represent the expected vessel length in the absence of inhibitor for comparison. D. Explants are treated with either 20 ng/mL FGF or 50 ng/mL VEGF for 10 days in the absence or presence of 2 μg/mL VEGFR2 blocking antibody. Error bars (S.E.M) represent data from a minimum of triplicate explants from two experiments.
(Rapraeger et al.)
Despite the effective block of angiogenesis by SSTN added at day 0, SSTN added at day 5 is surprisingly without effect (Fig. 1C). To question the timing of its inhibition, SSN92-119 was applied to aortic rings at day 0, the time of initial VEGF addition, or was added one, two or three days following stimulation of angiogenesis with VEGF. SSTN is effective when added on day 0, 1, or 2, but fails to inhibit when added at day 3 or later, the period of time when microvessels are actually forming and extending their length (Fig. 1B, C). Even 30 μM SSTN92-119, 10-fold higher than the concentration needed for maximal inhibition at day 0, is without effect when added at day 3. This inhibition by SSTN reflects its inactivation of αV-integrins known to couple with Sdc1, as αV-integrin blocking antibody blocks when added early, but fails to block when added at day 3 or later (data not shown). Outgrowth throughout the assay is dependent on VEGF, as outgrowth is blocked by VEGFR2 blocking antibody added at day 0 or day 3 or at later times (data not shown). Outgrowth is also blocked by IGF1R inhibitor added either early or late (data not shown), consistent with published findings that IGF1R is necessary for angiogenesis as it has a role in endothelial and mural cell outgrowth [21].
With αVβ3 or αVβ5 integrin seemingly confined to the early onset of angiogenesis, we surmised that further outgrowth was likely to depend on β1 integrins after this time, as other integrins – α2β1 and α5β1 in particular – are implicated along with αVβ3 and αVβ5 in angiogenesis [22, 23]. To test this hypothesis, we examined outgrowth from aortic ring explants subjected to either SSTN92-119 or β1 blocking integrin antibodies at times when outgrowth is either SSTN-sensitive (day 0) or SSTN-insensitive (day 5) (Fig. 1C). We find the same result whether the rings are stimulated with FGF-2 or VEGF, likely explained by the fact that FGF-induced angiogenesis is nonetheless mediated by VEGF (Fig. 1D), as FGF stimulates autocrine VEGF expression by the endothelial cells [24]. The experiments utilizing FGF are shown in Fig. 1C. Microvessels are clearly visible at day 5 of outgrowth in the absence of inhibitors and continue to grow longer between day 5 and day 10 (Fig. 1C and cartoon in Fig. 1A). SSTN treatment completely blocks outgrowth if added at day 0 (Fig. 1C, part c and d), but is without effect when added at day 5 (part e), as expected (cf. Fig. 1B). In contrast, addition of β1 integrin blocking antibody at day 5 blocks further outgrowth and causes microvessel regression (part h). Surprisingly, however, a different result is seen if β1 blocking antibody is added at day 0, where it has no effect on outgrowth over the ensuing 10 days (part g). Similarly, microvessels growing out in the presence of β1 blocking antibody since day 0 now become sensitive to SSTN added at day 5 and regress (part i). These findings suggest that whereas the onset of angiogenesis is absolutely dependent on Sdc1-coupled IGF1R and αV integrins, outgrowth transitions to a dependence on β1 integrins, which appear to feed back and inactivate the Sdc1-coupled mechanism.
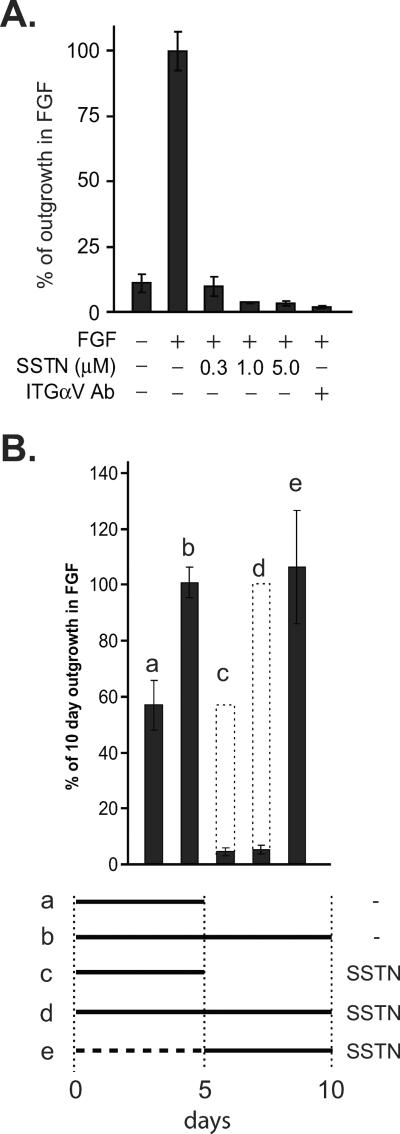
Similar findings are observed for aortic rings explanted to fibrin gels. Fibrin replicates the provisional matrix deposited as a clot in wounds and is a ligand for αVβ3 and αVβ5 integrins. We find that FGF-stimulated angiogenesis in fibrin is blocked by αV-integrin blocking antibody and is highly sensitive to SSTN 92–119, with over 90% inhibition seen even at 0.3 μM (Fig. 2A). Furthermore, SSTN displays the same temporal dependence in fibrin as collagen I, as it blocks outgrowth when added at day 0, but is without effect at day 5 (Fig. 2B).
Figure 2. SSTN blocks early onset of microvessel outgrowth in fibrin gels.
