Abstract
Many peptide growth factors, including epidermal growth factor receptor (EGFR) ligands, accelerate wound reepithelialization in vivo and in vitro. Furthermore, EGFR expression is transiently increased at wound margins, suggesting an active role for this receptor in wound repair. During reepithelialization of cutaneous wounds, keratinocytes display a phenotypic plasticity resembling aspects of epithelial-mesenchymal transformation (EMT). The transcription factor Slug is a regulator of EMT during development, and we reported previously that Slug expression is elevated in keratinocytes bordering cutaneous wounds in vivo, ex vivo, and in vitro. In this study we provide evidence that Slug expression is necessary for an EGFR-stimulated reepithelialization response. EGF induces Slug expression and the response to EGFR activation is more robust than to other receptor tyrosine kinase ligands. EGFR-stimulated reepithelialization is highly dependent on Slug, as demonstrated by the absence of EGF-stimulated outgrowth in explants derived from Slug null mice. In vitro reepithelialization stimulated by ectopic Slug expression was not impaired by an inhibitor of EGFR catalytic activity suggesting that Slug is a downstream mediator of this EGFR-stimulated response. Our findings provide evidence that Slug is an essential component of the pathway leading to EGFR-mediated epithelial outgrowth.
INTRODUCTION
Successful wound healing is a complex process involving cells of the epidermis, dermis, vasculature, and the immune system [Coulombe 2003; Arnoux et al, 2005]. A crucial component of wound repair is reepithelialization, whereby the epidermal defect is sealed and barrier function is reestablished [Coulombe, 2003; Arnoux et al, 2005]. During reepithelialization, migrating keratinocytes undergo numerous functional and phenotypic alterations reminiscent of epithelial to mesenchymal transformation (EMT), including retraction of Intermediate filaments, disruption of desmosomes and hemidesmosomes, alterations in the actin-based cytoskeleton, and loss of cell polarity [Coulombe, 2003; Arnoux et al, 2005]. These changes in keratinocyte morphology and behavior after injury are often referred to collectively as “keratinocyte activation” [Coulombe 1997, 2003; Freedberg et al, 2001; Arnoux et al, 2005]. Keratinocytes become activated in response to changes in the microenvironment upon injury and then become major participants in the repair process through secretion of various cytokines and growth factors that help orchestrate tissue repair [Freedberg et al., 2001; Coulombe 1997, 2003; Arnoux et al, 2005, Myers et al., 2007].
Ligands for the epidermal growth factor receptor (EGFR) are present in the wound environment and promote wound repair [Werner and Grose, 2003]. EGFR expression is elevated at the leading edge of healing wounds [Stoscheck et al., 1992; Wenczak et al., 1992] and experimental augmentation of EGFR expression improves wound healing in vivo [Nanney et al., 2000]. Furthermore, keratinocyte migration in vitro and epithelial outgrowth in vivo is decreased in EGFR null skin (Repertinger et al., 2004), indicating that the EGFR is important for optimal wound repair. Although regulation of keratinocyte migration and epithelial outgrowth by ligands for the EGFR has been described [Stoll et al., 1997, Hudson and McCawley, 1998; Repertinger et al., 2004; Li et al., 2006; Tokumaru et al. 2006], the key downstream effectors of EGFR required for epithelial outgrowth are not well characterized.
The similarities among wound repair, tumor invasion and developmental processes in the embryo are of interest based on the potential for shared regulatory pathways [Martin and Parkhurst, 2004; Stramer and Martin, 2005; Wood et al., 2002]. Slug expression is enhanced at the margins of healing wounds in vitro [Savagner et al., 2005; Ikuta and Kawajiri 2006], ex vivo and in vivo [Savagner et al., 2005]; and in vitro reepithelialization is markedly accelerated in keratinocytes that ectopically express Slug [Savagner et al., 2005]. Because Slug expression appears to be regulated by ras and mitogen-activated protein kinase cascades [Schmidt et al., 2005; Savagner, 2001; Conacci-Sorrell et al., 2003; Hudson et al. 2007] and the EGFR and Slug are both elevated at the margins of healing wounds [Stoscheck et al., 1992; Wenczak et al., 1992; Savagner et al., 2005; Ikuta and Kawajiri 2006], we investigated whether Slug is a downstream mediator of EGFR-regulated reepithelialization.
