Abstract
Background
As knowledge deepens about how new neurons are born, differentiate, and wire into the adult mammalian brain, growing evidence depicts hippocampal neurogenesis as a special form of neuroplasticity that may be impaired across psychiatric disorders. This review provides an integrated-evidence based framework describing a neurogenic basis for addictions and addiction vulnerability in mental illness.
Methods
Basic studies conducted over the last decade examining the effects of addictive drugs on adult neurogenesis and the impact of neurogenic activity on addictive behavior were compiled and integrated with relevant neurocomputational and human studies.
Results
While suppression of hippocampal neurogenic proliferation appears to be a universal property of addictive drugs, the pathophysiology of addictions involves neuroadaptative processes within frontal-cortical-striatal motivation circuits that the neurogenic hippocampus regulates via direct projections. States of suppressed neurogenic activity may simultaneously underlie psychiatric and cognitive symptoms, but also confer or signify hippocampal dysfunction that heightens addiction vulnerability in mental illness as a basis for dual diagnosis disorders.
Conclusions
Research on pharmacological, behavioral and experiential strategies that enhance adaptive regulation of hippocampal neurogenesis holds potential in advancing preventative and integrative treatment strategies for addictions and dual diagnosis disorders.
Keywords: addiction, dual diagnosis, hippocampus, learning and memory, neurogenesis, neuroplasticity
1. INTRODUCTION
First glimpsed as a dogma defying phenomenon 50 years ago (Altman, 1963), the birth of neurons in the adult mammalian brain is now widely accepted as an ordinary process with extraordinary implications (Eriksson et al., 1998; Kozorovitskiy and Gould, 2004). In describing what may be nature’s version of neural stem cell therapy, research on the generation and integration of new neurons in the adult hippocampus, a key structure implicated in most major mental disorders, provides a view of brain remodeling that many psychiatric patients may need to get better (Eisch et al., 2008).
Converging lines of evidence are now providing an increasingly clear picture implicating adult hippocampal neurogenesis in the pathophysiology of drug addiction, magnifying the public health relevance of this phenomena since substance disorders collectively represent the greatest cause of premature illness and death in the U.S. (Mokdad et al., 2004; Olive, 2011). This review integrates translational research outlining adult hippocampal neurogenesis in disease mechanisms of addiction and its comorbidity in mental illness. Starting with a brief summary of hippocampal neurogenesis and its putative role in psychiatric disorders based on several comprehensive reviews (Balu and Lucki, 2009; Eisch et al., 2008; Lledo et al., 2006; Ming and Song, 2005), we consider newer evidence supporting the following inter-related hypotheses: 1) addictive drugs suppress hippocampal neurogenesis; 2) low states of hippocampal neurogenesis heighten addiction vulnerability and/or severity; and 3) augmentation of neurogenic activity is an important therapeutic strategy for addictions and dual diagnosis disorders. Finally, we conclude with an exploration of key research implications and knowledge gaps that warrant further investigation for advancing addictions and integrated dual diagnosis treatment.
2. FUNDAMENTALS OF ADULT NEUROGENESIS
Neurogenesis is a process that encompasses the generation and development of individual neurons, and the re-population and structural revision of neural networks as a special form of neuroplasticity (Ming and Song, 2005; Schmidt-Hieber et al., 2004). As demonstrated in neural network modeling, neurogenesis works in conjunction with, and boosts ‘conventional’ synaptic plasticity, the basic mechanism of learning and memory, to provide a more efficient and reliable method for storing new data (Chambers et al., 2004; Luu et al., 2012). Neurogenesis begins with proliferation, the multiplication of stem cells, followed by differentiation and maturation weeks later, in which glial vs. neuronal phenotypic destinies are programed and fulfilled. Although new astroglial cells continue to divide throughout the adult mammalian brain, in only two structures, the subventricular zone (which supplies cells to the olfactory system), and the dentate gyrus (DG) of the hippocampus, do large quantities of cells become neurons (Ming and Song, 2005). In the young adult rat hippocampus, on the order of 103 nascent neurons are generated daily from stem cells, adding to 104 immature neurons that migrate into position within the DG granule cell layer which hosts a mature population of about 106 (Amrein et al., 2011; Lledo et al., 2006). There, young neurons ‘wire in’ by growing axo-dendritic connections with adjacent hippocampal compartments (Figure 1), acquiring physiological and neuroplastic properties of mature DG neurons (Lledo et al., 2006). At any developmental stage, neurogenic neurons could also die (Dayer et al., 2003). Because their information processing and neuroplastic capabilities are dependent on maturational stage (Schmidt-Hieber et al., 2004), their survival is of interest in understanding how they impact overall network function and cognition.
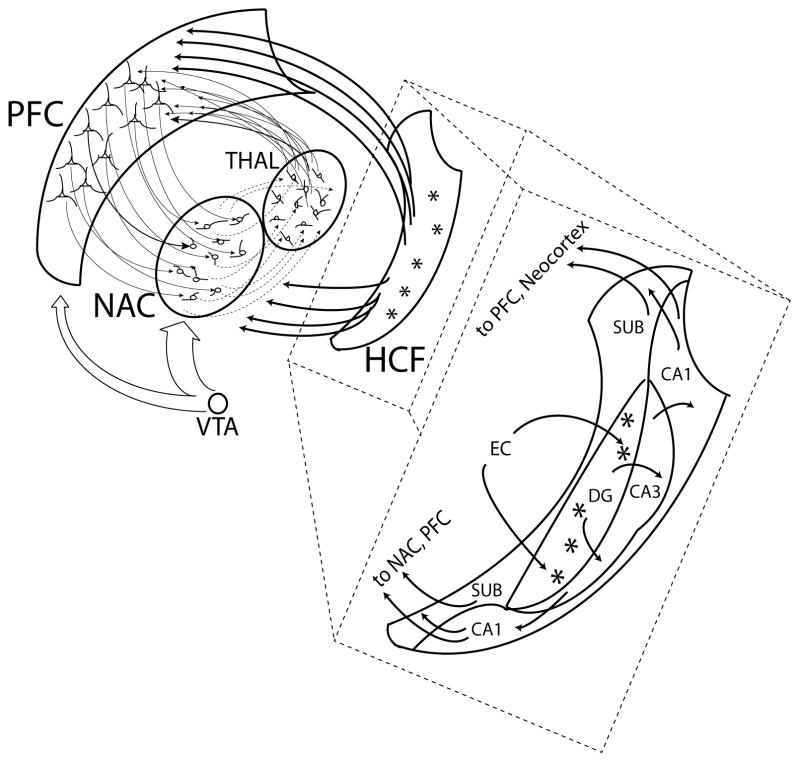
Figure 1. Prefrontal cortical-striatal-circuits and their modulation by hippocampal inputs with detailed circuit map of the neurogenic hippocampus.
Glutamatergic axonal projections are represented by solid arrows in and out of the Prefrontal Cortex (PFC) and hippocampal formation (HCF) and within HCF. Ventral Striatal GABAergic projections (from Nucleus Accumbens (NAC) to thalamus (THAL) (via pallidum, not shown)) are depicted as dotted arrows, with open arrows representing Dopamine projections from the Ventral Tegmental Area (VTA) to PFC and NAC. Prefrontal-cortical striatal-thalamocortical circuit loops flowing thorough the NAC, coordinate decision-making, motivational control and learning as facilitated by natural or drug-induced dopamine efflux from the VTA. Hippocampal afferents predominantly originating from the ventral sectors of the output layers of the HCF (Subiculum (SUB) and CA1 regions) project directly to PFC and NAC networks where motivational leaning and drug-reinforcement plasticity occurs. Within the HCF, information pertaining to contextual, episodic and temporal memory funnels in from the neocortex via the Enthorhinal cortex (EC), into the Dentate Gyrus (DG) where newly generated neurons (*) reside. Young DG neurons grow dendritic trees receiving EC inputs and send axonal projections to the CA3 layer, where information is packaged for output from the HCF via CA1/SUB layers.