A. Segments of mouse aorta explanted to fibrin gels in the presence of 20 ng/mL FGF are treated from Day 0 to Day 10 with a range of SSTN92-119 concentrations or 10 μg/mL αV-integrin blocking antibody (mAb M9), followed by quantification of microvessel outgrowth. B. Explants cultured in 20 ng/mL FGF are treated with SSTN92-119 (3 μM) at the times shown and total length of microvessel outgrowth shown as a percentage of FGF alone. Error bars (S.E.M) represent data from a minimum of triplicate explants from two experiments.
(Rapraeger et al.)
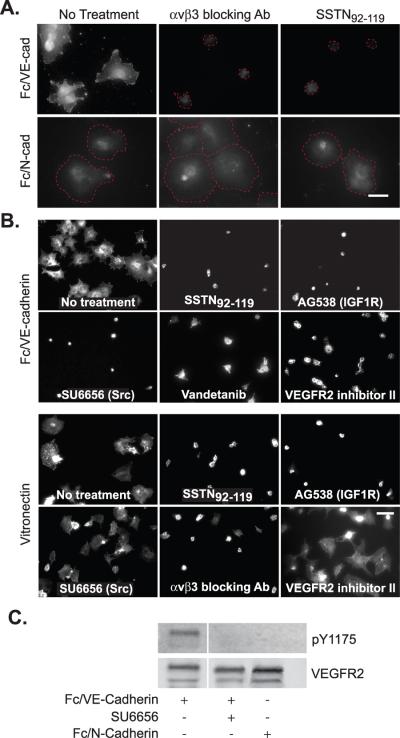
The outgrowth of endothelial cells from a resting vessel requires the disruption of VE-cadherin-rich AJs that maintain the cells as a confluent sheet. However, homotypic VE-cadherin interactions between endothelial cells must be required during outgrowth of new sprouts, as this process is known to be disrupted by VE-cadherin blocking antibodies. With this in mind, we noticed in adhesion assays on VN that the Sdc1-dependent activation of αVβ3 integrin was enhanced by endothelial cell-cell contact, which we reasoned might trace to either VE-cadherin or N-cadherin – both of which are highly expressed by endothelial cells [25]. To test the hypothesis that cadherin-mediated homotyic adhesion might activate the Sdc1-coupled ternary complex, we mimicked cell-cell adhesion by plating endothelial cells on substrata coated with either Fc/VE-cadherin or Fc/N-cadherin ectodomain chimeras (Fig. 3A). HMEC-1 human endothelial cells attach and spread on either ligand. However, the spreading in response to VE-cadherin engagement, and not N-cadherin, is blocked either by αVβ3 blocking antibody or by SSTN92–119 (Fig. 3A). Furthermore, staining of the cells with fluorescent fibrinogen, a ligand for active αVβ3 integrin [19], identifies active integrin only in cells spread on VE-cadherin and this binding is lost when the cells are treated with integrin blocking antibody or SSTN (Fig. 3A).
Figure 3. Spreading of vascular endothelial cells on Fc/VE-cadherin requires Src, active VEGFR2 and the Sdc1-coupled core activation complex.
A. HUVECs were plated on Fc/VE-cadherin or Fc/N-cadherin to mimic cell-cell adhesion in the presence of SSTN92-119 (3 μM) or 30 μg/mL LM609 blocking antibody to the αVβ3 integrin. Cells were fixed after 2 hr, then stained for 1 hr with fluorescent fibrinogen to detect active αVβ3 integrin. The cells that fail to bind fibrinogen are outlined by a dashed red line so that the extent of their spreading can be seen. Note that cells on Fc/N-cadherin spread regardless of the treatment, but fail to specifically bind the fibrinogen probe. Bar = 50 μm. B. HMEC-1 cells were plated either on Fc/VE-cadherin-coated or VN-coated plates in the presence of 3 μM SSTN92-119, 30 μg/mL αVβ3 integrin blocking antibody (LM609), 100 μM IGF1R inhibitor AG538, 10 μM Src inhibitor SU6656 or 10 μM of two different VEGFR2 inhibitors (vandetanib or VEGFR2 kinase inhibitor II). Cells were fixed after 2 hr and stained with rhodamine-phalloidin to observe the cells. Bar = 50 μm. C. HUVECs were serum-starved for 6 hr, then plated on Fc/VE-cadherin or Fc/N-cadherin in the presence or absence of Src inhibitor SU6656 (10 μM). Cells were lysed, immunoprecipitated with VEGFR2 antibody and analyzed on immunoblots for either total or active (pY1175) VEGFR2.
(Rapraeger et al.)
In the context of cell-matrix adhesion, such as endothelial cell binding to VN, activation of the αVβ3 integrin occurs when Sdc1 engages the VN matrix and clusters the ternary complex, leading to IGF1R transphosphorylation and activation of the kinase and the integrin [20]. To determine how VE-cadherin engagement activates the integrin, we screened a series of kinase inhibitors using the cell spreading assay. Spreading of HMEC-1 cells on Fc/VE-cadherin is blocked by IGF1R inhibitor (tyrphostin AG538) as we would expect due to the direct association of IGF1R with the integrin [20], but also by two different VEGFR2 inhibitors (vandetanib and VEGFR2 kinase inhibitor II) and the Src inhibitor SU6656 (Fig. 3B). In comparison, endothelial cell spreading on VN, in which the ternary complex is activated directly by Sdc1 engagement and clustering [20], is blocked by the IGF1R inhibitor but is unaffected by inhibition of c-Src or VEGFR2 (Fig. 3B). The findings obtained from cell spreading are supported by direct measurement of VEGFR2 activation, as plating cells on Fc/VE-cadherin leads to phosphorylation of Y1175 in VEGFR2, whereas plating cells on N-cadherin does not (Fig. 3C). Activation of VEGFR2 by VE-cadherin is also blocked by Src inhibitor, suggesting a role for Src in linking VEGFR2 activation to VE-cadherin stimulation (Fig. 3C).
