Abstract
OBJECTIVE
The purpose of our study was to correlate decrease in apparent diffusion coefficient (ADC) and increase in fractional anisotropy (FA) in various white matter (WM) regions using diffusion tenor imaging (DTI) within the first year of life.
MATERIALS AND METHODS
We performed DTI on 53 infants and measured FA and ADC within 10 WM regions important in brain development. For each region, we calculated the slope of ADC as a function of FA, the correlation coefficient (r) and correlation of determination (r2). We performed a group analysis of r values and r2 values for six WM regions primarily composed of crossing fibers and four regions primarily having parallel fibers. Upon finding that a strong correlation of FA with age existed, we adjusted for age and calculated partial correlation coefficients.
RESULTS
Slopes of FA versus ADC ranged from −1.00711 to −1.67592 (p < 0.05); r values ranged from −0.81 to −0.50 and r2 values from 0.25 to 0.66. The four greatest r2 values were within WM regions having large numbers of crossing fibers and the three lowest r2 values were in regions having predominantly parallel fibers. After adjusting for age, slopes ranged from −1.08095 to 0.09612 (p < 0.05 in five cases); partial correlation coefficients ranged from −0.49 to 0.03 and r2 values from 0.31 to 0.79. The highest partial correlation coefficients were then relatively equally distributed between the two types of WM regions.
CONCLUSION
In various regions, FA and ADC evolved with differing degrees of correlation. We found a strong influence of age on the relationship between FA and ADC.
Keywords: apparent diffusion coefficient, brain, development, diffusion, diffusion tensor imaging, fractional anisotropy, infant, white matter
Diffusion tensor imaging (DTI) is a widely accepted MRI method for assessing white matter (WM) integrity [1, 2]. This technique has been used to study WM in a variety of conditions, such as WM development in normal children, WM changes associated with normal brain aging, and demyelinating changes in a number of disease processes. Two primary types of measurements used in DTI are the apparent diffusion coefficient (ADC), which is a measurement of the rate of microscopic water motion without reference to any one direction, and fractional anisotropy (FA), which is one measure of anisotropy, i.e., the tendency for water to diffuse in one direction as opposed to randomly [2]. During the course of brain development, ADC has been shown to decrease as more barriers to random water motion (e.g., cell membranes, myelinated axonal projections, and extracellular molecules) increase in number [1-4]. At the same time, FA is known to decrease because of a number of factors including development of parallel, compact fiber tracts as well as myelination [2]. On the other hand, in normal aging as well as in brain degenerative processes, ADC is known to increase and FA is known to decrease [5, 6].
Although these paired, inverse relationships are known to exist between ADC change and FA change, little is known about the degree to which change in one metric correlates with the change in the other metric. Because many of the same factors that affect ADC also affect FA, it seems reasonable to assume that a strong correspondence between ADC change and FA change exists. However, this topic is relatively underexplored in the medical literature. Instead, solely comparisons of either ADC or FA with age are found [5, 7-9]. The purpose of this study was to assess the correlation of decrease in ADC and increase in FA in various brain regions over a discrete time period that is important in the development of the infant brain, i.e., within the first year of life, and to determine to what degree, if any, this correlation was affected by age.
Materials and Methods
Study Population
An initial group of 54 infants, from 0 to 52 weeks after normalization to a full gestational age of 40 weeks, underwent clinical MRI with DTI. The 54 infants were found to have normal MR examinations and did not show or develop developmental delay. Of the 54 infants, images from one infant were found to be missing sex and age information within the DICOM data, preventing analysis and leading to exclusion of this case from the study. Each subject was found to have no neurologic disease or abnormality clinically diagnosed after a 5-year period as assessed through investigation of electronic medical records. A waiver of informed consent was granted by the institutional review board at our institution for this study. Four 1.5-T scanners produced by the same manufacturer (Signa, GE Healthcare) and operating on the same imaging platform were used to obtain scans over a period of 14 months. Initial DTI results on these patients, obtained using a different analysis program by a different observer, have been previously published [10]. That study did not include correlation of ADC and FA values, which is the focus of this study.
