Abstract
Objective
To investigate whether proanthocyanidins (PA) is capable of improving dentin collagen’s biological stability through cross-linking within time periods that are clinically relevant.
Materials and methods
Demineralized dentin collagen slabs were treated with 3.75 wt% PA solution for 10 s, 1 min, 30 min, 60 min, 120 min, 360 min, and 720 min, respectively. The resultant cross-linked collagen samples were subject to digestion with 0.1% collagenase at 37 °C for 2 h, 6 h, 12 h, 24 h, 36 h, and 48 h. The percentage of weight loss after digestion was calculated to evaluate PA-treated collagen’s resistance toward enzymatic degradation. Fourier-Transformed Infrared (FTIR) spectroscopy was used to probe evidences of PA-collagen interactions after various periods of PA treatment.
Results
The collagenase digestion assay suggests that PA treatment as short as 10 s can enhance collagen’s resistance toward enzymatic challenge. The FTIR spectroscopy further verifies that PA is indeed incorporated into collagen regardless of treatment time, possibly via a mechanism involving the chemical interactions between PA and collagen.
Significance
This study confirmed that PA can effectively cross-link collagen and improve its biological stability in time periods as short as 10 s. The use of PA as a priming agent is therefore clinically feasible and is a promising approach to improving the durability of current dentin bonding systems.
Keywords: Dentin collagen, Cross-linking, Proanthocyanidins, Biodegradation, FTIR
1. Introduction
The use of adhesive systems is a common practice in current restorative dentistry to promote strong bonding between restorative materials and dentin substrates [1]. While the shortterm effectiveness of modern adhesive systems is well recognized [2], their long-term stability leaves a lot to be desired [3]. One of the leading causes for restoration failure is the breakdown of the adhesive/dentin (a/d) interface [3]. This interface, also known as the “a/d hybrid layer”, is composed of collagen fibrils and adhesive resin that form an interlocked entanglement, providing for the basis of bonding between the bulk adhesive and the underlying intact dentin [4]. Due to the incomplete penetration of adhesive monomers into the interfacial layer [5, 6], the collagen fibrils are not completely impregnated by resin as they were by hydroxyapatite mineral, rendering them susceptible to hydrolysis and enzymatic degradation. In addition, recent studies have shown that acid etching of dentin activates the otherwise dormant host-derived matrix metalloproteinases (MMPs) [7], increasing the risk of breakdown of the a/d hybrid layer.
As such, the improvement of collagen’s stability within the a/d hybrid layer has been an intriguing target for dentin bonding research. Previous studies have shown that collagen’s mechanical and biological stability can be enhanced after being treated with a variety of crosslinking agents [8-15]. A group of naturally-occurring polyphenolic compounds, proanthocyanidins (PA) [16] is of particular interest to us, because PA is not only capable of cross-linking dentin collagen, its non-toxicity is also a much coveted property that cannot be found in other cross-linking agents such as glutaraldehyde and carbodiimides [17]. It is generally believed that the protein-polyphenolics complexation is a result of non-covalent interactions including hydrogen bonding and hydrophobic effect[18, 19], while a few studies have also indicated the possibility of covalent bond formation under certain circumstances[20, 21]. There are two possible options when incorporating PA in current adhesive systems: as an additive to the adhesives, or as a primer. Green et al. tested the first option [11], but it was found to result in a less than optimum quality of the hybrid layer, presumably due to the low degree of double bond conversion caused by PA’s radical-scavenging ability. In addition, Hechler et al. evaluated the long term performance of both options with Single Bond adhesive (3M ESPE Dental Products, St. Paul, MN) [22], and it turns out that after 52 weeks’ exposure to collagenase digestion, the microtensile bond strength of dentin beams with PA as a primer is significantly higher than control, whereas that of dentin beams with PA as an additive shows no significant difference to control. This observation strongly supports the use of PA as a primer. The limitation of this study, however, is that the PA priming time has been set to 1 h, which is clinically irrelevant. As such, the purpose of the present work is to evaluate PA’s ability to cross-link and stabilize dentin collagen in clinically relevant time periods.