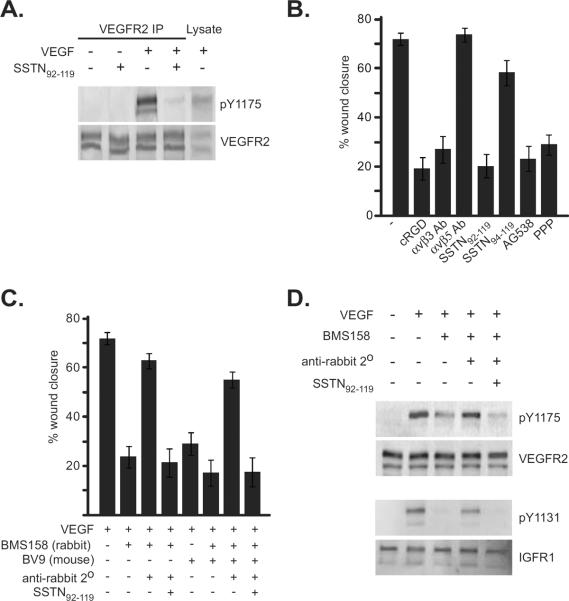
Activation-dependent cross-talk between VEGFR2 and αVβ3 involving Src has been shown previously, but the exact mechanism of this cross-talk remains unknown [15]. But our data suggest Sdc1 and IGF1R may be central to this process. To test this, we examined the effects of SSTN92–119 on VEGFR2 activation when serum-starved HUVECs are treated with VEGF. Whereas the receptor is activated by VEGF as expected in the absence of SSTN, as shown by pY1175, Y1175 phosphorylation is reduced to background levels in cells treated with the peptide (Fig. 4A).
Figure 4. SSTN blocks VEGF-mediated VEGFR2 phosphorylation.
A. HUVECs cultured on serum-coated dishes were serum-starved overnight, then treated for 10 min with or without 20 ng/mL VEGF in the presence or absence of 5 μM SSTN92-119. Immunoprecipitated VEGFR2 (or, whole lysate) was probed on western blots for anti-pY1175 indicative of active VEGFR2 or VEGFR2 mAb to detect total VEGFR2. B. HUVECs were grown to confluence in serum-containing medium, serum-starved for 6 hr, then stimulated to close a scratch wound by 20 ng/mL VEGF in the presence of 10 μM cycloRGDfV, 30 μg/mL αVβ3 integrin blocking antibody LM609 or αVβ5 blocking antibody P1F6, 3 μM SSTN92-119 or inactive SSTN94-119, or IGF1R inhibitors tyrphostin AG538 (10 μM) or picropodophyllin (30 nM). The percentage of wound closure after 18 hr is quantified. C. Closure of scratch wounds in confluent HUVEC monolayers is assessed in the presence of VE-cadherin blocking antibodies BMS158 (10 μg/mL/; rabbit polyclonal Ab) or BV9 (10 μg/mL; mouse mAb) and the dependence on Sdc1 by addition of SSTN92-119. Rescue of wound closure by clustered VE-cadherin is analyzed by adding 50 μg/mL rabbit-specific secondary antibody to target BMS158-decorated VE-cadherin, even in the continued presence of BV9 blocking antibody. Error bars represent S.E.M from triplicate measurements. D. HUVECs grown to confluence in complete MCDB-131 medium were starved for 6 hr in the presence or absence of VE-cadherin blocking antibody BMS158. Cells were then treated with 20 ng/mL VEGF and select cells pretreated with BMS158 were treated with BMS158 with or without secondary antibody to cluster BMS158-decorated VE-cadherin in the presence or absence of SSTN. Immunoprecipitated VEGFR2 or IGF1R was probed on Western blots for total receptor or for active receptor using anti-pY1175 (VEGFR2) or anti-pY1131 (IGF1R) antibodies.
(Rapraeger et al.)
These findings indicate that the Sdc1-coupled ternary complex, which depends on active IGF1R, not only causes integrin activation, but is functionally linked to VE-cadherin and VEGFR2 signaling in endothelial cells. To test the interdependence of these receptors in endothelial cell migration, we examined scratch wound closure of cells stimulated with VEGF. In support of our prior findings that wound closure depends on active ternary complex, closure of the scratch wounds was diminished by treatment of the cells with αVβ3 blocking antibody, cycloRGDfV peptide specific for this integrin, by the IGF1R inhibitors AG538 and PPP, and by SSTN92–119 but not SSTN94–119 (inactive peptide) [18](Fig. 4B). Closure was not dependent on the αVβ5 integrin, which is also expressed by these cells and blocked by SSTN [18], as wound closure is unaffected by P1F6 blocking antibody. We next tested whether wound closure also depends on homotypic adhesion by VE-cadherin. Indeed, cell migration is blocked by either of two anti-VE-cadherin blocking antibodies, BV9 or BMS158 (Fig. 4C). We then questioned whether this block occurs because physical association of the cells is required, or whether it requires the clustering of VE-cadherin that occurs during cell-cell adhesion and which we suspect activates the Sdc1-coupled ternary complex (cf. Fig. 3). To do this, we added secondary antibody to BMS158-blocked cells, reasoning that the blocking antibody would continue to prevent homotypic adhesion, but the secondary antibody would cluster the BMS158-decorated VE-cadherin monomers. Indeed, we find that clustering VE-cadherin restores wound closure, and this is blocked by SSTN92–119 (Fig. 4C) To rule out the possibility that the secondary antibody might be acting by preventing the function of the blocking antibody, we also conducted the experiment in the presence of BV9, a mouse mAb specific for blocking VE-cadherin, together with the BMS158 rabbit polyclonal antibody, as the anti-rabbit secondary antibody is specific only for BMS158. BV9 alone blocks wound closure, as does BMS158. But, the addition of both blocking antibodies together with the anti-rabbit secondary antibody to rescue VE-cadherin clustering via its interaction with BMS158 alone still restores wound closure and is blocked by SSTN (Fig. 4C).