Each infant underwent one MRI examination. The distribution of the infants’ gestational ages at birth was as follows: 32 weeks (n = 1), 35 weeks (n = 1), 36 weeks (n = 2), 37 weeks (n = 3), 38 weeks (n = 6), 39 weeks (n = 2), 40 weeks (n = 34), and 41 weeks (n = 4). During consideration of our inclusion and exclusion criteria, we consulted with a pediatric neurologist at our institution who has more than three decades of experience with multiinstitutional infant studies to discuss whether to include the premature infants in the study. The infants born at 38 weeks’ gestational age or later were considered to be so close to the standard 40 weeks’ gestational age that they were not deemed considerably different than infants born at 40 weeks. In addition, we were advised to include premature infants who did not meet the exclusion criteria of having adverse birth events or developing of any neurologic disability over a period of multiple years. The small number of infants who were born prematurely underwent imaging many weeks after birth, limiting the effects of prematurity in the study. After adjusting for premature birth, the infant born at 32 weeks’ gestational age was imaged 43 weeks postnatally, the infant born at 35 weeks’ gestational age was imaged 30 weeks postnatally, the two infants born at 36 weeks’ gestational age were imaged at 16 and 46 weeks postnatally, and the three infants born at 37 weeks’ gestational age were imaged at 9, 21, and 32 weeks postnatally.
The indications for obtaining clinical MRI included scalp or facial lesions, including scalp nevus (n = 14), increased head circumference (n = 6), possible tethered spinal cord (n = 4), nonaccidental trauma (n = 4), suspected visual abnormality (n = 4), irritability (n = 4), sleep apnea (n = 2), suspected encephalocele (n = 2), hearing deficit (n = 2), mild hypertonia (n = 2), congenital cardiac or abdominal abnormality (n = 2), cranial nerve palsy (n = 1), tachypnea (n = 1), aspiration (n = 1), microcephaly (n = 1), abnormal movements (n = 1), cranial bruit (n = 1), and congenital nystagmus (n = 1).
The age of each of the subjects in weeks was obtained from the medical record. Each age was normalized for our purposes to a full gestational age of 40 weeks postconception. For infants born prematurely, the adjusted postnatal age was calculated as follows: [postnatal age in weeks at imaging – (gestational age in weeks at birth – 40 weeks)]. Therefore, an infant born at 37 weeks’ gestational age and imaged 10 weeks after birth was given a normalized age of 7 weeks. The normalized age of infants born after 40 weeks’ gestation was calculated as follows: [postnatal age in weeks at imaging – (40 weeks – gestational age in weeks at birth)]. Therefore, an infant born at 41 weeks and imaged at 14 postnatal weeks was given a normalized age of 15 weeks. Adjusted postnatal ages at the time of imaging are as follows (reported in 4-week epochs): −2 to 0 weeks (n = 1), 1–4 weeks (n = 4), 5–8 weeks (n = 6), 9–12 weeks (n = 8), 13–16 weeks (n = 5), 17–20 weeks (n = 3), 21–24 weeks (n = 1), 25–28 weeks (n = 5), 29–32 weeks (n = 4), 33–36 weeks (n = 3), 37–40 weeks (n = 0), 41–44 weeks (n = 7), 45–48 weeks (n = 4), and 49–52 weeks (n = 2).
Imaging Parameters
All imaging was obtained using a 1.5-T clinical MRI scanner with a standard head coil. The DTI protocol consisted of a single-shot, spin-echo planar sequence with TR/TE, 12,000/101; field of view (FOV), 22 cm2; 128 × 64 matrix; and 6-mm continuous slices throughout the brain using two excitations. Six directions of diffusion gradients were used with a b value of 1,000 s/mm2, and another image with no diffusion gradient was obtained (b = 0 s/mm2). The entire brain was imaged.
In addition to the DTI protocol, each patient underwent unenhanced transverse T1-weighted imaging, intermediate-weighted imaging, and transverse T2-weighted imaging. A spin-echo, echo-planar imaging sequence was performed in the transverse plane for diffusion imaging using the following parameters: 12,000/100; inversion time, 2,200; diffusion gradient encoding in three orthogonal directions; b = 1,000 s/mm2; FOV, 20 × 40 cm; matrix size, 128 × 64 pixels; section thickness, 5 mm; section gap, 2.5 mm; and number of signals acquired, one.
The sequence parameters for T2-weighted imaging were as follows: 2,800/100; FOV, 22 cm; matrix size, 256 (frequency direction) × 192 (phase direction); slice thickness, 5 mm; slice gap, 2.5 mm; and two excitations. The sequence parameters for T1-weighted imaging were as follows: 500/14; FOV, 22 cm; matrix size, 256 (frequency direction) × 194 (phase direction); slice thickness, 5 mm; slice gap, 2.5 mm; and two excitations.