Most previous studies have chosen collagen’s mechanical properties to gauge PA’s stabilizing effect [9, 10, 12, 23-26]. This approach, however, inevitably calls for long treatment times for two reasons. First, during the biosynthesis of collagen, extensive lysyl oxidase-mediated covalent cross-linking is already existent [27], which is the reason why dentin collagen has superior mechanical strength even when untreated. Therefore, it is inherently difficult to further increase collagen’s mechanical performance by introducing more cross-links. Second and more importantly, mechanical tests are highly sensitive to specimen size and shape in addition to the intrinsic inter- and intra-tooth differences in dentin’s tubular structures and orientation [28]. Since all these factors are impossible to standardize, substantial noise is introduced to the test outcome [29]. As a result, longer periods of PA treatment become necessary, because in order to observe any significant changes to collagen’s mechanical properties, PA’s stabilizing effect must be big enough to overcome the high noise level. One article by Liu et al.[30] did examine the changes in dentin collagen’s ultimate tensile strength after being treated by PA for clinically favorable time periods (30 s, 60 s, and 120 s), but their specimen were all cut into hourglass shapes in order to reduce the noise caused by sample shape. Even by doing so, dentin collagen’s ultimate tensile strength showed no significant improvement regardless of treatment time, unless the concentration of PA was increased from 5% to 10% and 15%.
Apparently, researchers have gone to great lengths to examine collagen’s mechanical enhancement upon cross-linking. In contrast, we believe that although increased mechanical properties are certainly a manifestation of PA’s cross-linking capabilities, they are not our ultimate goal. Dentin collagen is already mechanically sound due to the innate extensive cross-links, thus the biological breakdown of collagen fibrils poses bigger threat than mechanical breakdown to the integrity of the a/d hybrid layer. In light of this, the present study aims to evaluate PA’s effect in clinically relevant durations (as short as 10 s and 1 min) on dentin collagen’s resistance toward enzymatic degradation. The null hypothesis tested is that PA could not enhance dentin collagen’s resistance toward enzymatic degradation in clinically relevant times. In addition, the FTIR spectroscopy is employed to investigate structural changes of collagen after PA treatment and to probe possible interactions between PA and collagen.
2. Materials and methods
2.1. Dentin collagen preparation
Extracted non-carious human molars stored in 0.96% (w/v) phosphate buffered saline (PBS) containing 0.002% sodium azide at 4 °C were used. All teeth were collected after obtaining the patient’s informed consent under a protocol approved by the University of Missouri-Kansas City Adult Health Sciences Institutional Review Board. A flat base was created by removing the roots of the teeth 2-3 mm below the cemento-enamel junction, using a water-cooled low-speed diamond saw (Buehler, Lake Bluff, IL). Then the teeth were attached to an aluminum block with cyanoacrylate adhesive (Zapit, Dental Ventures of America, Corona, CA), and the occlusal one-third to one-half was removed using the same saw.
These teeth were divided into two parts. Three teeth were used to make dentin films. Surface dentin of these teeth was cut into 10 μm thick films by means of a tungsten carbide knife mounted on a SM2500S microtome (Leica, Deerfield, IL). Six teeth were used to prepare dentin slabs. The same water-cooled low-speed saw was used to make perpendicular cuts into the dentin surface at 1.2 mm increments. A single cut was then made parallel to and about 1.2 mm beneath the surface dentin. This freed dentin slabs from the remaining dentin blocks. Because some tooth structure was removed due to the thickness (~0.35 mm) of the saw blade, the final dimensions of the slabs were approximately 0.8 × 0.8 mm, and the length varied with respect to their position (10-12 mm). These dentin films and slabs were fully demineralized by 0.5 M EDTA solution (pH = 7.4) to make dentin collagen samples and washed with deionized water 3 times.
2.2. Proanthocyanidins (PA) and collagenase solution preparation
The PA solution was prepared by adding a powdered grape seed extract (MegaNatural Gold, containing over 90% PA, Polyphenolics, Madera, CA) to deionized water to a concentration of 3.75 wt%.
For the collagenase solution, TESCA buffer was first prepared by the addition of 11.5 g N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid, 50 mg sodium azide and 53 mg CaCl2·2H2O into distilled water to a total volume of 1000 mL, followed by pH adjustment to 7.4. Then 1 g collagenase with a molecular weight of ~110 kDa (Collagenase Type I, Clostridiopeptidase A from Clostridium histolyticum, 125 U/mg, Sigma–Aldrich, St. Louis, MO) was dissolved in the TESCA buffer to a final concentration of 0.1% (w/v).
