Abstract
This study demonstrates the proof-of-principle of rapid surface modification of plasmonic nanostructured materials with oligonucleotides using low power microwave heating. Due to their interesting optical and electronic properties, silver nanoparticle films (SNFs, 2 nm thick) deposited onto glass slides were used as the model plasmonic nanostructured materials. Rapid surface modification of SNFs with oligonucleotides was carried out using two strategies (1) Strategy 1: for ss-oligonucleotides, surface hybridization and (2) Strategy 2: for ds-oligonucleotides, solution hybridization), where the samples were exposed to 10, 15, 30 and 60 seconds microwave heating. To assess the efficacy of our new rapid surface modification technique, identical experiments carried out without the microwave heating (i.e., conventional method), which requires 24 hours for the completion of the identical steps. It was found that SNFs can be modified with ss- and ds-oligonucleotides in 10 seconds, which typically requires several hours of incubation time for the chemisorption of thiol groups on to the planar metal surface using conventional techniques.
Keywords: Surface modification, oligonucleotides, plasmonics, plasmonic nanostructures, silver island films, gold nanoparticles, metal-enhanced fluorescence
1 Introduction
Plasmonics has emerged as one of the leading new technologies in biomedical research. This is mostly due to the ability of plasmonic (surface plasmon-supporting) nanostructured materials to manipulate and transport electromagnetic energy, which afford for the realization of more efficient biological and electronic processes. In this regard, Surface Plasmon Resonance (SPR) [1–4], Surface Enhanced Raman Scattering (SERS) [5–7] and Surface Plasmon Fluorescence Spectroscopy (SPFS) [8–10] are the most commonly utilized plasmonics technologies today. The interest in plasmonic nanostructured materials is also due to their size (in the order of biomolecules themselves) and their amenability to the attachment of biological materials. To create these bio-nano hybrid materials, one has to carryout surface modification procedures on the plasmonic nanostructured materials [11].
One can find several techniques in literature for the surface modification of plasmonic nanostructured materials with biological materials. These techniques include layer-by-layer assembly [11, 12], self-assembled monolayers [11,13], protein-antigen interactions [11,14], covalent attachment [11, 15–17], sol-gels [18]. One of the most commonly used biological materials in conjunction with plasmonic nanostructured materials are oligonucleotides due to their relevance in biological processes [19]. The attachment of oligonucleotides onto plasmonic nanostructured materials is carried out via covalent attachment [17, 20, 21]. In this regard, one of the ends of oligonucleotides are chemically modified with a thiol group and these thiolated oligonucleotides are then attached to plasmonic nanostructured materials via metal-thiol covalent bond in the presence of a salt [16, 22]. Typically, the attachment of thiolated oligonucleotides onto plasmonic nanostructured materials (i.e., gold nanoparticles in solution) takes up to 48 hours [17]. In recent years, surfactants were employed to speed up the surface modification process, which reduced this process down to 2 hours [19, 23]. More recently, Zhang et. al. [21] reduced the duration of solution-based surface modification process to 3 min (plus centrifugation time) by lowering the pH of the citrate buffer medium to 3.0.
In addition to the use of plasmonic nanostructured materials modified with thiolated oligonucleotides for solution-based applications, some applications such as SPFS [24–26], SPR [18, 27, 29] and metal-enhanced fluorescence (MEF) [30–32] employ plasmonic nanostructured materials deposited onto a solid substrate (i.e, glass, polymers, electrodes). Similar to surface modification of gold nanoparticles with thiolated oligonucleotides in solution, covalent attachment is the most widely used technique. In this regard, the surface modification process takes up to several hours due to the diffusion limited chemisorption of thiolated oligonucleotides onto plasmonic nanostructured materials deposited onto a solid substrate. Subsequently, there is still a need to minimize the duration of the surface modification of plasmonic nanostructured materials on solid substrates with oligonucleotide.
