Abstract
Objective
Synovitis is thought to be a secondary phenomenon in the osteoarthritis (OA) process and the menisci might be triggers of localized synovitis. The aim was to assess the cross-sectional associations of posterior horn meniscal damage with perimeniscal synovitis, and with synovitis posterior to the posterior cruciate ligament (PCL) using contrast enhanced (CE) MRI.
Design
The Multicenter Osteoarthritis (MOST) Study is a longitudinal observational study of subjects with or at risk for knee OA. Subjects are a subset of MOST who were examined with 1.5 T CE MRI and had semiquantitative synovitis (scored from 0–2 at 11 locations) and meniscal readings (scored with WORMS from 0–4 ) available. Logistic regression was used to assess the association of posterior meniscal damage and perimeniscal synovitis in the same compartment, and between posterior meniscal damage and synovitis posterior to the PCL.
Results
Three hundred and seventy seven knees were included (mean age 61.1 years ± 6.9, mean BMI 29.6 ± 4.9, 44.3% women). The odds for ipsi-compartmental perimeniscal synovitis were increased for knees with medial posterior horn meniscal damage (adjusted odds ratio [aOR] 2.5, 95% confidence intervals [95% CI] 1.3,4.8), but not for lateral damage (aOR 1.7, 95% CI 0.4,6.6). No positive associations were found for meniscal damage and presence of synovitis posterior to the PCL (aOR 0.9, 95% CI 0.6,1.5).
Conclusions
Meniscal damage of the posterior horns is associated with ipsi-compartmental perimensical synovitis. No associations were found for posterior horn meniscal damage with synovitis posterior to the PCL, which suggests that synovitis posterior to the PCL is likely to be triggered by different pathomechanisms.
Keywords: Osteoarthritis, Magnetic resonance imaging, Synovitis, Meniscal damage
Degenerative joints regularly demonstrate signs of synovial activation, i.e. inflammatory synovial thickening and joint effusion, even in the early phase of disease process [1–3]. The degree of synovitis correlates with radiographic disease stage, pain and may be a marker of structural change and clinical outcome [4–6]. The histological correlates of synovitis include synovial hyperplasia, fibrosis, thickening of the synovial capsule, activated synoviocytes and in some cases lymphocytic infiltrates [7].
In knee osteoarthritis (OA) synovitis is thought to be a secondary phenomenon triggered by release of detritus from joint structures including cartilage and also meniscal damage, as synovial cell response seems to play an important role in meniscal tear repair [8–10]. An association between meniscal damage and concomitant joint effusion has been described in the Framingham OA study and others have reported perimeniscal synovitis to be associated with meniscal tears [11,12]. However, this association is still under discussion. A recent study investigated the association between meniscal damage, meniscal extrusion and localized synovitis and found an association for medial meniscal extrusion and localized synovitis but not for lateral extrusion, and not for adjacent meniscal tears and other forms of damage [13].
To date, semiquantitative MRI assessment of synovitis in large studies of OA is usually performed on non-enhanced fluid-sensitive fat suppressed (fs) sequences [14]. Commonly, signal alterations in Hoffa’s fat pad are scored as synovitis surrogates [3], which have shown an association with pain severity, but have proven to represent only a non-specific marker when using contrast-enhanced MRI as the reference standard [15]. Comparing three different scoring systems to evaluate synovitis and joint effusion, Loeuille et al. reported that only scoring of contrast-enhanced T1-weighted images correlated with microscopically proven synovitis [16]. Recently, reliable instruments have been introduced that are able to assess synovitis in a semi-quantitative fashion at multiple sites within the knee joint on contrast-enhanced images [13,17].
An MRI-based analyses from the VIDEO study describing the anatomical distribution of synovitis in knee OA reported that the most prevalent location of any intraarticular synovitis in OA knees is around the posterior cruciate ligament (PCL), a finding unexplained [18]. The absolute most frequent location of meniscal damage is the posterior horn of the medial meniscus [19]. Synovitis posterior to the posterior cruciate ligament could be triggered by meniscal damage of the posterior horns, a neighboring anatomical location. Further, meniscal damage of the posterior horns might be an important trigger for predominantly posterior whole-knee synovitis.
Thus, the aims were to assess (1) if posterior horn meniscal damage is associated with ipsi-compartmental synovitis, (2) if posterior horn meniscal damage is associated with synovitis around the posterior cruciate ligament when compared to knees without posterior horns tears and (3) if posterior horn meniscal damage is associated with predominantly posterior whole-knee synovitis.
