Abstract
Background
Reduced cognitive insight has been associated with psychotic symptoms, in particular with the presence of delusions; however, there is little information about whether such reductions are present in at-risk individuals prior to the onset of threshold psychotic symptoms.
Method
We conducted a cross-sectional comparison of cognitive insight (as indexed by the Beck Cognitive Insight Scale; BCIS) in 62 help-seeking individuals at clinical high risk for psychosis (CHR), 59 individuals with schizophrenia-spectrum disorders (SCZ), and 37 healthy controls (HC). In patients, we evaluated associations of insight with positive symptoms, including later transition to psychosis in high-risk patients.
Results
Individuals with schizophrenia reported significantly higher self-certainty scores than the at-risk patients and healthy controls, with the at-risk patients scoring intermediate to the individuals with schizophrenia and controls. Similarly, Individuals with schizophrenia scored significantly higher on self-reflectiveness, with no differences between the at-risk patients and controls. In individuals with schizophrenia, delusions were significantly correlated with self-certainty. In at-risk patients, cognitive insight was not associated with positive symptom severity and did not differentiate those at-risk patients who later developed psychosis from those who did not. However, post-hoc analyses suggested that at-risk patients with marked unusual thought content (approaching threshold psychosis) had lower self-reflectiveness, whereas those with high suspiciousness had significantly higher self-certainty.
Conclusions
The findings are discussed in the context of normal developmental processes occurring during adolescence, their putative links to neurobiological functioning, and their implications for treatment and future research.
Keywords: Psychosis, High Risk, Psychosis Prodrome, Schizophrenia, Insight, Metacognition, Cognitive Insight, Self-Reflectiveness, Self-Certainty, Hallucinations, Delusions, Paranoia
Introduction
The ability to reflect on one’s own thoughts and beliefs, and modify them based on new information, is a core ingredient of healthy functioning and is at the heart of therapeutic approaches such as cognitive behavioral therapy1. Beck and colleagues posited that individuals with psychosis have difficulties examining and correcting their thoughts and beliefs. They hypothesized that these difficulties, which they labeled poor cognitive insight, may contribute to the development and maintenance of delusional beliefs1,2. A growing literature points to impaired cognitive insight, as indexed by the Beck Cognitive Insight Scale (BCIS), as a correlate of psychosis. The BCIS is comprised of two subscales: Self-Reflectiveness, which assesses introspection, and Self-Certainty, which appraises overconfidence in decisions and judgments2. Cognitive insight is defined as the difference between self-reflectiveness and self-certainty scores.
In schizophrenia, delusions have been associated with poor cognitive insight, in particular high self-certainty. Engh and colleagues3 found that severe delusions were associated with high self-certainty, independent of hallucinations. In contrast, hallucinations in the absence of delusions were associated with high self-reflectiveness and low self-certainty. Consistent with this, Warman and colleagues4 found higher self-certainty among schizophrenia patients with at least moderate delusions as compared to patients without delusions and healthy controls. Altogether, these studies suggest that high self-certainty is associated with delusions, rather than schizophrenia per se. In contrast, increased self-reflectiveness occurs with effective treatment of psychotic symptoms in schizophrenia 5, 6 (although specific symptom types are not known).
While reduced cognitive insight is associated with delusions, its role in delusion development is not known. Delusion-proneness as assessed by self-report in college students is associated with high levels of both self-certainty and self-reflectiveness7, which has been interpreted as self-certainty being key to proneness to delusions, whereas self-reflectiveness is protective against delusion formation8. In support of this model, first-episode psychosis patients with severe delusions have been found to have lower self-reflectiveness than patients with no or minimal delusions, but equivalent self-certainty.9
While a growing clinical literature suggests significantly reduced cognitive insight in schizophrenia related to delusions, important gaps in the literature remain unaddressed. First, it is not known whether reductions in insight exist in individuals at clinical high risk (CHR) for psychosis. Second, the relationship of cognitive insight to the sub-threshold psychotic symptoms that characterize CHR cohorts is not known. Third, it is not known if poor cognitive insight is a predictor of later transition to psychosis.
