Abstract
Functional magnetic resonance imaging (fMRI) was used to examine cognitive regulation of negative emotion in unmedicated Major Depressive Disorder (MDD). Twenty-four controls and 12 depressed adults used reappraisal to increase (real condition) and reduce (photo condition) the personal relevance of negative and neutral pictures during fMRI as valence ratings were collected; passive viewing (look condition) served as a baseline. Reappraisal was not strongly affected by MDD. Ratings indicated that both groups successfully reappraised negative emotional experience. Both groups also showed better memory for negative vs. neutral pictures two weeks later. Across groups, increased brain activation was observed on negative/real vs. negative/look and negative/photo trials in left dorsolateral prefrontal cortex (DLPFC), rostral anterior cingulate, left parietal cortex, caudate, and right amygdala. Depressive severity was inversely correlated with activation modulation in the left DLPFC, right amygdala, and right cerebellum during negative reappraisal. The lack of group differences suggests that depressed adults can modulate the brain activation and subjective experience elicited by negative pictures when given clear instructions. However, the negative relationship between depression severity and effects of reappraisal on brain activation indicates that group differences may be detectable in larger samples of more severely depressed participants.
Keywords: emotion regulation, major depressive disorder, default mode network, memory, fMRI
1. Introduction
Anhedonia and excessive sadness are cardinal symptoms of Major Depressive Disorder (MDD) (American Psychiatric Association, 2000). Emotional context insensitivity research demonstrates that these symptoms flatten the emotional landscape (Rottenberg, 2005; Rottenberg et al., 2005). In one study, healthy controls and depressed adults viewed amusing, sad, and neutral films (Rottenberg et al., 2005). Controls showed predictable changes in self-reported sadness and happiness, but the depressed group showed heightened sadness regardless of which film was presented. While blunted reactivity to positive stimuli in depression is widely known, it is noteworthy that depressed participants did not show increased sadness when viewing sad films (Rottenberg et al., 2005), a result linked to more severe depression and worse psychosocial function (Rottenberg et al., 2002). This finding indicates that depression truncates the range of negative emotional experience, which has clinical implications.
Emotional context insensitivity may have consequences for emotion regulation. Reappraisal—re-interpreting stimuli to modify their meaning—can modulate negative emotional experience (Ochsner et al., 2004) and supports successful interpersonal functioning (Gross and John, 2003). Furthermore, reappraisal does not impair explicit memory and may improve it, in contrast to the negative effects on memory associated with expressive suppression (Dillon et al., 2007; Hayes et al., 2010; Richards and Gross, 2000). Thus, reappraisal is widely considered an effective emotion regulation technique. Because depression restricts the range of emotional reactions, it may also limit the ability to reappraise emotional responses once they arise.
Behavioral support for this hypothesis is mixed. Studies in remitted depression (Ehring et al., 2010; Kanske et al., 2012) reported that instructed reappraisal reduced negative emotional experience. However, the use of remitted samples may have decreased the likelihood of detecting depression effects. Indeed, compared to controls, an unmedicated MDD sample reported greater difficulty cognitively reducing sadness, and the level of difficulty was correlated with depressive severity (Beauregard et al., 2006). Thus, reappraisal of negative emotional experience may be impaired in acute, unmedicated depression.
The neuroimaging literature is also mixed. One functional magnetic resonance imaging (fMRI) study found that medicated depressed adults could cognitively reduce amygdala activation elicited by negative pictures, although the degree of amygdala modulation was negatively correlated with depressive severity (Erk et al., 2010a). This contrasts with reports of blunted reappraisal effects on amygdala activation in both remitted (Kanske et al., 2012) and unmedicated depressed samples (Beauregard et al., 2006). Another study found no amygdala modulation during reappraisal in controls or unmedicated depressed adults (Johnstone et al., 2007), but reported right prefrontal cortex (PFC) hyperactivation in the depressed group. This is difficult to interpret, because another study reported right dorsolateral prefrontal cortex (DLPFC) hypoactivation during reappraisal in medicated depression (Erk et al., 2010a). Overall, effects of depression on reappraisal are not well-understood.
In light of this mixed evidence, we conducted an fMRI study of reappraisal in MDD. To maximize sensitivity to depression effects, we recruited an unmedicated sample experiencing a current major depressive episode and compared them to healthy controls. Participants reappraised their responses to negative and neutral pictures and provided trial-by-trial valence ratings to permit investigation of subjective experience. The primary hypothesis was that depressed participants would not be able to cognitively increase or reduce their negative emotional responses, as measured by valence ratings and brain activation.
The alternative hypothesis was that depression would have minimal effects on reappraisal because of the use of detailed instructions and cues. This prediction was motivated by a prior study in remitted students, which found no effects of depression on instructed reappraisal (Ehring et al., 2010). Importantly, this study also reported that the remitted group spontaneously engaged in an ineffective emotion regulation strategy (expressive suppression). This suggests that the remitted participants were able to reappraise effectively because they were given clear instructions and cues, and may not have done so otherwise.
We also examined explicit memory. Two weeks after the fMRI session, participants completed a recognition memory test for the negative and neutral pictures presented in the scanner. In controls, high confidence memory responses are typically more accurate for arousing vs. neutral material, an effect linked to amygdala activation at encoding (Canli et al., 2004; Dolcos et al., 2004). A prior study in a mostly medicated sample suggested that this mechanism is hyperactive in depression (Hamilton and Gotlib, 2008). Thus, we performed a subsequent memory analysis to test whether the MDD group showed stronger amygdala activation than controls during successful encoding of negative pictures. We also investigated whether memory was sensitive to reappraisal.
2. Methods
2.1. Procedures
2.1.1. Participants
Twenty-seven controls and 14 depressed individuals participated. Data from three controls and one depressed participant were excluded due to excessive head motion (> 4 mm or degrees incremental). A depressed participant with amygdala activation 5 SDs below the MDD mean was removed, leaving 24 controls and 12 depressed participants. Valence ratings were not recorded for one depressed participant. Twenty-two controls and all depressed participants completed a memory test two weeks later. Consent was obtained, consistent with an IRB-approved protocol. Participants were paid (MRI: $25/hour; memory: $10/hour) and debriefed.