PATIENTS AND METHODS
Study design and subjects
Subjects were participants in the Multicenter Osteoarthritis Study (MOST), a prospective study of 3026 persons aged 50–79 years with a goal of identifying risk factors for incident and progressive knee OA in a sample either with OA or at high risk of developing disease. Subjects were recruited from two US communities, Birmingham, Alabama and Iowa City, Iowa. Details of subject inclusion, exclusion and recruitment have been described previously [20,21]. The study protocol was approved by the institutional review boards at the University of Iowa, University of Alabama, Birmingham; University of California, San Francisco and Boston University Medical Campus, and written informed consent was obtained from all participants.
For the present study, an unselected subset of MOST subjects who volunteered to undergo a 1.5 T CE-MRI of one knee at the 30-month follow-up clinic visit was studied. Altogether 1295 subjects were approached at the two clinical centers (624 at Birmingham and 671 at Iowa City). Of these, 336 participants refused to undergo the 1.5 T CE MRI. Documented reasons were “unwillingness to receive injection” (n = 121), “no time/too busy” (n = 169) and “other reasons” (n = 46). Two hundred and fifty six subjects were excluded because they reported kidney disease, had an elevated serum creatinine level or did not receive a 1.0 T MRI at 30 months. Finally, 157 subjects that were approached and scheduled missed the 30-month visit for other reasons leaving 546 subjects that were examined with 1.5 T CE MRI.
CE-MRIs were obtained on one knee only. To choose the knee to image, radiographs were read and the knee with the lower KL grade was selected to avoid knees with severe OA and likely co-occurrence in these knees of structural features associated with pain. If the grade was the same for both knees, the dominant leg was chosen. The CE-MRI was performed on the same day or within 30 days of non CE-MRIs obtained in all MRI eligible subjects in the parent study. For subjects with renal disease, diabetes or over the age of 65, serum creatinine was determined and the glomerular filtration rate (GFR) calculated before intravenous gadolinium administration. Those subjects with renal insufficiency (GFR <30 ml/min) were excluded from the study.
Radiographs
All subjects underwent weight-bearing posteroanterior (PA) fixed flexion knee radiographs using the protocol by Peterfy et al. and a plexiglass positioning frame (SynaFlexer™) [22] at 30-month follow-up. A musculoskeletal radiologist (a non-author) and a rheumatologist (DTF), blinded to clinical data, graded radiographs according to the Kellgren–Lawrence (K/L) grade [23], followed by an adjudication process (by two non-authors and DTF).
MRI acquisition
In the MOST parent study, MR imaging was performed using a 1.0 T extremity-based OrthOne scanner (Oni Inc, Wilmington, MA) but CE scans were not advisable. Images were acquired using a circumferential extremity coil using fat-suppressed, fast spin echo, proton density-weighted sequence in two planes, sagittal (TR = 4800 ms, TE = 35 ms, 3.0 mm slice thickness, 0 mm interslice gap, FOV 14 × 14 cm2, matrix 288 × 192, NEX2); and axial (TR = 4700 ms, TE = 13.2 ms, 3.0 mm slice thickness, 0 mm interslice gap, FOV 14 cm, matrix 288 × 192, NEX2) and a short tau inversion recovery sequence (STIR) in the coronal plane (TR = 7820 ms, TE = 14 ms, TI = 100 ms, 3.0 mm slice thickness, 0 mm interslice gap, FOV 14 cm, matrix 256 × 256, NEX2).
For the purpose of this study, CE-MRIs were obtained with a 1.5 T system (Siemens MAGNETOM Symphony™, Malvern, PA) with a circumferential extremity coil. Axial and sagittal fat-suppressed T1-weighted CE sequences were acquired (TR = 600 ms, TE = 13 ms, 3.0 mm slice thickness, 0.3 mm interslice gap, FOV 16 × 16 cm2, matrix 512 × 512, ETL 1). Intravenous gadolinium (Magnevist (gadopentetate dimeglumine; Bayer Health-Care Pharmaceuticals, Bayer Schering Pharma AG, Berlin, Germany) or Omniscan (gadodiamide; GE Healthcare, New Jersey, USA)) was administered at a dose of 0.2 ml (0.1 mmol)/kg body weight. Two minutes after completing the injection of the gadolinium, sagittal sequences were obtained immediately followed by the axial sequences. The timing of scanning (2 min post injection) was chosen so that we can visualize the maximal synovial enhancement, and also to be able to complete acquisition of images before blurring of synovitis/effusion borderline occurred due to effusion enhancement from the periphery [24].
MRI interpretation
MRI readings were performed independently by two musculoskeletal radiologists (AG, FWR), with 9 and 7 years of experience respectively in semiquantitative MR assessment of knee OA, after an initial session of training and calibration with 20 test cases that were randomly-selected. Synovitis was scored using the axial and sagittal CE-MRI sequence, while effusion and bone marrow lesions were scored using the non CE-MRI sequences of the parent study. MR images were assessed using eFilm™ software (Version 2.0.0, Merge Healthcare, Milwaukee, WI). Readers were blinded to the subjects’ pain status.
