To the Editor
Despite the success of cytology-based (Pap) screening in the United States, over 12,000 women develop and 4,000 women die from cervical cancer each year,1 signaling important flaws in current practice. Paradoxically, a large proportion of women are over-screened,2 while at least 50% of cases occur among women who are infrequently or never screened.3 Guidelines have historically recommended screening early and frequently (e.g., annually) to offset the poor sensitivity of a single Pap test. However, a better understanding of the slow natural course of disease, the availability of highly sensitive tests to detect oncogenic human papillomavirus (HPV), the causal agent of cervical cancer, and evidence of adverse pregnancy outcomes associated with precancer treatment have triggered momentum towards less aggressive screening in the general population. Consensus guidelines issued this year now recommend screening no earlier than age 21 and no more frequently than every three years for routine cytology screening to minimize overuse and patient harms while maintaining high levels of cancer prevention.4,5 Because the impact of changing guidelines on cervical cancer will not be observed for several years, we utilized a mathematical simulation model to project the cost-effectiveness of routine cytology screening at different intervals.
Methods
The mathematical model simulates the natural history of cervical cancer in U.S. females based on data from epidemiological studies and cancer registries (eMethods, eTables 1 and 2, eFigure 1). Individuals enter the model and transition between clinically-relevant health states in monthly time steps. The model simulates detailed cervical cancer control strategies and tracks each woman’s health status and resource use to generate estimates of quality-adjusted life expectancy and lifetime costs of interventions. Strategies included routine cytology screening at one- to five-year intervals and a baseline scenario reflecting current U.S. screening rates.6 Costs included screening, diagnosis, and treatment of disease, patient time, and patient transportation (eTable 3). Incremental cost-effectiveness ratios were calculated (additional cost divided by the additional health benefit of a strategy compared to the next-less-costly strategy) and discussed in the context of $50,000 to $100,000 per quality-adjusted life year (QALY) gained, commonly-cited thresholds indicating good value for money in the United States.7
Results
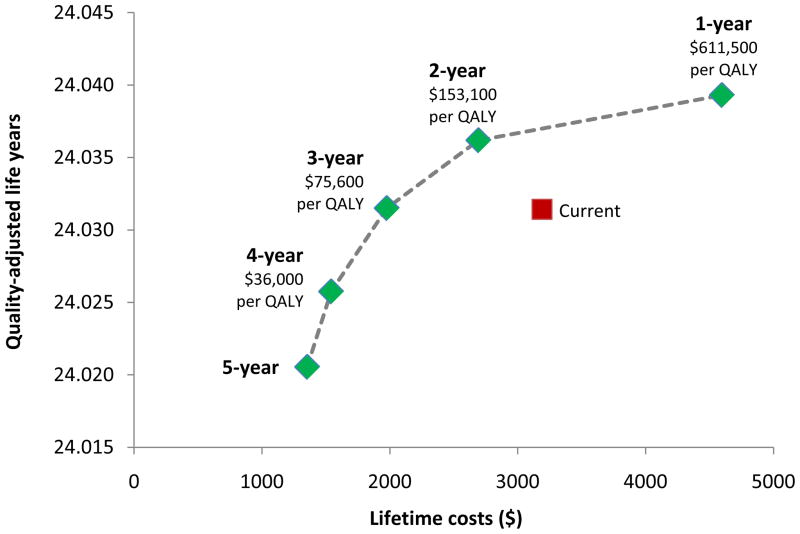
The Figure displays the projected outcomes for each screening scenario. As expected, both lifetime costs and health benefits increased as routine screening was administered more frequently. Importantly, screening all eligible women every 2 or 3 years was associated with similar or greater QALYs and lower costs than screening at current U.S. rates. For example, cytology testing every 3 years yielded a cost savings of $1,210 per woman and slightly higher QALYs compared with current screening.
Figure. Efficiency Frontier.
Quality-adjusted life years (QALYs), lifetime costs, and incremental cost-effectiveness ratios ($ per QALY gained) are displayed for routine cytology screening at intervals ranging from every 1 to 5 years (green diamonds) and current screening at variable rates (red square).
Annual and biennial screening had cost-effectiveness ratios that exceeded $150,000 per QALY gained. Screening every three years cost less than the upper threshold of $100,000 per QALY gained (i.e., $75,600 per QALY gained). Screening every four years cost less than the lower threshold of $50,000 per QALY gained ($36,000 per QALY gained), although this strategy resulted in lower health benefits than current screening.
Comment
Our analysis has two main findings: (1) compared to current practice, screening all-eligible women every two or three years can yield equal or greater health benefits at a significant cost-savings, and (2) routine screening more than every three years exceeds conventional thresholds for cost-effectiveness in the United States. Together, these findings support recent guidelines recommending routine cytology screening at three-year intervals.4,5
Investments in programs to achieve high coverage of three-year screening can be considerable, up to $1,200 per screen-eligible woman, before spending on cervical cancer screening reaches current levels. Programs, such as call/recall systems and community-based outreach – likely to be under $1,200 per woman – can focus on not only removing barriers for under-screened women, but also decreasing utilization in women who unnecessarily get annual routine screening. Attaining high coverage across all eligible women has the added advantage of promoting equity in health gains across sub-group populations, such as minorities and the uninsured, known to have high rates of cervical cancer incidence and mortality.
Our analysis has limitations. Because we did not explicitly model heterogeneous subgroups, our estimates may be conservative if improved access to screening leads to the reduction of cases that otherwise would have differentially worse outcomes and/or higher costs than average. We also did not assess the impact of improving compliance to diagnostic visits and access to timely treatment among women who are screened appropriately, efforts that are paramount to reducing cervical cancer burden in the United States.
We conclude that improving cervical cancer screening does not necessitate increased expenditures in the United States. Indeed, shifting away from the status quo, with at least half of women getting screened too frequently and over a quarter not enough, can likely reduce current expenditures without compromising on the tremendous health gains already achieved in cervical cancer prevention. This cost savings can be invested in more prudent ways to improve health, whether through cervical cancer prevention or other health interventions.
Supplementary Material
Acknowledgments
Funding/Support: The authors are supported in part by grants from the National Cancer Institute (U54 CA164336-01) and the Bill and Melinda Gates Foundation (30505) for related work in developing countries.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial disclosure: None reported
Conflict of Interest Disclosure: The authors declare no conflicts of interest.
References
- 1.American Cancer Society. [Accessed August 4, 2012];Cancer Facts and Figures. 2011 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf.
- 2.Roland KB, Soman A, Benard VB, Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. Am J Obstet Gynecol. 2011;205(5):447 e1–8. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007;45(2–3):93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;156:880–891. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics. Sample adult cancer file. Hyattsville, MD: U.S. Department Of Health and Human Services, Centers for Disease Control and Prevention; 2011. National Health Interview Survey, 2010. [Google Scholar]
- 7.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.