2.1.2. Stimulus selection
Three sets of 144 pictures (72 negative, 72 neutral) were used in the MRI session, as distracters in the memory test, and in an electroencephalography session following the memory test (data not presented). Assignment of picture sets to sessions was counterbalanced. Negative pictures included images from the International Affective Picture System (IAPS) (Lang et al., 2005) and the Internet depicting threatening animals, violence, drug use, accidents, painful medical procedures, poverty, and old age. Neutral pictures depicted people engaged in mundane activities.
2.1.3. Stimulus validation
Nine laboratory members (5 females) rated the pictures for valence (1 = negative, 9 = positive) and arousal (1 = calm, 9 = excited). Gender x Set x Picture Type ANOVAs revealed only effects of Picture Type for valence (negative: 2.62±0.60; neutral: 5.55±0.47; p = 0.001) and arousal (negative: 6.96±0.31; neutral: 4.14±0.80; p = 0.006). Thus, the pictures elicited the intended emotional responses in both genders.
2.1.4. Diagnostic interview
Eligibility was established using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (First et al., 2002). Depressed participants were unmedicated, met criteria for MDD, and had no history of psychosis. Comorbidity was mainly confined to anxiety disorders (see Results). Past psychotropic medication was allowed (no use in the past 2 weeks for benzodiazepines, 6 weeks for SSRIs, 6 months for dopaminergic drugs). Two depressed participants were attending psychotherapy sessions once or twice monthly; the other depressed participants were not in therapy. Five depressed participants reported past psychotherapy of varying duration (one month or less, n = 2; two years or less, n = 2; unclear, n = 1). Controls reported no current or past Axis I diagnosis. Participants were 18 - 64 years old and right-handed. None presented with neurological conditions or significant medical history, or met criteria for lifetime substance dependence or substance abuse in the last year.
2.1.5. Reappraisal task
The task was designed to modulate emotional experience and minimize demand characteristics. Trials included a cue word (“REAL”, “LOOK”, or “PHOTO”; duration: 1 s), a jittered inter-stimulus interval (ISI: 3-5 s), a negative or neutral picture (6 s), a second ISI (1.5-3 s), and a rating screen (3 s). The rating screen displayed self-assessment manikins (Lang et al., 2005) corresponding to five levels on a valence scale (1 = negative, 3 = neutral, 5 = positive). Participants pressed a button to rate their emotional state at trial end. A fixation cross was presented during the ISIs and inter-trial interval (2-11 s). Participants completed 12 practice trials after the interview and in the scanner to ensure comprehension. During scanning, they completed six blocks of 24 trials. Optimal trial sequences were determined with optseq (Dale, 1999). The cues were explained after the interview and at the outset of the MRI session. To maximize experimental control, we constrained the reappraisal technique by emphasizing self-focused reappraisal rather than situation-focused reappraisal, in which participants reinterpret negative situations in order to envision more positive outcomes (Ochsner et al., 2004). Specifically, in response to the real cue, participants were asked to mentally place themselves in scenes as though they were happening now, and vividly imagine all the sensations that would be experienced. This was intended to intensity negative emotional experience. By contrast, the photo cue was designed to dampen responses to negative pictures by increasing the sense of psychological distance (Kross and Ayduk, 2008). Thus, in response to the photo cue, participants were told to imagine that scenes were old, posed photographs being viewed from a distance. In response to the look cue, participants viewed pictures without controlling their responses. The instructions emphasized imagery rather than emotion regulation to limit demand characteristics1.
The task was programmed in E-Prime (Psychology Software Tools, Inc; Sharpsburg, PA). Behavioral data were analyzed with SPSS version 19.0.0 software (IBM; Armonk, NY).
2.1.6. Questionnaires
To assess depressive and anxious symptoms, habitual use of emotion regulation strategies, and mental imagery, the following self-report measures were administered after scanning: the Beck Depression Inventory-II (BDI-II: Beck et al., 1996), Emotion Regulation Questionnaire (ERQ: Gross and John, 2003), Mood and Anxiety Symptom Questionnaire (MASQ: Watson et al., 1995), Ruminative Responses Scale (RRS: Nolen-Hoeksema and Morrow, 1991; Treynor et al., 2003), and Vividness of Visual Imagery Questionnaire (VVIQ: Marks, 1973). The Wechsler Test of Adult Reading (WTAR: Green et al., 2008; Psychological Corporation, 2001) provided an IQ estimate.
2.1.7. MRI acquisition
MRI data were collected on a 3 T magnet (Siemens, USA; 12-channel head coil). Sessions included an auto-align localizer (van der Kouwe et al., 2005), a T1-weighted MPRAGE structural image (1.2 mm3 voxels; 144 slices; TR = 2.2 s; TE1/2/3/4 = 1.54/3.36/5.18/ 7.01 ms; flip angle = 7 degrees), and T2*-weighted images sensitive to blood oxygen level-dependent contrast, acquired during the reappraisal task (3.0 mm3 voxels; 46 slices; TR = 3 s; TE = 30 ms; flip angle = 85 degrees; transverse acquisition).
2.1.8. Recognition memory test
The 144 “old” pictures from the MRI session plus 144 “new” distracters were presented. Participants indicated whether pictures were old or new, and rated their confidence (high, medium, low) in each decision. There was no time limit for either response. The picture sequence was random, and the BDI-II was re-administered.
2.2. Data analysis
2.2.1. Questionnaires
Scale scores were computed for the MASQ (General Distress: Depression [MASQ-GDD], Anhedonic Depression [MASQ-AD], General Distress: Anxiety [MASQ-GDA], and Anxious Arousal [MASQ-AA]), the RRS (RRS-Brooding, RRS-Reflection, RRS-Depression), and the ERQ (habitual use of reappraisal [ERQ-R] and expressive suppression [ERQ-S]). Total scores were computed for the BDI-II, VVIQ, and WTAR. WTAR scores were age-normed. Group differences were assessed by two-tailed t-test.
2.2.2. Valence ratings
Ratings were entered into a Group x Gender x Cue x Picture Type ANOVA. For all ANOVAs, Greenhouse-Geisser corrected p-values are reported when sphericity was violated. Exploratory analyses investigated whether reappraisal efficacy, assessed with [negative/real – negative/photo] valence rating difference scores, was correlated with BDI-II, RRS-Brooding, RRS-Reflection, or ERQ-R scores.