Synovitis was defined as enhancing thickened synovium (>2 mm) and was evaluated at 9 sites of the joint, i.e. the medial and lateral parapatellar recess, suprapatellar, infrapatellar, intercondylar, medial and lateral perimeniscal, and adjacent to the anterior (ACL) and posterior to the posterior cruciate ligaments in all subjects. If knees presented with Baker’s cysts or loose bodies, these two sites were scored in addition. Three sites were considered as posterior anatomical loations, i.e. perimeniscal medial, perimeniscal lateral and around the PCL. Synovial thickness was scored semiquantitatively based on the maximal thickness in any slice at each site as follows: grade 0 if <2 mm, grade 1 if 2–4 mm, and grade 2 if >4 mm.
A reliability exercise was performed on 50 randomly chosen examinations. The weighted kappa values for the individual sites were 0.67–1.00 for reader 1 and 0.63–1.00 for reader 2. Inter-observer reliability (kappa) for the individual sites ranged 0.67–0.92. The intraclass correlation coefficient (ICC) of summed synovitis score from 11 sites for intra-reader reliability was 0.98 (95% CI 0.97, 0.99) for reader 1, 0.96 (0.91, 0.98) for reader 2 and the inter-reader reliability was 0.94 (0.88, 0.97) [17].
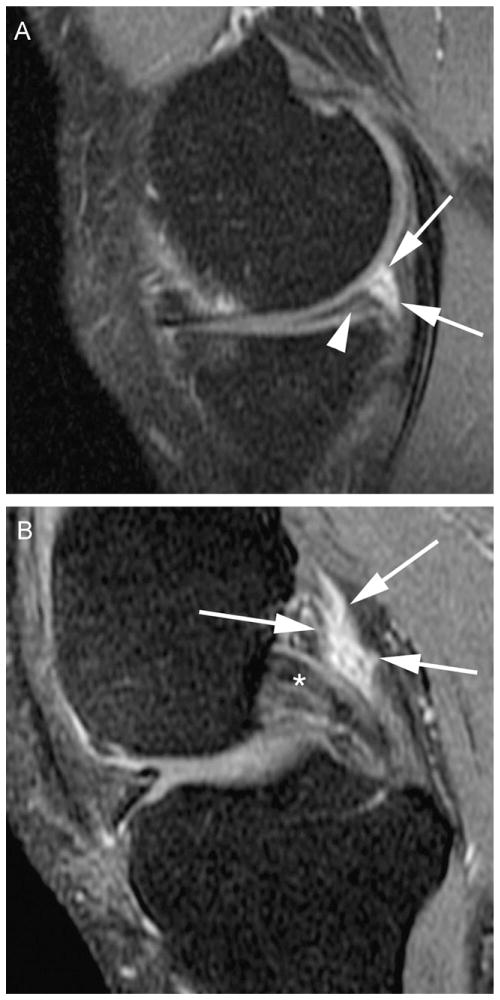
Meniscal status was graded according to the Whole-Organ MRI Score (WORMS) method using the 1.0 T extremity MRIs [25]. The anterior horn, body segment, and the posterior horn of the medial and lateral menisci were scored separately from 0 to 4: 0 = intact; 1 = minor radial or parrot-beak tear; 2 = non-displaced tear; 3 = displaced tear; 4 = complete maceration or destruction (inter-observer reliability for meniscal scoring weighted-kappa 0.79, 95% confidence intervals [0.59,0.97]). The readers regarded intrameniscal signal as a meniscal tear only when it communicated with the meniscal inferior or superior margin on atleast two slices. Intrameniscal signal alterations that did not fulfill the aforementioned criteria of a tear were scored as no tear (Grade 0) (Fig. 1).
Figure 1.
Examples of posterior synovitis. (A) Horizontal medial posterior horn meniscal tear (arrowhead) and marked (grade 2) surrounding perimeniscal synovitis (arrows). (B) Marked (grade 2) synovitis posterior to the posterior cruciate ligament (arrows).
Statistical analysis
Synovitis was defined as predominantly posterior in location when any of the three posterior locations had a synovial thickness score of grade 2 and no other sites had scores of grade 2. A synovitis score ≥1 was defined as synovitis presence in an individual location. A separate analysis was performed focusing on severe (= grade 2) synovitis only.
Logistic regression was used to assess the association of posterior horn meniscal damage (predictor) and ipsi-compartmental perimeniscal synovitis (outcome). In addition we examined the association between mensical damage (predictor) and synovitis posterior to the PCL and predominantly posterior whole knee synovitis (outcome). Adjustment was performed for age, gender, radiographic OA and body mass index. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Three hundred and seventy seven subjects were included that were examined with 1.0 T extremity MRI and 1.5 T CE MRI that could be read for whole knee synovitis and meniscal status. Mean age was 61.1 years (± 6.9), on average the participants were overweight (mean body mass index 29.6 (± 4.9) and 44.3% were women.
