Abstract
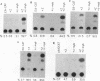
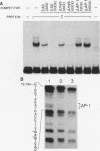
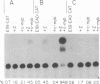
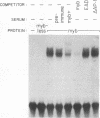
Regulation of replicative functions in the Epstein-Barr virus (EBV) genome is mediated through activation of a virally encoded transcription factor, Z (BZLF1). We have shown that the Z gene product, which binds to AP-1 sites as a homodimer and has sequence similarity to c-Fos, can efficiently activate the EBV early promoter, BMRF1, in certain cell types (i.e., HeLa cells) but not others (i.e., Jurkat cells). Here we demonstrate that the c-myb proto-oncogene product, which is itself a DNA-binding protein and transcriptional transactivator, can interact synergistically with Z in activating the BMRF1 promoter in Jurkat cells (a T-cell line) or Raji cells (an EBV-positive B-cell), whereas the c-myb gene product by itself has little effect. The simian virus 40 early promoter is also synergistically activated by the Z/c-myb combination. Synergistic transactivation of the BMRF1 promoter by the Z/c-myb combination appears to involve direct binding by the Z protein but not the c-myb protein. A 30-bp sequence in the BMRF1 promoter which contains a Z binding site (a consensus AP-1 site) is sufficient to transfer high-level lymphoid-specific responsiveness to the Z/c-myb combination to a heterologous promoter. That the c-myb oncogene product can interact synergistically with an EBV-encoded member of the leucine zipper protein family suggests c-myb is likely to engage in similar interactions with cellularly encoded transcription factors.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender T. P., Kuehl W. M. Differential expression of the c-myb proto-oncogene marks the pre-B cell/B cell junction in murine B lymphoid tumors. J Immunol. 1987 Dec 1;139(11):3822–3827. [PubMed] [Google Scholar]
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lipsick J. S., Baluda M. A. Antibodies to the evolutionarily conserved amino-terminal region of the v-myb-encoded protein detect the c-myb protein in widely divergent metazoan species. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4685–4689. doi: 10.1073/pnas.83.13.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990 Jul;64(7):3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Gruffat H., Manet E., Calender A., Sergeant A. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J Virol. 1989 Feb;63(2):615–623. doi: 10.1128/jvi.63.2.615-623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Jenson H., Seibl R., Wolf H., Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987 Dec;61(12):3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. A., Leahy J., Hardwick J. M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990 Jan;64(1):313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Moore T. L., Kuehl W. M., Bender T., Ting J. P. Functional analysis of c-Myb protein in T-lymphocytic cell lines shows that it trans-activates the c-myc promoter. Mol Cell Biol. 1990 Nov;10(11):5747–5752. doi: 10.1128/mcb.10.11.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Rowe D. T., Rooney C. M., Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989 Jan;8(1):127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A., LaMontagne K., Reavis D., Stober-Grässer U., Lipsick J. S. Determinants of sequence-specific DNA-binding by p48v-myb. Oncogene. 1991 Feb;6(2):265–273. [PubMed] [Google Scholar]
- Giot J. F., Mikaelian I., Buisson M., Manet E., Joab I., Nicolas J. C., Sergeant A. Transcriptional interference between the EBV transcription factors EB1 and R: both DNA-binding and activation domains of EB1 are required. Nucleic Acids Res. 1991 Mar 25;19(6):1251–1258. doi: 10.1093/nar/19.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Kirchgens C., Edwards C. F., Young L. S., Rowe M., Forster A., Rabbitts T. H., Rickinson A. B. Epstein-Barr virus-transformed human precursor B cell lines: altered growth phenotype of lines with germ-line or rearranged but nonexpressed heavy chain genes. Eur J Immunol. 1987 Aug;17(8):1199–1207. doi: 10.1002/eji.1830170818. [DOI] [PubMed] [Google Scholar]
- Gruffat H., Manet E., Rigolet A., Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Dec 11;18(23):6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K. M., Reakes C. F., Watson R. J. Characterization of the sequence-specific interaction of mouse c-myb protein with DNA. EMBO J. 1990 Jan;9(1):161–169. doi: 10.1002/j.1460-2075.1990.tb08092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C. E., Lipsick J. S. Structural and functional domains of the myb oncogene: requirements for nuclear transport, myeloid transformation, and colony formation. J Virol. 1988 Jun;62(6):1981–1988. doi: 10.1128/jvi.62.6.1981-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez C. E., Lipsick J. S. trans activation of gene expression by v-myb. Mol Cell Biol. 1990 May;10(5):2285–2293. doi: 10.1128/mcb.10.5.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Golay J., Frampton J., Nakano T., Ness S. A., Graf T. Mutations in v-myb alter the differentiation of myelomonocytic cells transformed by the oncogene. Cell. 1990 Dec 21;63(6):1289–1297. doi: 10.1016/0092-8674(90)90424-d. [DOI] [PubMed] [Google Scholar]