2.2.3. Recognition memory: emotion analysis
A Group x Gender x Confidence (high, low) x Picture Type ANOVA was conducted on [hit rate - false alarm rate] scores for old items. False alarm rates were subtracted from hit rates because emotion tends to increase both, thus considering only hit rates can inflate estimates of improved memory (Sharot et al., 2005; Dougal and Rotello, 2007). To avoid spurious results, only data from participants with at least 10 high confidence negative hits and 10 negative misses were analyzed (controls: n = 17; MDD: n = 11). Repeating this analysis with all participants yielded identical results (Supplementary Material).
2.2.4. Recognition memory: reappraisal analysis
A Group x Gender x Cue x Picture Type ANOVA was conducted on hit rates. False alarms were not subtracted as there is no independent measure of false alarms for the cue conditions within each picture type.
2.2.5. fMRI pre-processing
Pre-processing involved: discarding five volumes collected at the onset of each run to ensure stable longitudinal magnetization; slice-time and motion-correction using the FSL tools slicetimer and mcflirt (Jenkinson et al., 2002); segmentation of brain tissue (Smith, 2002); coregistration; normalization to MNI152 templates; re-sampling to 2 mm3 voxels; and spatial smoothing (6 mm FWHM).
2.2.6. fMRI: reappraisal and subsequent memory analyses
The general linear model (GLM) implemented in SPM8 (Wellcome Department of Cognitive Neurology, London, UK) was used for statistical analysis. Onset times and durations for the cues, pictures, and rating screen were convolved with a canonical hemodynamic response function, and nuisance regressors accounted for run-to-run fluctuations in mean image intensity. The data were high-pass filtered (cut-off period: 128 s). The GLM returns least squares parameter estimates (“beta weights”) for conditions of interest, which were used in separate reappraisal and subsequent memory analyses.
The reappraisal analysis consisted of a Group x Reappraisal Condition (negative/real, negative/look, negative/photo) ANOVA (Urry et al., 2009). The main effect of Reappraisal Condition was expected to reveal increased activation on negative/real vs. negative/photo trials in regions implicated in emotional arousal (amygdala; Ochnser et al., 2004), self-referential processing (medial PFC; Mitchell et al., 2005), and mental imagery (parietal cortex; Farah, 1984), with negative/look trials eliciting intermediate activation. No predictions were made regarding the main effect of Group. However, Group x Reappraisal Condition interactions were expected in prefrontal areas thought to implement reappraisal, as well as in sub-cortical regions whose activation is affected by reappraisal, namely the amygdala. Weaker modulation of brain activation by reappraisal was expected in the MDD group.
Given the link between amygdala activation and subsequent memory for emotional stimuli in controls, as well as evidence of amygdala hyperactivation during negative picture encoding in depressed adults (Hamilton and Gotlib, 2008), the subsequent memory analysis focused on negative pictures. Encoding responses were binned according to eventual memory status, and a [high confidence negative hits – negative misses] contrast identified brain regions whose activation was linked to accurate memory. This contrast was computed in each group separately, and a between-groups t-test investigated whether amygdala activation was stronger in the MDD group.
2.2.7. fMRI: whole-brain regressions
Activation in a [negative/real – negative/photo] contrast was regressed against BDI scores in the MDD group to identify brain regions where the range of activation modulation during reappraisal was negatively correlated with depression severity. The [negative/real – negative/photo] contrast was used to maximize the likelihood of identifying effects of depression on emotional flexibility. Negative correlations were expected in the amygdala and DLPFC (Erk et al., 2010a; Siegle et al., 2002, 2007). To identify regions that tracked shifts in subjective experience, this contrast was also regressed against [negative/photo – negative/real] valence rating difference scores. In this analysis, stronger effects of reappraisal on subjective experience (bigger valence drops from the photo to real trials) are positively correlated with larger effects in the [negative/real – negative/photo] contrast.
2.2.8. fMRI: multiple comparisons correction
The voxelwise p-value was 0.005. Inferences were made after multiple comparisons correction using Gaussian Random Fields. Only clusters significant at p < 0.05 (corrected) are reported unless otherwise noted. Given a priori interest, contrasts in the amygdala were corrected for multiple comparisons over the amygdala mask in the Wake Forest University PickAtlas (Maldjian et al., 2003). MarsBaR was used to extract beta weights for additional analysis (Brett et al., 2002).
3. Results
3.1. Clinical data
Data on the number and timing of Major Depressive Episodes (MDE) are provided in Table 1. Four depressed participants had co-morbid anxiety (two had social anxiety disorder; one had social anxiety and panic disorder; one had social anxiety, panic disorder, and specific phobia), and another met criteria for binge eating disorder.
Table 1.
Demographics and Self-Report Data
| Variable | Controls | Depressed | t / χ2 | p |
|---|---|---|---|---|
| MRI Session | ||||
| Number of MDEs | -- | 2.33 (1.56) | -- | -- |
| Age at first MDE | -- | 18.58 (7.65) | -- | -- |
| Gender | 12 f, 12 m | 7 f, 5 m | 0.22 | 0.637 |
| Age (years) | 34.42 (14.93) | 31.00 (8.20) | 0.89 | 0.382 |
| Education (years) | 15.88 (1.51) | 15.33 (2.06) | 0.90 | 0.376 |
| BDI-II (fMRI session) | 1.63 (2.34) | 25.83 (10.94) | −7.58 | < 0.001 |
| BDI-II (memory session)* | 1.18 (2.65) | 21.42 (10.10) | −6.81 | < 0.001 |
| MASQ-GDD | 13.29 (2.05) | 37.33 (10.24) | −8.06 | < 0.001 |
| MASQ-AD | 47.38 (12.24) | 83.08 (8.97) | −8.95 | < 0.001 |
| MASQ-GDA | 13.17 (2.06) | 25.00 (5.31) | −7.45 | < 0.001 |
| MASQ-AA | 18.38 (1.58) | 27.33 (10.88) | −2.84 | 0.016 |
| RRS-Brooding | 7.42 (2.08) | 12.58 (3.53) | −5.54 | < 0.001 |
| RRS-Depression | 17.67 (5.06) | 32.25 (6.52) | −7.40 | < 0.001 |
| RRS-Reflection | 9.46 (4.01) | 11.33 (3.60) | −1.37 | 0.181 |
| ERQ-Reappraisal | 30.96 (4.36) | 27.83 (8.74) | 1.17 | 0.262 |
| ERQ-Suppression | 12.13 (4.03) | 14.67 (4.58) | −1.71 | 0.097 |
| VVIQ | 29.25 (9.86) | 33.50 (9.89) | −1.22 | 0.232 |
| WTAR-standardized score† | 117.00 (7.17) | 102.30 (13.83) | 2.54 | 0.028 |
Note. f = female; m = male; BDI = Beck Depression Inventory II; MASQ = Mood and Anxiety Symptoms Questionnaire (GDD = General Distress: Depressive symptoms, AD = Anhedonic Depression, GDA = General Distress: Anxious Symptoms, AA = Anxious Arousal); RRS = Ruminative Responses Scale; ERQ = Emotion Regulation Questionnaire; VVIQ = Vividness of Visual Imagery Questionnaire; WTAR = Wechsler Test of Adult Reading.