In this work, we present a new, one-step and rapid surface modification technique for the attachment of thiolated oligonucleotides onto plasmonic nanostructured materials on solid substrates. Our technique is based on the combined use of plasmonic nanostructured materials and low power microwave heating as depicted in Scheme 1. In this regard, microwave heating of a solution containing a thiolated oligonucleotides placed on SNFs resulted in selective heating of the solution and a subsequent thermal gradient (up to 4°C) between the solution and SNFs. The resultant thermal gradient induced the rapid transfer of thiolated oligonucleotides to SNFs, which was completed in 10 seconds. The presence of thiolated oligonucleotides on SNFs after microwave heating was confirmed by measuring the fluorescence emission from fluorophore-labeled oligonucleotides complimentary to the thiolated oligonucleotides. The efficacy of our new rapid surface modification technique was assessed by carrying out identical experiments without the microwave heating (i.e., conventional method), which required 24 hours for the completion of identical steps. A close match in fluorescence emission measured from samples prepared using 10 second microwave heating and conventional method carried out for 24 hours was observed. Control experiments, where the thiolated oligonucleotides were omitted from the surface showed a significantly low background fluorescence emission. These results prove that our new rapid surface modification technique can be used for the attachment of ss- and ds-oligonucleotides onto SNFs in 10 seconds.
Scheme 1.

(online color at: www.annphys.org) Schematic depiction of rapid surface modification of plasmonic nanostructured materials (P) with thiolated oligonucleotides. The presence of thiolated oligonucleotides on plasmonic nanostructured materials was confirmed with a fluorophore-labeled oligonucleotide with complementary strand to that of thiolated oligonucleotide. The samples were washed after each step to remove unbound materials.
2 Materials and methods
2.1 Materials
Succinic anhydride, Ethylene diamine tetraacetic acid (EDTA), Borax anhydrous, 1-methyl-2-pyrrolidinone, HEPES buffer solution, premium quality Aminopropylsilane (APS)-coated glass slide (75 × 25 mm), Silicone isolator (12 well, 2.0 mm diameter, 1.5 mm deep) and p53 specific ss-oligonucleotides (gadd45, DNA repair [33]): single stranded (ss)-thiolated oligonucleotides: [3′-thiol-(CH2)6-GTA-CAG-AAC-ATG-TCT-AAG-CAT-GCT-GGG-GAC- 5′] and Alexa 488 labeled-oligomer [5′-CAT-GTC-TTG- TAC-AGA-TTC-GTA-CGA-CCC-CTG-(A488)-3′] were obtained from Sigma-Aldrich. Physical constants for the oligonucleotides used in this study are: melting temperature: 63°C (basic) and 72.1°C (salt adjusted), ΔH = 243.1 Kcal/mol). Potassium chloride was obtained from Fisher Scientific. Silver target (57 mm diameter × 0.1 mm thick), which was used for deposition of SNFs was obtained from Electron Microscopy Sciences. All solutions were prepared using deionized water obtained from Millipore Direct Q3 system.
2.2 Methods
(1) Deposition of SNFs on APS-coated glass slides
SNFs was deposited onto APS-coated glass slides using a sputter coater (EMS 150R, equipped with film thickness monitor) obtained from Electron Microscopy Sciences (PA, USA). In this regard, a silver target (57 mm diameter × 0.1 mm thick) was used. The thickness of the SNFs was adjusted to 2.0 nm using the instruments film thickness monitor.
(2) Oligomer solution preparation
For the experiments carried out in this study, the following solutions were prepared. A 5 μM stock solution of ss-thiolated oligonucleotides and a 5 μM stock solution of target ss-A488-labeled were prepared in 5 mM HEPES buffer (pH 7.5 with final concentration of 100 mM KCl, 0.25 mM EDTA). The initial temperature of the stock solutions was adjusted to 25°C before use in all experiments.
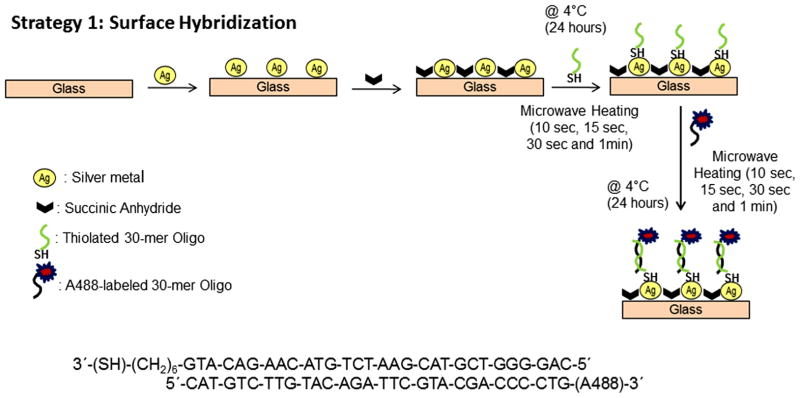
(3) Rapid and conventional surface modification of SNFs with ss-thiolated oligonucleotides: strategy (1)
This two-step procedure, also called “surface hybridization”, is carried out using a conventional method at 4°C (no microwave heating) and the new rapid method using microwave heating as depicted in Scheme 2:
Scheme 2.