RESULTS AND DISCUSSION
EGFR activation promotes epithelial outgrowth and Slug expression
Epithelial cell migration from murine skin explants is a model for examining the reepithelialization component of wound repair [Mazzalupo et al., 2002; Savagner et al., 2005]. Using this model, epithelial outgrowth from explants was limited when maintained in medium containing 10% fetal bovine serum (FBS) but lacking EGF [Fig. 1]. Explant outgrowth was markedly increased when 1 nM EGF was included in the culture medium, and the response was lost in the presence of an inhibitor of EGFR catalytic activity [Fig. 1]. These findings indicate that EGFR activation is a potent stimulator of epithelial outgrowth from mouse skin explants.
Figure 1. EGF receptor activation promotes ex vivo reepithelialization.

Explants from wild type mice were cultured for 5 or 7 days as described in Materials and Methods. Explants were maintained in basal medium without EGF or in basal medium supplemented with EGF (1 nM) with or without 5 μM AG1478. Epithelial outgrowth was measured from digital images. Values represent the mean of 2–4 explants per mouse per treatment group from a total of 4 individual mice −/+ standard deviation. *: p<0.05 EGF treatment compared to untreated control cultures.
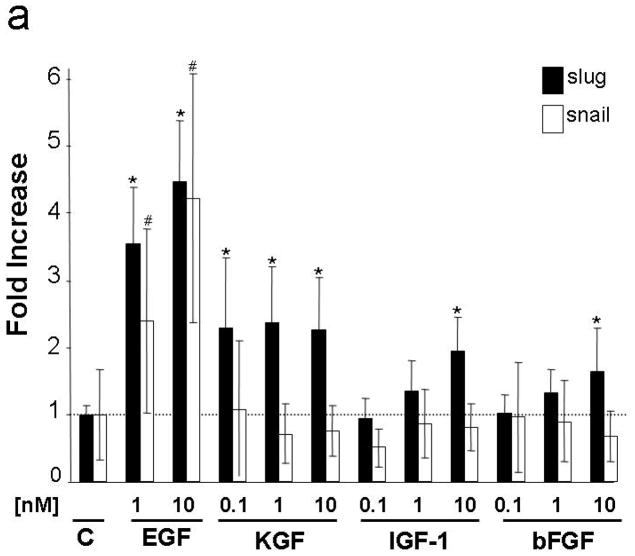
Because Slug is upregulated at the margins of healing wounds [Savagner et al., 2005] and elevated Slug expression promotes epithelial outgrowth [Savagner et al., 2005; Chandler et al., 2007], we determined if EGF induces Slug expression. EGF stimulated Slug mRNA expression by 4.5-fold in vitro (Fig. 2a). Keratinocyte growth factor (KGF) induced Slug expression by nearly 2.5-fold, and modest increases (<2-fold) were observed following treatment with insulin-like growth factor-1 (IGF-1) or basic fibroblast growth factor (bFGF). Interestingly, this finding is consistent with results obtained in human skin explants where EGF, IGF-1 and FGF were all identified as important mitogens, but explant outgrowth was substantially greater with EGF [Bhora et al., 1995]. Because the EGFR ligand transforming growth factor (TGF)-α is reported to be a more robust migration stimulus than EGF (Hudson and McCawley, 1998; Li et al., 2006) we tested both ligands for their ability to induce Slug protein expression. EGF and TGF-α induced Slug within 4h and maximal response was observed at 24h of treatment (Fig. 2b). Induction of Slug was nearly 2-fold greater with TGF-α than EGF (10nM, 24h treatment, n=4).