Given the evidence for reduction in cognitive insight in schizophrenia, and its association with delusions, and the lack of studies exploring development of delusions in relation to cognitive insight, our aims were: 1) to confirm previous findings linking self-certainty and delusions in individuals with schizophrenia; 2) to investigate whether cognitive insight is reduced in CHR patients; 3) to assess whether reduction in cognitive insight, if present, is related to sub-threshold positive symptoms, in particular unusual thought content or attenuated delusional ideation; and 4) to investigate whether cognitive insight reduction predicts later development of psychotic disorder in CHR patients.
Method
Participants
We conducted a cross-sectional comparison of cognitive insight among help-seeking individuals at CHR for psychosis, individuals with schizophrenia, and comparably aged healthy controls. Data were collected for 62 help-seeking individuals at CHR for psychosis who were enrolled at the Center of Prevention and Evaluation (COPE), a psychosis clinical-high-risk research program located at the New York State Psychiatric Institute at Columbia University. Data for additional 22 COPE participants were excluded or not available due to incompletion of the baseline assessment due to scheduling difficulties (n=15), study discontinuation/withdrawal (n=4), and information indicating transition to psychosis prior to baseline (n=3). No CHR patients refused participation. Among the sixty-two CHR individuals who completed the baseline assessments, forty-three were medication-free (69%), seven (11%) were taking anti-psychotic medications (three on risperidone, two on quetiapine, and one on aripiprazole), twelve (19%) were taking antidepressants (four on sertraline, four on bupropion, two on fluoxetine, two on venlafaxine), and nine (14%) were taking other psychotropic medications. Thirty-seven of the sixty-two CHR individuals (60%) received psychotherapy, primarily supportive psychotherapy.
Data for fifty-nine individuals with schizophrenia-spectrum disorders were obtained from two longitudinal studies focusing on autonomic regulation and recovery from psychosis conducted at the NYSPI. Data for an additional eleven individuals with schizophrenia were excluded or not available due to study discontinuation/withdrawal (n=9) and clinical exacerbation (n=2). Fifty individuals had a DSM-IV diagnosis of schizophrenia (85%), eight had schizoaffective disorder (14%), and one had a diagnosis of schizophreniform disorder (1%). Medication information was available for 51 of the 59 participants with schizophrenia. Average antipsychotic medication prescribed, as indexed by chlorpromazine equivalence, was 235.86 (SD=259.68). Information about provision of psychotherapy among the participants with schizophrenia was not available.
Data were obtained for thirty-seven healthy controls recruited as part of the three aforementioned studies. All participants provided written consent and the studies were approved by the Institutional Review Board of the New York State Psychiatric Institute.
Measures
Clinical high risk status and assessment of sub-threshold positive symptoms were established using the Structured Interview for Prodromal Symptoms and the Scale of Prodromal Symptoms (SIPS/SOPS10), a semi-structured diagnostic interview that identifies individuals at CHR for psychosis based on: 1) presence of attenuated psychotic symptoms; 2) brief intermittent psychotic symptoms; and/or 3) genetic risk (1st degree family member) with a recent decline in functioning (30% decline in GAF during the previous twelve months). The SIPS/SOPS was administered by trained raters and scored by consensus with an expert psychiatrist involved in the development of the instrument (CC), who has established excellent interrater reliability (kappa=1.00 for prodromal category and .7–.9 ICC’s for dimensional ratings of positive and other symptoms) with other prodromal research programs (PRIME program, Yale, CT; RAP program, Zucker Hillside Hospital, NY). Previous studies utilizing SIPS/SOPS with individuals at CHR for psychosis have reported a 30% likelihood of developing psychosis within 30 months11. Participants at COPE are evaluated prospectively using the SIPS/SOPS every three months for up to four years to evaluate prodromal symptom severity and potential transition to psychotic disorder.