2.3 PA treatment, collagenase treatment and analysis of collagen slabs
Demineralized dentin collagen slabs were divided into 8 groups randomly (3 slabs per group). The slabs were treated by 3.75 wt% PA solution for 0 (control), 10 s, 1 min, 30 min, 60 min, 120 min, 360 min, and 720 min, respectively. Prior to and after PA treatment, each slab was rinsed with excessive deionized water 3 times, and then dried in a desiccator for 24 h. Dry slabs were weighed carefully using an electronic balance (AG285, Mettler Toledo, Columbus, OH), after which they were re-expanded in distilled water for 1 h, and digested by the collagenase solution for 2 h. Slabs were rinsed, dried and weighed again. The percentage weight loss was calculated with respect to the weight of the slab after PA treatment. This weigh-and-digest procedure was repeated for several times so that the digesting time was 2 h, 6 h, 12 h, 24 h, 36 h and 48 h. All treated slabs were individually incubated at 37 °C with shaking in wells of a plastic cell culture plate (Costar 24, Corning, Lowell, MA) holding ~2.5 mL of collagenase solution per well. The collagenase solution was changed every 24 h for treatment times of 36 h and 48 h. Statistical analysis was done with two-tailed unpaired Student’s t-tests, and significance level was set to 5% (p < 0.05).
2.4 PA treatment and FTIR spectra of collagen films
Demineralized dentin collagen films were divided into 8 groups randomly, each group containing 4 films. The films were treated by PA solution, rinsed, and dried in the same way as above. The spectra of dry films were taken by an infrared spectrometer (Spectrum One, Fourier transform infrared spectrophotometer, Perkin-Elmer, Waltham, MA) at a resolution of 4 cm-1. The films were made to attach the diamond crystal top-plate of an Attenuated Total Reflectance (ATR) accessory (Perkin-Elmer, Waltham, MA) with a gauge force of 100. The ATR crystal is zinc selenide (ZnSe) with a transmission range between 650 and 4000 cm-1.
3 Results
When collagen samples are treated with PA solution, their weight continues to rise as the treatment time increases (Figure 1). At the clinically relevant times (10 s and 1 min), the sample’s average weight is not significantly different from control. Weight gain at 30 min is significantly higher than at 10 s and 1 min (p < 0.05), and weight gains at 2 h, 6 h and 12 h are all significantly higher than at 30 min (p < 0.05), although there is no significant difference among themselves (P > 0.05). After being digested with collagenase solution, the weight loss of the PA-treated collagen samples is shown in Figure 2. As can be seen, samples that are treated with PA for the shortest time (10 s) show significantly reduced weight loss after up to 12 h of exposure to collagenase challenge, and if the PA treatment time is elongated to 1 min, the resultant collagen samples can afford significantly enhanced resistance to degradation after 48 h.
Figure 1.
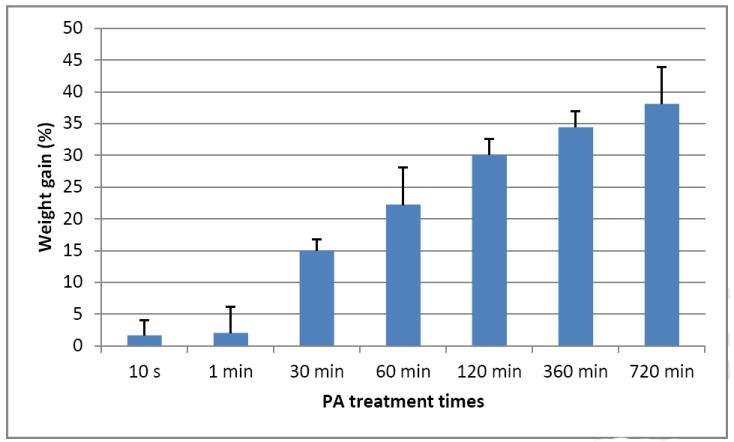
Percent weight gain after collagen was treated with PA solution for different times. The bars indicate the average of 3 separate samples and the whisker standard deviation.
Figure 2.
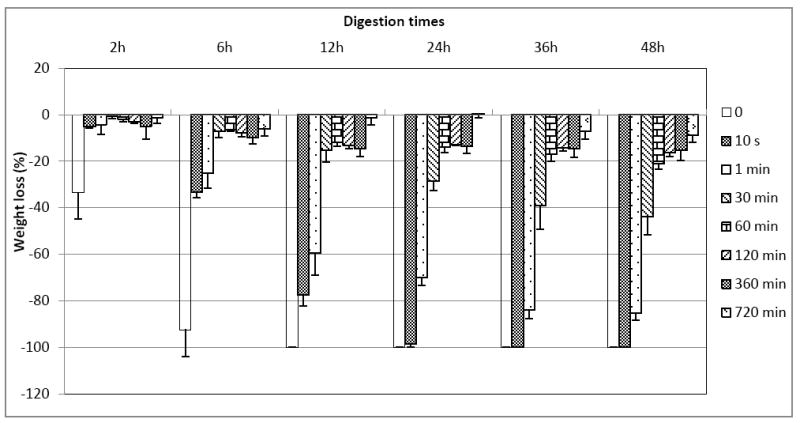
Percent weight loss of collagen samples that were treated with PA for 0 min (control), 10 s, 1 min, 30 min, 60 min, 120 min, 360 min and 720 min, after being digested with 0.1% collagenase solution for 2 h, 6 h, 12 h, 24 h, 36 h, and 48 h. The bars indicate the average of 3 separate samples and the whisker standard deviation.