Memory session data are from 22 controls (11 f, 11 m) and 12 depressed participants (7 f, 5 m).
WTAR data from two non-native English speaking participants in the MDD group were not analyzed. Data are frequency counts or mean (SD).
3.2. Demographics and questionnaires
There were no group differences in age, education, or gender (Table 1). The MDD group reported more brooding and anxious/depressive symptoms than controls, but there were no differences in VVIQ, reflection, or habitual use of reappraisal or expressive suppression. Controls had higher WTAR scores.
3.3. Valence ratings
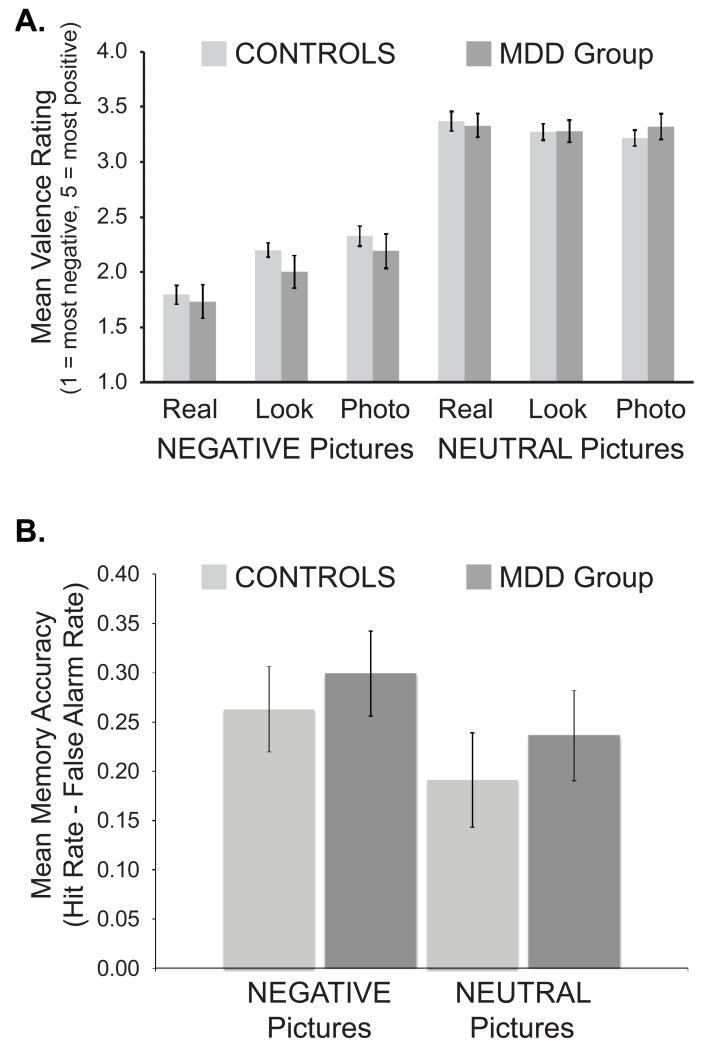
Reappraisal affected responses to negative pictures while having weak effects on responses to neutral pictures, but this was not influenced by MDD (Figure 1A; see caption for statistics). Valence was lowest on negative/real trials, intermediate on negative/look trials, and highest on negative/photo trials. There were no significant correlations between BDI-II, RRS-Brooding, RRS-Reflection, or ERQ-Reappraisal scores and [negative/real – negative/photo] rating difference scores.
Figure 1.
Behavioral results. (A) Valence ratings. There was a Cue x Picture Type interaction, F(2, 62) = 19.66, p < 0.001, that did not vary by Group (Group x Cue x Picture Type, F(2, 62) = 1.18, p = 0.32). A Cue effect was seen on negative trials, F(2, 62) = 22.79, p < 0.001, but not neutral trials (F(2, 62) = 2.60, p = 0.08). Valence ratings were lowest on negative/real trials, intermediate on negative/look trials, and highest on negative/photo trials (t(34) values > 2.69, ps < 0.02). (B) Memory accuracy for pictures remembered with high confidence. Accuracy was characterized by a Confidence x Picture Type interaction, F(1, 24) = 8.71, p = 0.007, but this did not interact with Group (Group x Confidence x Picture Type, F < 1). Accuracy was higher for negative (0.28±0.16) vs. neutral (0.21±0.18) pictures recognized with high confidence, t(27) = 3.57, p = 0.001, but not low confidence, t(27) < 1, p = 0.60 (data not shown). Error bars denote standard error of the mean.
3.4. Recognition memory: emotion analysis
A beneficial effect of emotion on high confidence responses was observed, but was not affected by depression (Figure 1B). Accuracy was higher for negative vs. neutral pictures remembered with high confidence. No effects involving Group were significant.
3.5. Recognition memory: reappraisal analysis
No effects of reappraisal on memory were found.