All participants completed a diagnostic evaluation using the Diagnostic Interview for Genetic Studies (DIGS)12, a semi-structured diagnostic interview and medical records review used to collect diagnostic and course of illness information for mood, psychotic, and substance use DSM-IV Axis I disorders. The DIGS interview was administered by clinical research interviewers holding a Master’s degree or higher. A team of clinical psychologists and psychiatrists convened and discussed information obtained from the DIGS and came to a consensus diagnosis for each participant. Schizophrenia-related symptoms were assessed in the schizophrenia group using the Scales for Assessment of Positive Symptoms (SAPS)13.
Cognitive insight was measured using the Beck Cognitive Insight Scale (BCIS)2, a self-report questionnaire. Typically, the BCIS was administered within a week of the SIPS/SOPS or SAPS symptom ratings. The BCIS is comprised of two subscales: Self-Reflectiveness and Self-Certainty. Cognitive insight is represented by the BCIS Index score, which is obtained by subtracting self-certainty from self-reflectiveness. The two-factor structure of the BCIS has been confirmed in seven studies14. Among the eight studies that investigated the internal consistency (IC) of the BCIS, six reported Cronbach’s alpha of 0.7 for the self-reflectiveness and five for self-certainty. Two studies each reported IC’s between 0.6 and 0.7, respectively14.
Procedures
The structured diagnostic and clinical interviews, and the cognitive insight measure, were administered to the schizophrenia cohort as part of baseline assessments, typically within one week of admission to the study. The high-risk participants completed the symptoms and cognitive insight measures as part of their COPE baseline assessments, typically within one week of admission to the program. Data on healthy controls were obtained from their participation in the baseline assessments of the aforementioned studies.
Statistical Analyses
Participants were compared in terms of demographics and IQ. Within the schizophrenia and high-risk cohorts, potential associations of cognitive insight measures with symptoms were examined. We set alpha at 0.05 for the hypothesized association of self-certainty with delusions in schizophrenia patients. The Holm’s Sequential Bonferroni procedure was used to reduce Type I error for other correlational analyses. Differences among the groups were assessed using Analysis of Covariance (ANCOVA), with clinical status (healthy, high-risk, schizophrenia) entered as the independent variables, the cognitive insight variables (self-reflectiveness, self-certainty, or BCIS Index) as the dependent variables, and potential demographic variables as covariates. Follow-up pairwise comparisons were conducted with the Holm’s Sequential Bonferroni procedure used to reduce risk for Type I error. This study has sufficient power to detect medium to large effect sizes (Cohen’s d = 0.6)
Results
The sample’s demographic and clinical data are presented in Table 1. To determine if any of the demographic variables were related to the self-reflectiveness, self-certainty, or the BCIS Index, the effects of age, education, race, ethnicity, and sex were examined. Age was associated with self-reflectiveness (r=.20, p=.02) and BCIS Index scores (r=.18; p=.03), and was therefore entered as a covariate in the ANCOVA analyses for all three cognitive insight variables. No associations were found for other demographic variables with any of the BCIS variables; likewise, among schizophrenia and CHR patients, no association of antipsychotic medication exposure (as indexed by chlorpromazine equivalents) was found for any of the BCIS variables.
Table 1.