Representative FTIR spectra of PA, untreated dentin collagen and PA-treated dentin collagen are presented in Figure 3. Peaks characteristic of collagen were identified and assigned as have been well documented in the literature, including N-H stretching at ~3300 cm-1 for the amide A, C-H stretching at 3071 cm-1 for the amide B, C=O stretching at ~1633 cm-1 for the amide I, out-of-phase combination of N-H bending and C-N stretching at ~1544 cm-1 for the amide II, CH2 scissoring at ~1450 cm-1, and in-phase combination of N-H bending and C-N stretching ~1235 cm-1 for the amide III [31, 32]. Some of PA’s peaks overlap with those of collagen and therefore cannot be clearly identified in the spectra of PA-treated collagen, except one group of peaks in the range of 900 cm-1 to 700 cm-1 that can be assigned to the C-H bending of phenyl rings plentiful in PA. A closer look at the FTIR spectra ranging from 1800 cm-1 to 1200 cm-1 revealed that the most prominent changes between untreated and treated collagen occurred in the amide II region and at ~1400 cm-1, regardless of treatment time. As can be seen in Figure 4, even a very brief PA treatment (10 s) induced the appearance of a shoulder at ~1530 cm-1 in the amide II region, and a significant reduction in the peak intensity at ~1400 cm-1.
Figure 3.
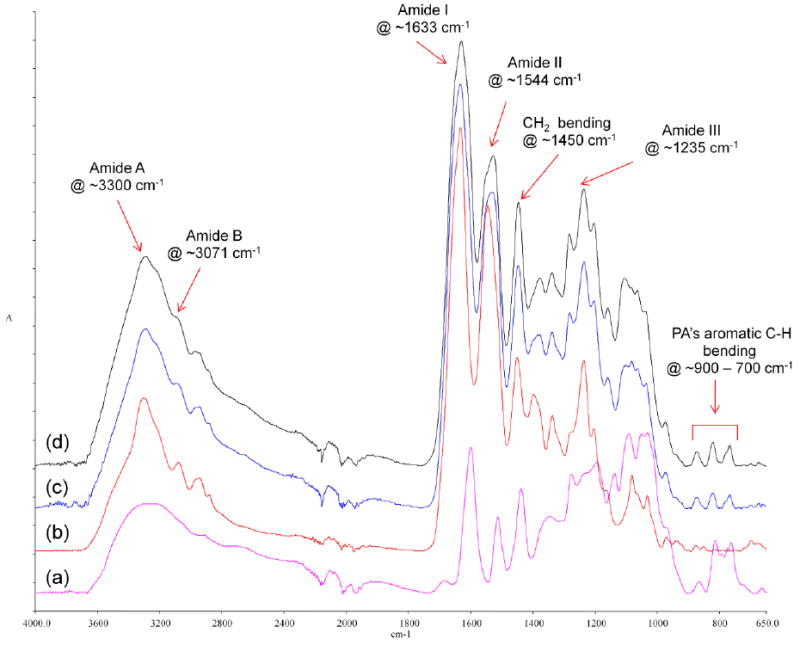
FTIR spectra of (a) PA powder (purple), (b) untreated collagen (red), (c) collagen treated with PA for 10 s (blue), and (d) collagen treated with PA for 720 min (black).
Figure 4.