3. NEUROGENESIS IMPLICATED IN MENTAL ILLNESS AND DUAL DIAGNOSIS DISORDERS
Animal and human studies implicate abnormal or maladaptive hippocampal neurogenic activity in the pathogenesis of a range of psychiatric disorders, particularly those associated with hippocampal dysfunction and abnormal psychosocial stress responses (Eisch et al., 2008). These disorders, including schizophrenia, post-traumatic stress disorder (PTSD), major depression and cluster B personality syndromes are highly comorbid with substance use disorders (Kessler, 2004). Similarly, in the general population, mild forms of cognitive dysfunction occurring in combination with impulsive-anxious personality traits are endophenotypic markers of addiction vulnerability (Ersche et al., 2012). This section presents cellular, neuroimaging and cognitive evidence linking neurogenesis with the pathogenesis of mental illnesses that frequently present as dual diagnosis disorders.
Impaired hippocampal neurogenesis has been discovered and replicated across animal models of mental illnesses spanning schizophrenia, PTSD, depression and personality disorder syndromes, even after the face, construct and predictive validities of these models had already been established (Table 1; Borcel et al., 2008; Buwalda et al., 2010; DeCarolis and Eisch, 2010; Duan et al., 2007; Garza et al., 2012; Heine et al., 2004; Hulshof et al., 2011; Ibi et al., 2008; Jayatissa et al., 2006; Kempermann et al., 2002; Kikusui et al., 2009; Lagace et al., 2010; Lieberwirth et al., 2012; Liu et al., 2006, 2008; Maeda et al., 2007; Mirescu et al., 2004; Pieper et al., 2005; Rizzi et al., 2007; Stranahan et al., 2006). As is thought to be the case for the human disorders, these models are heterogeneous in phenotypes and construct etiologies, but share common themes of deranged neuroplasticity or neural network integrity especially involving the hippocampus (Kempermann et al., 2008). Indeed, adult hippocampal neurogenesis is regulated by a host of cellular processes, genetic determinants, and neurochemcial stimuli known to modulate development and morphology of neurons and the course of mental illnesses (Balu and Lucki, 2009; DeCarolis and Eisch, 2010, 2008).
TABLE 1.
Adult Hippocampal Neurogenesis in Animal Models of Mental Illness
| Mental Illness Model | Experimental Intervention | Neurogenic Effects | Author, Year |
|---|---|---|---|
| Schizophrenia/Depression | Environmental Deprivation | ↓P/S | Kemperman, 2002 |
| Schizophrenia/Depression | DISC1 gene manipulation | ↑D* | Duan, 2007 |
| Schizophrenia | NPAS3 gene manipulation | ↓P | Pieper, 2005 |
| Schizophrenia | PCP, chronic exposure | ↓P | Liu, 2006 |
| Schizophrenia | PCP, chronic exposure | ↓P | Maeda, 2007 |
| PTSD/Depression | Chronic Unpredictable. Stress | ↓P/S | Heine, 2004 |
| PTSD/Depression | Chronic Unpredictable Stress | ↓P | Liu, 2008 |
| PTSD/Depression | Chronic Unpredictable Stress | ↓S | Borcel, 2008 |
| PTSD/Depression | Chronic Unpredictable Stress | ↓P | Garza, 2012 |
| PTSD/Depression | Chronic Mild Stress | ↓P | Jayatissa, 2006 |
| Depression | Social Defeat | ↓P | Bu walda, 2010 |
| Depression | Social Defeat | ↓P | Lagace, 2010 |
| Depression | Social Isolation | ↓P | Stranahan, 2006 |
| Depression | Social Isolation | ↓P | Ibi, 2008 |
| Depression | Social Isolation | ↓P/S/D | Lieberwirth, 2012 |
| Personality Disorders | Maternal Deprivation | ↓P | Mirescu, 2007 |
| Personality Disorders | Maternal Deprivation | ↓P/S | Rizzi, 2007 |
| Personality Disorders | Maternal Deprivation | ↓P/S | Kikusui,2009 |
| Personality Disorders | Maternal Deprivation | ↓P | Hulshof, 2011 |
↑ increase
↓ decrease
P=Proliferation
D=Differentiation (e.g. into neurons)
S=survival
In this study the primary finding was abnormal and premature morphological development and incorporation of new neurons into local circuitry.
Surges or sustained high levels of corticosteroids are biological correlates of major psychosocial distress and environmental change that can provoke symptom onset or destabilization across a wide range of psychiatric syndromes. Corticosteroid surges produce atrophic connectivity among neurons throughout the neocortex and hippocampus, and in the hippocampus they can kill neurons and suppress DG neurogenesis (Gould et al., 2000; McEwen, 2007). Subsequently, analogous with the regenerative capacity of a mowed lawn to regrow, neurogenic activity in the DG is renewed upon waning of corticosteroid surges (Cameron and Gould, 1994; Gould et al., 1997). Generally, many neural insults, whether neurochemical, electrical or mechanical, delivered to brain regions connected with, or intrinsic to the DG, are initially destructive but secondarily provocative of neurogenesis (Gould et al., 2000; Lledo et al., 2006). More relevant to recovery from stress-sensitive psychiatric disorders involving cognitive dysfunction, hippocampal neurogenesis is up-regulated by antidepressants (Boldrini et al., 2009; Malberg et al., 2000), lithium (Chen et al., 2000), electroconvulsive therapy (Scott et al., 2000), environmental enrichment (Kempermann et al., 1997) and exercise (Kerr and Swain, 2011).
Neuroimaging has consistently characterized hippocampal atrophy across schizophrenia, post-traumatic stress disorder, major depression and cluster B personality syndromes, potentially reflecting declines in neurogenesis and closely related neuroplastic and information bearing elements (e.g., axodendritic connectivity and neuropil; Bremner et al., 1997; Csernansky et al., 2002; Schmahl and Bremner, 2006 ; Sheline et al., 1996; Teicher et al., 2012). Accordingly, human postmortem studies are beginning to describe decreases in markers of hippocampal neurogenesis in schizophrenia and depressive disorders (Lucassen et al., 2010; Reif et al., 2006). Corresponding to these subtle biomarkers, mild cognitive deficits consistent with hippocampal dysfunction in short and long-term memory storage, contextual-spatial memory, and episodic memory retrieval pervade these disorders (Small et al., 2011).
Current imaging technologies have yet to explore relationships between neurogenesis and cognition in humans while the development of specific biomarkers and imaging ligands that can robustly quantify neurogenic neurons is still in its early stages (Manganas et al., 2007). However, animal studies examining individual differences or experimentally-induced neurogenesis deficits have characterized alterations in hippocampal-dependent cognition like that in mental illness (Eisch et al., 2008). This body of evidence is somewhat mixed, suggesting the functional subtlety of hippocampal neurogenesis. The balance of data suggests that decrements in neurogenesis impacts some, but is not catastrophic to most domains of hippocampal learning and memory (Becker et al., 2009). Thus, the role of neurogenic activity may be more about optimizing hippocampal function for certain styles of learning and memory retrieval, than it is simply about turning the hippocampus on or off, or the brain ‘happy’ vs. ‘depressed’ (Chambers and Conroy, 2007; Petrik et al., 2012). Although low levels of neurogenesis do not guarantee the presence of, or by any means represent the sole cause of a given mental illness, neurogenesis seems helpful to behavioral adaptation (Petrik et al., 2012). Animal and neurocomputational studies suggest that neurogenic proliferation, cell survival and death work in concurrent or alternate phases, to create a dynamic modulation of DG neuronal populations over time, changing the style and optimal learning attributes of hippocampal networks according to evolving environmental challenges (Chambers and Conroy, 2007; Glasper et al., 2012). In turn, one or more parameters of neurogenic activity may show abnormal or impaired regulation in response to environmental challenges, potentially playing some role in determining differential subtyping of psychiatric disorders.
In sum, investigating how neurogenesis is involved in drug addiction, makes sense given that neurogenesis is implicated across psychiatric disorders with elevated substance disorder vulnerability (Kessler, 2004). Neurobiologically, major forms of mental illnesses and addiction vulnerability are intimately inter-related, and in some aspects inseparable disease processes (Chambers et al., 2007, 2001). Clinically, mental disorders and addictions often unfold as intertwined, chronic conditions punctuated by episodes of symptom worsening (binging/relapse) and resolution (recovery; O’Brien et al., 2004). Exacerbations of these dual diagnoses are associated with novel or destabilizing social/environmental contexts, accompanying psychological stress, struggles to adapt, and re-exposure to addictive drugs (Sinha, 2008). So, although a large heterogeneity of dual diagnosis conditions exist as defined by differential combinations of mental illnesses and addictions, this heterogeneity shares common clinical-pathological themes (e.g., stress and novelty sensitivity, cognitive dysfunction), and anatomical pathways rooted in hippocampal dysfunction. As reviewed next, new findings position neurogenesis at the heart of an integrative anatomy of dual diagnosis, in which a bidirectional relationship between hippocampal neurogenic activity and the effects of reinforcing drugs may underpin the balance of disease worsening vs. recovery in addiction and dual diagnosis disorders.