Demographic and Clinical Information
| Group | Mean | SD | Statistic | p | |
|---|---|---|---|---|---|
|
| |||||
| Age | Healthy Controls | 22.3 | 4.6 | F(2,156)=46.77 | <.001 |
| High Risk | 19.7 | 3.6 | |||
| Schizophrenia | 28.8 | 7.5 | |||
|
| |||||
| Sex (Male / Female) | Healthy Controls | 19/18 | -- | χ2(2,156)=8.97 | =.01 |
| High Risk | 48/14 | ||||
| Schizophrenia | 33/26 | ||||
|
| |||||
| IQ Score (Full Scale) | Healthy Controls | 111.8 | 14.0 | F(2,156)=1.69 | NS |
| High Risk | 107.1 | 17.3 | |||
| Schizophrenia | 103.4 | 13.8 | |||
|
| |||||
| Racial Group: | Healthy Controls | High Risk | Schizophrenia | ||
| Asian | 4 (11%) | 5 ( 8%) | 7 (12%) | ||
| Black/African-American | 7 (19%) | 13 (21%) | 9 (15%) | ||
| Caucasian | 24 (65%) | 29 (47%) | 39 (66%) | ||
| Multiracial | 2 ( 5%) | 15 (24%) | 4 ( 7%) | ||
| Ethnicity: | |||||
| Hispanic | 9 (24%) | 22 (36%) | 13 (22%) | ||
| Non-Hispanic | 28 (76%) | 40 (64%) | 46 (78%) | ||
|
| |||||
| SAPS Scores (Average Global): | Healthy Controls | High Risk | Schizophrenia * | ||
| Hallucinations | -- | -- | 2.71 (2.23) | ||
| Delusions | -- | -- | 2.88 (1.54) | ||
| Bizarre Behavior | -- | -- | .82 (1.26) | ||
| Positive Formal Thought Dis. | -- | -- | 1.39 (1.47) | ||
| SIPS Scores (Total): | |||||
| Positive | -- | 12.61 (4.45) | -- | ||
| Negative | -- | 13.05 (6.27) | -- | ||
| Disorganization | -- | 7.33 (3.50) | -- | ||
| General | -- | 9.31 (4.25) | -- | ||
N = 158 (Schizophrenia=62, High Risk for Psychosis=59, Healthy Controls=37); IQ Score – based on the Wechsler Adult Intelligence Scale (WAIS) or Wechsler Intelligence Scale for Children (WISC); SAPS - Scale for Assessment of Positive Symptoms (range 0–5; 5=most severe); SIPS - Structured Interview for Prodromal Symptoms;
n=51.
Our first aim was to confirm previous findings linking self-certainty and delusions in individuals with schizophrenia. Concurrent symptom data (within one week) were available for 51 of the 59 participants with schizophrenia. Consistent with previous reports, self-certainty was significantly correlated with severity of delusions (r=.31, p=.03). Post-hoc analyses indicated that delusions were inversely associated with self-reflectiveness (r=−.29, p=.04), as well as the BCIS index (r=−.47, p<.001).
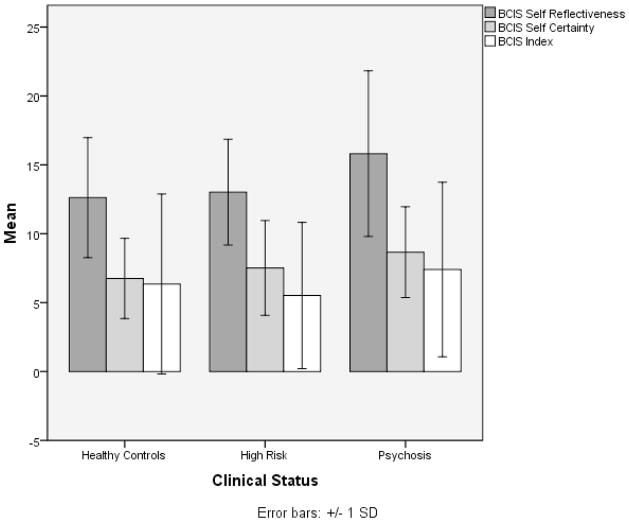
Next, we conducted three one-way analyses of covariance (ANCOVA), with clinical status entered as the independent variable, the cognitive insight variables as the dependent variables, and age as a covariate. The results of these tests are presented in Table 2. The ANCOVA was significant for self-certainty (F(2,154)=5.71, p<.004), indicating significant differences among the three groups’ ratings, with clinical status accounting for 7% of the variance, controlling for age. The schizophrenia group had the highest mean self-certainty ratings (M=8.66, SD=3.30), followed by the high-risk group (M=7.52, SD=3.44), and then healthy controls (M=6.76, SD=2.91). Follow-up pairwise comparisons were conducted with the Holm’s Sequential Bonferroni procedure used to control for Type I error. There were significant differences between the schizophrenia patients and both the healthy control (p=.001, d=1.43) and CHR (p=.010, d=.98) groups, with no differences between healthy control and high-risk groups (p=.26; d =.59; See Figure 1).