(online color at: www.ann-phys.org) Schematic depiction of Strategy 1: Surface Hybridization. Thiolated ss-oligomers are deposited onto SNFs with succinic anhydride at 4°C for 24 hours or microwave heating (10 sec, 15 sec, 30 sec and 1 min). Alexa 488-labeled target ss-oligomers are hybridized on the surface at 4°C for 24 hours or microwave heating (10 sec, 15 sec, 30 sec and 1 min).
Step 1: attachment of ss-thiolated oligonucleotides onto SNFs.
Step 2: surface hybridization of target ss-A488-labeled oligonucleotides with sequence complimentary to the ss-thiolated oligonucleotides on SNFs.
The surface modification of SNFs with ss-thiolated oligonucleotides using a conventional method was carried out using previously published procedure. [34] In this regard, a 30 μl stock solution of ss-thiolated oligonucleotides was incubated on SNFs for a period of 24 hours at 4°C. Rapid surface modification of SNFs with ss-thiolated oligonucleotides was carried out microwave heating of the SNFs immediately after placing the 30 μl stock solution of ss-thiolated oligonucleotides on SNFs for 10 sec, 15 sec, 30 sec and 1 minute. The presence of ss-thiolated oligonucleotides attached to SNFs was verified by surface hybridization of target ss-A488-labeled oligomer with sequence complimentary to the ss-thiolated oligonucleotides on SNFs. In this regard, a 30 μl stock solution of ss-A488-labeled oligonucleotides was incubated on SNFs for a period of 24 hours at 4°C or microwave heated for 10 sec, 15 sec, 30 sec and 1 minute. In each step of the procedure described above, SNFs were rinsed with deionized water to remove the unbound materials.
(4) Rapid and conventional surface modification of SNFs with double-stranded (ds)-oligonucleotides: strategy 2
In this “solution hybridization” procedure, the following steps were carried out as depicted in Scheme 3:
Scheme 3.

(online color at: www.ann-phys.org) Schematic depiction of Strategy 2: Solution Hybridization. Thiolated ss-oligomers and Alexa 488-labeled target ss-oligomers are mixed in an equimolar ratio. DNA hybridization in solution is carried out for 24 hours at 4°C, followed by the deposition of ds-DNA onto the silver nanoparticle-deposited onto glass slides coated with succinic anhydride at 4°C or using microwave heating (10 sec, 15 sec, 30 sec and 1 min).
Step 1: hybridization of target ss-A488-labeled oligonucleotides (30 μl) with sequence complimentary to the ss-thiolated oligonucleotides (30 μl) in solution at room temperature (25°C) for 60 min.
Step 2: attachment of ds-oligonucleotides (30 μl) onto SNFs at 4°C (for 24 hours) or using microwave heating (for 10 sec, 15 sec, 30 sec and 1 minute).
In all the experiments used in this study, the power setting of the microwave oven (Emerson Model No: MW87845B, 0.7 cu. ft, 700 W output power) was set at 2. To prevent the non-specific absorption and binding of oligonucleotides onto the non-silvered surface, [34] SNFs were incubated in freshly prepared succinic anhydride solution (0.156 M in 1-methyl-2-pyrrolidone, 20 mM borate buffer pH 8.0) for 15 mins at room temperature, followed by rinsing with deionized water. In each step of the procedure described above, SNFs were rinsed with deionized water to remove the unbound materials.
In order to determine the extent of non-specific binding of target ss-A488-labeled oligonucleotides on SNFs, control experiments, where a solution of target ss-A488-labeled oligonucleotides were incubated on SNFs (without ss-thiolated oligonucleotides at 4°C (for 24 hours) or using microwave heating (for 10–60 sec), were carried out as shown in Scheme 4.