4. ADDICTIVE DRUG SUPPRESSION OF NEUROGENESIS
4.1 Suppression of neurogenic proliferation transcends addictive drug types
All of the major addictive drugs exert at least part of their reinforcing actions through a common pathway involving dopamine (DA) release via projections from the ventral tegmental area (VTA) into the Nucleus Accumbens (NAC) (used here as synonymous with the ventral striatum, Figure 1; Di Chiara and Imperato, 1988; Wise, 1998). This parsimony of action partly explains how nicotine, alcohol, opiates, cocaine, amphetamines, and cannabis, although producing such diverse or opposing intoxication profiles, can all lead to the same syndrome of addiction described by the same DSM criteria for substance dependence (DSM-IV-TR, 2000).
Research conducted over the last decade has characterized another parsimonious theme in addiction biology in parallel the DA story: addictive drugs also share a capacity to reduce hippocampal neurogenic activity. At least 3 replications of decreased neurogenic proliferation with nicotine, ethanol, opiates, cocaine, and amphetamines have been demonstrated in rodents (Table 2; Abrous et al., 2002; Alen et al., 2010; Arguello et al., 2009, 2008; Barr et al., 2010; Cho et al., 2007; Crews et al., 2006; Dominguez-Escriba et al., 2006; Eisch et al., 2000; Fischer et al., 2008; Garcia-Fuster et al., 2010; He et al., 2005; Hernandez-Rabaza et al., 2010; Herrera et al., 2003; Jiang et al., 2005; Kahn et al., 2005; Kochman et al., 2006; Lagace et al., 2006; Mackowiak et al., 2007; Mandyam et al., 2008; Mao and Wang, 2001; Mudo et al., 2007; Nixon and Crews, 2002, 2004; Nixon et al., 2008; Noonan et al., 2008; Recinto et al., 2011; Scerri et al., 2006; Shingo and Kito, 2005; Teuchert-Noodt et al., 2000; Yamaguchi et al., 2004, 2005), with additional replications emerging in primates (Taffe et al., 2010). Notably, these findings are overall quite similar to the effects of mental illness models on hippocampal neurogenic proliferation (Table 1), which may help explain how addictive drug use typically worsens rather than improves psychiatric syndromes, lending support to arguments that dual diagnosis disorders represent disease synergies rather than reflecting “self-medicating” efficacies of addictive drugs (Chambers, 2009, 2001).
TABLE 2.
Effects of Addictive Drugs on Adult Hippocampal Neurogenesis in Rodents
| Drug | Neurogenic Effects by Dosing | Cell Death Effects* | Author, Year | ||
|---|---|---|---|---|---|
| Acute | Chronic | Withdrawal | |||
| Nicotine | ↓P | ↑ | Abrous, 2002 | ||
| Nicotine | ↓D/S | Shingo, 2005 | |||
| Nicotine | ↓P | Scerri, 2006 | |||
| Nicotine | 0P | Mudo, 2007 | |||
| Alcohol | ↓P | ↓P/S | Nixon, 2002 | ||
| Alcohol | ↓S | ↑ | Herrera, 2003 | ||
| Alcohol | ↓P | ↑P | Nixon, 2004 | ||
| Alcohol | ↓P/S | ↑ | He, 2005 | ||
| Alcohol | ↓P/S | Crews, 2006 | |||
| Alcohol | ↑P | Nixon, 2008 | |||
| Alcohol | ↓S | Hernandez-Rabaza, 2010 | |||
| Alcohol | ↓P | Alen, 2010 | |||
| Opioids | 0P | ↓P/S | Eisch, 2000 | ||
| Opioids | ↓P | Mandyam, 2004 | |||
| Opioids | ↓P/D | ↑P | Kahn, 2005 | ||
| Opioids | 0P | ↓P | Fisher, 2008 | ||
| Opioids | ↓P/D | ↑ | Arguello, 2008 | ||
| Opioids | ↓P | Arguello, 2009 | |||
| Cocaine | 0P | ↓P | Yamaguchi, 2004 | ||
| Cocaine | 0P | ↓P | Yamaguchi, 2005 | ||
| Cocaine | ↓P | ↓P | 0 | Dominguez-Escriba, 2006 | |
| Cocaine | ↓P | ↑P/D | 0 | Noonan, 2008 | |
| Cocaine | 0P | ↓P/S | ↓P/S | Garcia-Fuster, 2010 | |
| Amphetamine (meth) | ↓P | 0 | Teuchert-Noodt, 2000 | ||
| Amphetamine | 0P | Mao, 2001 | |||
| Amphetamine (meth) | ↑P/D | ↓P/D/S | ↑ | Mandyam, 2008 | |
| Amphetamine | 0P/S | ↓S | 0 | Barr, 2010 | |
| Amphetamine(meth) | ↓P | ↑P/S | ↑ | Recinto, 2011 | |
| Methylphenidate | 0P | ↓S | Lagace, 2006 | ||
| MDMA | ↓P | Cho, 2007 | |||
| MDMA | ↓S | Hernandez-Rabaza, 2010 | |||
| Cannabinoids | ↑P/D | synthetic | Jiang, 2005 | ||
| Cannabinoids | 0P | D9-THC | Kochman, 2006 | ||
| Cannabinoids | 0P/D | synthetic | Mackowiak, 2007 | ||
| Cannabinoids | ↓P | synthetic | Alen, 2010 | ||
0 =no change found
↑ increase
↓ decrease
P=Proliferation
D=Differentiation (e.g. into neurons)
S=survival
Drug shown to kill neurons as evidenced by markers that may label either old or young DG neurons All data restricted to rodent models
Just as there is variation in how different addictive drugs produce their DA-releasing effects (Di Chiara and Imperato, 1988; Wise, 1998), there is heterogeneity in how they impact neurogenesis. Across addictive drugs, down regulation of proliferation is most consistently observed, with more varied effects on neuronal differentiation, survival and death (Table 2). With certain brain insults, some addictive drugs known to suppress proliferation, may prevent injury or death, illustrating that a drug’s effects on neurogenesis do not necessarily reflects its effects on health or survival of mature neurons (Zhuang et al., 2005; Zohar et al., 2006). In general, while the severity of a drug’s anti-proliferative effects are dependent on the chronicity and magnitude of dosing, different addictive drugs likely follow different dose functions on proliferation (Abrous et al., 2002; Fischer et al., 2008; Mandyam et al., 2008; Shingo and Kito, 2005; Yamaguchi et al., 2004).
Whereas the acute DA-releasing activity of addictive drugs encodes a reinforcement signal and facilitates other neuroplastic changes thought to lead to, or maintain addiction (Kalivas and O’Brien, 2008; Kalivas and Volkow, 2005), the suppression of neurogenic activity by addictive drugs may be a biomarker of one form of neuroplastic change underlying the early stages of addiction. In contrast to their acute DA releasing activities, which do not necessarily change with repeated dosing, or require an addicted phenotype, the neurogenic effects of addictive drugs predominantly require chronic dosing, as with a behaviorally sensitized or addicted phenotype. Moreover, combinations of addictive drugs appear to produce greater effects in altering neurogenic activity than what single drugs produce (Alen et al., 2010; Hernandez-Rabaza et al., 2010), suggesting that neurogenic suppression could play a role in cross-sensitization and greater severity of addictive syndromes with poly-substance dependence (Anthony and Petronis, 1995). There also appears to be a consistent effect across drugs including alcohol, cocaine, methamphetamine, and opioids, where cessation of use, as a prerequisite for recovery, permits rebounding of neurogenic activity (Crews and Nixon, 2009; Kahn et al., 2005; Liu et al., 2006; Nixon and Crews, 2004; Noonan et al., 2008; Recinto et al., 2011).