Table 2.
Mean, Standard Deviation, and One-Way Analyses of Covariance (ANCOVA) for the Effects of Clinical Status on Cognitive Insight Variables Controlling for Age
| Variable | Group | Mean | SD | F | p | d | Pairwise Comparisons |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Self-Reflectiveness | Healthy Controls | 12.62 | 4.36 | 4.89 | .009 | .59 | SCZ > HC SCZ > CHR |
| High Risk | 13.02 | 3.84 | |||||
| Schizophrenia | 15.81 | 6.23 | |||||
|
| |||||||
| Self-Certainty | Healthy Controls | 6.76 | 2.91 | 5.71 | .004 | .62 | SCZ > HC SCZ > CHR |
| High Risk | 7.52 | 3.44 | |||||
| Schizophrenia | 8.66 | 3.30 | |||||
|
| |||||||
| BCIS Index | Healthy Controls | 6.33 | 6.63 | .29 | NS | NS | |
| High Risk | 5.20 | 5.20 | |||||
| Schizophrenia | 7.45 | 6.56 | |||||
N = 158 (Schizophrenia=62, High Risk for Psychosis=59, Healthy Controls=37); BCIS – Beck Cognitive Insight Scale; SCZ schizophrenia; CHR – clinical high risk for psychosis; HC – healthy controls.
Figure 1. A Comparison of Mean Cognitive Insight Variable Scores Across Clinical Status.
There were significant differences between the schizophrenia patients and both the healthy control (p=.001, d=1.43) and CHR (p=.010, d=.98) groups, with no differences between healthy control and high-risk groups (p=.26; d =.59; See Figure 1).
Similarly, there were significant differences among the three groups’ self-reflectiveness ratings (F(2,154)=4.89, p<.009), with clinical status accounting for 6% of the variance, controlling for age. The schizophrenia group had the highest mean self-reflectiveness (M=15.81, SD=6.01), with smaller means for the CHR (M=13.02, SD=3.84), and the healthy controls (M=12.62, SD=4.36). Follow-up pairwise comparisons conducted with the Holm’s Sequential Bonferroni procedure indicated significant differences in the means between the schizophrenia group and both the healthy control (p=.004, d=1.15) and CHR (p=.009, d=1.06) groups, but no difference was found between the healthy control and high-risk groups (p=.64, .d=.17). There were no significant differences found among the three groups for the BCIS index ratings.
Our third aim was to assess whether cognitive insight was related to attenuated or sub-threshold delusional ideation in CHR participants. Delusional-type ideation, as indexed by SIPS symptoms of unusual thought content, was not found to be associated with self-certainty (r=.10, p=.44), self-reflectiveness (r=.01, p=.97), or the BCIS index (r=−.07, p=.60) in CHR patients. Likewise, suspiciousness was not found to be associated with self-certainty (r=.12, p=.34), self-reflectiveness (r=.07, p=.59), or the BCIS index (r=−.03, p=.83) in this group.
Finally, we investigated whether cognitive insight is associated with transition to psychosis among CHR patients. Thirteen (21%) of the 62 CHR patients eventually developed psychosis (average follow-up 20.03 months; SD=15.32 months). No group differences were detected in self-certainty between those who later developed threshold psychosis and those who did not (t=.30, p=.77, df=60; d=.08). Similarly, no group differences were detected in the self-reflectiveness (t=.10, p=.92, df=60; d=.03) or the BCIS index scores (t=.27, p=.78, df=60; d=.07). As most CHR patients do not develop psychosis11, we conducted a post-hoc analysis to evaluate any differences in cognitive insight between CHR individuals with near-threshold delusional ideation and those with moderate/low/no delusional ideation. Our rationale was that “severe but not psychotic” attenuated delusions (defined by the SIPS/SOPS as characterized by a relative lack of skepticism, with doubt elicited primarily by external contrary evidence, and by impact on functioning), would be more similar in phenomenology to threshold delusions of “fixed, false ideas” in the absence of intact reality testing10.