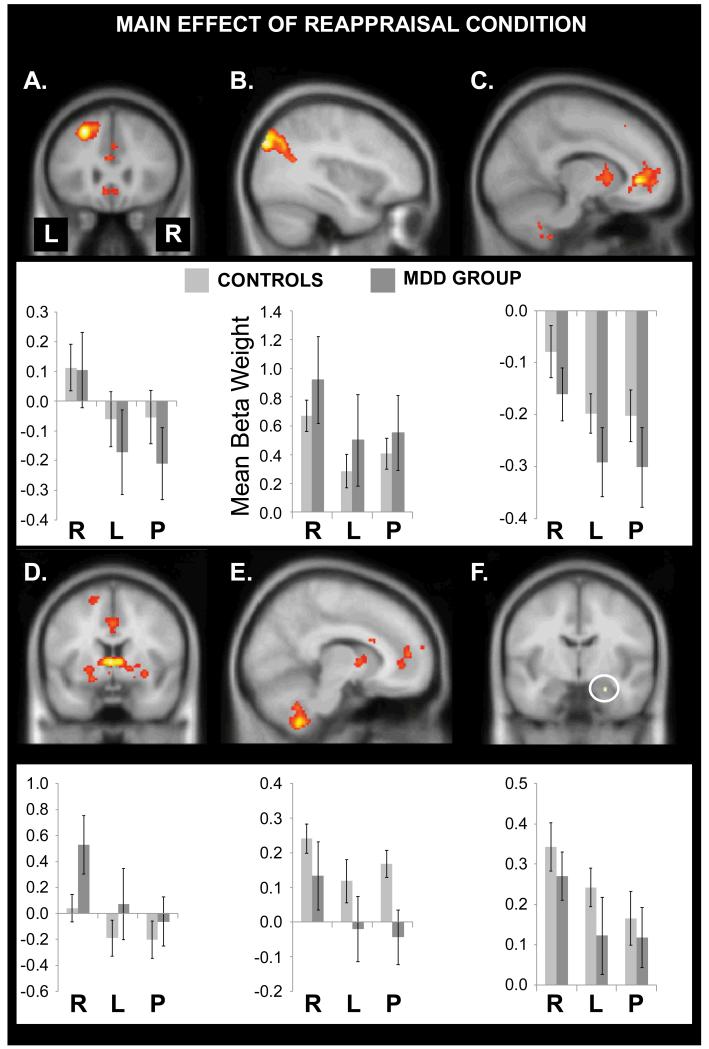
3.6. fMRI: reappraisal model
As shown in Figure 2 and Table 2, the main effect of Reappraisal Condition revealed activation in the left DLPFC, left parietal cortex, rostral anterior cingulate cortex (rACC) extending into medial PFC, caudate, and the right amygdala, with a trend in the right cerebellum. To decompose these results, beta weights were extracted from spherical ROIs (8 mm radius) centered on the peak voxel in each region and submitted to Group x Reappraisal Condition ANOVAs. For the right amygdala, activation was simply extracted from the 5 significant voxels.
Figure 2.
Main effects of Reappraisal Condition. Reappraisal modulated brain activation elicited by negative pictures in (A) left DLPFC, (B) left parietal cortex, (C) rostral anterior cingulate extending into medial PFC, (D) anteroventral caudate, (E) the cerebellum, and (F) the right amygdala. The y-axes indicate the size of the mean beta weights for controls (light gray bars) and depressed participants (dark gray bars); the x-axes indicate the reappraisal condition (R = real, L = look, P = photo). Error bars show the standard error of the mean. No significant group differences were observed.
Table 2.
Effects of Reappraisal and Group on fMRI Activation Elicited by Negative Pictures
| Region | x | y | z | Voxels | Z-score | FWE- corrected p-value |
|---|---|---|---|---|---|---|
| Main Effect of Reappraisal Condition | ||||||
| Left middle frontal gyrus | −26 | 26 | 46 | 821 | 5.71 | 0.001 |
| Left lateral occipital cortex (superior division) |
−36 | −84 | 36 | 1742 | 5.42 | 0.004 |
| Rostral anterior cingulate | −12 | 38 | 0 | 2220 | 5.34 | 0.005 |
| Caudate (anteroventral) | −2 | 4 | 4 | 856 | 5.33 | 0.005 |
| Right cerebellum | 10 | −52 | −54 | 545 | 4.78 | 0.067 |
| Right amygdala* | 24 | −8 | −22 | 5 | 3.36 | 0.023 |
| Main Effect of Group | ||||||
| No significant activations. | ||||||
| Group x Reappraisal Condition Interaction | ||||||
| No significant activations. | ||||||
Note. *The p-value for this cluster reflects multiple comparison correction using the structurally-defined bilateral amygdala mask from the Wake University PickAtlas. For all other regions, p-values are given for the peak voxel and reflect multiple comparison correction over the whole brain.
The main effect of Reappraisal Condition was significant in each region (F(2, 68) values > 5.77, ps < .01). As depicted in Figure 2 (bar graphs), in every ROI activation was stronger on negative/real vs. negative/look trials (t(35) values > 2.31, ps < 0.03) and negative/photo trials (t(35) values > 4.20, ps < 0.001). Activation did not differ between negative/look and negative/photo trials in any region (t(35) values < 1.52, ps > 0.13). Thus, reappraisal effects were observed in expected regions and driven by increased activation on negative/real trials.
Contrary to the primary hypothesis, and in favor of the alternative hypothesis, no brain region showed a significant Group x Reappraisal Condition interaction or main effect of Group (Table 2). To protect against Type II error, an exploratory amygdala ROI analysis looked for any voxels showing a Group x Reappraisal Condition interaction, but none were found. Psychophysiological interaction analyses were conducted to determine if functional connectivity of the right amygdala, left DLPFC, or rACC differed across the negative/real and negative/photo conditions, but no group differences emerged (Supplementary Material). Thus, effects of reappraisal on brain activation were similar across groups.