The Kellgren Lawrence grades of the examined knees were: K/L 0: 233 knees (62.0%), K/L 1: 61 knees (16.2%), K/L 2: 41 knees (10.9%), K/L 3: 34 knees (10.9%) and K/L4: 7 knees.
Any medial perimeniscal synovitis was observed in 65 knees (17.2%), any lateral perimeniscal synovitis in 109 knees (28.9%). Eighty knees (21.2%) exhibited a maximum perimeniscal synovitis score (either medial or lateral) of a grade 1 and 50 knees (13.3%) of a grade 2. A grade 1 synovitis score around the posterior cruciate ligaments was seen in 80 knees (21.2%) and a grade 2 synovitis around the PCL was observed in 106 knees (28.1%). Twenty eight knees (7.4%) showed predominantly posterior whole-knee synovitis.
Any meniscal damage of the medial posterior horn (grades ≥1) was observed in 123 knees (32.6%) and in 10 knees (2.7%) meniscal damage of the posterior horn of the lateral meniscus was seen. Two hundred and fourty eight knees (65.8%) did not show any damage of the medial or lateral posterior horns, 19 knees (5.0%) had a maximum score of 1 (in either the medial or lateral posterior horn), 64 knees (17.0%) a grade 2, 45 knees (11.9%) a grade 3 and 1 knee (0.3%) had a maximum score of a grade 4 lesion—i.e. complete maceration. The detailed frequency distribution of meniscal damage of the posterior horns and synovitis at the three posterior locations according to K/L grade is presented in Table 1.
Table 1.
Frequencies of Posterior Horn Meniscal Damage and Posterior Synovitis According to Radiographic OA Severity
| Radiographic OA Status (K/L Grade) | K/L 0 N (%)a |
K/L 1 | K/L 2 | K/L 3 | K/L 4 | All Grades N (%)b |
|---|---|---|---|---|---|---|
| Posterior horn meniscal damagec | ||||||
| 0 | 184 (74.5) | 34 (13.8) | 20 (8.1) | 6 (2.4) | 3 (1.2) | 247 (65.5) |
| 1 | 10 (52.6) | 4 (21.1) | 3 (15.8) | 2 (10.5) | 0 (0.0) | 19 (5.0) |
| 2 | 27 (42.2) | 16 (25.0) | 12 (18.8) | 9 (14.1) | 0 (0.0) | 64 (17.0) |
| 3 and 4 | 12 (26.1) | 7 (15.2) | 6 (13.0) | 17 (37.0) | 4 (8.7) | 46 (12.2) |
| Synovitis around posterior hornd | ||||||
| 0: <2 mm | 165 (7.1) | 40 (16.3) | 23 (9.4) | 15 (6.1) | 3 (1.2) | 246 (65.3) |
| 1: 2–4 mm | 48 (60.0) | 11 (13.8) | 11 (13.8) | 10 (12.5) | 0 (0.0) | 80 (21.2) |
| 2: >4 mm | 20 (40.0) | 10 (20.0) | 7 (14.0) | 9 (18.0) | 4 (8.0) | 50 (13.3) |
| Synovitis posterior to the PCL | ||||||
| 0: <2 mm | 137 (72.1) | 33 (17.4) | 11 (5.8) | 9 (4.7) | 0 (0.0) | 190 (50.4) |
| 1: 2–4 mm | 42 (52.5) | 12 (15.0) | 17 (21.3) | 7 (8.8) | 2 (2.5) | 80 (21.2) |
| 2: >4 mm | 54 (50.9) | 16 (15.1) | 13 (12.3) | 18 (17.0) | 5 (4.7) | 106 (28.1) |
% in regard to meniscal/synovitis grade in rows.
In regard to all knees: n = 377.
Medial only, lateral only, or both, medial and lateral.
Either medial or lateral or both.
Risk of any medial perimensical synovitis was increased for knees with medial posterior horn meniscal damage (adjusted odds ratio [OR] 2.5 95% confidence interval [CI] 1.3,4.8). As shown in Table 2 this association could be observed for grades 2–4 meniscal damage but not for grade 1. No positive associations were observed for lateral posterior horn meniscal damage and any lateral perimeniscal synovitis (adjusted OR 1.7, 95% CI 0.4,6.6). Considering severe synovitis only, risk of ipsi-compartmental posterior synovitis was markedly increased for both, medial and lateral posterior meniscal damage (adjusted OR 3.8 95% CI 1.1,13.0 and 5.4, 95% CI 1.3,22.7 respectively Table 3). No statistically significant associations were found for knees with any (medial or lateral) posterior meniscal damage and presence of any or severe synovitis posterior to the PCL. Table 4 gives a detailed overview of medial and lateral meniscal damage and severe synovitis posterior to the PCL. Further, no statistically significant associations were observed for posterior meniscal damage and predominantly posterior whole-knee synovitis (adjusted OR 2.1, 95% CI 0.9,5.1) using knees without posterior meniscal damage as the reference (Table 5).