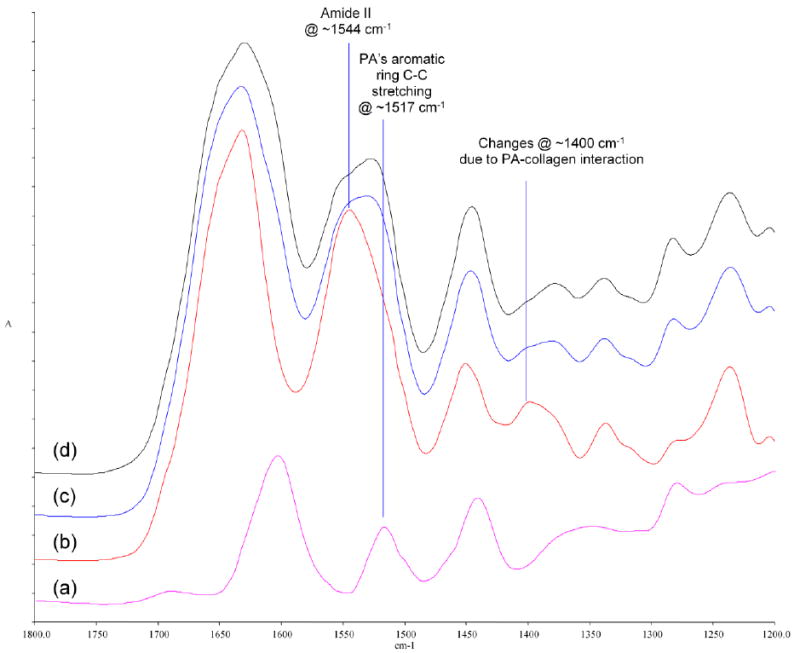
Pronounced FTIR spectral changes in the amide II region and at ~1400 cm-1 when collagen is treated with PA. (a) spectrum of PA powder (purple); (b) spectrum of untreated collagen (red); (c) spectrum of PA-treated collagen for 10 s (blue); (d) spectrum of PA-treated collagen for 720 min (black).
To further elucidate the interactions between PA and collagen, the spectrum of untreated collagen was subtracted from the spectra of collagen samples that were treated with PA for the shortest (10 s) and longest time (720 min), respectively. Using the amide II peak as the internal standard for subtraction, the resultant difference spectra are demonstrated in Figure 5 along with the spectrum of pure PA powder. No obvious change in peak location or intensity was observed as the treatment time increased from 10 s to 720 min, and all but one peak (~1663 cm-1) could find corresponding counterparts in the spectrum of PA powder.
Figure 5.
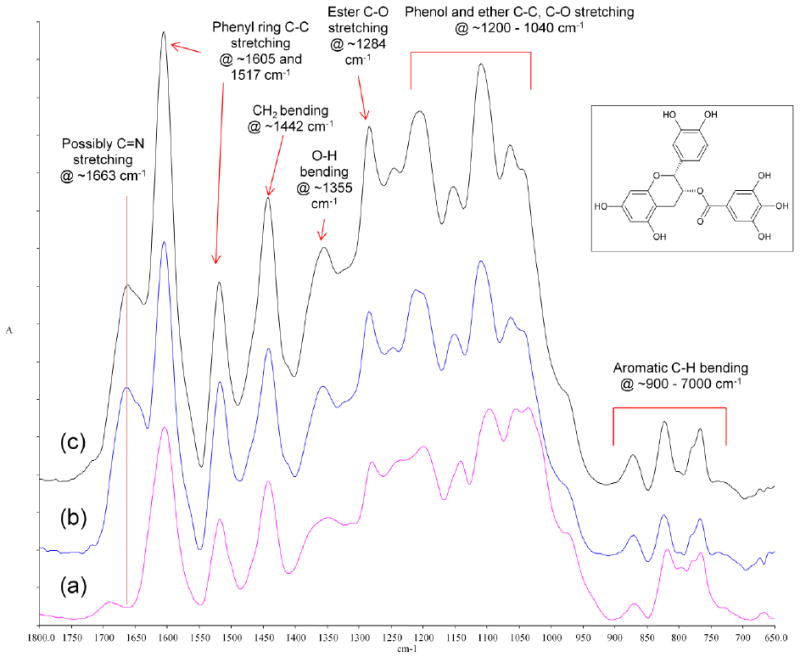
Overlay of (a) the spectrum of PA powder (purple), (b) the difference spectra of collagen treated with PA for 10 s (blue) and (c) for 720 min (black) with the spectrum of untreated collagen subtracted. Insert: structure of epicatechin gallate, one of the building blocks of PA.
4 Discussion
The results of weight gain (Figure 1) coincide with previous findings on collagen’s mechanical properties after different PA treatment times. More specifically, our results indicate that an in-significant amount of PA was incorporated into collagen upon brief exposure (10 s and 1 min) to PA solution, and the added weight of PA became significant only when the treatment time exceeded 30 min. Similarly, no significant enhancement was found in collagen’s ultimate tensile strength after short terms (30 s, 60 s and 120 s) of PA cross-linking [30], whereas longer treatment times (> 10 min) led to significant increases in elastic modulus [10, 12] and ultimate tensile strength [33]. These observations point to a positive correlation between the amount of cross-linking agent incorporated and the strengthening of collagen matrix.