To test whether the dependence of cell migration upon VE-cadherin clustering reflects its role in activating VEGFR2 and IGF1R, we probed for activation of these kinases (Fig. 4D) using phospho-specific antibodies (p1175 for VEGFR2 and p1131 for IGF1R). Neither kinase is active in the absence of VEGF, but both are stimulated by addition of VEGF to confluent HUVECs. However, this activation is prevented by preincubation with BMS158 to disrupt VE-cadherin homotypic adhesion, but is restored by clustering the BMS158-decorated cadherin with a secondary antibody. This is blocked by SSTN92–119, implicating the Sdc1-coupled IGF1R in this mechanism.
DISCUSSION
Sdc1 plays the role of central organizer in a ternary receptor complex consisting of Sdc1, the αvβ3 or αvβ5 integrin, and IGF1R. Its organizing function depends on a site in the extracellular domain (amino acids 92–119) of the receptor that serves to capture the integrin and receptor tyrosine kinase. Although assembly of the ternary complex does not appear to activate either IGF1R or integrin directly, IGF1R signaling and subsequent integrin activation does occur upon clustering of the complex via Sdc1 engaging the extracellular matrix, or upon direct stimulation with IGF1. However, disruption of the ternary complex by SSTN92–119, a peptide mimic of the interaction site in Sdc1, blocks integrin activation by IGF1R, even in response to IGF1. Because of its effect on this mechanism, the SSTN peptide becomes a powerful tool to detect Sdc1- and IGF1R-dependent integrin activation in biological systems. In this study, SSTN defines an important role for Sdc1-coupled IGF1R in VE-cadherin- and VEGF-stimulated activation of the αVβ3 integrin that appears necessary for endothelial cell dissemination during angiogenesis.
It is well known that among the earliest steps in angiogenesis is VEGF-stimulated breakdown of VE-cadherin-rich AJs, a step that occurs during the SSTN-sensitive period that we have identified in this study. Disruption of VE-cadherin function using blocking antibodies or gene-targeting approaches in mice leads to increased vascular permeability [3, 4, 26–28], defining its important role in AJ formation. Addition of blocking antibodies to cultured endothelial cells also causes AJ breakdown [3], an approach that we utilized in this work, and enhances the migration of endothelial cells into a scratch wound in the absence of VEGF or FGF [3], ostensibly by freeing them from their neighbors. But although freeing endothelial cells from their neighbors may aid migration, blocking VE-cadherin antibodies are also known to disrupt angiogenesis in response to VEGF or FGF in a variety of models, including tube formation in collagen gels, corneal angiogenesis, and tumor- induced angiogenesis, with the latter being particularly susceptible [4, 29]. That finding is duplicated here using VEGF- or FGF-stimulated aortic ring explants or endothelial cells stimulated to close scratch wounds by VEGF. Thus, transient VE-cadherin-mediated adhesion between migrating endothelial cells appears to be required for VEGF-stimulated migration. To our knowledge, studies have not previously questioned whether this homotypic adhesion is required to physically link the cells together, perhaps providing the opportunity for other cell-cell interactions to stimulate migration, or whether it is actually the clustering of VE-cadherin and subsequent signaling induced by its homotypic adhesion that is required. Our findings suggest that the clustering is required, as VEGF-stimulated migration can be rescued by antibodies that cluster the receptor but still prevent homotypic adhesion. We find that this clustering restores the activation of VEGFR2 in response to VEGF, and restores activation of IGF1R – both of which are inhibited if VE-cadherin is blocked. This dependence on IGF1R downstream of VE-cadherin clustering is one of the key findings of this work.
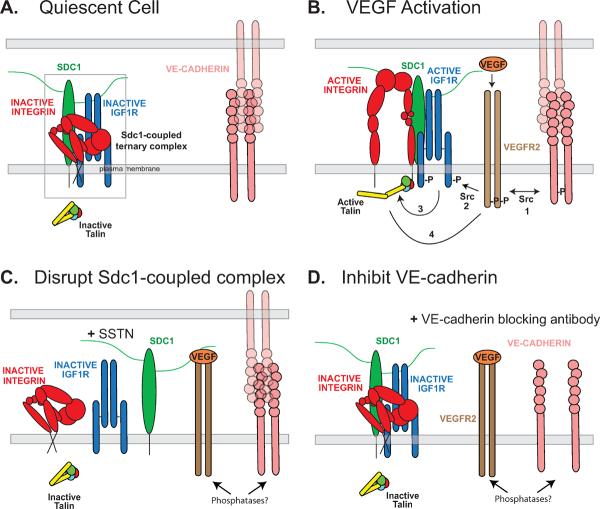
As shown in the model in Figure 5, IGF1R coupled to Sdc1 is required for activation of the αVβ3 integrin and for VEGFR2 signaling. At the early time during which SSTN inhibition is observed during aortic ring angiogenesis, the αvβ3 integrin likely localizes to VE-cadherin-rich cell-cell junctions [30, 31] where active VEGFR2 is also localized and aids in c-Src-mediated AJ breakdown. The αVβ3 integrin has been implicated in angiogenesis in a variety of models [8–10, 12, 18, 32]. Its expression is upregulated on activated endothelial cells [33, 34] and its signaling mediates endothelial cell migration into the stromal matrix, and promotes their proliferation and survival [9–11, 15, 35–37]. Several studies have used a gene knockout approach to test the importance of the αV or β3 integrin in vasculogenesis and angiogenesis in vivo, but those studies may be misleading due to compensatory mechanisms activated in response to integrin loss (see review [5]). However, using a gene replacement strategy to knock in a phosphorylation-defective β3 mutant (Y747F/Y759F) but otherwise retain integrin expression, Byzova's group has shown that the mutant mice display angiogenesis defects consistent with numerous in vitro studies [14]. These constructs have also been used to show an interdependence between αVβ3 integrin activation and VEGF-dependent activation of VEGFR2 that is mediated by Src kinase [15], supplementing prior work from Bussolino's group [16].