Generation of ADC and FA Maps
ADC and FA maps were generated with DtiStudio (National Institutes of Health, mristudio.org). For computation of diffusion tensors, raw data were transferred onto a workstation containing the DtiStudio software and processed. The six independent, directional elements of the diffusion tensor, Dxx, Dyy, Dzz, Dxy, Dxz, and Dyz, were calculated for each voxel using a previously described method and based on the following equation: In [A(b) / A (b = 0)] = ∑i∑jbijDij, where bij is the component of the i row and j column of the diffusion gradient matrix b; A(b) is the resulting echo intensity for a gradient sequence with directions and magnitudes of the diffusion-sensitizing gradients described by the b matrix; A(b = 0) is the echo intensity when b is the zero matrix (no diffusion gradient); and Dij is the corresponding component of the diffusion tensor matrix D [11]. After the diffusion tensors were calculated, the eigenvalues were determined through diagonalization of the tensor matrix. The following equation was used to acquire ADC values:
where G is the amplitude of the pulsed diffusion gradient, γ is the gyrometric ratio, Δ is the interval between the diffusion gradients, δ is the duration of the diffusion gradients, S (G) is the signal strength with the diffusion gradient on, S (0) is the signal strength with the pulsed diffusion gradient off [12]. FA was used as the index of anisotropy because of its rotational invariancy, its excellent gray matter to WM contrast, and its high contrast-to-noise ratio [13]. In addition, FA is the most widely used index of anisotropy in recent literature, facilitating comparison with data from other studies. FA represents the anisotropic portion of overall diffusion. The equation for FA is as follows:
where Ei = the three eigenvalues, and d = (E1 + E2 + E3) / 3 [14]. Fractional anisotropy values range from 0 to 1, where 0 represents perfectly isotropic diffusion and 1 represents extremely anisotropic diffusion [12]. The FA values are unitless because they are a ratio of diffusion coefficients. Calculations for FA were performed for each voxel and displayed as an FA map.
Regions of Interest
A single observer drew standard (60 ± 4 mm2) regions of interest (ROIs) on FA maps using DtiStudio, with side-by-side reference to corresponding T1-weighted images, T2-weighted images, and color maps. The T1-weighted and T2-weighted images were viewed using ImageJ software (freeware). The analyst identified the splenium using the T1-weighted and T2-weighted images in ImageJ and then proceeded to identify and draw the splenium using color maps and the FA map in DtiStudio. FA and ADC were obtained for all 53 patients through use of the DtiStudio program. In DtiStudio, the ROI was drawn using the FA and color maps and then automatically transferred to the ADC map, which generated the values for each of these parameters (Figs. 1-3). ROIs were obtained from axial images from the center of the diencephalon where the anterior–posterior diameter of the splenium was greatest as assessed by T1- and T2-weighted images, FA maps, and color maps.
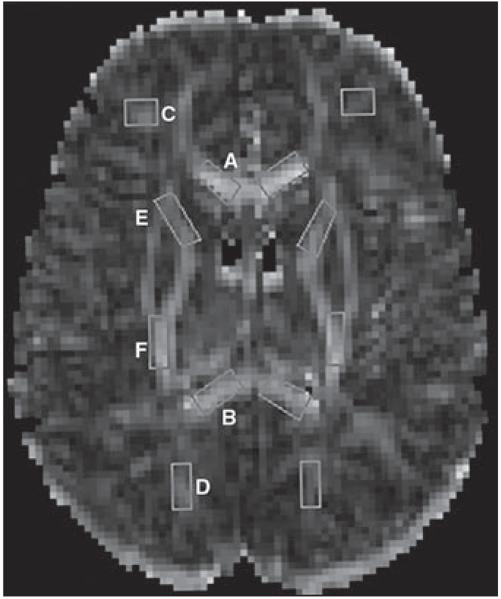
Fig. 1.
Fractional anisotropy map shows regions of interest used for following locations: A = genu of corpus callosum, B = splenium of corpus callosum, C = inferior frontal white matter (WM), D = inferior parietal WM, E = anterior limb of internal capsule, and F = posterior limb of internal capsule.
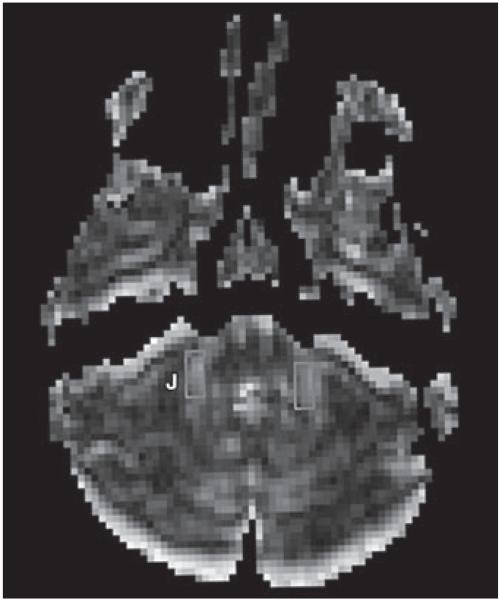
Fig. 3.