Figure 2. Induction of Slug and Snail by growth factors.
(a) SCC 12F cells were grown to confluence, rinsed with phosphate-buffered saline and placed in serum free medium containing 0.1% (w/v) bovine serum albumin for 48h prior to addition of the indicated concentrations of growth factors. RNA was collected after 2 h and mRNA levels were measured as described in Materials and Methods. Values were normalized to GAPDH with the baseline level of Slug (filled bars) or Snail (open bars) in untreated cells defined as 1.0 (dotted line) and other values expressed as fold increase. Data shown represent the mean of 3 independent samples, each analyzed in 3 separate PCR reactions, −/+ standard deviation. *= p<0.05 compared to Slug level in control group; #= p<0.05 compared to Snail level in control group. (b) SCC12F cells were grown as in (a), treated with 1 nM EGF or 1 nM TGF-α for the indicated times (upper panels) or with the indicated concentrations of EGF or TGF-α for 24h (lower panels). Slug protein was detected by immunoblot analysis. Data shown are representative of three independent experiments. (c) Explants from wild type mice were treated as indicated for 5 days (AG=5 μM AG1478). Activity of the β-galactosidase-Slug fusion protein (blue stain) was detected by histochemistry.
EGFR activation also stimulated Slug expression in mouse skin explants. In Slug–lacZ mice, the Slug locus has been inactivated by an in-frame insertion of the β-galactosidase gene into the zinc finger coding region of the Slug gene, thus Slug expression can be monitored by detecting β-galactosidase activity (Jiang et al. 1998). EGFR-stimulated Slug expression was also apparent at sites of epithelial outgrowth of Slug −/+ explants as determined by expression of a Slug-β-galactosidase fusion protein (Fig. 2c) and measurement of Slug mRNA levels in keratinocytes migrating from the explant (data not shown). Slug expression was greatly reduced in explants grown in complete medium containing FBS when EGFR activation was blocked by AG1478 (Fig. 2c). These findings indicate that Slug is an EGFR responsive gene and suggest that Slug is a candidate downstream mediator of EGFR-dependent reepithelialization.
EGF dependent epithelial outgrowth is highly dependent on Slug expression
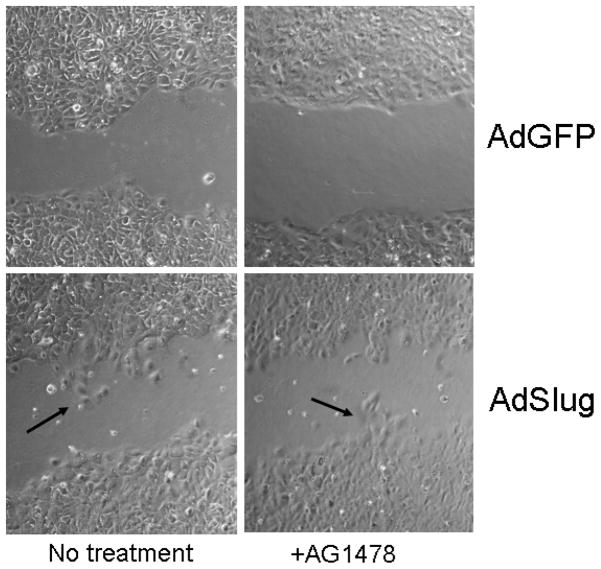
To test whether Slug is required for EGFR-stimulated keratinocyte outgrowth, we compared ex vivo reepithelialization in explants derived from wild type, heterozygous (not shown) or Slug null mice (Fig. 3). Time dependent and EGFR activation-dependent epithelial outgrowth was observed in explants isolated from wild type and heterozygous mice with some evidence for a partial defect in the heterozygotes at the time of most active outgrowth (5 day outgrowth area: Slug +/+ 0.252 −/+ 0.003 vs. Slug −/+ 0.144 −/+ 0.027 cm2). Little outgrowth was observed in Slug null explants. Although EGF effectively enhanced epithelial outgrowth in explants isolated from wild type mice, exogenous EGF did not promote this response in explants derived from Slug null mice (Fig. 3). As further evidence that Slug is downstream of EGFR activation, inhibition of EGFR by AG1478 did not disrupt migration of SCC12F cells expressing exogenous Slug (Fig. 4). The defect in outgrowth does not appear to be due to changes in cell proliferation. Quantification of Ki-67-positive cells in the intact epidermis did not reveal a significant difference between wild type and knockout epidermis (12.04 ± 3.32 versus 16.08 ± 7.07, p = 0.112 using the Student t test), nor were significant differences in Ki-67 staining evident at the wound margins of wild type and Slug mice (manuscript in preparation).