When dentin collagen samples were challenged by collagenase solution, the result (Figure 2) demonstrates that PA treatment for 10 s and 1 min did lead to significantly reduced weight loss after 12 h and 48 h, respectively. In contrast, at 12 h, the weight loss for non-crosslinked collagen was 100%. Therefore, the null hypothesis was rejected. This suggests that although PA did not improve collagen’s mechanical strength in clinically relevant periods [30], it can effectively cross-link collagen and increase its resistance toward enzymatic degradation. As a matter of fact, for the collagen samples treated with PA for 10 s only, even after 48 h of collagenase digestion, there was still a very thin membrane remaining. The weight of the membrane, however, was too low for the balance to detect or rule out as non-noise. It seems that 10 s was too short a time for PA to diffuse into the collagen slabs (0.8 mm thick) and interact with the entirety of it. Fortunately, this is not an issue if we are to use PA as a primer in clinical situations, because the demineralized layer after acid etching is only a few microns thick. Based on the above, PA can fulfill the role of a priming agent satisfactorily, and its use as a primer will be studied further in the future. In addition, in relation to the long-term degradation, 48 h period used in this study might not be enough. Our next target is certainly to examine whether the 10 s application translates to a more durable adhesive/dentin interface during longer time period.
The FTIR spectra of untreated and treated collagen samples were investigated in more detail to explore the interactions between the two species. As mentioned earlier, pronounced changes occurred in the amide II region and at ~1400 cm-1 (Figure 4) regardless of PA treatment time. In the amide II region, a shoulder emerged at ~1530 cm-1, which is attributed to the aromatic ring C-C stretching in PA at ~1517 cm-1. This assignment is confirmed by the difference spectra between untreated and treated collagen as discussed in the following paragraph. No literature has clearly assigned the peak at ~1400 cm-1 to specific vibration modes, but experimental evidences have indicated that it could be due to the CH2 bending from glycine residues [34], as well as the symmetric stretching of carboxylate side chains (COO-) from glutamate and aspartate residues [35, 36]. Specifically in Boryskina’s work [34], the side-chain-free collagen mimic poly(glycine-proline-proline) tripeptide featured a peak at ~1410 cm-1, which exhibited no shift when the hydration medium was changed from H2O to D2O. In comparison, such a medium change caused a red shift in collagen’s peak at ~1405 cm-1 to ~1413 cm-1, which was reported to happen when glutamate and aspartate ion was solvated in D2O instead of H2O [37]. Therefore, we assigned the peak of collagen at ~1400 cm-1 to the combined effect of glycine CH2 bending mode and side chain carboxylate symmetric stretching mode. Now the question is, when collagen is treated with PA, is the reduction of peak intensity at ~1400 cm-1 caused by the simple protonation of side chain carboxylates due to the acidic nature of PA solution (pH = 4.6), or by some other interaction between PA and collagen? The first possibility was ruled out when we compared the IR spectra of untreated collagen before and after being immersed in an acetate buffer with the same pH (pH = 4.6) as PA solution for 1 h. No difference was observed in terms of the location or intensity of the peak at ~1400 cm-1 (data not shown). Therefore, the reduction at ~1400 cm-1 must be due to some other interaction between PA and collagen. Interestingly, it was noted that the diminishment of the ~1400 cm-1 peak could also be caused by dehydration for type I collagen [34]. In addition, according to Miles et al, the cross-linking of collagen results in the dehydration of collagen fibers, and dehydration is the real and sole reason for collagen’s enhanced stability upon cross-linking [38]. Therefore, it can be inferred that the PA treatment indeed cross-linked collagen, and the cross-linking interaction led to the dehydration of collagen fibers, which in turn caused the drop in peak intensity at ~1400 cm-1 as well as the improved resistance toward collagenase digestion (Figure 2). The fact that the emergence of the shoulder at ~1530 cm-1 and the fall of the peak intensity at ~1400 cm-1 took place after even 10 s of PA treatment (Figure 4) indicates that a PA treatment time as short as 10 s could lead to the cross-linking of dentin collagen.