- Kenney S., Holley-Guthrie E., Mar E. C., Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989 Sep;63(9):3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Arnold H., Biedenkapp H. Activation of transcription by v-myb: evidence for two different mechanisms. Genes Dev. 1989 Oct;3(10):1582–1589. doi: 10.1101/gad.3.10.1582. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 1987 Sep;6(9):2719–2725. doi: 10.1002/j.1460-2075.1987.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Packham G., Cook A., Farrell P. J. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991 Feb;6(2):195–204. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane T., Ibanez C., Garcia A., Graf T., Lipsick J. Transformation by v-myb correlates with trans-activation of gene expression. Mol Cell Biol. 1990 Jun;10(6):2591–2598. doi: 10.1128/mcb.10.6.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Li R., Knight J. D., Jackson S. P., Tjian R., Botchan M. R. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell. 1991 May 3;65(3):493–505. doi: 10.1016/0092-8674(91)90467-d. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990 Jun;64(6):2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Lipsick J. S., Boyle W. J. c-myb protein expression is a late event during T-lymphocyte activation. Mol Cell Biol. 1987 Sep;7(9):3358–3360. doi: 10.1128/mcb.7.9.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part II. Myb. Genes Dev. 1990 Dec;4(12B):2235–2241. doi: 10.1101/gad.4.12b.2235. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G., Nakagoshi H., Nagase T., Nomura N., Date T., Ueno Y., Ishii S. DNA binding activity and transcriptional activator function of the human B-myb protein compared with c-MYB. J Biol Chem. 1990 Jun 5;265(16):9280–9284. [PubMed] [Google Scholar]
- Nakagoshi H., Nagase T., Ueno Y., Ishii S. Transcriptional trans-repression by the c-myb proto-oncogene product. Nucleic Acids Res. 1989 Sep 25;17(18):7315–7324. doi: 10.1093/nar/17.18.7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina Y., Nakagoshi H., Imamoto F., Gonda T. J., Ishii S. Trans-activation by the c-myb proto-oncogene. Nucleic Acids Res. 1989 Jan 11;17(1):107–117. doi: 10.1093/nar/17.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura N., Takahashi M., Matsui M., Ishii S., Date T., Sasamoto S., Ishizaki R. Isolation of human cDNA clones of myb-related genes, A-myb and B-myb. Nucleic Acids Res. 1988 Dec 9;16(23):11075–11089. doi: 10.1093/nar/16.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham G., Economou A., Rooney C. M., Rowe D. T., Farrell P. J. Structure and function of the Epstein-Barr virus BZLF1 protein. J Virol. 1990 May;64(5):2110–2116. doi: 10.1128/jvi.64.5.2110-2116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C. M., Rowe D. T., Ragot T., Farrell P. J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989 Jul;63(7):3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney C., Taylor N., Countryman J., Jenson H., Kolman J., Miller G. Genome rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9801–9805. doi: 10.1073/pnas.85.24.9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H., Kanei-Ishii C., Nagase T., Nakagoshi H., Gonda T. J., Ishii S. Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5758–5762. doi: 10.1073/pnas.86.15.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Lüscher B., Eisenman R. N. A second c-myb protein is translated from an alternatively spliced mRNA expressed from normal and 5'-disrupted myb loci. Mol Cell Biol. 1989 Dec;9(12):5456–5463. doi: 10.1128/mcb.9.12.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto T., Ogura H., Sato H., Umeda R., Hatano M. Isolation of transforming and early antigen-inducing Epstein-Barr virus from nasopharyngeal carcinoma hybrid cells (NPC-KT). J Natl Cancer Inst. 1985 Jan;74(1):57–60. [PubMed] [Google Scholar]
- Taylor N., Flemington E., Kolman J. L., Baumann R. P., Speck S. H., Miller G. ZEBRA and a Fos-GCN4 chimeric protein differ in their DNA-binding specificities for sites in the Epstein-Barr virus BZLF1 promoter. J Virol. 1991 Aug;65(8):4033–4041. doi: 10.1128/jvi.65.8.4033-4041.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C. J., Cohen P. S., Israel M. A. Regulation of c-myb expression in human neuroblastoma cells during retinoic acid-induced differentiation. Mol Cell Biol. 1988 Apr;8(4):1677–1683. doi: 10.1128/mcb.8.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Tice-Baldwin K., Fink G. R., Arndt K. T. BAS1 has a Myb motif and activates HIS4 transcription only in combination with BAS2. Science. 1989 Nov 17;246(4932):931–935. doi: 10.1126/science.2683089. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Hayday A. C., Keating A. Electric field-mediated DNA transfer: transient and stable gene expression in human and mouse lymphoid cells. Mol Cell Biol. 1986 Feb;6(2):703–706. doi: 10.1128/mcb.6.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urier G., Buisson M., Chambard P., Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989 May;8(5):1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- Weber B. L., Westin E. H., Clarke M. F. Differentiation of mouse erythroleukemia cells enhanced by alternatively spliced c-myb mRNA. Science. 1990 Sep 14;249(4974):1291–1293. doi: 10.1126/science.2205003. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Gorse K. M., Clarke M. F. Alternative splicing of the human c-myb gene. Oncogene. 1990 Aug;5(8):1117–1124. [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]