Scheme 4.
(online color at: www.ann-phys.org) (Top)-Schematic depiction of control experiment. Thiolated ss-oligomers were omitted in the control. (A) Alexa 488-labeled target ss-oligomers are deposited onto the silver nanoparticle-deposited glass slides coated with succinic anhydride at 4°C for 24 hours and (B) using microwave heating (10 sec, 15 sec, 30 sec and 1 min). (Bottom)-Schematic depiction of the experimental setup.
(5) Fluorescence measurements and real-color photographs
In this work, all fluorescence measurements were using an in-house build setup, equipped with a Fiber Optic Spectrometer (Jaz, Ocean Optics, Inc., FL, USA), laser 473 nm (BW&Tek, Inc., DE, USA), fiber optic connections and reflective mirrors as shown in Scheme 4). Samples were excited at a 45° angle and the fluorescence emission was detected through a 473 nm razor-edge emission filter (Thorlabs, USA). All fluorescence measurements were carried out in 2 minutes, where the laser beam is blocked in between measurements (actual laser exposure time is 4 sec for each measurement), to minimize the photo-destruction of the fluorophores. In this regard, all fluorescence measurements are consistent with respect to laser exposure and detection periods. Real color photographs of A488-labeled oligonucleotides on SNFs were taken with a 5 MP digital camera through the same emission filter as used to record the emission spectra.
(6) In-situ temperature measurements of water on SNFs during microwave heating
Thirty microliters of deionized water was placed on the wells of SNFs modified with a silicon isolator (identical to that used in the DNA hybridization assays). The temperature of the water was measured using an infrared thermometer (Klein Tools, IR1000) through a 5 mm opening drilled on top the microwave oven (same microwave oven used in Sect. 2). The opening was positioned in a way that several wells of the silicon isolator were visible during the entire 60 sec continuous microwave heating. Thermal reading of the water on a single was recorded every 5 seconds without interrupting the microwave heating. The room temperature (initial reading at time = 0 sec) was 25.8°C. The size of the laser spot was adjusted to the size of one well to read the average temperature of the water.
3 Results and discussion
Since the methods described in this work are based on the use of silver nanoparticle films (SNFs) in conjunction with microwave heating, the characterization of SNFs were carried out first. Our goal was to deposit SNFs as a semi-continuous thin film using physical deposition method (2 nm thick according to the film thickness monitor of the instrument used) onto glass slides, which affords for reproducible and consistent thickness of silvered surfaces on the same and different glass slides. Our research group [11, 14, 35, 36] and others [32, 37] have also used SNFs prepared deposited by chemical methods (i.e., Tollen’s reaction scheme), where the reproducibility of SNFs can vary from batch to batch. Figure 1A shows the absorption spectrum of SNFs, which displays a broad spectrum at wavelengths longer than a typical surface resonance peak for silver nanoparticles (400–450 nm). The broadening in the absorption spectrum suggests that the inter-particle distance is less than the size of the silver nanoparticles themselves, i.e., approaching a semi-continuous thin film.
Figure 1.
(online color at: www.annphys.org) (A) Absorption spectrum of SNFs, (B) Real-color photograph of SNFs as deposited and used in the experiments, (C) SEM image of 2 nm thick SNFs (D) SEM image SNFs after creating a groove on the surface with a micro-needle to demonstrate the semi-continuous nature of the SNFs.
To further investigate whether the SNFs are deposited as semi-continuous thin films as intended, a real-color photograph (Fig. 1B) and SEM images (Fig. 1C and 1D) of SNFs were taken. A typical real-color photograph of SNFs shows that SNFs are semi-transparent, as also suggested by the absorption spectrum given in Fig. 1A. It was previously shown that, when exposed to microwave radiation, continuous thin metal films tend to accumulate electric field and spark [38], which makes them unusable in microwave-accelerated techniques. In order to avoid sparking of the SNFs and being destroyed during microwave heating, SNFs were removed from the selected regions of the glass surface and kept only within the confines of the wells (2 mm diameter) of a silicon isolator, as shown in Fig. 1B. In this scheme, SNFs were intact even after multiple microwave heating process for 2 minutes. The use of silicon isolators afforded for the multiple bioassays or assessment of repeated samples to be carried out in a confined volume of 30 μl (volume of each well) on the same surface. Figs. 1C and 1D show the SEM images of SNFs. In these images, SNFs appears as to have smooth surface with several dark spots due the lack of silver nanoparticle coverage. The semi-continuous nature of the SNFs was demonstrated by creating a deep groove (diagonal dark spot running along the left side of the image) on the surface of SNFs using a micro-needle, as shown in Fig. 1D.