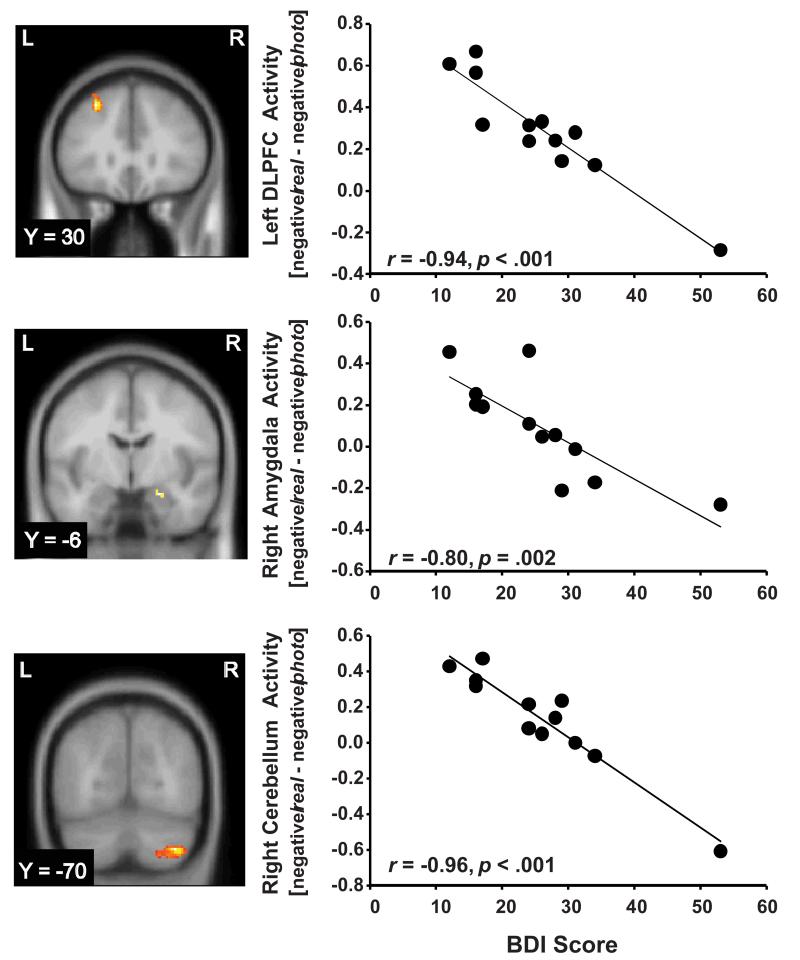
3.7. fMRI: correlations with BDI-II
Regressing the [negative/real –negative/photo] contrast against BDI-II scores in the MDD group revealed negative correlations in the left DLPFC, right amygdala, and right cerebellum (Figure 3). Increased depressive severity was associated with weaker effects of reappraisal on brain activation in these regions. To test the specificity of these relationships, identical analyses were performed with MASQ-GDA and MASQ-AA scores; no significant findings emerged, providing evidence that these correlations were specific to depressive symptoms rather than general psychological distress.
Figure 3.
Negative correlations in the MDD group between BDI scores and activity in the left DLPFC (peak voxel: −28, 30, 46; Z = 4.50; 306 voxels; cluster p = 0.001; r(10) = −0.94, p < 0.001), right amygdala (peak voxel: 22, −6, −18; Z = 3.15; 7 voxels; cluster p = 0.051; r(10) = −0.80, p = 0.002), and right cerebellum (peak voxel: 36, −70, −44; Z = 4.86; 226 voxels; cluster p = 0.011; r(10) = −0.96 p < 0.001) in the [negative/real – negative/photo] contrast. Excluding the subject with the highest BDI score did not substantially weaken the correlations (DLPFC: r(9) = −0.87, p = 0.001; amygdala: r(9) = −0.78, p = 0.005; cerebellum: r(9) = −0.88, p = 0.001).
3.8. fMRI: correlation with valence ratings
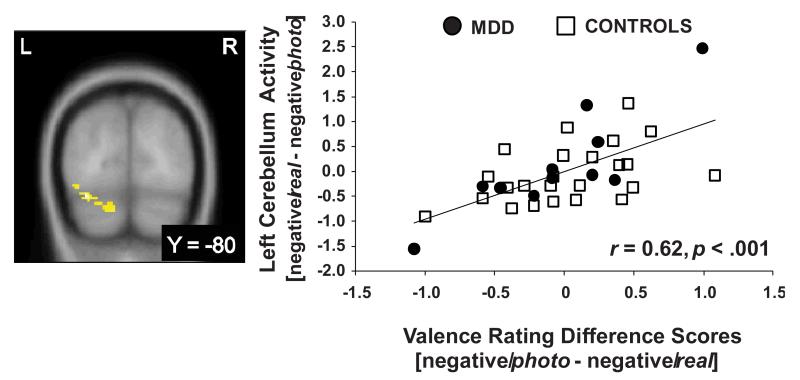
Regressing the [negative/real – negative/photo] contrast against [negative/photo – negative/real] valence rating scores revealed a correlation in the left cerebellum (Figure 4).
Figure 4.
Positive correlation between left cerebellum activity in the [negative/real – negative/photo] contrast and [negative/photo – negative/real] valence rating difference scores, across groups (peak voxel: −34, −80, −20; Z = 3.98, 363 voxels; cluster p = 0.009; r(33) = 0.62, p < 0.001). Increased brain activation is positively correlated with a stronger shift in subjective experience. Both brain activation and ratings scores are mean centered.
3.9. fMRI: subsequent memory model
No significant clusters were seen when the [high confidence negative hits – negative misses] contrast was computed separately in each group, and no significant group differences emerged. When the data were collapsed across groups, the peak activation was just dorsal to the right amygdala (peak: 20, 2, −12; Z = 4.14; 106 voxel cluster). A structural ROI analysis confirmed right amygdala activation (peak: 20, −6, −20; Z = 3.92; 40 voxels; cluster p = 0.01).
4. Discussion
MDD is characterized by truncated emotional reactions (Rottenberg, 2005; Rottenberg et al., 2005), and we hypothesized that this lack of emotional flexibility would limit reappraisal. Prior studies have reported mixed findings, but some evaluated medicated (Erk et al., 2010a) or remitted (Ehring et al., 2010; Kanske et al., 2012) samples, possibly underestimating depression effects. Thus, we tested an unmedicated MDD group. Contrary to expectations, reappraisal reliably affected valence ratings and brain activation in the MDD group. This supports the alternative hypothesis that depressed participants can reappraise negative emotions if given detailed instructions and cues. The findings echo studies indicating that, although depressed individuals often perform poorly on unstructured tasks, they can exhibit normative performance if supported (Ehring et al., 2010; Hertel and Rude, 1991).
However, this conclusion is tempered by negative correlations between BDI-II scores and reappraisal effects in the left DLPFC, right amygdala, and right cerebellum (Figure 3). These data are consistent with work implicating the cerebellum in emotion regulation (Schutter and van Honk, 2009) and linking DLPFC and amygdala dysfunction to depression (Siegle et al., 2002, 2007). Moreover, they dovetail with previously reported negative relationships between depressive severity and right amygdala modulation during reappraisal (Erk et al., 2010a), as well as between depressive severity and difficulty regulating sadness (Beauregard et al., 2006). These correlations suggest that despite the use of detailed instructions, more severe depression had a negative effect on brain systems implicated in reappraisal, although it was not large enough to support a group difference. Future studies should recruit larger samples of more severely depressed individuals, and may wish to take additional steps to maximize the paradigm’s sensitivity to depression (see section 4.4).