For each symptom, we therefore divided CHR participants by severe ideation (“severe but not psychotic”; SIPS score of 5 on a 1–6 scale, where 6=psychosis) vs. moderate/mild/no ideation (score ≤4) based on scores for the SIPS unusual thought content and suspiciousness items. CHR patients with near-threshold persecutory ideation (n=5) had significantly higher self-certainty scores compared to those with moderate/low/no (n=57) suspiciousness (t=2.15, p=.03, df=60; d=0.6; M=10.60, SD=3.58 vs. M=7.25, SD=3.32); by contrast, there were no significant differences found by suspiciousness severity among CHR patients in self-reflectiveness and BCIS index scores. CHR individuals with severe (but not psychotic) unusual thought content (n=10) had significantly lower self-reflectiveness scores compared to those with moderate/low/no (n=52) unusual thought content (t=2.81, p=.009; df=60; d=0.7; M=11.00, SD=2.05 vs. M=13.40, SD=3.99); by contrast there were no significant differences found for self-certainty and BCIS index ratings. Rate of transition to psychosis was significantly greater in CHR patients with severe vs. moderate/low “unusual thought content” (60% vs. 13.5%, ψ2=10.962, p=.004), suggesting that in this group in particular, self-reflectiveness may be relevant for psychosis risk and a particular treatment target. On the other hand, self-reflectiveness was not a predictor of transition to psychosis in the risk cohort as a whole.
Discussion
This is the first known study to investigate cognitive insight in individuals at CHR for psychosis, together with individuals with schizophrenia and healthy controls. Our results replicate findings from previous reports, indicating an association between higher self-certainty scores and more severe delusions among individuals with schizophrenia3, 4. Similarly, the inverse association between self-reflectiveness scores and delusion severity is consistent with findings from a study of patients experiencing first episode psychosis9, as well as treatment studies, in which changes in self-reflectiveness scores were associated with improvement in psychosis5, 6.
Our findings of significantly higher self-reflectiveness among the schizophrenia group compared to the CHR and healthy control groups are somewhat counterintuitive and are more difficult to interpret. While Warman and colleagues reported similar self-reflectiveness ratings among patients with delusions and healthy controls4, among first-episode schizophrenia patients, higher self-reflectiveness was found for patients without delusions vs. those with delusions15, supporting Beck and Warman’s (2004) original hypothesis. One potential explanation for the discrepant findings may be related to the age differences between the investigated samples. In studies that did not find differences in self-reflectiveness between clinical cohorts with psychosis and healthy controls, the mean ages of the healthy control groups were 21 years (SD = 5.5)4 and 21 years (SD = 6.5)7. Similarly, in our study the mean age of the healthy and high-risk groups were 22 years (SD = 4.6, range 13–39, 16% were age 18 or younger) and 19 years (SD = 3.6, range 13–26, 41% were age 18 or younger), respectively. In contrast, in studies that reported higher self-reflectiveness in healthy individuals vs. psychosis related samples, the mean ages of the healthy participants were higher - 23 years15, 32 years3, and 38 years2. The ability for self-reflection improves as part of normal brain maturation, reflected in maturation of the anterior medial prefrontal and posterior cingulate brain regions16–18. As these regions are not fully functionally mature until late adolescence19, the lack of self-reflectiveness differences in the studies that utilized large groups of adolescents or very young adults may be due to developmental maturation, rather than psychopathological reductions in self-reflectiveness per se. Another potential explanation may be rooted in symptom profiles of patients in the various studies - Engh et al.3 reported that patients with hallucinations, but no delusions, had significantly higher self-reflectiveness scores compared to patients with delusions. Specificity to type of symptom may also explain the lack of differences among schizophrenia and bipolar patients, and healthy controls, reported by Engh and colleagues20. A third possibility is that the schizophrenia patients are being treated, at least in terms of antipsychotics, whereas such medication treatment is far less common in our CHR cohort.