Table 2.
Cross-sectional Association of Medial Posterior Horn Meniscal Damage and any Medial Perimensical Synovitis.
| Medial Posterior Horn Meniscal Damage (Predictor)
|
Any Medial Perimensical Synovitis (Outcome)
|
Crude OR OR (95% CI) p-value |
Adjusted ORa OR (95% CI) p-value |
||
|---|---|---|---|---|---|
| Total number of knees | <2 mm (%) | ≥2 mm (%) | |||
| Absence | (N = 253) | 221 (87.4) | 32 (12.7) | 1.0 (reference) | 1.0 (reference) |
| Presence | (N = 123) | 90 (73.2) | 33 (26.8) | 2.5 (1.5,4.3) | 2.5 (1.3,4.8) |
| 0.0009 | 0.007 | ||||
| 0 | (N = 253) | 221 (87.4) | 32 (12.7) | 1.0 (reference) | 1.0 (reference) |
| 1 | (N = 18) | 13 (72.2) | 5 (27.8) | 2.6 (0.9,7.9) | 2.7 (0.8,8.5) |
| 0.08 | 0.095 | ||||
| 2 | (N = 60) | 44 (73.3) | 16 (26.7) | 2.5 (1.3,4.9) | 2.6 (1.2,5.5) |
| 0.008 | 0.015 | ||||
| 3 and 4 | (N = 45) | 33 (73.3) | 12 (26.7) | 2.5 (1.2,5.3) | 2.1 (0.8,5.5) |
| 0.017 | 0.133 | ||||
Adjusting for age, sex, BMI, and radiographic OA.
Table 3.
Cross-sectional Association of Medial/Lateral; Posterior Horn Meniscal Damage and Ipsi-Compartmental Severe Perimensical Synovitis.
| Medial Posterior Horn Meniscal Damage (Predictor)
|
Medial Severe Perimensical Synovitis (Outcome)
|
Crude OR OR (95% CI) p-value |
Adjusted ORa OR (95% CI) p-value |
||
|---|---|---|---|---|---|
| Total number of knees | ≤ 4 mm (%) | >4 mm (%) | |||
| Absence | (N = 253) | 246 (97.2) | 7 (2.8) | 1.0 (reference) | 1.0 (reference) |
| Presence | (N = 123) | 114 (92.7) | 9 (7.3) | 2.8 (1,7.6.0) | 3.8 (1.1,13.0) |
| 0.049 | 0.037 | ||||
| 0 | (N = 253) | 246 (97.2) | 7 (2.8) | 1.0 (reference) | 1.0 (reference) |
| 1 | (N = 18) | 17 (94.4) | 1 (5.6) | 2.1 (0.2,17.7) | 2.2 (0.2,21.1) |
| 0.511 | 0.506 | ||||
| 2 | (N = 60) | 55 (91.7) | 5 (8.3) | 3.2 (1.0,10.4) | 4.9 (1.2,19.5) |
| 0.055 | 0.026 | ||||
| 3 and 4 | (N = 45) | 42 (93.3) | 3 (6.7) | 2.5 (0.6,10.1) | 3.3 (0.5,20.6) |
| 0.197 | 0.205 | ||||
| Lateral posterior horn meniscal damage (predictor)
|
Lateral severe perimensical synovitis (outcome)
|
Crude OR | Adjusted OR | ||
|---|---|---|---|---|---|
| Total number of knees | ≤ 4 mm (%) | >4 mm(%) | OR (95% CI) p-value | OR (95% CI) p-value | |
| Absence | (N = 367) | 325 (88.6) | 42 (11.4) | 1.0 (reference) | 1.0 (reference) |
| Presence | (N = 10) | 6 (60.0) | 4 (40.0) | 5.1 (1.4,19.0) | 5.4 (1.3,22.7) |
| 0.014 | 0.022 | ||||
| 0 | (N = 367) | 325 (88.6) | 42 (11.4) | 1.0 (reference) | 1.0 (reference) |
| 1 | (N = 1) | 1 (100) | 0 (0.0) | n/a | n/a |
| 0.99 | 0.99 | ||||
| 2 | (N = 7) | 4 (57.1) | 3 (42.9) | 5.8 (1.3,26.7) | 6.1 (1.2,32.0) |
| 0.025 | 0.031 | ||||
| 3 and 4 | (N = 2) | 1 (50.0) | 1 (50.0) | 7.7 (0.5,125.6) | 6.7 (0.2,249.0) |
| 0.15 | 0.30 | ||||
Adjusting for age, sex, BMI, and radiographic OA.
Table 4.