4.1. Depression and modulation of subjective experience by reappraisal
Trial-by-trial valence ratings indicated that all participants could reappraise negative emotional experience. Across groups, valence ratings were lowest on negative/real trials, intermediate on negative/look trials, and highest on negative/photo trials (Figure 1a). These results are consistent with prior studies (Beauregard et al., 2006; Sheline et al., 2009) and confirm reliable effects of reappraisal on negative emotional experience in acute, unmedicated depression. Similar effects have been reported in remitted samples (Ehring et al., 2010; Kanske et al., 2012). Thus, depression does not appear to strongly affect reappraisal-based modulation of self-reported negative experience.
This evidence of effective reappraisal in the MDD group is encouraging and reminiscent of the efficacy of cognitive therapy for depression (Beck et al., 1979; Gloaguen et al., 1998). However, this study was not designed with clinical practice in mind, and the “distancing” technique used in the photo condition differs substantially from the methods used to challenge automatic negative thinking in cognitive therapy (e.g., hypothesis-testing). Building strong links between research on reappraisal and clinical practice thus remains an important goal.
4.2. Effects of reappraisal on brain activation and the default mode network
Across groups, reappraisal modulated activation in the left DLPFC, left parietal cortex, rACC/medial PFC, and right amygdala. Left DLPFC activation may reflect the generation and maintenance of reappraisal plans in working memory (Curtis and D’Esposito, 2003). Neurological data link generation of visual images to left posterior parietal cortex (Farah, 1984), thus left parietal activation may index the use of imagery to achieve reappraisal goals. Modulation of rACC/medial PFC activation during self-focused reappraisal is consistent with the established role of these regions in self-referential processing (Mitchell et al., 2005; Phan et al., 2004), and reappraisal-based shifts in amygdala activation may reflect changes in subjective experience.
At a systems level—and with the exception of the left DLPFC—the brain regions activated by reappraisal strongly resemble the default mode network (DMN; Buckner et al., 2008; Habas et al., 2009; Raichle et al., 2001). Indeed, inspection of the [negative/real – negative/photo] contrast collapsed across the groups (data not shown) reveals the regions in Figure 3 plus right parietal cortex and precuneus, yielding considerable overlap with the DMN (Buckner et al. 2008). Although the DMN is the focus of intense interest, its role in emotion regulation has not been emphasized. We propose that self-focused reappraisal should reliably activate the DMN, because the DMN supports self-relevant mental simulations (Buckner et al., 2008) and self-focused reappraisal entails mentally reframing events to modify their personal relevance and emotional impact. Furthermore, reappraisal often requires two processes— envisioning future scenarios and deploying theory of mind—that robustly activate the DMN (Buckner et al., 2008).
The rACC data in Figure 3 highlight the link between the DMN and reappraisal. Although DMN regions can show positive activations, the network was originally recognized because midline cortical regions showed consistent deactivation during task-based stimulus processing relative to passive control conditions (Raichle et al., 2001). In the current study, the rACC showed this response profile: all conditions yielded deactivations vs. fixation. Furthermore, predicting the order of reappraisal condition effects in this region is straightforward based on the DMN literature. DMN activation supports self-focused mentation, and when external stimuli are processed, this inward-directed mentation is reduced, leading to deactivation relative to baseline. Therefore, when reappraisal is used to increase the personal relevance of stimuli, rACC deactivation should be reduced because self-referential processing is ongoing. This is evident in Figure 3, as the negative/real condition yielded the weakest rACC deactivation.
This implies that stronger deactivation from baseline should be seen when reappraisal is used to de-emphasize self-referential processing. This hypothesis was not confirmed, as rACC deactivation was not stronger in the negative/photo vs. the negative/look condition. This reflects the limitations of the photo condition rather than a problem with conceptualization of DMN function, as no region showed differential activation on negative/look vs. negative/photo trials. These results raise an important caveat: although the real and photo cues modulated valence ratings, only the real cue reliably influenced brain activation. Thus, the fMRI results only support inferences about emotional flexibility and amplification of negative emotional experience.
This pattern of reappraisal results—stronger effects in the “increase” vs. the “decrease” condition—has been observed in studies using fMRI (Urry et al., 2006) and eyeblink startle responses (Dillon and LaBar, 2005), but it may appear to contrast with reports of increased lateral PFC activation and reduced amygdala activation when reappraisal is used to decrease negative emotional experience (e.g., Ochsner et al., 2004). However, even these studies suggest that the “distancing” technique used in the photo condition does not powerfully affect brain activation. For example, Ochsner and colleagues (2004) reported that when reappraisal was used to decrease negative emotion, bilateral PFC regions (along with many other regions) were more strongly activated during situation-focused vs. self-focused reappraisal. By contrast, only small sectors in the cingulate and left parietal cortex showed stronger activation during self-focused reappraisal. Similarly, Kross et al. (2009) elicited negative emotion in healthy volunteers and instructed them to feel the negative emotion as normal or reduce it, either by analyzing its causes or using a mindfulness-based acceptance strategy. Both the “analyze” and “accept” strategies reduced negative emotional experience, but neither elicited stronger activation in any brain region than the “feel” condition. The current study found the same pattern: the negative/photo condition reduced negative emotional experience, but the negative/real condition had a stronger effect on brain activity.
Intriguingly, cerebellum activation emerged as positively correlated with shifts in subjective experience (Figure 4), consistent with a growing appreciation of cerebellar contributions to emotional responses. Although effects of cerebellar lesions on emotional responding are often subtle, they can lead to disinhibition and flat affect (Levisohn et al., 2000; Schmahmann and Sherman, 1998). Moreover, a transcranial magnetic stimulation study linked cerebellar inhibition to increased negative mood after a reappraisal task (Schutter and van Honk, 2009). The present study extends these findings by indicating that cerebellar activation is related to modulation of subjective experience during reappraisal.
4.3. Memory
As expected, memory accuracy was higher for confidently remembered negative vs. neutral pictures, and confidently remembered negative pictures elicited stronger right amygdala activation at encoding than negative misses. However, depression did not affect these results, and the amygdala result only emerged only when all participants were considered. This is consistent with a meta-analysis indicating that depression leaves memory for negative material intact (Burt et al., 1995). The memory advantage for negative vs. neutral material was not stronger in depressed participants vs. controls.