Fractional anisotropy map shows placement of regions of interest for evaluation of middle cerebellar peduncle (J).
We then categorized WM regions according to whether they were likely to have a relatively large number of crossing fibers or a relatively small number of crossing fibers. We assigned the following regions to the category presumed to have a relatively large proportion of crossing fibers: inferior frontal WM, central WM, superior parietal WM, superior frontal WM, inferior parietal WM, and anterior limb of the internal capsule. We assigned the following regions to the category presumed to have a relatively small proportion of crossing fibers: genu, splenium, posterior limb of the internal capsule, and middle cerebellar peduncle. The regions of interest for each WM region are depicted in Figures 1-3. The designation of the anterior limb of the internal capsule into the predominantly crossing fiber category is based on the following rationale. Although many fibers in the anterior limb of the internal capsule course in the rostrocaudal direction, a large number of fibers diverge from the craniocaudad axis into the basal ganglia; hence, it was designated as having a relatively large number of crossing fibers.
Data Analysis
We calculated the slope of ADC as a function of FA for each ROI using linear regression. We then calculated the correlation coefficient (r) and the correlation of determination (r2) for all regions before correcting for age using linear regression. The r2 value is an estimation of the amount of the variation in the dependent variable that can be explained by the covariates in a model [15]. Our initial statistical analysis showed age to be a significant predictor for ADC and, on that basis, we adjusted models predicting ADC from FA for age. Thus, we calculated the partial correlation coefficient and the correlation of determination (r2) for all regions after correcting for age using regression models. Finally, we compared partial correlation coefficients and r2 values for the WM regions thought to have many crossing fibers and for the WM regions thought to have predominantly parallel fibers.
Results
Slopes of ADC Versus FA
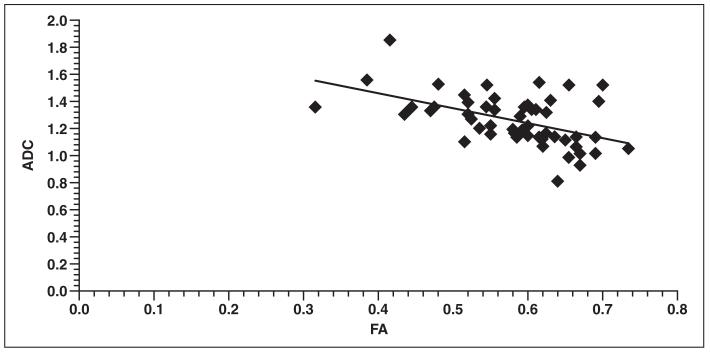
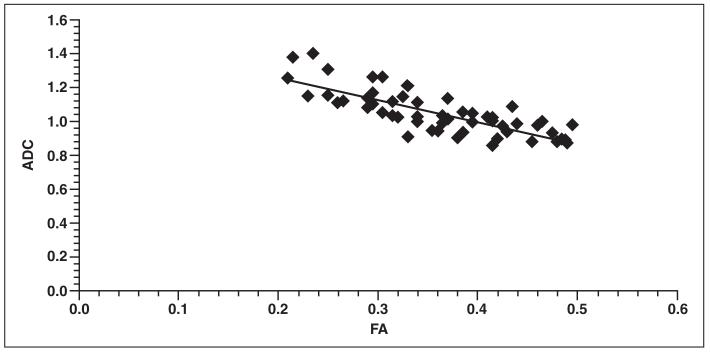
The results of the comparisons are outlined in Table 1, which indicates that before adjusting for age, slopes of FA versus ADC ranged from −1.00711 to −1.67592 (mean, −1.251944), of which all were statistically significant. All slopes before correction for age had a negative value. A plot of ADC as a function of FA for a representative region with predominantly parallel fibers, the genu of the corpus callosum, is shown in Figure 4. Such a plot for a representative region with a large number of crossing fibers, the superior parietal WM, is seen in Figure 5.
TABLE 1. Results of Models Predicting Apparent Diffusion Coefficient (ADC) from Fractional Anisotropy (FA).