Since the extent of DNA denaturation depends on the increase in temperature of the solution that DNA is in, the increase in temperature of water placed inside a well of the silicon isolator during microwave heating was investigated. Figure 2 shows the actual temperature reading of water during 60 sec of continuous microwave heating of the SNFs. The temperature of the water for 10, 15, 30 and 60 seconds (corresponding to the microwave heating period used in rapid surface modification experiments) was 26.7, 27.1, 27.9 and 30°C, respectively. The implications of these observations are discussed in conjunction with the data collected after the hybridization of fluorophore-labeled oligonucleotides with the thiolated oligonucleotides on SNFs.
Figure 2.

(online color at: www.ann-phys.org) Actual temperature reading of a 30 ml water drop placed inside a well of the silicon isolator during microwave heating up to 1 minute.
Subsequent to the characterization of SNFs and the temperature measurements during microwave heating, SNFs with silicon isolators shown in Fig. 1B (labeled as “used”) were employed in rapid surface modification and DNA hybridization experiments. It is important to note that the presence of thiolated oligonucleotides was confirmed by measuring the fluorescence emission from Alexa 488-labeled target oligonucleotides after the DNA hybridization procedure on SNFs. Control experiments, where the thiolated oligonucleotides were omitted from the surfaces, were used to assess the extent of background fluorescence due to the non-specific binding of Alexa 488-labeled target oligonucleotides to SNFs. In addition, to assess the efficacy of microwave-accelerated surface modification of SNFs with thiolated oligonucleotides, the identical procedures were repeated at 4°C without microwave heating. It is also important to note the concentration of the thiolated and of Alexa 488-labeled target oligonucleotides was kept in excess as compared to the binding capacity of the SNFs surface to maintain a constant concentration gradient between the surface and the solution during microwave heating. We note that microwave heating results in temperature gradient induced mass transfer as indicated in Scheme 1.
Figure 3 shows the emission spectra of Alexa 488-labeled target oligonucleotides after rapid surface modification and rapid DNA hybridization on SNFs for (1) control experiment (no thiolated oligonucleotides), (2) strategy 2 (ds-oligonucleotides, solution hybridization), and (3) strategy 1 (ss-oligonucleotides, surface hybridization). Real-color photographs of the fluorescence emission from the surfaces used in the different surface modification strategies and control samples after microwave heating. Since the aim of this study is demonstrate the proof-of-principle of rapid surface modification of SNFs with ss- and ds-oligonucleotides using microwave heating, it is important to discuss the observations for these oligonucleotides separately and in conjunction with each other. As shown in Fig. 3, the fluorescence emission maximum peak intensities (at 518 nm) measured from SNFs for Strategy 1 decreased slightly as the duration of continuous microwave heating is increased from 10 sec to 60 sec. The largest emission peak intensity in Strategy 1 was observed from samples microwave heated for 10 seconds. In Strategy 2, where ds-oligonucleotides is first hybridized in solution and attached to SNFs using microwave heating, the fluorescence emission peak intensities slightly lower than those measured from Strategy 2. In addition, the fluorescence emission peak intensities measured from surfaces using Strategy 2 also showed a slight decrease as the duration of microwave heating is increased. In control experiments, where the thiolated oligonucleotides were omitted, the fluorescence emission peak intensities were significantly lower as compared to the experiments carried out with thiolated oligonucleotides, which can attributed to the efficacy of procedure employed to reduce the non-specific adsorption of oligonucleotides. Real-color photographs visually demonstrate that the fluorescence emission from control experiments is significantly lower than those measured from samples used in both rapid surface modification strategies.
Figure 3.