Cross-sectional Association of Medial/Lateral Posterior Horn Meniscal Damage and Severe Synovitis Posterior to the PCL
| Medial Posterior Horn Meniscal Damage (Predictor)
|
Severe Synovitis Posterior to the PCL (Outcome)
|
Crude OR OR (95% CI) p-value |
Adjusted ORa OR (95% CI) p-value |
||
|---|---|---|---|---|---|
| Total number of knees | ≤ 4 mm (%) | >4 mm (%) | |||
| Absence | (N = 253) | 188 (74.3) | 65 (25.7) | 1.0 (reference) | 1.0 (reference) |
| Presence | (N = 123) | 82 (66.7) | 41 (33.3) | 1.4 (0.9,2.3) | 1.2 (0.7,2.1) |
| 0.129 | 0.552 | ||||
| 0 | (N = 253) | 188 (74.3) | 65 (25.7) | 1.0 (reference) | 1.0 (reference) |
| 1 | (N = 18) | 10 (94.4) | 8 (5.6) | 2.3 (0.9,6.1) | 2.2 (0.8,6.2) |
| 0.093 | 0.130 | ||||
| 2 | (N = 60) | 42 (70.0) | 18 (30.0) | 1.2 (0.7,2.3) | 1.1 (0.6,2.2) |
| 0.508 | 0.756 | ||||
| 3 and 4 | (N = 45) | 30 (66.7) | 15 (33.3) | 1.4 (0.7,2.8) | 0.9 (0.4,2.1) |
| 0.295 | 0.723 | ||||
| Lateral posterior horn meniscal damage (predictor) | |||||
| Total number of knees | |||||
| Absence | (N = 367) | 266 (72.5) | 101 (27.5) | 1.0 (reference) | 1.0 (reference) |
| Presence | (N = 10) | 5 (50.0) | 5 (50.0) | 2.6 (0.7,9.3) | 2.9 (0.8,11.4) |
| 0.134 | 0.121 | ||||
| 0 | (N = 367) | 266 (72.5) | 101 (27.5) | 1.0 (reference) | 1.0 (reference) |
| 1 | (N = 1) | 0 (100) | 1 (0.0) | n/a | n/a |
| 0.988 | 0.987 | ||||
| 2 | (N = 7) | 4 (57.1) | 3 (42.9) | 2.0 (0.4,8.9) | 2.2 (0.5,11.0) |
| 0.381 | 0.324 | ||||
| 3 and 4 | (N = 2) | 1 (50.0) | 1 (50.0) | 2.6 (0.2,42.3) | 1.6 (0.1,48.4) |
| 0.500 | 0.786 | ||||
Adjusting for age, sex, BMI, and radiographic OA.
Table 5.
Cross-sectional Association of any Posterior Horn Meniscal Damage and Predominantly Posterior Synovitis.
| Posterior Horn Meniscal Damage (Either Medial, Lateral or Both) | Predominantly Posterior Synovitis
|
Crude OR
|
Adjusted OR
|
|
|---|---|---|---|---|
| No (%) | Yes (%) | OR (95% CI) p-value | OR (95% CI) p-value | |
| Absence (N = 248) | 234 (94.4) | 14 (5.7) | 1.0 (reference) | 1.0 (reference) |
| Presence (N = 129) | 115 (89.2) | 14 (10.9) | 2.0 (0.9,4.4) | 2.1 (0.9,5.1) |
| 0.074 | 0.108 | |||
| Grade 0 (N = 248) | 234 (94.4) | 14 5.65 | 1.0 (reference) | 1.0 (reference) |
| Grade 1 (N = 19) | 17 (89.5) | 2 (10.5) | 2.0 (0.4,9.3) | 2.0 (0.4,10.1) |
| 0.399 | 0.394 | |||
| Grade 2 (N = 64) | 57 (89.1) | 7 (10.9) | 2.0 (0.8,5.3) | 2.2 (0.8,6.1) |
| 0.141 | 0.145 | |||
| Grade 3 and 4 (N = 46) | 41 (89.1) | 5 (10.9) | 2.0 (0.7,5.9) | 2.0 (0.5,7.3) |
| 0.196 | 0.310 | |||
DISCUSSION
In this large cohort of subjects with or at risk of OA we could demonstrate an association of MRI-detected ipsi-compartmental meniscal damage of the posterior horns with medial posterior perimeniscal synovitis emphasizing the role of meniscal pathology in regard to localized posterior inflammatory manifestations of OA. For the lateral compartment these associations were not as strong, a consequence of low frequencies of meniscal pathology in the lateral compartment, but still significant for severe localized perimeniscal synovitis. No associations were found for posterior horn meniscal tears with synovitis posterior to the PCL and with predominantly posterior whole-knee synovitis, which suggests that presence of PCL synovitis is likely to be triggered by different pathomechanisms than meniscal damage.