Figure 5. Model - Role of Sdc1-coupled core activation complex in VE-cadherin- and VEGF-mediated αvβ3 integrin activation and sustained VEGFR2 signaling.
A. Sdc1, IGF1R, and αvβ3 integrin are assembled as a ternary receptor complex (box) in the AJs of quiescent endothelial cells, along with VE-cadherin engaged in homotypic cell-cell adhesion. IGF1R is inactive and fails to initiate inside-out signaling leading to activation of talin, which is necessary for integrin activation [54]. The integrin is shown in its inactive conformation [55]. B. Activation of endothelial cells by VEGF enhances VEGFR2 localization to AJs and its functional coupling mediated by c-Src to VE-cadherin (step 1) and the Sdc1-coupled ternary complex (step 2). Although this leads to AJ breakdown, transient VE-cadherin engagement during outward migration of the endothelial cells is necessary to stimulate VEGFR2, as well as for activation of VEGFR2 by VEGF. Src activation downstream of active VEGFR2 leads to αVβ3 integrin activation [15], but this requires IGF1R coupled to the integrin via Sdc1. Src may couple the receptor complexes together, causing clustering of the ternary complex with the VE-cadherin and leading to IGF1R transphosphorylation, or Src may phosphorylate and activate IGF1R directly. Active IGF1R initiates an inside-out signaling mechanism, which remains to be defined, causing activation of talin and the αVβ3 integrin (step 3). Completing this co-stimulatory mechanism, active integrin is necessary for VEGF stimulation of VEGFR2 (step 4)[15, 16]. C. SSTN92–119 competitively disrupts the ternary complex and displaces IGF1R from the integrin. This prevents integrin activation [20] and its role in VEGFR2 activation, ostensibly due to regulation of phosphatase localization and activity. D. The addition of VE-cadherin blocking antibodies, which disrupt the AJ, nonetheless prevent transient homotypic VE-cadherin engagement necessary for VEGFR2 activation, blocking activation of the Sdc1-coupled ternary complex and αVβ3 integrin.
(Rapraeger et al.)
Our work also replicates this interdependence between VEGFR2 and αVβ3 integrin and a role for c-Src, but also identifies Sdc1 and IGF1R, e.g., the coupling of αVβ3 integrin to Sdc1 and IGF1R to form a core ternary receptor complex, and its coupling to VE-cadherin as being central to this mechanism. Src is not required to activate the Sdc1 ternary complex during matrix adhesion, nor is VEGFR2. However, activation of the complex by VE-cadherin clustering requires VEGFR2 and c-Src, in addition to IGF1R – three kinases in all – to activate the integrin. Src binds to the VE-cadherin cytoplasmic domain, and active Src is known to have a role linking VEGFR2 to VE-cadherin. This may explain the activation of VEGFR2 by VE-cadherin engagement observed in our study. But Src is also known to be activated downstream of VEGFR2, leading to phosphorylation of AJ components [2]. It is possible that it is c-Src that phosphorylates IGF1R in the Sdc1-coupled ternary complex leading to active integrin (Fig. 5), as Src is known to phosphorylate IGF1R [38]. Thus, Src may act both upstream and downstream of VEGFR2 to link homotypic VE-cadherin engagement to IGF1R and integrin in activated endothelial cells. It seems likely that the actions of Src lead to the assembly of a signaling scaffolding that incorporates the cytoplasmic domains of these receptors, e.g., engaging phosphorylation sites on the integrin, IGF1R, VEGFR2 and VE-cadherin that enhance and sustain the signaling mechanism. The αVβ3 integrin is known to immunoprecipitate with VEGFR2 once stimulated with VEGF [15, 16, 39], perhaps an earmark of such a complex. It is possible that the cytoplasmic domain of the syndecan, although not strictly required for IGF1R-mediated activation of the integrin, may have a role in the assembly of this intracellular scaffolding as well, as it can also be a target for c-Src [40]. This may place additional significance on the displacement of Sdc1 from these receptors by SSTN. One role of this cytoplasmic scaffolding is likely to be regulation of phosphatases that would otherwise inactivate the integrin, IGF1R and VEGFR2, thus preventing further cell migration and re-establishing stable AJs. There are multiple examples of active integrins playing a key role in sequestering phosphatases, including αVβ3 integrin sustaining active IGF1R by sequestering SHP-2 [41–46].
IGF1R has been implicated previously in angiogenesis [21], but the not in the central Sdc1-coupled role linked to VEGFR2 or VE-cadherin that we demonstrate here. Clemmons's group has shown a prominent role for IGF1R in vascular smooth muscle cells and demonstrated a strong link between IGF1R and αVβ3 integrin, showing that ligand occupancy of the αVβ3 integrin diverts the phosphatase SHP-2 from the IGF1R receptor and sustains IGF1R signaling [41, 43]. IGF-1 also stimulates endothelial cell migration and tube formation in collagen gels in vitro [3, 47]. But the SSTN sensitive period defined here outlines a specific and new role for IGF1R during the early onset of angiogenesis in which the receptor functions via its association with Sdc1 to regulate VEGFR2 and VE-cadherin dependent activation of αVβ3 integrin.