| WM Region | Unadjusted | Adjusted for Agea | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Slope of FA vs ADC |
p | Coefficient of Determination r2 |
Correlation r | Slope of FA vs ADC |
p | Coefficient of Determination r2 |
Partial Correlation |
|
|
| ||||||||
| Inferior frontal WM | −1.59469 | < 0.001 | 0.64 | −0.80 | −0.97412 | < 0.001 | 0.71 | −0.49 |
| Middle cerebellar peduncle | −1.16537 | < 0.001 | 0.30 | −0.55 | −1.08095 | 0.002 | 0.31 | −0.41 |
| Central WM | −1.09051 | < 0.001 | 0.54 | −0.74 | −0.67688 | 0.018 | 0.57 | −0.33 |
| Superior parietal WM | −1.30190 | < 0.001 | 0.61 | −0.78 | −0.49575 | 0.019 | 0.73 | −0.32 |
| Posterior limb of internal capsule | −1.00711 | < 0.001 | 0.50 | −0.71 | −0.6044 | 0.020 | 0.53 | −0.32 |
| Superior frontal WM | −1.37823 | < 0.001 | 0.66 | −0.81 | −0.38377 | 0.068 | 0.79 | −0.26 |
| Inferior parietal WM | −1.20142 | < 0.001 | 0.42 | −0.65 | −0.26579 | 0.121 | 0.76 | −0.22 |
| Anterior limb of internal capsule | −1.00918 | < 0.001 | 0.50 | −0.70 | −0.28647 | 0.219 | 0.61 | −0.17 |
| Genu of corpus callosum | −1.09512 | < 0.001 | 0.25 | −0.50 | −0.18875 | 0.570 | 0.43 | −0.08 |
| Splenium of corpus callosum | −1.67592 | < 0.001 | 0.40 | −0.63 | 0.09612 | 0.813 | 0.62 | 0.03 |
Note—Coefficients of determination (r2) are based on regression models. Partial correlation coefficient is correlation between FA and ADC, adjusted for age. For statistically significant tests, the p values are shown in bold.
WM = white matter. Names of WM regions with predominantly parallel fibers are in italics.
Initial statistical tests showed that age was a significant predictor of ADC. On this basis, models predicting ADC from FA were subsequently recalculated after adjusting for age.
Fig. 4.
Graph shows plot of fractional anisotropy (FA) values compared with apparent diffusion coefficient (ADC) values within genu of corpus callosum for 53 infants in study. After adjusting for age, slope was −0.18875 (p = 0.570), with r = −0.08 and r2 = 0.43.
Fig. 5.
Graph shows plot of fractional anisotropy (FA) values compared with apparent diffusion coefficient (ADC) values within superior parietal white matter for 53 infants in study. After adjusting for age, slope was −0.49575 (p = 0.019), with r = −0.32 and r2 = 0.73.
On adjusting for age, slopes of FA versus ADC were lower in all cases and ranged from to −1.08095 to 0.09612 (mean, −0.4857278). The greatest slope was seen in the middle cerebral peduncle followed closely by the pericallosal WM, indicating a large rate of ADC decrease with increasing FA values. At the other end of the spectrum, the slope for the genu (−0.18875) was very low. The age-corrected slope for the splenium was actually slightly positive at 0.09612. The slopes were statistically significant for five ROIs. Among the four WM regions considered to have predominantly parallel fibers, mean slope was −0.44449 (range, −1.08095 to 0.09612). Among the six WM regions considered to have a large number of crossing fibers, mean slope was −0.51379 (range, −0.97412 to −0.26579).
Correlation Coefficients
As Table 1 indicates, before adjusting for age, r values ranged from −0.81 to −0.50. The four greatest r values were found within WM regions presumed to have a large number of crossing fibers. The three lowest r values were found in WM regions presumed to have predominantly parallel fibers. The coefficient of determination (i.e., r2) values ranged from 0.25 to 0.66.
After adjusting for age, partial correlation coefficients ranged from −0.49 to 0.03. However, unlike the results based on analysis uncorrected for age, some of the highest partial correlation coefficients were found in WM regions presumed to have predominantly parallel fibers (e.g., the middle cerebellar peduncle and the posterior limb of internal capsule). The r2 values ranged from 0.31 to 0.79, with the five highest r2 found in the WM regions thought to have predominantly crossing fibers. The combination of higher r2 and lower partial correlations of FA with ADC after adjustment for age indicated a strong effect of age on the relationship of FA to ADC. Thus, for example, for the superior frontal WM region, in the model that was not adjusted for age, 66% of the variation in ADC is accounted for by FA; the remaining 34% is accounted for by unknown factors. However, in the model adjusted for age, 79% of the variation in ADC is accounted for by FA and age. Alternatively, we see that the correlation r between FA and ADC for the superior frontal WM region was −0.81, but the partial correlation, after adjusting for age, was lower, at −0.26, again indicating the strong effect of age on the relationship between FA and ADC. Similar changes were seen for all other WM regions.