4.2 Mechanisms of neurogenic effects of addictive drugs
Although it is now clear, after multiple replications spanning drug classes, that addictive drugs generally suppress neurogenic proliferation, we do not yet completely understand how they do this, and whether they do it via similar or distinct mechanisms. While the hypothalamic-pituitary-adrenal (HPA) system is a key regulator of neurogenesis, and some addictive substances can directly influence corticosteroid levels, addictive drugs do not generally exert their anti-neurogenic effects via the HPA axis (Eisch et al., 2000). This implies that addictive drugs can exert their effects via non-HPA axis pathways while interfering with the adaptive regulation of neurogenesis that corticosteroids provide in healthy brains. Since addictive drugs produce anti-neurogenic effects in parallel to corticosteroid surges corresponding to stress, the idea that drug use ‘self-medicates’ the anti-neurogenic effects of stress seems unlikely.
If not generally via the HPA, how do addictive drugs modulate neurogenesis? Another attractive possibility is through their DA actions. DA neurons do innervate the hippocampus, where DA receptors are present, and DA receptor blockade can increase hippocampal proliferation (Dawirs et al., 1998). Also, DA agonists can inhibit neurogenesis of DA neuronal precursors in the midbrain, although it is unclear if this is relevant for glutamatergic DG neurons (Berg et al., 2011a). However, contrary to the idea that drug-induced DA plays the only or leading role in neurogenic suppression, DA receptors are much less abundant and functionally significant in the hippocampus compared to frontal cortical-striatal circuits. Moreover, DA receptors are most dense not in the DG, but in the non-neurogenic CA1 dendritic fields where they modulate neuroplasticity between CA1 neurons and projections from the Entorhinal cortex and CA3 (Otmakhova and Lisman, 1999). Given these findings, investigations looking beyond DA into cellular-signaling mechanisms that are both altered by addictive drugs and influence neurogenesis, suggest that many biological pathways are involved, some of which are shared across addictive drugs and others not (Balu and Lucki, 2009). For example, certain addictive drugs can invoke or exacerbate oxidative stress, mitochondrial dysfunction, cell death or cell cycling pathways (Arguello et al., 2008; Cunha-Oliveira et al., 2008). Molecules involved in supporting or hindering neurogenesis including brain-derived neurotrophic factor (BDNF), interleukin 1 beta or vascular endothelial growth factor (VEGF), could be influenced by some addictive drugs but not others (Arguello et al., 2009). G-protein coupled receptor systems, which do seem to be modulated across addictive drug types via cascades that may or may not rely on DA, endogenous opioid or cannabinoid transmission, are especially promising mechanisms involved in regulating cell turnover, growth and maturation (Sargeant et al., 2008).
5. LOW NEUROGENIC STATES AND ADDICTION VULNERABILITY
Having reviewed evidence that addictive drugs suppress neurogenesis, we now turn to evidence suggesting a bi-directionality of causality in this association, i.e., that states of low hippocampal neurogenic activity also increase addiction vulnerability. As previously mentioned, psychiatric disorders considered most likely to involve abnormalities in hippocampal neurogenesis, e.g., depression, PTSD, cluster B personality disorders and schizophrenia (Eisch et al., 2008), characteristically also encompass substance disorder comorbidity (Kessler, 2004). Although these disorders involve many different symptom domains and etiological and developmental aspects, they do share at least 3 hippocampus-related attributes: 1) disturbances in HPA regulation and stress reactivity; 2) hippocampal atrophy, and 3) disturbances in hippocampal –dependent learning and memory. Ample data, including large volumes of animal modeling studies (Table 1), suggest these attributes represent an array of inter-related problems, all potentially associated with deficits in hippocampal neurogenesis; extreme surges in corticosteroid levels can shrink dendritic arbors or kill hippocampal neurons contributing to hippocampal volume loss (Bremner et al., 1997; McEwen, 2007). In turn, loss of hippocampal network vitality not only impairs learning and memory, but impairs hippocampal feedback regulation of HPA activity (Herman et al., 1989). Similarly, suppression of neurogenic activity is linked with impaired hippocampal-dependent cognition and network changes that underpin hippocampal atrophy (Czeh and Lucassen, 2007), while also producing deficient regulation of the HPA axis (Schloesser et al., 2009). How then might the other key attribute shared across dual diagnosis disorders, addiction vulnerability, be linked to the other three hippocampal-related parameters and neurogenesis?
5.1 Experimental Suppression of neurogenesis increases addictive behavior
Groundbreaking experiments have demonstrated that artificial suppression of adult hippocampal neurogenesis, occurring before drug exposure, directly increases cocaine self-administration delivered either as fixed doses or escalating work-effort contingencies (Noonan et al., 2010). This up-tick in addiction vulnerability is not accompanied by alterations in non-specific locomotion or food reinforcement, suggesting specificity to drug-learning. Similarly, neurogenic suppression occurring after animals acquire cocaine self-administration makes them more resistant to extinguishing drug-seeking in the absence of drug (Noonan et al., 2010). Importantly, the addiction-enhancing effects of suppressed neurogenesis are not specific or limited to particular causes of neurogenic suppression. A form of low frequency electrical stimulation that disables the hippocampus and suppresses neurogenic proliferation, enhances cocaine-primed re-instatement of drug-seeking after animals have previously been extinguished from drug-seeking behavior (Deschaux et al., in press). Furthermore, the recovery from cocaine-induced neurogenic suppression associated with extinction training is also blocked by the same low frequency stimulation. Corroborating an inverse connection between neurogenesis vs. drug-motivated learning and drug exposure, methamphetamine self-administration produces a cumulative dose effect on decreasing hippocampal proliferation while increasing the effect of a future drug dose in causing relapse to drug-seeking (Recinto et al., 2011). Notably, these methamphetamine effects are accompanied by decreased spatial and working memory function, as a correlate of deficient neurogenic activity. In yet more experiments, combined exposure to alcohol and cannabinoid (CB1) agonists produce greater alcohol-seeking and neurogenic suppression, than either alcohol or CB1 agonists alone (Alen et al., 2010).
Together, these studies demonstrate that suppression of hippocampal neurogenesis, whether induced by prior drug history or other interventions, enhances drug reinforcement learning while simultaneously impairing other aspects of hippocampal dependent learning and memory. Hence, suppression of hippocampal neurogenesis by x-irradiation impairs hippocampal dependent contextual memory, including fear conditioning (Wojtowicz, 2006), but spares cocaine-conditioned place preference (Brown et al., 2010). These seemingly contradictory trends, the impairment of one domain of learning with spared or augmented learning in another, likely represent important clues about the role of the neurogenic hippocampus in addiction and dual diagnosis. These clues inform the design of a translational model incorporating basic neuroscience data considered next. This model begins to explain how low states of hippocampal neurogenesis and mild cognitive dysfunction, whether generated by underlying mental illness, prior addictive drug exposure, or their combination, evokes a change in cortical-striatal circuit function that optimizes learning associated with drug reinforcement at the expense of learning and maintaining more adaptive natural-reward focused behaviors.
5.2 Neuroanatomy and mechanisms relating neurogenesis and addiction vulnerability
A wealth of human and animal data (see comprehensive reviews (Chambers et al., 2007; Kalivas and Volkow, 2005) illuminate decision-making, motivational control and drug-related motivational learning as key functions of prefrontal cortical–ventral striatal circuits (Figure 1). In turn, these circuits and their motivational functions are modulated by the status of the hippocampus (Chambers et al., 2005, 2001, 2010). At the focal point of this anatomy, the NAC is the epicenter of information processing and memory that generates an animal’s motivational–behavioral repertoire. Responsive to changing environmental demands that require revisions of motivated behavior, synaptic connectivity within the NAC network is subject to DA-facilitated neuroplasticity (Nestler, 2004; Volkow, 2004). As waves of DA release induced by natural reinforcers or their cues permeate this structure, the motivational repertoire is updated to optimally direct and sequence adaptive behaviors in face of environmental change (Chambers et al., 2007). But with drug-induced DA efflux, the motivational repertoire is increasingly oriented to efficiently pursue and use drug (Self, 1998).
Hippocampal influence on NAC-based motivational learning is mediated via glutamate-bearing axons projecting directly from the ventral hippocampus to NAC and prefrontal cortical networks (O’Donnell and Grace, 1995; Pennartz et al., 1994). However, the hippocampus does not project directly to VTA neurons responsible for DA release into the NAC. With this architectural plan, hippocampal dysfunction does not catastrophically disrupt the essential apparatus for motivational learning (i.e., striatal/mesolimbic DA circuits). But, a state of active vs. impoverished hippocampal input to cortical-striatal circuits nevertheless seems to determine which type of motivational learning the cortical-striatal assembly is most able to support: learning associated with a) a rapidly changing contextual map of complex, often subtle and temporally remote natural environmental reinforcers vs. b) relatively simple and immediate drug reinforcers (Chambers, 2008, 2007). Under these circumstances, both the history of drug-induced DA efflux into the NAC and the concurrent record of functional interactions between the hippocampus and prefrontal cortical-striatal circuits conspire to determine the course and severity of neuroadaptations produced by addictive drug exposure (Chambers et al., 2010; Goto and Grace, 2005, 2008).