SSTN92–119 effectively blocks angiogenesis in several angiogenesis models and dramatically reduces tumor-induced angiogenesis [18, 20, 48]. Somewhat surprisingly, however, our current findings show that Sdc1-coupled IGF1R and the αVβ3 integrin are relegated to an early time point in angiogenesis. SSTN is an effective inhibitor only initially, during which time mural cells grow out from aortic explants but new microvessels are not yet observed. Prior work has shown that mural cell outgrowth is not dependent on the Sdc1-coupled ternary complex, whereas outgrowth of the endothelial cells is blocked by SSTN [18]. This requirement for the coupling of IGF1R to the αVβ3 integrin by Sdc1 during the initial phase of endothelial cell dissemination likely reflects its role in VEGFR2 stimulation that depends on transient VE-cadherin engagement. After this initial time, outgrowth is dependent on β1 integrins, coinciding with the loss of SSTN sensitivity. This suggests that active β1 integrins feed back to irreversibly inactivate the function of the ternary complex. If the action of β1 integrins is prevented, then microvessel outgrowth continues to rely on the Sdc1-coupled mechanism and remains sensitive to SSTN. Although outside the scope of our work, which is focused on the roles of Sdc1, this will likely be important to understanding how the efficacy of angiogenesis inhibitors may depend on the timing of their application during the angiogenesis process. It also raises questions about whether the role of Sdc1 and IGF1R is more extensive in pathological angiogenesis, or is also confined to a defined temporal window.
This work emphasizes the potential of proteoglycans, and syndecans in particular, as pharmacological targets in cancer and other diseases [49]. Sdc1 is expressed in various cancers, is overexpressed in tumor-associated stroma and has been implicated in angiogenesis (reviewed in [49]). Shedding of syndecan extracellular domains is also enhanced in wound healing and during tumor progression, often coupled to the expression of heparanase (reviewed in [50]). Although there is no evidence to date for such an hypothesis, it is possible that shed Sdc1 ectodomain, containing the active site that couples IGF1R to αVβ3 or αVβ5 integrin, serves to hyperactive this mechanism in multiple cell types within the tumor microenvironment. As such, targeting the syndecan directly, with SSTN, immunotherapy or other novel types of drugs, may be a promising approach for cancer therapy.
In summary, we have identified a role for Sdc1-coupled IGF1R in the complex interplay between VE-cadherin, VEGFR2 and the αVβ3 integrin that initiates endothelial cell dissemination during VEGF-stimulated angiogenesis. The coupling of IGF1R to the αVβ3 integrin, which depends on Sdc1 and is prevented by SSTN92–119, is a central regulator downstream of VE-cadherin and VEGFR2 that serves to activate the integrin and sustain VEGFR2 signaling necessary for endothelial cell migration.
MATERIALS AND METHODS
Materials
Integrin antibodies used were P5D2 (mouse β1), M9 (mouse αv), LM609 (human αVβ3) and P1F6 (human αVβ5) from Millipore (Billerica, MA). Goat anti-human VEGFR2 antibody AF357 was purchased from R&D Systems (Minneapolis, MN) and rabbit anti-pY1175 VEGFR2 antibody (mAb 19A10) from Cell Signaling Technology (Danvers, MA). Mouse mAb BV9 (blocking human VE-cadherin) was purchased from GenWay Biotech (San Diego, CA) and rabbit polyclonal BMS158 (blocking human VE-cadherin) from eBioscience, (San Diego, CA). mAb BV13 (blocking mouse VE-cadherin) was from eBioscience, San Diego, CA. All secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
SSTN peptides (80–90% pure) originally described in Beauvais et al. [18] were purchased from GenScript Corporation (Scotch Plains, NJ). Fc/VE-cadherin and Fc/N-cadherin were from R&D Systems. IGF1R inhibitors picropodophyllin and tyrphostin AG538, VEGFR2 Tyrosine Kinase Inhibitor II, and Src inhibitor SU6656 were from EMD Millipore Chemicals (Billerica, MA). The VEGFR2 inhibitor Vandetanib (ZD6474) was from LC Laboratories, (Woburn, MA). CycloRGDfV peptide was from Enzo Life Sciences (Farmingdale, NY). FGF-2 and VEGF (VEGF165) were from PeproTech, Rocky Hill, NJ. Rat tail Type I collagen and human vitronectin (VN) were purified as described previously [18, 20]. Thrombin, fibrinogen and all other reagents and chemicals were from Sigma Aldrich (St. Louis, MO).
Cell culture
All cell lines were cultured at 37°C and 92.5% air/7.5% CO2. SV40-T immortalized human dermal microvascular endothelial cells (HMEC-1) were kindly provided by Drs. Ades and Candal (Center for Disease Control, Atlanta, GA) and Dr. Lawley (Emory University) [51]. HUVECs were obtained from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD). The endothelial cells were cultured in MCDB-131 medium (Mediatech, Manassas, VA) supplemented with 5 mM L-glutamine, 20 mM NaHCO3, 10 ng/mL Epidermal Growth Factor, 1 μg/mL Hydrocortisone, Bovine Brain Extract with heparin and antibiotics (SingleQuot kit from Lonza, Walkersville, MD) and 15% FBS (Atlanta Biologicals, Lawrenceville, GA).
Aortic ring outgrowth assay
All animal experiments were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Mouse aortic ring outgrowth assays were conducted as described in either type I collagen gels (1.6 mg/mL) or fibrin gels (3 mg/mL) in basal MCDB-131 medium containing 2.5% mouse serum. Medium containing FGF-2 (20 ng/mL) or VEGF (50 ng/mL) and blocking antibodies or SSTN peptide was refreshed every 2 d. Total vessel outgrowth was summed for each ring after 5 days and 10 days of outgrowth using Metamorph v6.1 (MDS Analytical Technologies, Downingtown, PA).