Synovitis in OA is thought to be a secondary phenomenon in the OA process due to debris of intraarticular tissue damage [8]. Synovitis alters the balance of cartilage matrix degradation and repair, leading to excess production of the proteolytic enzymes responsible for cartilage breakdown. Cartilage alteration in turn amplifies synovial inflammation, creating a vicious circle [26]. To date, most imaging based studies investigating synovitis in OA have focused on the peripatellar region and only sparse data is available on other synovitis locations [14,15,27]. Recently a semiquantitative scoring system has been introduced that allows for evaluation of synovitis at 11 different locations [17]. While five of these locations are focusing on the peripatellar region, the remaining locations are assessing the articular subregions around the cruciate ligaments, perimeniscal regions posteriorly, around loose bodies and in popliteal cysts. The system has shown good reliability and severe synovitis and synovitis in the peripatellar regions was associated with pain [17]. One study focused on perimeniscal synovitis assessment on coronal images and described associations with medial meniscal extrusion and degree of synovitis using both, semiquantitative and quantitative approaches [13]. These authors did not find significant associations with any meniscal damage or extrusion in the lateral compartment. However, the findings of Grainger et al. are only marginally comparable to ours as the authors did not assess the most common location of meniscal damage, i.e. the posterior horns of the medial meniscus, the focus of the current study.
It has been reported previously, that the commonest site of synovitis in OA is posterior to the PCL [18]. The intercondylar notch seems to play an important role for joint integrity. Around 20% of subjects with knee OA exhibit ACL disruptions without recalling trauma to the knee joint, which seems to represent an independent risk factor for consequent cartilage loss [27]. Work by Stein et al. from the Osteoarthritis Initiative found a strong association of femoral notch stenosis with prevalent ACL tears cross-sectionally [28]. Chronic friction within the notch might lead to debris and detritus triggering localized periligamentous synovitis around the ACL and PCL [29]. However, pathology of the PCL and its relation to disease activity and progression has not been explored in detail. We had hypothesized that despite cruciate ligament pathology, posterior meniscal pathology might explain some of the common synovitis occurrence posterior to the PCL, which we could not confirm in the present analysis. Thus, the high frequency of synovitis posterior to the PCL in OA knees still needs to be explained and we speculate that other pathomechanisms are responsible than meniscal damage. PCL pathology in OA is rare and although not systematically assessed, it seems unlikely that degeneration of the PCL is responsible for this common presence of periligametous synovial inflammation. The PCL is a very strong ligament uncommonly affected by incidental or traumatic pathology [28,29].
Although synovitis around the ACL and PCL is often observed in OA, synovitis in these locations does not seem to be associated with knee pain [17]. A recent study by Baker et al. [30] examined the relation of synovitis to pain using the scoring system proposed by Rhodes et al. [27] and found a generally stronger relation with pain than reported by others, which may also be due to the focus on parapatellar synovitis.
We were not able to elucidate a cause–effect relationship between meniscal damage and localized synovitis as our study design was cross-sectional in nature. Longitudinal studies using CE MRI are missing at present but may be able to further clarify.
We did not observe an association between high grade medial posterior horn meniscal damage and ipsi-compartmental severe synovitis, which seems unexpected. One likely reason could be that high grade meniscal damage is more commonly observed in knees with advanced articular tissue damage including severe cartilage loss. In these cases cartilage debris might be the paramount trigger of synovial activation in comparison to meniscal damage. In addition, loss of meniscal substance, a common finding for grade 3 and 4 lesions, may lead to a decrease in synovial activation in this region. In the lateral compartment only 2 knees exhibited grade 3 and 4 meniscal damage and thus the low frequency of these changes explains the negative results.
As no coronal contrast enhanced images were obtained, we were not able to analyze associations of meniscal damage in the body segment and perimeniscal synovitis around the body. We can only speculate if similar associations might be observed. The only publication available on this topic could not confirm such an association [13].
CE MRI is mandatory to assess detailed whole knee synovitis as non-enhanced studies are only able to visualize a composite of effusion and synovitis that cannot be differentiated from each other and thus was termed “effusion-synovitis” recently [31]. The MRI finding of hyperintensity in Hoffa’s fat pad has been used as a surrogate for whole knee synovitis in several studies but has been proven to be only a non-specific imaging feature [15]. Further, posterior synovitis cannot be assessed by this surrogate. Recent work by Loeuille et al. showed that only contrast enhanced MRI correlates with histology proven inflammatory synovial infiltrates [16].
Summarizing our findings we could show that damage of the posterior horns of the medial and lateral meniscus is associated with ipsi-compartmental posterior perimeniscal synovitis suggesting that local structural damage may cause synovitis nearby. No associations were found for posterior horn meniscal tears with synovitis posterior to the PCL and predominantly posterior whole-knee synovitis. This suggests that presence of PCL synovitis is likely to be triggered by different pathomechanisms than meniscal damage.