Neuroanatomical and functional data illustrative of the relationship between frontal cortical-striatal circuits and the hippocampus have provided new insights into the neurobiological origins of addiction vulnerability in mental illness (Chambers et al., 2001). Converging lines of preclinical investigations conducted in the 1990’s predicted that perturbation of hippocampal regions that project to the prefrontal cortex and NAC would produce a complex neuropsychiatric syndrome with impaired hippocampal-mediated cognition with enhancement of addictive drug-mediated behavioral learning (Chambers et al., 2001). In testing this hypothesis, using the same psychostimulant drugs that would be used a decade later for demonstrating the inverse relationship between hippocampal neurogenesis and addiction vulnerability (Noonan et al., 2010; Recinto et al., 2011), adult rats with neonatal ventral hippocampal lesions (NVHL) were shown to have both hippocampal-dependent cognitive deficits analogous with that in depression, PTSD or schizophrenia (Chambers et al., 1996), and increased addiction vulnerability to cocaine (Chambers and Self, 2002) and methamphetamine (Brady et al., 2008). NVHLs were subsequently shown increase consumption of alcohol solutions (Berg et al., 2011b) mirroring data where the cumulative history of alcohol self-administration is inversely proportional to hippocampal neurogenic activity (Alen et al., 2010).
NVHLs represent an approach that is more damaging and permanent for the hippocampus and neurogenesis than other experimental interventions (x-irradiation, drugs) that impact neurogenic activity. However, the status and functionality of neurogenic activity is intimately linked with hippocampal anatomy beyond the neurogenic DG including non-neurogenic cell populations and axodendritic elements of entorhinal cortex, CA3, CA1, and subiculum. Stimuli that regulate neurogenic activity or cell death in the DG (e.g., corticosteroids, exercise, environmental enrichment, anti-depressant drugs) have extensive effects throughout the hippocampus. Generally, factors that up-regulate neurogenesis tend to bulk up the structural complexity of networks throughout the hippocampus, while those that downgrade neurogenesis or kill neurons, tend to degrade them (Czeh and Lucassen, 2007). With these observations in mind, it is useful to study the NVHL model and others, like chronic-unpredictable stress, or human contexts such as early childhood maltreatment, that encompass robust structural and functional changes throughout the hippocampus (Baram et al., 2012; McEwen, 2007; Teicher et al., 2012), to understand what low states of neurogenic activity and related pathological states of hippocampal function may mean for pre-frontal cortical-ventral striatal circuits where addiction pathology primarily occurs.
Both NVHLs and chronic unpredictable stress produce structural and functional changes in prefrontal cortical-ventral striatal circuits that underlie a shift toward an addiction vulnerability phenotype. Both interventions produce losses of prefrontal cortical axodendric complexity and grey matter volume corresponding to increases in impulsive styles of approach, or self-administration of rewards (Chambers et al., 2010; Dias-Ferreira et al., 2009; McEwen, 2007; Tseng et al., 2009). In NVHL animals, these dynamics are reflected in decreased volume and neural activation in the prefrontal cortex that corresponds inversely, with amplification of the capacity of prior cocaine history to increase cocaine-induced neural activation in the striatum and long-term behavioral sensitization (Chambers et al., 2010). Similarly, in chronic-unpredictably stressed rats, prefrontal cortical neuronal atrophy is matched inversely by overgrowth of neuronal dendritic trees in the striatum (Dias-Ferreira et al., 2009). In an experimental approach that can be viewed as a hybrid of the early developmental insult of NVHLs and adult stress models, perinatal rats subjected to maternal deprivation (modeling childhood abuse/neglect as a key etiological dynamic in human mood and personality disorders) show enhanced vulnerability to cocaine and alcohol self-administration in adulthood (Huot et al., 2001; Matthews et al., 1999) and adult-age decrements in hippocampal function, cognition, and neurogenic proliferation (Heim et al., 2010; Mirescu et al., 2004). Taken together, these data suggest a thematic effect of hippocampal impairment on prefrontal cortical-striatal circuitry that is fairly non-specific to the scope or etiological ingredients of the hippocampal impairment. Differential forms of hippocampal network failure (e.g., potentially spanning different forms of dual diagnosis disorders in humans) appear to produce similar secondary effects on prefrontal cortical-striatal network function underpinning impulsive behavior, augmentation of DA-mediated striatal-based habit learning, and ultimately increased addiction vulnerability. For instance, NVHL rats show low expression patterns of brain-derived neurotrophic factor (BDNF) in hippocampal and cortical-striatal circuits (Ashe et al., 2002), indicative of low capacity for neuroplastic change), while cocaine exposure increases BDNF expression in the NAC, indicating pathological increases in neuroplasticity (Graham et al., 2007). Given that addictive drugs also augment dendritic complexity in striatal regions associated with habit formation (Robinson and Kolb, 2004), a state of low hippocampal functionally, often encompassing suppressed neurogenic activity, may prime conditions where the neuroplastic effects of addictive drugs more efficiently recruit striatal circuits leading to the habit formation of drug-seeking and taking.
6. TOWARD A NEUROGENIC MODEL OF ADDICTION AND DUAL DIAGNOSES
Moving from this understanding of hippocampal dysfunction in the pathogenesis of dual diagnosis toward a neurogenic model of addiction, it is important to consider that the functional and neuroplastic status of the hippocampus, as reflected by its neurogenic activity, and its modulation by the HPA-axis, is not static but is time-variable in reaction to environmental change (Glasper et al., 2012). As demonstrated by computational modeling of neurogenic-facilitated learning, neurogenesis may be flexibly regulated, according to the degree of environmental novelty the brain is challenged with adapting to (Chambers and Conroy, 2007). So, whether in a transient trough of neurogenic activity due to normal stressful contextual change, or more so, in an abnormally severe or sustained suppression of neurogenic activity and hippocampal plasticity, the influence of hippocampal projections on prefrontal cortical-striatal circuits, encompassing the ability of these axons to facilitate neuroplastic change and produce neurotrophic effects pertaining to complex vs. simple reward motivation in those rostral circuits, is diminished. As suggested in Figure 2, a brain in a low (e.g., mental illness) vs. high neurogenic state may become more susceptible to the DA-effects of addictive drugs to sculpt and recruit striatal circuits to direct drug-seeking and taking behavior. Then, driven by these dynamics, a low neurogenic state brain more rapidly accumulates a greater cumulative addictive drug dose history, producing even greater suppression of neurogenic activity, suggestive of a vicious cycle of accelerating drug use in addiction’s progression.
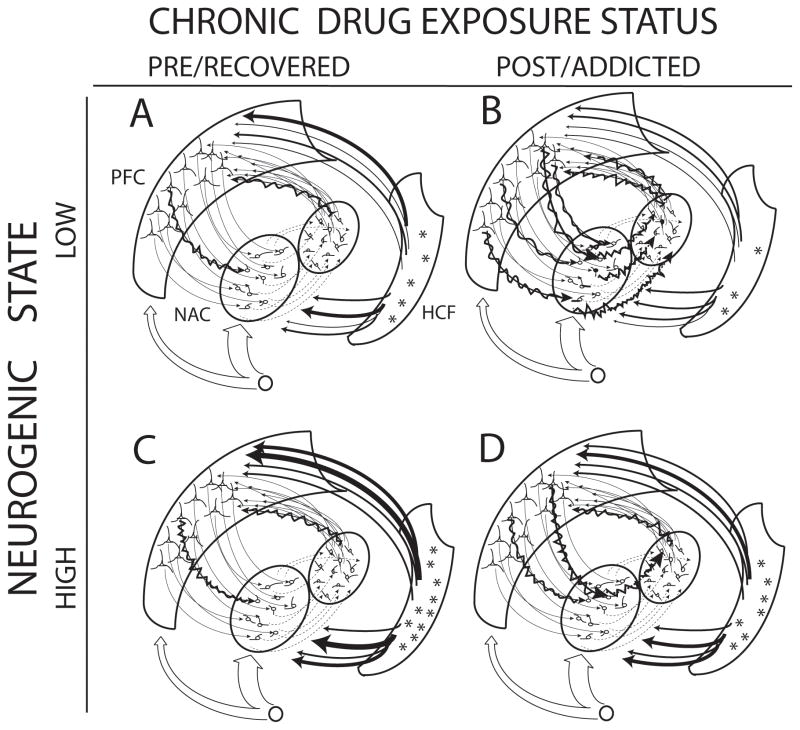
Figure 2. Prefrontal cortical-striatal-hippocampal circuits involved in a neurogenic basis of addiction and dual diagnosis disease progression vs. recovery.