The most important finding of the present study is that individuals at CHR for psychosis displayed self-certainty, self-reflectiveness and BCIS Index ratings comparable to healthy individuals, consistent with their relatively intact ability to evaluate and modify their beliefs. The presence of intact insight suggests that individuals at CHR for psychosis may potentially benefit from Cognitive-Behavioral Therapy (CBT) or other psychological therapies that actively target exploration and modification of beliefs 21,22,23 in CHR patients.
Our findings also indicate that in CHR patients, self-certainty, self-reflectiveness, and BCIS Index scores were not associated with severity of delusional-type ideation, as indexed by suspiciousness and unusual thought content. These findings stand in contrast to reported associations in schizophrenia3,4,6,24, including in our sample, as well as in individuals with a first episode psychosis9. Likewise, there were no significant differences in cognitive insight between high-risk individuals who later developed psychotic disorder and those who did not, although our study would only have the power to detect large differences in transition rates. These data together suggest that poor insight does not precede or predict psychosis.
However, comparisons of at-risk individuals with near-threshold delusional-type symptoms with those with less severe ideation indicate differences in cognitive insight. Specifically, individuals with “severe but not psychotic” unusual thought content displayed significantly lower self-reflectiveness than those with mild symptoms. In contrast, individuals with severe suspiciousness had higher self-certainty, indicating a relatively intact ability to examine and reflect on internal experiences, but limited ability to reappraise and modify them. However, given the exploratory nature of these findings and the null findings from the correlational, between-group, and “predictor of transition” analyses, these findings should be considered very preliminary until replicated in a larger study.
The main limitation of the study is its size, such that only medium to large effect sizes could be detected. Future studies should entail longitudinal evaluation of both symptoms and insight in CHR patients, to determine how these co-evolve, and evaluate potential biomarkers of insight (i.e. hippocampal volume for self-certainty25.) A putative biomarker of self-reflectiveness for longitudinal CHR cohort and treatment studies is activation of the anterior cingulate in behavioral paradigms that evaluate focus on self versus others26–30. Another limitation is the lack of detailed information about provision of psychotherapy among the CHR and schizophrenia group. As previous reports indicated that CBT for psychosis might influence BCIS variables among individuals with schizophrenia, the lack of differences among the groups may have been influenced by such treatment. However, given the relatively poor dissemination CBT for psychosis in the U.S. (Kimhy et al., in press) 31, it is unlikely that many of our subjects with schizophrenia received this treatment.
Finally, our samples included large and unavoidable differences between groups with regard to use of antipsychotic medications and age, confounds which cannot be easily controlled using an ANCOVA. Likewise, the examination of associations between chlorpromazine equivalents and BCIS scores may be influenced by numerous confounds such as symptom severity preceding prescription in the CHR group, and floor/ceiling effects in the schizophrenia group.
Acknowledgments
This work was supported by grants 1K23MH077653 (DK), 1K23MH66279 (CC) and 1K24MH01699 (DM) from the National Institute of Mental Health, Bethesda, MD. We would like to thank Julia Vakhrusheva, Samira Khan and Rachel Chang for their help in the preparation of this manuscript.