(online color at: www.ann-phys.org) Emission spectra of Alexa 488-labeled target ss-oligomers after microwave-accelerated hybridization on silvered surface for strategy 1, strategy 2, and control experiment. Inset- real-color photographs fluorescence emission from surfaces prepared using different strategies and control experiments. Microwave heating at 10 sec (A), 15 sec (B), 30 sec (C), and 1 minute (D). The measurements were the mean spectra of six separate surface locations from three different runs.
In order to compare the efficacy of our new rapid surface modification technique with the conventional method, the identical experimental schemes described above were carried out without microwave heating at 4°C for 24 hours and the results are shown in Fig. 4A. The fluorescence emission peak intensities from samples using both conventional surface modification strategies were similar, which implies that the extent of oligonucleotides on SNFs were similar. In addition, the fluorescence emission peak intensity in the control experiments was significantly lower than those measured from samples used in both conventional surface modification strategies. Figure 4B shows the comparison of the fluorescence emission intensity at 518 nm from the samples prepared using conventional and our new rapid surface modification technique and the corresponding control experiments. The fluorescence emission intensities measured from samples using rapid surface modification technique (labeled as MW in Fig. 4B) were slightly lower than those measured samples used in both conventional surface modification technique (labeled as @4°C in Fig. 4B). The closest matching of fluorescence emission intensities measured from samples using rapid surface modification technique with that measured using conventional surface modification technique was at 10 sec microwave heating. In this comparison, the error ranges were <10% and up to 20% for rapid and conventional surface modification techniques, respectively. In addition, the background fluorescence in these experiments was similar.
Figure 4.

(online color at: www.ann-phys.org) Emission spectra Alexa 488-labeled target ss-oligomers after surface and solution hybridization on silvered surface at 4°C for strategy 1, strategy 2, and control experiment, insert-real color photographs of fluorescence emission from surfaces used in strategy 1, strategy 2, and control after incubation at 4°C for a period of 24 hours. The measurements were the average of 6 spectra collected from six separate surface locations from three different runs. The surfaces were NH2-blocked (succinic anhydride) (A). Fluorescence emission intensity of Alexa 488-labeled target ss-oligomers at 518 nm on silvered surface at 4°C and microwave heating 10 sec, 15 sec, 30 sec, and 1 minute (B).
As Fig. 4B shows and mentioned previously in the discussion of Fig. 3, there is a slight decrease in the fluorescence emission intensity measured from samples using our rapid surface modification technique as the duration of the microwave heating is increased. This observation can be attributed to the increase in temperature of the medium (26.7, 27.1, 27.9 and 30°C, for 10, 15, 30 and 60 seconds of microwave heating, respectively). That is, an increase in temperature of the medium containing oligonucleotides due to microwave heating increases the extent of dissociation of oligonucleotides, and results in the removal of Alexa 488-labeled target oligonucleotides from SNFs and a decrease in fluorescence emission. Subsequently, the trend in the decrease in fluorescence emission intensity measured from samples using rapid surface modification technique corresponds to the trend in increase in the temperature of the medium due to microwave heating. Although not measured, it is thought that there is a significant increase in the temperature of SNFs in the immediate surroundings caused microwave heating, [39, 40] which contributed to the dissociation of oligonucleotides from the surface. It is also important to note that the extent of background fluorescence emission was at least 4-fold lower than the fluorescence emission intensity from other samples, which is an acceptable ratio in the fluorescence-based applications [32].
The observations made in this study have the following implications:
Rapid surface modification of SNFs (and other plasmonic nanostructured materials, i.e., gold, copper, iron, aluminum, zinc, tin) with ss- and ds-thiolated oligonucleotides can be carried out in 10 sec as compared to 24 hours typically required for conventional surface modification technique. SNFs and other plasmonic nanostructured materials can be used in luminescence-based and / or colorimetric bioassays. Although not investigated in this study, SNFs and other plasmonic nanostructured materials mentioned here show metal-enhanced fluorescence (MEF) [11, 35, 36, 41]. In a MEF-based biosensing scheme, an increase in fluorescence emission and a decrease in lifetime of fluorophores are observed, which afford for lower detection limits and improved fluorophores photostability [31, 37, 42].