In (A) the low neurogenic state prior to chronic addictive drug exposure (e.g. as in mental illness), relatively low cell proliferation in the HCF (denoted by few *) corresponds to relatively low neuroplastic influence and/or novel information being relayed via HCF projections to PFC/NAC (denoted by sparse ‘active’ hippocampal projections symbolized by thick solid arrows). After chronic addictive drug exposure (A to B), more prefrontal cortical-striatal circuit neurons and their connections are recruited into representing drug seeking-related motivational programming (more jagged arrows), while neurogenic activity in the HCF is further suppressed, and active HCF modulation of cortical-striatal plasticity is diminished further (no thick hippocampal projection arrows). In contrast, for the relatively high neurogenic state (C), the same chronic drug exposure (C to D) may be less efficient in recruiting prefrontal cortical-striatal circuits in representing drug seeking-related motivational programming compared to the low neurogenic state (A). Thus higher neurogenic activity corresponding to more actively influential, novel-information bearing HCF projections to PFC/NAC (symbolized by more thick projection arrows from HCF), may protect against addiction, by supporting motivational responsiveness to complex, natural reinforcers. In the high neurogenic state (C), a greater initial volume of neurogenic activity could provide greater resilience to the adverse effects of neurogenic suppression by addictive drugs. In addiction and dual diagnosis treatments that augment hippocampal neurogenic activity during recovery, extremely low neurogenic brains (B) might progress more efficiently to a more fully recovered state (e.g. to (C) via (D)). Similarly, preventative or recovery maintenance treatments may shift the brain from (A) to (C) to confer baseline reductions in addiction vulnerability prior to drug exposure.
Investigating the impact of hippocampal functionality and neurogenesis on prefrontal-cortical-striatal circuits will increase our understanding of how addiction vulnerability endophenotypes are associated with low vs. high neurogenic states in various forms of mental illness. Available evidence suggests that while psychological stress and corresponding HPA activation suppresses neurogenic activity and hippocampal plasticity, the brain is attempting to prepare for, but is not yet adapted to novel environmental contexts (Freeman, 2003; McEwen, 2007). In this putative low neurogenic and mental illness risk-state, cortical-striatal circuits may be hyper-responsive to novel environments, consistent with novelty hyper-reactivity in animal models and clinical contexts of addiction vulnerability (Belin et al., 2011; Chambers et al., 2003; Tseng et al., 2009). Given the role of the neurogenic hippocampus in encoding time and temporal relationships between salient events (Aimone et al., 2009) and the importance of prefrontal-cortical-striatal-hippocampal communication in recalling past and predicting future event sequences (Buckner, 2010; Sadeh et al., 2011), further studies are needed to determine if a low neurogenic state contributes to a shortened temporal-event horizon. If so, low neurogenic states may be linked with motivational blindness to distant future goals as a common trait of dual diagnosis patients, corresponding to discounting of delayed rewards (Bobova et al., 2009), a well characterized dimension of impulsivity and marker of addiction vulnerability (Bickel et al., 2011, 2010). Further investigations are needed to determine if and how these endophenotypes (e.g., impulsivity, novelty hyper-responsivity, stress over-reactivity) are contributed to by low-neurogenic states in the hippocampus. Accordingly, low states of hippocampal neurogenesis may be linked with enhanced motivational salience of primitive reinforcers (e.g., sex, gambling, drugs) and impaired ability to organize behavior to pursue and acquire more complex, temporally distant reinforcers.
7. EXPLORING NEUROGENIC AUGMENTATION FOR TREATING ADDICTION AND DUAL DIAGNOSES
Further studies examining the neurophysiological and neuroplastic interactions between hippocampus and cortical-striatal circuits in brains shifting from low vs. high neurogenic states will be important to understanding how addiction severity is dynamically modulated by mental illness course. Nevertheless, the findings integrated in this review suggests the potential of therapeutic strategies for addictions and dual diagnosis patients that can shift individuals from low to high neurogenic states (Figure 2). Several lines of translational evidence are already emerging in support of this model. For example, genetic knockout of the mu opioid receptor increases survival of hippocampal neurogenic neurons (Harburg et al., 2007), while the opioid receptor blocker naltrexone, the only FDA-indicated medicine for treating addictions to two different substances (alcohol and opioids; Tiihonen et al., 2012), up-regulates hippocampal neurogenic activity in post-natal development (Zagon and McLaughlin, 1986) and adulthood (Persson et al., 2004). In dual diagnosis schizophrenia, the atypical antipsychotic agent clozapine appears to be uniquely efficacious compared to the typical agent haloperidol, in treating both schizophrenia and co-morbid addictions (Green, 2005, 2003), mirroring preclinical data showing that clozapine has greater activity in up-regulating hippocampal neurogenic proliferation compared to haloperidol (Balu and Lucki, 2009; Halim et al., 2004; Maeda et al., 2007). Other potential pro-neurogenic agents, which may influence neurogenic activity in different ways, are important to investigate. For instance, pharmacological blunting of HPA axis activation via corticotropin-releasing factor receptor antagonism may reduce the capacity of stressful environmental changes to increase sensitivity to drug reward (Nader et al., 2012), possibly by preventing entry into low neurogenic states. Meanwhile, the pro-neurogenic effects of lithium, could relate to its effects in decreasing impulsivity (Chen et al., 2000; Kovacsics et al., 2009). How and if bupropion, the only FDA-approved dual diagnosis medication (i.e., indicated for both an addiction and a mental disorder), up-regulates neurogenesis remains to be explored. Similarly, electroconvulsive (ECT) therapy, also a potent pro-neurogenic stimulus (Scott et al., 2000) has not been sufficiently studied as an addiction treatment. Notably, although anti-depressant drugs up-regulate neurogenic activity (Malberg et al., 2000), they have not generally proven useful for treating addictions as monotherapies. Collectively, these observations suggest that many different cellular pathways may be therapeutically manipulated to up-regulate neurogenic activity, but with differential efficacies for treating psychiatric disorders, addictions, and their co-morbidities.
More work is needed to explore how pharmacological regulation of proliferation vs. differentiation vs. survival may differentially impact addictive disease (Frielingsdorf and Kuhn, 2007), and how these pathways may depend on concurrent positive environmental experiences and psychotherapeutic approaches. Psychotherapies are mainstays of addictions and mental disorder treatment and likely depend on the brain’s capacity for neuroplastic change (Grosjean, 2005). Similarly, the action of enriched social experience and environmental contexts to enhance neural connectivity and promote neurogenesis parallels their effects in promoting resistance to the reinforcing effects of addictive drugs (Kempermann et al., 2002; Nader et al., 2012; Solinas et al., 2010). Sustained exercise, also a potent pro-neurogenic stimulus, is being studied with increasing rigor as an effective addiction and dual diagnosis treatment (Taylor et al., 2007).
In addition to motivating research needed to determine how particular combinations of pro-neurogenic psychotherapeutic and pharmacologic therapies may be effective depending on their timing of deployment at different stages of addiction recovery, or in the context of different forms of mental illness, a neurogenic theory of dual diagnosis also provides a basis for understanding how certain drug exposures or environmental experiences that impede neurogenesis may be detrimental to recovery. For instance, over-prescribing of opioid medications (Richardson et al., 2012) or criminal incarceration with solitary confinement (Hartwell, 2004) occur at disproportionate rates in dual diagnosis patient populations, potentially worsening illness severities and increasing treatment resistance. Eventually, this neurogenic therapeutics frontier, as informed by a more systematic understanding of clinical contexts where neurogenic up-regulation is most beneficial, could lead to advanced interventional approaches that implant pro-neurogenic stimuli, molecules, or neural stems cells into the hippocampi of dual diagnosis patients.