Discussion
In this study, we set out to determine the degree to which, in a population of normal neonates, decrease of ADC values with advancing age correlated with increasing FA values and to what degree, if any, this correlation was influenced by age. We found variable degrees of correlation between FA and ADC. Before correcting for age, the greater degree of correlation was seen in WM regions that we designated as having a higher proportion of crossing fibers. However, after adjusting for age, the partial correlation coefficients for comparison of FA and ADC within each WM region were substantially lower than the correlations between FA and ADC before controlling for age. Furthermore, we found the WM regions in which the greater degree of correlation (as judged by the partial correlation coefficients) relatively equally distributed between regions in which predominantly parallel fibers were thought to be present and those in which predominantly crossing fibers were thought to exist. Thus, we found that the relationship between FA and ADC is strongly influenced by age.
Very little has been written on the correlation of FA changes and ADC changes. As a result, little is known about the degree to which ADC in a region will change relative to a change in FA. A broad estimate of such changes can be found in one recent article. That study focused on the corpus callosum of adult brains in which investigators examined the time course of mean ADC values in adults of varying ages and compared them with the time course of mean FA values [5]. The study found that the age of maximum mean FA values differed from the age of minimum mean ADC values according to the WM region being evaluated [5]. These differences were usually on the order of 5 years or more. However, that study differed from our study in that it examined mean values over a population, whereas our study correlated specific FA and ADC values in the same WM region in individual patients.
As some investigators have noted, ADC values and FA values likely represent two very different processes and, in fiber tracts without a high degree of organization, ADC values may more closely reflect WM maturation than anisotropy values [1]. Factors that have been implicated as influential in ADC decreases during brain maturation include an increased concentration of macromolecules and a greater membrane surface-to-cell volume ratio resulting from cellular proliferation [4].
Initial interest in anisotropy values placed much emphasis on the role of myelination in determining anisotropy indices [16]. For instance, in a mouse model of dysmyelination in which the animals lack proper myelin (the so-called shiverer mouse), relative anisotropy was decreased as well as the more specific metric of water diffusion perpendicular to the long axis of fibers (so-called radial diffusivity) [17]. However, a number of studies have shown that FA values are not highly dependent on degree of myelination. For instance, substantial anisotropy can be present even in the absence of myelination [2]. In addition, demyelination can be present but poorly reflected in anisotropy values. For instance, investigations with the shiverer mouse have shown that differences in ADC values compared with controls are much larger than differences in FA values [18]. Anisotropy appears to be related to a large number of factors other than myelination, including cellular membranes, cell density, and tissue hydration [1, 3]. In one study, investigators found that, during the course of development of the optic nerve in rats, ADC along the long axis of the nerve increased but (despite the process of myelination) ADC perpendicular to the nerve did not change [19]. Anisotropy thus increased while myelination was occurring but was not related to it. Instead, the increase in longitudinal ADC (and subsequent rise in anisotropy) was due to progressive decrease in tortuosity of axons and removal of barriers to water diffusivity in the longitudinal axis (e.g., cell membranes and myelin sheaths) that existed due to axonal tortuosity.
The changes in ADC and FA in early brain maturation have recently been summarized as representing three major processes, which occur in the following sequence: fiber organization into fascicles, proliferation and maturation of glial cell bodies and intracellular compartments, and myelination [9]. The first process, fiber organization, which produces structured arrays of fibers that are to some degree parallel, occurs largely in utero in humans. This fact is evidenced by the presence of anisotropy in late intrauterine life and premature infants [8, 20]. This process would be expected to increase anisotropy values without substantially affecting ADC. The second process includes maturation of glial cell bodies and their processes as well as development of the cytoskeleton and various intracellular structures (e.g., microtubules), which predominantly serve to provide a widespread increase in barriers to water diffusion and, thus, a decrease in ADC without substantially influencing anisotropy measurements. The third process refers to the development of the myelin envelope around axons, which increases anisotropy to some extent but also decreases ADC. Because of decreased diffusion in the direction perpendicular to the myelin sheath and relatively unchanged diffusion along the long axis of the axon, overall water diffusion decreases.
The processes outlined provide a framework for a potential explanation for the varying degrees of correlation of FA change and ADC change in infancy. Depending on the degree to which WM development in a specific region is governed by the process of glial cell proliferation and development of intracellular compartments or by myelination, ADC decrease proceeds at a faster rate than FA increase, or vice versa. For instance, during infancy, numerous adhesion and signaling molecules that link adjacent membranes likely develop. This process would have the effect of limiting the volume of extracellular space in which water molecules diffuse. The expected result would be a decrease in ADC values without much change in FA values. To the extent that this process might be more pronounced in one WM region than another (e.g., in regions with many crossing fibers as opposed to regions with few crossing fibers), regional disparities in changes in ADC relative to FA might be seen. As another example, in regions with many crossing fibers, myelination could proceed without much change in FA values (because of no net increase in anisotropic diffusion of water); however, the increase in myelination would presumably again decrease the extracellular volume in which water molecules could diffuse and produce a decrease in ADC. Further regional variations are seen in the fact that exuberant corticocortical connections are developing in peripheral brain regions, which proceed for many months after birth; this process is occurring at a point in time when callosal fiber growth has stopped and, in fact, retraction of exuberant fibers in the corpus callosum has begun [20].