Cell Attachment and Spreading Assay
Cell attachment and spreading assays on VN were conducted as described previously [52, 53]. Assays using Fc/VE-cadherin and Fc/N-cadherin (R&D Systems) as ligand were performed in the same manner. Briefly, nitrocellulose-coated wells were incubated with cadherin fusion protein (10 μg/mL in PBS) overnight at 4°C, blocked in PBS containing 1% BSA, and washed in PBS. Endothelial cells were suspended in 5 mM Tris/5 mM EDTA (pH 7.4) in physiological saline, washed in Hepes (50 mM, pH 7.4)-buffered basal MCDB-131 medium containing 0.1% heat-denatured BSA, and plated in the same medium for 2 hr with or without inhibitors, followed by rinsing in PBS and fixation in PLP fixative [24]. Fixed cells were either incubated with 180 μg/mL fluorescent fibrinogen (Invitrogen Molecular Probes) to detect active αVβ3 integrin, or with a 1:50 dilution of rhodamine-phalloidin (Life Technologies, Grand Island, NY) to label cellular F-actin for ease of viewing. Images were acquired using either a Nikon PlanFluor 10× (0.5 NA), PlanApo 20× (0.75 NA) or PlanApo 63× (1.4 NA) objective on a Microphot-FX microscope (Nikon, Inc., Garden City, NY) equipped with an Image-Point cooled CCD camera system or using a PlanApo 20× (0.75 NA) objective and a Roper Scientific Photometrics CoolSnap ES camera on a Nikon Eclipse TE2000U microscopy system.
Scratch wound assay
Scratch wound assays were conducted as described in Beauvais et al [20]. Briefly, confluent monolayers of HUVECs were serum-starved for 6 hr in basal MCBD-131 medium. A scratch wound was introduced and the monolayers were washed in basal medium and photographed (T0). Migration was then stimulated by the addition 20 ng/mL VEGF (+/− inhibitors) for 18 hr.
VEGFR2 and IGF1R Immunoprecipitation
HUVECs plated on either Fc/VE-cadherin, Fc/N-cadherin or serum-coated dishes were serum-starved then treated for 10 min with or without 20 ng/mL VEGF in the presence or absence of SSTN92–119 peptide. In certain cases, cells were pre-treated for 6 hr with VE-cadherin blocking antibody (BMS158) to disrupt cell-cell junctions or with Src inhibitor (SU6656) for 60 min prior to stimulation with VEGF (+/− donkey anti-rabbit 2° Ab). Immunoprecipitated VEGFR2 (goat polyclonal, AF357) or IGF1R (mouse mAb JBW902) was detected on Western blots by probing for active VEGFR2 (rabbit anti-pY1175, mAb 19A10), total VEGFR2 (AF357), active IGF1R (rabbit polyclonal anti-pY1131) or total IGF1R (mouse anti-human IGF1R, clone 33255) followed by an alkaline phosphatase (AP)-conjugated secondary antibody. Visualization of immunoreactive bands was performed using ECF reagent (GE Healthcare Life Sciences, Piscataway, NJ) and scanned on a Typhoon Trio Variable Mode Imager (GE Healthcare Life Sciences).
ACKNOWLEDGMENTS
This work was supported by funds to A.C.R. from the National Cancer Institute (R01-CA118839, R01-CA109010 and R01-CA139872) and American Heart Association (09GRNT2250572). O.R.M. was supported as a member of the University of Wisconsin School of Medicine and Public Health - Spelman College Summer Research Opportunity Program. The authors thank the University of Wisconsin Carbone Cancer Center for the use of its shared services, supported by the National Cancer Institute (P30 CA014520).
ABBREVIATIONS
- AJ
adherens junction
- Fc/N-cadherin
immunoglobulin Fc/N-cadherin extracellular domain fusion protein
- Fc/VE-cadherin
immunoglobulin Fc/VE-cadherin extracellular domain fusion protein
- FGF
fibroblast growth factor
- HMEC-1
Human dermal microvascular endothelial cell-1
- HUVEC
Human umbilical vein endothelial cell
- IGF1R
insulin-like growth factor-1 receptor
- N-cadherin
neural cadherin
- Sdc1
Syndecan-1
- SSTN
synstatin
- VE-cadherin
Vascular endothelial cadherin
- VEGF
vascular endothelial cell growth factor
- VEGFR2
VEGF tyrosine kinase receptor 2
- VN
vitronectin
REFERENCES
- 1.Robinson AS, Vreysen MJ, Hendrichs J, Feldmann U. Enabling technologies to improve area-wide integrated pest management programmes for the control of screwworms. Medical and veterinary entomology. 2009;23(Suppl 1):1–7. doi: 10.1111/j.1365-2915.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 2.Robinson DS. Interleukin-5, eosinophils and bronchial hyperreactivity. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 1993;23:1–3. doi: 10.1111/j.1365-2222.1993.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 3.Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97:1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- 4.Corada M, Zanetta L, Orsenigo F, Breviario F, Lampugnani MG, Bernasconi S, Liao F, Hicklin DJ, Bohlen P, Dejana E. A monoclonal antibody to vascular endothelial-cadherin inhibits tumor angiogenesis without side effects on endothelial permeability. Blood. 2002;100:905–911. doi: 10.1182/blood.v100.3.905. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SD, Hodivala-Dilke KM. The role of beta3-integrins in tumor angiogenesis: context is everything. Current opinion in cell biology. 2011;23:630–637. doi: 10.1016/j.ceb.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Serini G, Napione L, Arese M, Bussolino F. Besides adhesion: new perspectives of integrin functions in angiogenesis. Cardiovasc Res. 2008;78:213–222. doi: 10.1093/cvr/cvn045. [DOI] [PubMed] [Google Scholar]