Although this review focuses on a straightforward hypothesis where low states of neurogenesis may underlie certain forms of dual diagnosis, some disorders that elevate addiction risk could alternatively involve inappropriately regulated or excessive neurogenic activity. For instance, adolescence is a neurodevelopmental stage that relative to adulthood, is characterized by increased impulsivity, heightened novelty reactivity and heighted addiction vulnerability (Chambers et al., 2003). However, neurogenic activity is at its highest level soon after birth and declines through adolescence into adulthood (Amrein et al., 2011; Knoth et al., 2010). It is possible that the robust neurogenic activity of the adolescent hippocampus cannot provide for, or serve as a biomarker of protection against addiction, because the overall functional significance of neurogenesis for addiction vulnerability depends on the maturational status of the prefrontal cortex (Chambers et al., 2003). Moreover, addictive drug suppression of neurogenic activity is greater in adolescence compared to adulthood (McClain et al., 2011), so that adolescent drug exposure may deliver a relatively greater age-dependent risk of acquiring addiction (Crews et al., 2006). Accordingly, growing evidence suggests that the way the brain regulates neurogenic activity, and, conversely, how neurogenic activity effects the functionality of the brain as a whole, is dependent on the maturity and health of regions that directly interact with the hippocampus, such as the amygdala, hypothalamus and prefrontal cortex (Eisch and Petrik, 2012). New neuroimaging technologies (Manganas et al., 2007) and other approaches to assessing levels or temporal patterns of hippocampal neurogenic activity in humans will be key to exploring potential variations of a neurogenic theory of dual diagnosis, and more concretely, in determining treatment needs of individual patients.
8. CONCLUSION
Available evidence presents a framework for understanding how neurogenic activity in the adult hippocampus operates as a special form of neuroplasticity involved the pathogenesis of mental illnesses, addictions and their comorbidities. This area of research exemplifies how psychiatric neuroscience is evolving beyond strict categorical disease models based on single neurotransmitters, genes, or nature vs. nurture arguments, to new conceptual frontiers that embrace complexity, the integration of disease models and psychotherapeutic and pharmacological approaches to understanding and improving brain function. Ongoing research on how pathological regulation of neurogenic activity, occurring as a marker of mental illness and addictive drug exposure, may in turn produce a deleterious shift in addiction vulnerability and/or severity, shows promise in generating new leads on advanced diagnostic approaches and integrative treatments for dual diagnosis disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alen F, Mouret A, Viveros MP, Llorente R, Lepousez G, Lledo PM, Lopez-Moreno JA. Converging action of alcohol consumption and cannabinoid receptor activation on adult hippocampal neurogenesis. Int J Neuropsychopharmacol. 2010;13:191–205. doi: 10.1017/S1461145709991118. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Amrein I, Isler K, Lipp HP. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur J Neurosci. 2011;34:978–987. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR. Early-onset drug use and risk of later drug problems. Drug Alcohol Depend. 1995;40:9–15. doi: 10.1016/0376-8716(95)01194-3. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Fischer SJ, Schonborn JR, Markus RW, Brekken RA, Eisch AJ. Effect of chronic morphine on the dentate gyrus neurogenic microenvironment. Neuroscience. 2009;159:1003–1010. doi: 10.1016/j.neuroscience.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157:70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe PC, Chlan-Fourney J, Juorio AV, Li XM. Brain-derived neurotrophic factor (BDNF) mRNA in rats with neonatal ibotenic acid lesions of the ventral hippocampus. Brain Res. 2002;956:126–135. doi: 10.1016/s0006-8993(02)03176-1. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A, Stern H. Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry. 2012;169:907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Macqueen G, Wojtowicz JM. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: effects of interference, stress and depression. Brain Res. 2009;1299:45–54. doi: 10.1016/j.brainres.2009.07.095. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DA, Kirkham M, Wang H, Frisen J, Simon A. Dopamine controls neurogenesis in the adult salamander midbrain in homeostasis and during regeneration of dopamine neurons. Cell Stem Cell. 2011a;8:426–433. doi: 10.1016/j.stem.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Berg SA, Czachowski CL, Chambers RA. Alcohol seeking and consumption in the NVHL neurodevelopmental rat model of schizophrenia. Behav Brain Res. 2011b;218:346–349. doi: 10.1016/j.bbr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Mueller T, Jones BA, Christensen DR. The behavioral economics of drug dependence: towards the consiliance of economics and behavioral neuroscience. In: Self DW, Staley JK, editors. Behavioral Neuroscience of Drug Addiction. Springer; New York: 2010. pp. 320–334. [DOI] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcel E, Perez-Alvarez L, Herrero AI, Brionne T, Varea E, Berezin V, Bock E, Sandi C, Venero C. Chronic stress in adulthood followed by intermittent stress impairs spatial memory and the survival of newborn hippocampal cells in aging animals: prevention by FGL, a peptide mimetic of neural cell adhesion molecule. Behav Pharmacol. 2008;19:41–49. doi: 10.1097/FBP.0b013e3282f3fca9. [DOI] [PubMed] [Google Scholar]
- Brady AM, McCallum SE, Glick SD, O’ Donnell P. Enhanced methamphetamine self-administration in a neurodevelopmental rat model of schizophrenia. Pschopharmacology. 2008;200:205–215. doi: 10.1007/s00213-008-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Ryu V, Herzog T, Czaja K, Dong Y. Reducing hippocampal cell proliferation in the adult rat does not prevent the acquisition of cocaine-induced conditioned place preference. Neurosci Lett. 2010;481:41–46. doi: 10.1016/j.neulet.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48. C21–28. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Buwalda B, van der Borght K, Koolhaas JM, McEwen BS. Testosterone decrease does not play a major role in the suppression of hippocampal cell proliferation following social defeat stress in rats. Physiol Behav. 2010;101:719–725. doi: 10.1016/j.physbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Chambers RA. Impulsivity, dual diagnosis, and the structure of motivated behavior in addiction. Behav Brain Sci. 2008;31:443–444. [Google Scholar]
- Chambers RA. A nicotine challenge to the self-medication hypothesis in a neurodevelopmental animal model of schizophrenia. J Dual Diagn. 2009;5:139–148. doi: 10.1080/15504260902869808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Bickel WK, Potenza MN. A scale-free systems theory of motivation and addiction. Neurosci Biobehav Rev. 2007;31:1017–1045. doi: 10.1016/j.neubiorev.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Conroy SK. Network modeling of adult neurogenesis: shifting rates of neuronal turnover optimally gears network learning according to novelty gradient. J Cogn Neurosci. 2007;19:1–12. doi: 10.1162/jocn.2007.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Jones RM, Brown S, Taylor JR. Natural reward related learning in rats with neonatal ventral hippocampal lesions and prior cocaine exposure. Psychopharmacology. 2005;179:470–478. doi: 10.1007/s00213-004-2042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmaclogy. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical-striatal integration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol Psychiatry. 2010;67:788–792. doi: 10.1016/j.biopsych.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Cho KO, Kim SK, Rhee GS, Kwack SJ, Cho DH, Sung KW, Kim SY. Chronic 3,4-methylenedioxymethamphetamine treatment suppresses cell proliferation in the adult mouse dentate gyrus. Eur J Pharmacol. 2007;566:120–123. doi: 10.1016/j.ejphar.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Hildebrandt K, Teuchert-Noodt G. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 1998;105:317–327. doi: 10.1007/s007020050061. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaux O, Vendruscolo L, Schlosburg J, Diaz-Aguilar L, Yuan CJ, Sobieraj JC, George O, Koob GF, Mandyam CD. Hippocampal neurogenesis protects against cocaine-primed relapse. Addict Biol. doi: 10.1111/adb.12019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- DSM-IV-TR. Diagnostic and Statistical Manual of Mental Disorders. 4. American PSychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson TB, Alborn A, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocapus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer SJ, Arguello AA, Charlton JJ, Fuller DC, Zachariou V, Eisch AJ. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administration paradigm. Neuroscience. 2008;151:1217–1224. doi: 10.1016/j.neuroscience.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Neurodynamic models of brain in psychiatry. Neuropsychopharmacology. 2003;28(Suppl 1):S54–63. doi: 10.1038/sj.npp.1300147. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Kuhn HG. Adult neurogenesis-a reality check. Debates Neurosci. 2007;1:33–41. [Google Scholar]