In light of these considerations, the manner by which age would be expected to strongly influence the correlation of FA and ADC (as shown by our findings) becomes evident. It is well-recognized that, especially in childhood, FA and ADC are both highly age dependent. So, too, are the microstructural developmental processes described earlier, e.g., glial proliferation, myelination, and the founding and expansion of many new neuronal connections. As the brain develops, these age-dependent microstructural changes, in concert, highly influence both FA and ADC. Even though the influence of these microstructural developments is exerted at different degrees in different locations, the overall influence of age is a dominant feature.
Both FA and ADC are scalar quantities derived from the same diffusion tensor and are deterministic functions of the three eigenvalues. Each combination of eigenvalues results in a distinctive ADC. However, for a given FA value, many possible combinations of eigenvalues exist that can give rise to that FA. Thus, the correlation between FA and ADC is expected, in general, to be weak. This relationship is consistent with our observation that, when the influence of age is accounted for, the partial correlation coefficient between FA and ADC is relatively low. During the process of normal brain maturation, WM development follows a specific, genetically encoded trajectory that results in a network of fiber pathways formed by myelinated axons. Along this specific trajectory, the principle eigenvalues (λ1, λ2, and λ3) of the diffusion tensors in these fibers also evolve in a specific pattern: diffusivity along the long axis of axons (λ1 or so-called longitudinal, or axial, diffusivity) remains relatively stable, and diffusivity in the direction perpendicular to the (λ2 and λ3 or so-called radial diffusivity) decreases. This specific pattern corresponds to a decreasing ADC and an increasing FA as well as an increased correlation between FA and ADC.
The natural asynchrony of maturation of various WM fiber bundles throughout the brain is another feature that likely plays a role in regional differences in degrees in correlation of FA and ADC values. For instance, it is worth noting that the four regions that we presumed to have predominantly parallel fibers are located in more caudal locations relative to regions with largely crossing fibers. It is widely recognized that human myelination progresses in a caudal to rostral direction, beginning in the first trimester in the spinal cord and continuing into the third decade in the intracortical fibers of the cerebral cortex [21, 22]. The most rapid changes in myelination occur in the corpus callosum and corticospinal tracts (including the posterior limb of internal capsule) in the period between midgestation and the end of the second postnatal year, i.e., an age range in which our study population falls. Myelination is generally believed to rapidly reduce the three eigenvalues of the diffusion tensor, thus reducing the ADC. Although the myelination process also has an effect on FA values, as noted previously a relatively large degree of anisotropy is present even in unmyelinated axons [2]. Furthermore, marked differences in ADC values (rather than FA values) are seen between animals with normal myelination and animals lacking myelin [18]. Thus, even in the phase of myelination, ADC changes figure substantially.
Compared with the caudal fiber tracks mentioned previously, many changes other than myelination predominate in the more rostral brain regions, which are expected to have fibers oriented at multiple orientations even at mature stages during infancy. The association fibers of the parietal and temporal areas are among the last regions to myelinate. At this early stage of development, the fiber structures are not yet fully organized. The existence of diffusion anisotropy implies some element of restriction of diffusion perpendicular to the fiber tracts, which could be related to an increase in fiber diameter, axonal membrane changes, or perhaps most importantly, the early wrapping of axons by oligodendroglial processes. All of these processes also contribute to lowering of ADC values, in part by reducing extracellular water volume. Depending on whether one of these two processes is dominant at any one time, change in FA may or may not be well correlated with ADC.
In summary, our findings suggest that in various WM regions, and perhaps during various stages of WM development, FA and ADC evolve with differing degrees of correlation. Our observation of WM heterogeneity regarding the correlation between FA and ADC suggests that declines in ADC and increases in FA during brain development can be influenced by multiple factors, including early neurogenesis, fiber organization, and myelination. Importantly, the relationship between FA and ADC is strongly influenced by the effect of age. It is worth noting that our study is restricted to infants within the first year, when the developmental asynchrony within WM is still relatively easy to observe. Further studies that include older children or adult populations in which WM is fully matured but yet to go through age-related demyelination would be valuable to further validate our hypothesis. We recognize that, in populations in which FA values and ADC values are not as strongly influenced by age as in the brain of the newborn infant, the influence of age on the correlation of FA and ADC values is likely to be much less prominent than in our study population. We suggest that further study of the relationship between FA and ADC in older populations may be fruitful to determine whether the effect of age on FA and ADC changes is lessened.