- 7.Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 8.Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J. Biol. Chem. 2000;275:21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 9.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 10.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 12.Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, Cheresh DA. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin. Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 13.Wayner EA, Orlando RA, Cheresh DA. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 1991;113:919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrinvascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570–580. doi: 10.1161/CIRCRESAHA.107.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alphavbeta3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Exp. Cell Res. 2003;286:219–232. doi: 10.1016/s0014-4827(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 20.Beauvais DM, Rapraeger AC. Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. Journal of cell science. 2010;123:3796–3807. doi: 10.1242/jcs.067645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:435–444. doi: 10.1161/01.ATV.0000105902.89459.09. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TJ, Stranc MF. The anatomy of the medial canthal ligament. British journal of plastic surgery. 1970;23:1–7. doi: 10.1016/s0007-1226(70)80002-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am. J. Path. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. The Journal of cell biology. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 28.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. Journal of cell science. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 29.Liao F, Li Y, O'Connor W, Zanetta L, Bassi R, Santiago A, Overholser J, Hooper A, Mignatti P, Dejana E, Hicklin DJ, Bohlen P. Monoclonal antibody to vascular endothelial-cadherin is a potent inhibitor of angiogenesis, tumor growth, and metastasis. Cancer Res. 2000;60:6805–6810. [PubMed] [Google Scholar]
- 30.Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. The Journal of biological chemistry. 2006;281:19631–19644. doi: 10.1074/jbc.M600301200. [DOI] [PubMed] [Google Scholar]
- 31.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4:e4449. doi: 10.1371/journal.pone.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buerkle MA, Pahernik SA, Sutter A, Jonczyk A, Messmer K, Dellian M. Inhibition of the alpha-V integrins with a cyclic RGD peptide impairs angiogenesis, growth and metastasis of solid tumours in vivo. Br J Cancer. 2002;86:788–795. doi: 10.1038/sj.bjc.6600141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Max R, Gerritsen RR, Nooijen PT, Goodman SL, Sutter A, Keilholz U, Ruiter DJ, De Waal RM. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Sepp NT, Li LJ, Lee KH, Brown EJ, Caughman SW, Lawley TJ, Swerlick RA. Basic fibroblast growth factor increases expression of the alpha v beta 3 integrin complex on human microvascular endothelial cells. J Invest Dermatol. 1994;103:295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- 35.Stupack DG, Cheresh DA. Apoptotic cues from the extracellular matrix: regulators of angiogenesis. Oncogene. 2003;22:9022–9029. doi: 10.1038/sj.onc.1207110. [DOI] [PubMed] [Google Scholar]
- 36.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 2001;155:459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JE, Kulik G, Jelinek T, Reuter CW, Shannon JA, Weber MJ. Src phosphorylates the insulin-like growth factor type I receptor on the autophosphorylation sites. Requirement for transformation by src. The Journal of biological chemistry. 1996;271:31562–31571. doi: 10.1074/jbc.271.49.31562. [DOI] [PubMed] [Google Scholar]
- 39.Borges E, Jan Y, Ruoslahti E. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- 40.Ott VL, Rapraeger AC. Tyrosine phosphorylation of syndecan-1 and -4 cytoplasmic domains in adherent B82 fibroblasts. The Journal of biological chemistry. 1998;273:35291–35298. doi: 10.1074/jbc.273.52.35291. [DOI] [PubMed] [Google Scholar]
- 41.Clemmons DR, Maile LA. Interaction between insulin-like growth factor-I receptor and alphaVbeta3 integrin linked signaling pathways: cellular responses to changes in multiple signaling inputs. Mol Endocrinol. 2005;19:1–11. doi: 10.1210/me.2004-0376. [DOI] [PubMed] [Google Scholar]
- 42.Ling Y, Maile LA, Badley-Clarke J, Clemmons DR. DOK1 mediates SHP-2 binding to the alphaVbeta3 integrin and thereby regulates insulin-like growth factor I signaling in cultured vascular smooth muscle cells. The Journal of biological chemistry. 2005;280:3151–3158. doi: 10.1074/jbc.M411035200. [DOI] [PubMed] [Google Scholar]
- 43.Maile LA, Clemmons DR. Regulation of insulin-like growth factor I receptor dephosphorylation by SHPS-1 and the tyrosine phosphatase SHP-2. The Journal of biological chemistry. 2002;277:8955–8960. doi: 10.1074/jbc.M109258200. [DOI] [PubMed] [Google Scholar]
- 44.Mitola S, Brenchio B, Piccinini M, Tertoolen L, Zammataro L, Breier G, Rinaudo MT, den Hertog J, Arese M, Bussolino F. Type I collagen limits VEGFR-2 signaling by a SHP2 protein-tyrosine phosphatase-dependent mechanism 1. Circ Res. 2006;98:45–54. doi: 10.1161/01.RES.0000199355.32422.7b. [DOI] [PubMed] [Google Scholar]
- 45.Mattila E, Auvinen K, Salmi M, Ivaska J. The protein tyrosine phosphatase TCPTP controls VEGFR2 signalling. Journal of cell science. 2008;121:3570–3580. doi: 10.1242/jcs.031898. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells. Circ Res. 2008;102:1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigematsu S, Yamauchi K, Nakajima K, Iijima S, Aizawa T, Hashizume K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocrine journal. 1999;46(Suppl):S59–62. doi: 10.1507/endocrj.46.suppl_s59. [DOI] [PubMed] [Google Scholar]
- 48.Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 50.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876–3889. doi: 10.1111/j.1742-4658.2010.07798.x. [DOI] [PubMed] [Google Scholar]
- 51.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 52.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates alpha(v)beta(3) integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC. Syndecan-1 regulates alpha(v)beta(5) integrin activity in B82L fibroblasts. J. Cell Sci. 2006;119:2445–2456. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- 54.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–1806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 55.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–511. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]