When we subtracted the spectra of untreated collagen from those of treated collagen (Figure 5), the resultant difference spectra could further verify the incorporation of PA in collagen after various treatment times. Most of the peaks in the difference spectra could be accounted for in that of PA powder, including peaks at ~1605 cm-1 and ~1517 cm-1 for phenyl ring C-C stretching, at ~1442 cm-1 for CH2 bending, at ~1355 cm-1 for O-H bending, at ~1284 cm-1 for ester C-O stretching, between 1200 cm-1 and 1040 cm-1 for C-C stretching and various phenol and ether C-O stretching, and between 900 cm-1 and 700 cm-1 for aromatic C-H bending [39]. The relative intensity of the peaks, however, does not match between the difference spectra and PA powder spectra. Such a discrepancy may be because PA powder is a mixture of polyphenol species, and each species has its own unique reactivity toward collagen. As a result, the composition of PA that is incorporated in collagen during cross-linking is different from that of PA powder, which is why we chose the spectra of untreated collagen instead of PA powder to subtract in the first place. There is one peak at ~1663 cm-1 that could not be located in PA powder, and this peak is speculated to be the evidence of covalent interaction between PA and collagen. Such a covalent interaction between polyphenols (such as PA) and protein was postulated in a few studies before[20, 21], and it involves the auto-oxidation of catechol moieties to ortho-quinone groups, which are available for Schiff base formation with free amine groups on proteins [40]. Therefore, this additional peak at ~1663 cm-1 could be due to the imine (C=N) stretching of the Schiff base formed between PA and collagen. The emergence of such a peak for collagen treated with PA for only 10 s could be another indication that PA effectively cross-links collagen in clinically relevant time periods.
5 Conclusion
In this study, we investigated dentin collagen’s resistance to biological degradation after being treated with PA solution for various time periods, and we used FTIR spectroscopy to examine the nature of interactions between collagen and PA. Based on the result of degradation study, PA can effectively cross-link collagen and increase its biological stability in clinically relevant times. Based on the FTIR results, PA is incorporated in collagen after treatment as short as 10 s, and the mechanism may include the formation of imine C=N bonds. These findings are one step forward for the use of PA in clinical settings.
Acknowledgments
This investigation was supported in part by USPHS Research Grants R15-DE021023 and R01-DE021431-01A1 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892. The authors would also like to thank Dr. Ying Liu at the School of Dentistry, University of Missouri-Kansas City for her generous help in statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albers HF. Tooth-colored restoratives: principles and techniques. 9. Hamilton, Ontario: BC Decker Inc; 2002. [Google Scholar]
- 2.Inoue S, Vargas MA, Abe Y, Yoshida Y, Lambrechts P, Vanherle G, HidehikoSano, Meerbeek B. Microtensile bond strength of eleven contemporary adhesives to dentin. Journal of Adhesive Dentistry. 2001;3(3):237–245. [PubMed] [Google Scholar]
- 3.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: Methods and results. Journal of Dental Research. 2005;84(2):118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 4.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. Journal of Biomedical Materials Research. 1982;16(3):265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. Journal of Biomedical Materials Research. 2002;59(1):46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. Journal of Dental Research. 2003;82(2):141–145. doi: 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- 7.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, Visintini E, Cadenaro M, Tay FR, Dorigo EDS, Pashley DH. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dental Materials. 2010;26(6):571–578. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL, Yamaychi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2007;80(1):268–272. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 9.Bedran-Russo AKB, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2008;86(2):330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 10.Castellan CS, Pereira PN, Grande RHM, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dental Materials. 2010;26(10):968–973. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, Wang Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. Journal of Dentistry. 2010;38(11):908–915. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellan CS, Bedran-Russo AK, Karol S, Pereira PNR. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4(7):1343–1350. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu C, Wang Y. Cross-linked demineralized dentin maintains its mechanical stability when challenged by bacterial collagenase. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2011;96(B(2)):242–248. doi: 10.1002/jbm.b.31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu C, Wang Y. Collagen Cross Linking Increases Its Biodegradation Resistance in Wet Dentin Bonding. Journal of Adhesive Dentistry. 2011;13 doi: 10.3290/j.jad.a21494. Pre-print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogullari-Dirihan R, Hoshika T, Cadenaro M, Breschi L, Vallittu P, Tay FR, Pashley DH. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs, in vitro. Journal of Dental Research. 2012;91(2):192–196. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie - International Edition. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 17.Fine AM. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Alternative Medicine Review. 2000;5(2):144–151. [PubMed] [Google Scholar]