One can add thiolated oligonucleotides to a solution containing the target oligonucleotides and attach the resultant ds-oligomer to SNFs surfaces. This scheme can be especially useful in the DNA hybridization assays for the rapid detection of small amounts of target oligonucleotides in the presence of other oligonucleotides in a solution [31, 38].
Although it is not the subject of investigation in this study, based on the observation that ds-oligonucleotides can rapidly be attached to SNFs, one can extend the technique described here to a 3-piece DNA hybridization assay [30]. In this regard, after attaching ss-thiolated oligonucleotides to SNFs and a fluorophore-labeled oligonucleotides that recognizes both a target oligonucleotides and the thiolated oligonucleotides at the same time can be rapidly hybridized on SNFs. In this regard, work is underway to use this technique for the rapid and sensitive detection of wild-type p53 protein and the mutated p53 protein recovered from ovarian cells.
4 Conclusions
The proof-of-principle of rapid surface modification of a model plasmonic nanostructured material (SNFs) with ss- and ds-oligonucleotides based on the selective heating of the solution containing the oligonucleotides placed on SNFs using low power microwave heating was demonstrated. Optical spectroscopy and SEM images showed SNFs were deposited as semi-continuous films that afforded for reproducible and consistent thickness of silvered surfaces on the same and different glass slides. The temperature of the solution containing thiolated oligonucleotides was increased up to 30°C during 60 seconds of microwave heating. Rapid surface modification of SNFs with ss- and ds-oligonucleotides was carried out using 10, 15, 30 and 60 seconds of microwave heating. The presence of thiolated oligonucleotides after microwave heating on SNFs was verified by measuring the fluorescence emission from fluorophore-labeled oligonucleotides complimentary to the thiolated oligonucleotides on SNFs. A decrease in fluorescence emission from these surfaces was observed as the duration of the microwave heating was increased, which was attributed to the removal of Alexa 488-labeled target oligonucleotides from SNFs due to the increase in the extent of dissociation of oligonucleotides. To assess the efficacy of the new rapid surface modification technique, the identical assays were carried out without microwave heating (conventional method). A close match in fluorescence emission measured from samples prepared using 10 second microwave heating and conventional method carried out for 24 hours, which implies that one can carry our surface modification of SNFs with ss- and ds- oligomers in 10 seconds using our new surface modification technique.
Acknowledgments
The project described was supported by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
References
- 1.Zheng R, Cameron BD. Expert Rev Mol Diagn. 2012;12:5. doi: 10.1586/erm.11.91. [DOI] [PubMed] [Google Scholar]
- 2.Linman MJ, Yu H, Chen X, Cheng Q. Methods Mol Biol. 2012;808:183. doi: 10.1007/978-1-61779-373-8_13. [DOI] [PubMed] [Google Scholar]
- 3.de Mol NJ. Methods Mol Biol. 2012;800:33. doi: 10.1007/978-1-61779-349-3_4. [DOI] [PubMed] [Google Scholar]
- 4.Pimkova K, et al. Anal Bioanal Chem. 2012;402:381. doi: 10.1007/s00216-011-5395-3. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Han H, Luo Z. Analyst. 2012;137:1259. doi: 10.1039/c2an15997j. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, et al. Anal Bioanal Chem. 2012;402(3):1093. doi: 10.1007/s00216-011-5577-z. [DOI] [PubMed] [Google Scholar]
- 7.Guven B, Boyaci IH, Tamer U, Calik P. Analyst. 2012;137:202. doi: 10.1039/c1an15629b. [DOI] [PubMed] [Google Scholar]
- 8.Murakami T, et al. Anal Biochem. 2012;421:632. doi: 10.1016/j.ab.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Feng CL, et al. Macromol Rapid Commun. 2011;32:679. doi: 10.1002/marc.201000788. [DOI] [PubMed] [Google Scholar]