- Garcia-Fuster MJ, Perez JA, Clinton SM, Watson SJ, Akil H. Impact of cocaine on adult hippocampal neurogenesis in an animal model of differential propensity to drug abuse. Eur J Neurosci. 2010;31:79–89. doi: 10.1111/j.1460-9568.2009.07045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Mol Psychiatry. 2012;17:790–808. doi: 10.1038/mp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper ER, Schoenfeld TJ, Gould E. Adult neurogenesis: optimizing hippocampal function to suit the environment. Behav Brain Res. 2012;227:380–383. doi: 10.1016/j.bbr.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cereb Cortex. 2008;18:1407–1414. doi: 10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea L, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Green AI. Schizophrenia and comorbid substance use disorder: effects of antipsychotics. J Clin Psychiatry. 2005;66(Suppl 6):21–26. [PubMed] [Google Scholar]
- Green AI, Burgess ES, Dawson R, Zimmet SV, Strous RD. Alcohol and cannabis use in schizophrenia: effects of clozapine vs. risperidone. Schizophr Res. 2003;60:81–85. doi: 10.1016/s0920-9964(02)00231-1. [DOI] [PubMed] [Google Scholar]
- Grosjean B. From synapse to psychotherapy: the fascinating evolution of neuroscience. Am J Psychother. 2005;59:181–197. doi: 10.1176/appi.psychotherapy.2005.59.3.181. [DOI] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29:1063–1069. doi: 10.1038/sj.npp.1300422. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144:77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell S. Triple Stigma: Persons with mental illness and substance abuse problems in the crminal justice system. Crim Justice Policy Rev. 2004;15:84–99. [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, Renau-Piqueras J, Canales JJ. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addict Biol. 2010;15:413–423. doi: 10.1111/j.1369-1600.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacol (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, Yamada K. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Jayatissa MN, Bisgaard C, Tingstrom A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Normand E, Manzoni OJ. Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur J Neurosci. 2005;21:493–500. doi: 10.1111/j.1460-9568.2005.03883.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug Addiction as a pathology of staged neuroplasticity. Neuoropsychophramacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The Neural Basis of Addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kerr AL, Swain RA. Rapid cellular genesis and apoptosis: effects of exercise in the adult rat. Behav Neurosci. 2011;125:1–9. doi: 10.1037/a0022332. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of dual diagnosis. Biol Psychiatry. 2004;56:730–737. doi: 10.1016/j.biopsych.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34:762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochman LJ, dos Santos AA, Fornal CA, Jacobs BL. Despite strong behavioral disruption, Delta9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse dentate gyrus. Brain Res. 2006;1113:86–93. doi: 10.1016/j.brainres.2006.07.080. [DOI] [PubMed] [Google Scholar]
- Kovacsics CE, Gottesman II, Gould TD. Lithium’s antisuicidal efficacy: elucidation of neurobiological targets using endophenotype strategies. Annu Rev Pharmacol Toxicol. 2009;49:175–198. doi: 10.1146/annurev.pharmtox.011008.145557. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24:6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry. 2006;60:1121–1130. doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Suzuki T, Seki T, Namba T, Tanimura A, Arai H. Effects of repeated phencyclidine administration on adult hippocampal neurogenesis in the rat. Synapse. 2006;60:56–68. doi: 10.1002/syn.20275. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yu J, Mao-Ying QL, Mi WL, Li B, Wang YQ, Wang J, Wu GC. Repeated clomipramine treatment reversed the inhibition of cell proliferation in adult hippocampus induced by chronic unpredictable stress. Pharmacogenomics J. 2008;8:375–383. doi: 10.1038/sj.tpj.6500485. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Luu P, Sill OC, Gao L, Becker S, Wojtowicz JM, Smith DM. The role of adult hippocampal neurogenesis in reducing interference. Behav Neurosci. 2012;126:381–391. doi: 10.1037/a0028252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak M, Chocyk A, Markowicz-Kula K, Wedzony K. Acute activation of CB1 cannabinoid receptors transiently decreases PSA-NCAM expression in the dentate gyrus of the rat hippocampus. Brain Res. 2007;1148:43–52. doi: 10.1016/j.brainres.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, Takuma K, Yamada K. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci. 2007;103:299–308. doi: 10.1254/jphs.fp0061424. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Gliogenesis in the striatum of the adult rat: alteration in neural progenitor population after psychostimulant exposure. Brain Res Dev Brain Res. 2001;130:41–51. doi: 10.1016/s0165-3806(01)00195-x. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacol (Berl) 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- McClain JA, Hayes DM, Morris SA, Nixon K. Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J Comp Neurol. 2011;519:2697–2710. doi: 10.1002/cne.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup JS, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145:470–483. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Nader J, Claudia C, Rawas RE, Favot L, Jaber M, Thiriet N, Solinas M. Loss of environmental enrichment increases vulnerability to cocaine addiction. Neuropsychopharmacology. 2012;37:1579–1567. doi: 10.1038/npp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell PO, Grace AA. Synaptic interactions among excitatroy afferents to the nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Charney DS, Lewis L, Cornish JW, Post RM, Woody GE, Zubieta JK, Anthony JC, Blaine JD, Bowden CL. Priority actions to improve the care of persons with co-occurring substance abuse and other mental disorders: a call to action. Biol Psychiatry. 2004;56:703–713. doi: 10.1016/j.biopsych.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Olive MF, editor. Drug Addiction and Adult Neurogenesis. Research Signpost; Kerala, India: 2011. [Google Scholar]
- Otmakhova NA, Lisman JE. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci. 1999;19:1437–1445. doi: 10.1523/JNEUROSCI.19-04-01437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, Groenewegen HJ, Lopez da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioral, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Persson AI, Naylor AS, Jonsdottir IH, Nyberg F, Eriksson PS, Thorlin T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur J Neurosci. 2004;19:1847–1855. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predicts memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.315. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Richardson LP, Russo JE, Katon W, McCarty CA, DeVries A, Edlund MJ, Martin BC, Sullivan M. Mental health disorders and long-term opioid use among adolescents and young adults with chronic pain. J Adolesc Health. 2012;50:553–558. doi: 10.1016/j.jadohealth.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi S, Bianchi P, Guidi S, Ciani E, Bartesaghi R. Neonatal isolation impairs neurogenesis in the dentate gyrus of the guinea pig. Hippocampus. 2007;17:78–91. doi: 10.1002/hipo.20247. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A. Cooperation between the hippocampus and the striatum during episodic encoding. J Cogn Neurosci. 2011;23:1597–1608. doi: 10.1162/jocn.2010.21549. [DOI] [PubMed] [Google Scholar]
- Sargeant TJ, Miller JH, Day DJ. Opioidergic regulation of astroglial/neuronal proliferation: where are we now? J Neurochem. 2008;107:883–897. doi: 10.1111/j.1471-4159.2008.05671.x. [DOI] [PubMed] [Google Scholar]
- Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacol (Berl) 2006;184:540–546. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl C, Bremner JD. Neuroimaging in borderline personality disorder. J Psychiatr Res. 2006;40:419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Self DW. Neural substrates of drug craving and relapse in drug addiction. Ann Med. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J Neural Transm. 2005;112:1475–1478. doi: 10.1007/s00702-005-0370-2. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Thiriet N, Chauvet C, Jaber M. Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol. 2010;92:572–592. doi: 10.1016/j.pneurobio.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci USA. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 2000;107:133–143. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Krupitsky E, Verbitskaya E, Blokhina E, Mamontova O, Fohr J, Tuomola P, Kuoppasalmi K, Kiviniemi V, Zwartau E. Naltrexone implant for the treatment of polydrug dependence: a randomized controlled trial. Am J Psychiatry. 2012;169:531–536. doi: 10.1176/appi.ajp.2011.11071121. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Li T-K. Drug addiction: the neurobiology of behavior gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16:261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann NY Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]
- Zagon IS, McLaughlin PJ. Opioid antagonist-induced modulation of cerebral and hippocampal development: histological and morphometric studies. Brain Res. 1986;393:233–246. doi: 10.1016/0165-3806(86)90025-8. [DOI] [PubMed] [Google Scholar]
- Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA. Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology. 2005;48:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Zohar O, Getslev V, Miller AL, Schreiber S, Pick CG. Morphine protects for head trauma induced cognitive deficits in mice. Neurosci Lett. 2006;394:239–242. doi: 10.1016/j.neulet.2005.10.099. [DOI] [PubMed] [Google Scholar]