- 18.Oh HI, Hoff JE, Armstrong GS, Haff LA. Hydrophobic interaction in tannin-protein complexes. Journal of Agricultural and Food Chemistry. 1980;28(2):394–398. [Google Scholar]
- 19.Baxter NJ, Lilley TH, Haslam E, Williamson MP. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry. 1997;36(18):5566–5577. doi: 10.1021/bi9700328. [DOI] [PubMed] [Google Scholar]
- 20.Beart JE, Lilley TH, Haslam E. Polyphenol interactions. Part 2. 1 covatent binding of procyanidins to proteins during acid-catalysed decomposition; observations on some polymeric proanthocyanidins. Journal of the Chemical Society, Perkin Transactions. 1985;2(9):1439–1443. [Google Scholar]
- 21.Haslam E, Lilley TH. Interactions of natural phenols with macromolecules. Progress in clinical and biological research. 1986;213:53–65. [PubMed] [Google Scholar]
- 22.Hechler B, Yao X, Wang Y. Proanthocyanidins Alter Adhesive/Dentin Bonding Strengths when Included in a Bonding System. American Journal of Dentistry. 2012 Accepted. [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ammar A, Drummond JL, Bedran-Russo AK. The use of collagen cross-linking agents to enhance dentin bond strength. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2009;91(1):419–424. doi: 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacEdo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. Journal of Dental Research. 2009;88(12):1096–1100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dos Santos PH, Karol S, Bedran-Russo AK. Long-term nano-mechanical properties of biomodified dentin-resin interface components. Journal of Biomechanics. 2011;44(9):1691–1694. doi: 10.1016/j.jbiomech.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dos Santos PH, Karol S, Bedran-Russo AKB. Nanomechanical properties of biochemically modified dentin bonded interfaces. Journal of Oral Rehabilitation. 2011;38(7):541–546. doi: 10.1111/j.1365-2842.2010.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulmes DJS. Collagen Diversity, Synthesis and Assembly. In: Fratzl P, editor. Collagen: Structure and Mechanics. Springer Potsdam; Germany: 2008. pp. 15–41. [Google Scholar]
- 28.Meira JBC, Ballester RY, Lima RG, De Souza RM, Driemeier L. Geometrical aspects on Bi-material microtensile tests. Journal of the Brazilian Society of Mechanical Sciences and Engineering. 2005;27(3):310–313. [Google Scholar]
- 29.Van Noort R, Noroozi S, Howard IC, Cardew G. A critique of bond strength measurements. Journal of Dentistry. 1989;17(2):61–67. doi: 10.1016/0300-5712(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu R, Fang M, Xiao Y, Li F, Yu L, Zhao S, Shen L, Chen J. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. Journal of Materials Science: Materials in Medicine. 2011;22(11):2403–2411. doi: 10.1007/s10856-011-4430-4. [DOI] [PubMed] [Google Scholar]
- 31.Barth A, Zscherp C. What vibrations tell us about proteins. Quarterly Reviews of Biophysics. 2002;35(4):369–430. doi: 10.1017/s0033583502003815. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Acharya G, Lee CH. Effects of dialdehyde starch on calcification of collagen matrix. Journal of Biomedical Materials Research Part A. 2011;99(3):485–492. doi: 10.1002/jbm.a.33209. [DOI] [PubMed] [Google Scholar]
- 33.Bedran-Russo AKB, Castellan CS, Shinohara MS, Hassan L, Antunes A. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomaterialia. 2011;7(4):1735–1741. doi: 10.1016/j.actbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boryskina OP, Bolbukh TV, Semenov MA, Gasan AI, Maleev VY. Energies of peptide-peptide and peptide-water hydrogen bonds in collagen: Evidences from infrared spectroscopy, quartz piezogravimetry and differential scanning calorimetry. Journal of Molecular Structure. 2007;827(1-3):1–10. [Google Scholar]
- 35.Barth A. The infrared absorption of amino acid side chains. Progress in Biophysics and Molecular Biology. 2000;74(3-5):141–173. doi: 10.1016/s0079-6107(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 36.Parikh SJ, Kubicki JD, Jonsson CM, Jonsson CL, Hazen RM, Sverjensky DA, Sparks DL. Evaluating glutamate and aspartate binding mechanisms to rutile (α-TiO2) via ATR-FTIR spectroscopy and quantum chemical calculations. Langmuir. 2011;27(5):1778–1787. doi: 10.1021/la103826p. [DOI] [PubMed] [Google Scholar]
- 37.Tackett JE. FT-IR characterization of metal acetates in aqueous solution. Applied Spectroscopy. 1989;43(3):483–489. [Google Scholar]
- 38.Miles CA, Avery NC, Rodin VV, Bailey AJ. The Increase in Denaturation Temperature Following Cross-linking of Collagen is Caused by Dehydration of the Fibres. Journal of Molecular Biology. 2005;346:551–556. doi: 10.1016/j.jmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Larkin P. Infrared and Raman Spectroscopy; Principles and Spectral Interpretation. Waltham, MA: Elsevier; 2011. [Google Scholar]
- 40.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]