References
- 1.Beck AT, Warman DM. Cognitive Insight: Theory and Assessment. In: Amador XF, David AS, editors. Insight and Psychosis. 2. London: Oxford University Press; 2004. pp. 79–87. [Google Scholar]
- 2.Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophrenia research. 2004;68:319–29. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 3.Engh JA, Friis S, Birkenaes AB, et al. Delusions are associated with poor cognitive insight in schizophrenia. Schizophrenia bulletin. 2010;36:830–5. doi: 10.1093/schbul/sbn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophrenia research. 2007;90:325–33. doi: 10.1016/j.schres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Granholm E, McQuaid JR, McClure FS, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. The American journal of psychiatry. 2005;162:520–9. doi: 10.1176/appi.ajp.162.3.520. [DOI] [PubMed] [Google Scholar]
- 6.Bora E, Erkan A, Kayahan B, Veznedaroglu B. Cognitive insight and acute psychosis in schizophrenia. Psychiatry Clin Neurosci. 2007;61:634–9. doi: 10.1111/j.1440-1819.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 7.Warman DM, Martin JM. Cognitive insight and delusion proneness: An investigation using the Beck Cognitive Insight Scale. Schizophrenia research. 2006;84:297–304. doi: 10.1016/j.schres.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophrenia bulletin. 2004;30:1005–22. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 9.Buchy L, Malla A, Joober R, Lepage M. Delusions are associated with low self-reflectiveness in first-episode psychosis. Schizophrenia research. 2009;112:187–91. doi: 10.1016/j.schres.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia bulletin. 2003;29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 11.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 14.Riggs SE, Grant PM, Perivoliotis D, Beck AT. Assessment of Cognitive Insight: A Qualitative Review. Schizophrenia bulletin. 2010 doi: 10.1093/schbul/sbq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchy L, Torres IJ, Liddle PF, Woodward TS. Symptomatic determinants of insight in schizophrenia spectrum disorders. Compr Psychiatry. 2009;50:578–83. doi: 10.1016/j.comppsych.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MK, Reeder JA, Raye CL, Mitchell KJ. Second thoughts versus second looks: an age-related deficit in reflectively refreshing just-activated information. Psychol Sci. 2002;13:64–7. doi: 10.1111/1467-9280.00411. [DOI] [PubMed] [Google Scholar]
- 19.Pfeifer JH, Masten CL, Borofsky LA, Dapretto M, Fuligni AJ, Lieberman MD. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–38. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engh JA, Friis S, Birkenaes AB, et al. Measuring cognitive insight in schizophrenia and bipolar disorder: a comparative study. BMC Psychiatry. 2007;7:71. doi: 10.1186/1471-244X-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison AP, French P, Parker S, et al. Three-year follow-up of a randomized controlled trial of cognitive therapy for the prevention of psychosis in people at ultrahigh risk. Schizophrenia bulletin. 2007;33:682–7. doi: 10.1093/schbul/sbl042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addington J, Epstein I, Liu L, French P, Boydell KM, Zipursky RB. A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia research. 2011;125:54–61. doi: 10.1016/j.schres.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Kimhy D, Corcoran C. Use of Palm computer as an adjunct to cognitive-behavioural therapy with an ultra-high-risk patient: a case report. Early intervention in psychiatry. 2008;2:234–41. doi: 10.1111/j.1751-7893.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granholm E, McQuaid JR, McClure FS, et al. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. The Journal of clinical psychiatry. 2007;68:730–7. doi: 10.4088/jcp.v68n0510. [DOI] [PubMed] [Google Scholar]
- 25.Buchy L, Czechowska Y, Chochol C, et al. Toward a model of cognitive insight in first-episode psychosis: verbal memory and hippocampal structure. Schizophrenia bulletin. 2010;36:1040–9. doi: 10.1093/schbul/sbp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimhy D, Goetz R, Yale S, Corcoran C, Malaspina D. Delusions in individuals with schizophrenia: factor structure, clinical correlates, and putative neurobiology. Psychopathology. 2005;38:338–44. doi: 10.1159/000089455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 28.Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Brain Res Rev. 2003;43:224–30. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Sabri O, Erkwoh R, Schreckenberger M, Owega A, Sass H, Buell U. Correlation of positive symptoms exclusively to hyperperfusion or hypoperfusion of cerebral cortex in never-treated schizophrenics. Lancet. 1997;349:1735–9. doi: 10.1016/S0140-6736(96)08380-8. [DOI] [PubMed] [Google Scholar]
- 30.Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychological medicine. 2004;34:591–6. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- 31.Kimhy D, Tarrier N, Essock S, Malaspina D, Cabaniss D, Beck AT. Cognitive Behavioral Therapy for Psychosis: Dissemination and Training Practices in the United States. Psychosis: Psychological, Social and Integrative Approaches. doi: 10.1080/17522439.2012.704932. (in press) [DOI] [Google Scholar]