- 10.Huang CJ, Dostalek J, Sessitsch A, Knoll W. Anal Chem. 2011;83:674. doi: 10.1021/ac102773r. [DOI] [PubMed] [Google Scholar]
- 11.Abel B, Akinsule A, Andrews C, Aslan K. Nano Biomed Eng. 2011;3:184. doi: 10.5101/nbe.v3i3.p184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brett CMA, et al. Electrochem Commun. 2006;8:1665. [Google Scholar]
- 13.Phadtare S, et al. Biotechnol Prog. 2004;20:1817. doi: 10.1021/bp049792h. [DOI] [PubMed] [Google Scholar]
- 14.Addae SA, et al. Nano Biomed Eng. 2010;2:155. doi: 10.5101/nbe.v2i3.p155-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, et al. Carbon. 2010;48:4504. [Google Scholar]
- 16.Herne TM, Tarlov MJ. J Am Chem Soc. 1997;119:8916. [Google Scholar]
- 17.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 18.Brennan JD, Luckham RE. Analyst. 2010;135:2028. doi: 10.1039/c0an00283f. [DOI] [PubMed] [Google Scholar]
- 19.Hurst SJ, Lytton-Jean AK, Mirkin CA. Anal Chem. 2006;78:8313. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson KA, et al. J Nanosci Nanotechnol. 2002;2:397. doi: 10.1166/jnn.2002.110. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Servos MR, Liu J. J Am Chem Soc. 2012;134:7266. doi: 10.1021/ja3014055. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Servos MR, Liu J. Langmuir. 2012;28:3896. doi: 10.1021/la205036p. [DOI] [PubMed] [Google Scholar]
- 23.Zu Y, Gao Z. Anal Chem. 2009;81:8523. doi: 10.1021/ac901459v. [DOI] [PubMed] [Google Scholar]
- 24.Touahir L, et al. Biosens Bioelectron. 2010;25:2579. doi: 10.1016/j.bios.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Chu LQ, Forch R, Knoll W. Angew Chem Int Ed Engl. 2007;46:4944. doi: 10.1002/anie.200605247. [DOI] [PubMed] [Google Scholar]
- 26.Kambhampati D, Nielsen PE, Knoll W. Biosens Bioelectron. 2001;16:1109. doi: 10.1016/s0956-5663(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 27.Nelson BP, et al. Anal Chem. 2001;73:1. doi: 10.1021/ac0010431. [DOI] [PubMed] [Google Scholar]
- 28.Nakatani K, Sando S, Saito I. Nat Biotechnol. 2001;19:51. doi: 10.1038/83505. [DOI] [PubMed] [Google Scholar]
- 29.Kukanskis K, et al. Anal Biochem. 1999;274:7. doi: 10.1006/abio.1999.4241. [DOI] [PubMed] [Google Scholar]
- 30.Aslan K, et al. Analyst. 2007;132:1130. doi: 10.1039/b707876e. [DOI] [PubMed] [Google Scholar]
- 31.Aslan K, Malyn SN, Geddes CD. Biochem Biophys Res Commun. 2006;348:612. doi: 10.1016/j.bbrc.2006.07.093. [DOI] [PubMed] [Google Scholar]
- 32.Malicka J, Gryczynski I, Lakowicz JR. Biochem Biophys Res Commun. 2003;306:213. doi: 10.1016/S0006-291X(03)00935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg RL, Veprintsev DB, Bycroft M, Fersht AR. J Mol Biol. 2005;348:589. doi: 10.1016/j.jmb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Aslan K, Malyn SN, Bector G, Geddes CD. Analyst. 2007;132:1122. doi: 10.1039/b708069g. [DOI] [PubMed] [Google Scholar]
- 35.Abel B, Aslan K. Nano Biomed Eng. 2012;4:23. doi: 10.5101/nbe.v4i1.p23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslan K. Nano Biomed Eng. 2010;2:1. doi: 10.5101/nbe.v2i1.p1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aslan K, Badugu R, Lakowicz JR, Geddes CD. J Fluoresc. 2005;15:99. doi: 10.1007/s10895-005-2515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aslan K, Previte MJ, Zhang Y, Geddes CD. J Immunol Methods. 2008;331:103. doi: 10.1016/j.jim.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Whittaker AG, Mingos DMP. J Chem Soc, Dalton Trans. 1993;16:2541. [Google Scholar]
- 40.Aslan K, Geddes CD. Anal Chem. 2005;77:8057. doi: 10.1021/ac0516077. [DOI] [PubMed] [Google Scholar]
- 41.Grell TA, Paredes E, Das SR, Aslan K. Nano Biomed Eng. 2010;2:165. doi: 10.5101/nbe.v2i3.p165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aslan K, et al. Curr Opin Biotechnol. 2005;16:55. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]