We found no effects of reappraisal on memory. This might reflect the 2-week delay following encoding, as positive effects of reappraisal on memory have been reported at delays of one hour or less (Dillon et al., 2007; Richards and Gross, 2000), but not one year (Erk et al., 2010b). Another critical factor concerns the reappraisal strategy and activation of the left ventrolateral PFC (VLPFC). Deep processing of verbal stimuli elicits left VLPFC activation (Fletcher et al., 2003; Otten et al., 2001) and supports explicit memory (Craik and Tulving, 1975). When participants use situation-focused reappraisal to reinterpret negative stimuli in more favorable ways, stronger left VLPFC activation is seen than when they use self-focused reappraisal (Ochsner et al., 2004). This is noteworthy because an fMRI study found a positive effect of reappraisal on memory after two weeks delay that was linked to left VLPFC and hippocampal activation (Hayes et al., 2010). Thus, reappraisal may affect memory via left VLPFC activation, which was not observed here.
4.4. Limitations and considerations for future studies
This study is limited by the small MDD sample and by the fact that the photo cue did not reliably modulate brain activation, restricting inferences about neural systems involved in the reduction of negative emotion. Future studies should consider taking four steps to address these limitations. First, larger samples of more severely depressed participants are needed. Second, it would be valuable to replace the broadly negative stimulus set used here with depressogenic stimuli organized around themes of sadness and hopelessness (Watkins et al., 1992). Third, it may be useful to induce negative mood prior to the reappraisal task, as this impairs emotion regulation in healthy volunteers (Berna et al., 2010) and may be especially potent in depressed adults. Similarly, presenting reappraisal cues mid-way through emotional stimulus presentation, rather than before, may increase task difficulty for depressed participants. Fourth, situation-focused reappraisal may be better suited for probing emotion regulation in depression than self-focused reappraisal. As noted earlier, situation-focused reappraisal more consistently activates lateral PFC regions that may be hypofunctional in depression. Moreover, situation-focused reappraisal likely requires greater suppression of DMN activity, which may be impaired in depression (Anticevic et al., 2012). Indeed, one study of situation-focused reappraisal already reported weak DMN suppression in depressed adults (Sheline et al., 2009).
4.5. Conclusion
This study suggests that unmedicated, depressed adults can reappraise negative emotions if provided with clear instructions. However, severe depression was associated with weak reappraisal effects in the DLPFC, amygdala, and cerebellum, suggesting that group differences in these regions may be evident with larger, more severely depressed samples.
Supplementary Material
Acknowledgements
This work was supported by an individual post-doctoral Ruth S. Kirschstein National Research Service Award granted by the National Institute of Mental Health to D.G.D. (F32MH081394-02) and R01 MH068376 awarded to D.A.P. The authors wish to thank Dr. Randy Buckner for assistance implementing MRI data collection and analysis, Nancy Hall Brooks for diagnostic assessment of all participants, and Sunny Dutra for assistance with subject recruitment and testing.
Footnotes
See Supplementary Material for verbatim instructions.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed TR. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. Guilford Press; NY: 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biological Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:S497. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao A, Brewer J, Gabrieli JDE, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, LaBar KS. Startle modulatin during conscious emotion regulation is arousal-dependent. Behavioral Neuroscience. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Ritchey M, Johnson BD, LaBar KS. Dissociable effects of conscious emotion regulation strategies on explicit and implicit memory. Emotion. 2007;7:354–365. doi: 10.1037/1528-3542.7.2.354. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychonomic Bulletin and Review. 2007;14:423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnulle J, Fischer S, Gross JJ. Emotion regulation and vulnerability to depression: Spontaneous vs. instructed use of emotion suppression and reappraisal. Emotion. 2010;10:563–572. doi: 10.1037/a0019010. [DOI] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. Journal of Neuroscience. 2010a;30:15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, von Kalckreuth A, Walter H. Neural long-term effects of emotion regulation on episodic memory processes. Neuropsychologia. 2010b;48:989–996. doi: 10.1016/j.neuropsychologia.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Farah MJ. The neurological basis of mental imagery: A componential analysis. Cognition. 1984;18:245–272. doi: 10.1016/0010-0277(84)90026-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fletcher PC, Stephenson CM, Carpenter TA, Donovan T, Bullmore ET. Regional brain activations predicting subsequent memory success: an event-related fMRI study of the influence of encoding tasks. Cortex. 2003;39:1009–1026. doi: 10.1016/s0010-9452(08)70875-x. [DOI] [PubMed] [Google Scholar]
- Gloaguen V, Cottraux J, Cucherat M, Blackburn IM. A meta-analysis of the effects of cognitive therapy in depressed patients. Journal of Affective Disorders. 1998;49:59–72. doi: 10.1016/s0165-0327(97)00199-7. [DOI] [PubMed] [Google Scholar]
- Green RE, Melo B, Christensen B, Ngo LA, Monette G, Bradbury C. Measuring premobid IQ in traumatic brain injury: an examination of the validity of the Wechsler Test of Adult Reading. Journal of Clinical and Experimental Neuropsychology. 2008;30:163–172. doi: 10.1080/13803390701300524. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Keller K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry. 2008;63:1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, LaBar KS. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Ayduk O. Facilitating adaptive emotional analysis: distinguishing distanced-analysis of depressive experiences from immersed-analysis and distraction. Personality and Social Psychology Bulletin. 2008;34:924–938. doi: 10.1177/0146167208315938. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Kraft R, Burdette J. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marks DF. Visual imagery differences in the recall of pictures. British Journal of Psychology. 1973;64:17–24. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho S-H, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Psychological Corporation . Wechsler Test of Adult Reading manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: the cognitive costs of keeping one’s cool. Journal of Personality and Social Psychology. 2000;79:410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Rottenberg J. Mood and emotion in major depression. Current Directions in Psychological Science. 2005;14:167–170. [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schutter D, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8:28–34. doi: 10.1007/s12311-008-0056-6. [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences, U.S.A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Fischl B, Schmitt F, Salat DH, Harder M, Sorenson AG, Dale AM. On-line automatic slice positioning for brain MR imaging. Neuroimage. 2005;27:222–230. doi: 10.1016/j.neuroimage.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Mathews A, Williamson DA, Fuller RD. Mood-congruent memory in depression: emotional priming or elaboration? Journal of Abnormal Psychology. 1992;101:581–586. doi: 10.1037//0021-843x.101.3.581. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104:15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.