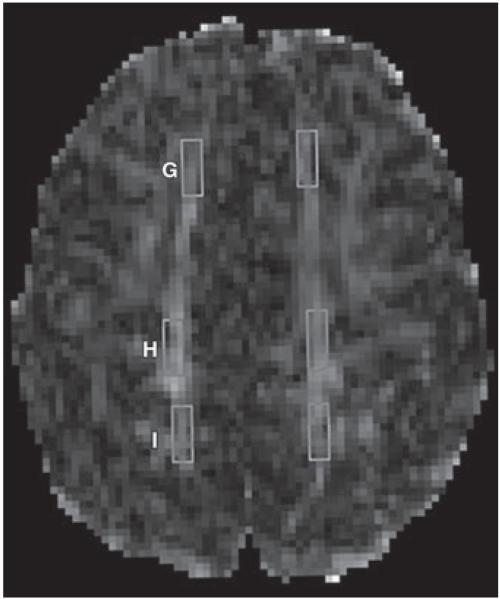
Fig. 2.
Fractional anisotropy map shows locations of subcortical white matter (WM) regions of interest used in study: G = superior frontal WM, H = central WM, I = superior parietal WM.
References
- 1.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulieu C, Allen PS. Determinants of anisotropic water diffusion in nerves. Magn Reson Med. 1994;31:394–400. doi: 10.1002/mrm.1910310408. [DOI] [PubMed] [Google Scholar]
- 3.Virta A, Barnett A, Pierpaoli C. Visualizing and characterizing white matter fiber structure and architecture in the human pyramidal tract using diffusion tensor MRI. Magn Reson Imaging. 1999;17:1121–1133. doi: 10.1016/s0730-725x(99)00048-x. [DOI] [PubMed] [Google Scholar]
- 4.Baratti C, Barnett AS, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210:133–142. doi: 10.1148/radiology.210.1.r99ja09133. [DOI] [PubMed] [Google Scholar]
- 5.Lebel C, Caverhill-Godkewitsch S, Beaulieu C. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage. 2010;52:20–31. doi: 10.1016/j.neuroimage.2010.03.072. [DOI] [PubMed] [Google Scholar]
- 6.Engelter ST, Provenzale JM, Farrelly MK, Petrella JR, MacFall JR. The effect of aging on the apparent diffusion coefficient of normal-appearing white matter. AJR. 2000;175:425–430. doi: 10.2214/ajr.175.2.1750425. [DOI] [PubMed] [Google Scholar]
- 7.McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during childhood with diffusion-tensor imaging. AJR. 2002;179:1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- 8.Partridge SC, Mukherjee P, Henry RG, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Dubois J, Dehaene-Lambertz G, Perrin M, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provenzale JM, Liang L, DeLong D, White LE. Diffusion-weighted and diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. AJR. 2007;189:476–486. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- 11.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 12.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, DiChiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 13.Hasan KM, Alexander AL, Narayana PA. Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magn Reson Med. 2004;51:413–417. doi: 10.1002/mrm.10682. [DOI] [PubMed] [Google Scholar]
- 14.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, et al. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Mittlböck M, Heinzl H. A note on R2 measures for Poisson and logistic regression models when both models are applicable. J Clin Epidemiol. 2001;54:99–103. doi: 10.1016/s0895-4356(00)00292-4. [DOI] [PubMed] [Google Scholar]
- 16.Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. AJNR. 2002;23:803–808. [PMC free article] [PubMed] [Google Scholar]
- 17.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 18.Tyszka JM, Readhead C, Bearer EL, Pautler RG, Jacobs RE. Statistical diffusion tensor histology reveals regional dysmyelination effects in the shiverer mouse mutant. Neuroimage. 2006;29:1058–1065. doi: 10.1016/j.neuroimage.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi M, Ono J, Harada K, Maeda M, Hackney DB. Diffusional anisotropy in cranial nerves with maturation: quantitative evaluation with diffusion MR imaging in rats. Radiology. 2000;216:881–885. doi: 10.1148/radiology.216.3.r00se41881. [DOI] [PubMed] [Google Scholar]
- 20.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Trapp BD, Moench T, Pulley M, Barbosa E, Tnnekoon G, Griddin J. Spatial segregation of mRNA encoding myelin-specific proteins. Proc Natl Acad Sci USA. 1987;84:7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamholz J, Toffenetti J, Lazzarini RA. Organization and expression of the human myelin basic protein gene. J Neurosci Res. 1988;21:62–70. doi: 10.1002/jnr.490210110. [DOI] [PubMed] [Google Scholar]