Acknowledgments
The MOST study is supported by NIH grants from the National Institute on Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820).
Footnotes
AUTHOR CONTRIBUTIONS
- All authors were involved in the conception and design of the study, or acquisition of data, or analysis and interpretation of data.
- All authors contributed to drafting the article or revising it critically for important intellectual content.
- All authors gave their final approval of the manuscript to be submitted.
Additional contributions:
- Analysis and interpretation of the data: JN, TY.
- Drafting of the article: FWR, MN, DTF, AG.
- Provision of study materials or patients: MN, DTF, CEL, AG, FWR.
- Statistical expertise: JN, TY, MN, DTF.
- Obtaining of funding: MN, DTF, CEL, JT.
- Collection and assembly of data: MN, DTF, CEL, JT, AG, FWR.
Responsibility for the integrity of the work as a whole, from inception to finished article, is taken by F. Roemer, MD (first author; froemer@bu.edu) and A. Guermazi, MD, PhD (last author; ali.guermazi@bmc.org).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindblad S, Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987;30(10):1081–8. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- 2.Ostergaard M, Stoltenberg M, Lovgreen-Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imaging-determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum. 1997;40(10):1856–67. doi: 10.1002/art.1780401020. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13(2):177–83. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 4.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7. [PubMed] [Google Scholar]
- 5.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Krasnokutsky S, Belitskaya-Levy I, Bencardino J, Samuels J, Attur M, Regatte R, et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63(10):2983–91. doi: 10.1002/art.30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach HI, Aigner T, Soder S, Haag J, Welkerling H. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 2007;8(2):271–82. doi: 10.2174/138945007779940160. [DOI] [PubMed] [Google Scholar]
- 8.Aigner N, Van der Kraan P, Van den Berg W. Osteoarthritis and Inflammation—inflammatory changes in osteoarthritis synoviopathy. In: Buckwalter J, Lotz M, Stoltz J-F, editors. Osteoarthritis, inflammation and degradation: a continuum. Amsterdam: IOS Press; 2007. pp. 219–235. [Google Scholar]
- 9.Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15:281–6. doi: 10.1016/s0749-8063(99)70035-6. [DOI] [PubMed] [Google Scholar]
- 10.Itsuiki J, Ochi M, Ikuta Y. Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy. 1994;10:659–66. doi: 10.1016/s0749-8063(05)80065-9. [DOI] [PubMed] [Google Scholar]
- 11.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage. 2009;17(6):748–53. doi: 10.1016/j.joca.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Haddad G, Kumar R, Pamplona R, Alavi A. PET/MRI depicts the exact location of meniscal tear associated with synovitis. Eur J Nucl Med Mol Imaging. 2006;33(4):507–8. doi: 10.1007/s00259-005-0034-x. [DOI] [PubMed] [Google Scholar]
- 13.Grainger AJ, Rhodes LA, Keenan AM, Emery P, Conaghan PG. Quantifying perimeniscal synovitis and its relationship to meniscal pathology in osteoarthritis of the knee. Eur Radiol. 2007;17(1):119–24. doi: 10.1007/s00330-006-0282-6. [DOI] [PubMed] [Google Scholar]
- 14.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant HK, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66(12):1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s fat pad: evaluation on unenhanced MR images as a measure of patellofemoral synovitis in osteoarthritis. Am J Roentgenol. 2009;192(6):1696–700. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 16.Loeuille D, Sauliere N, Champigneulle J, Rat AC, Blum A, Chary-Valckenaere I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19(12):1433–9. doi: 10.1016/j.joca.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011;70(5):805–11. doi: 10.1136/ard.2010.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roemer FW, Javaid MK, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18(10):1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359(11):1108–15. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felson DT, Niu J, Guermazi A, Hunter DJ, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56(9):2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 21.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772–80. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostergaard M, Klarlund M. Importance of timing of post-contrast MRI in rheumatoid arthritis: what happens during the first 60 min after IV gadolinium-DTPA? Ann Rheum Dis. 2001;60(11):1050–4. doi: 10.1136/ard.60.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology. 2005;44(12):1569–73. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 28.Voos JE, Mauro CS, Wente T, Warren RF, Wickiewicz TL. Posterior cruciate ligament: anatomy, biomechanics, and outcomes. Am J Sports Med. 2012;40(1):222–31. doi: 10.1177/0363546511416316. [DOI] [PubMed] [Google Scholar]
- 29.Fanelli GC, Beck JD, Edson CJ. Current concepts review: the posterior cruciate ligament. J Knee Surg. 2010;23(2):61–72. doi: 10.1055/s-0030-1267466. [DOI] [PubMed] [Google Scholar]
- 30.Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema MD, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis. 2010;69(10):1779–83. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]