Figure 3. EGF-dependent epithelial outgrowth is dependent on Slug expression.

Explants from sex and age matched wild type (WT) or homozygous Slug null (KO) littermates were cultured in complete medium containing 5 nM EGF (+) or complete medium containing 5 μM AG1478 to inhibit EGF receptor activity (−). Epithelial outgrowth was measured as described in Materials and Methods and the legend to Figure 1. *= p<0.05 comparing activated EGFR versus inactivated EGFR at corresponding time point.
Figure 4.
Inhibition of EGFR does not disrupt keratinocyte outgrowth in cells expressing exogenous Slug. SCC 12F cells were infected with either AdSlug or AdGFP as described in Materials and Methods. An in vitro wound was introduced into cultures treated with 5μM AG1478 or untreated, and outgrowth into the wounded area was monitored by phase contrast microscopy. As reported previously, AG1478 inhibited outgrowth in control cells (Savagner et al., 2005), but note that the Slug associated keratinocyte outgrowth (arrows) was evident even when EGFR activity was inhibited by AG1478.
Interestingly, epithelial outgrowth in Slug null mice was impaired despite EGF stimulation of Snail expression (Fig. 2a), illustrating that Slug and Snail may play distinct roles in the epidermis. A recent publication highlights divergent transcriptional programs regulated by Slug and Snail despite their significant structural similarities [Moreno-Bueno et al., 2006].
Several lines of evidence suggest an important role for Slug/Snai2 in normal adult epidermis. Slug is expressed in adult skin predominantly in hair follicles and interfollicular epithelium adjacent to hair follicles [Parent et al., 2004]. Slug expression coincides with outgrowth from wound margins [Savagner et al., 2005; Ikuta and Kawajiri, 2006] and ectopic expression of Slug promotes epithelial outgrowth of keratinocytes [Savagner et al., 2005] and corneal epithelium [Chandler et al., 2007]. Furthermore, Slug expression is greatly reduced at the margins of non-healing corneal ulcers [Chandler et al., 2007]. Collectively, these findings suggest that Slug expression plays a positive role in wound reepithelialization. Our findings provide evidence that Slug is an essential component of the pathway leading to EGF-stimulated epithelial outgrowth and supports the conclusion that Slug contributes to regenerative processes in adult tissues.
MATERIALS AND METHODS
Cell Culture Studies
The nontumorigenic human keratinocyte cell line (SCC 12F) was generously provided by Dr. William A. Toscano, Jr. (University of Minnesota, Minneapolis, MN) and maintained in a medium consisting of 50% Dulbecco’s modified Eagle’s medium and 50% Hams-F12 medium (Sigma, St. Louis, MO) containing the antibiotics penicillin and streptomyocin, 5% glutamine, and 5% fetal bovine serum (Gibco, Gaithersburg, MD) as described previously (Hudson et al., 2007). To minimize basal expression of Slug and Snail for induction studies, confluent cultures were maintained for two days in serum-free medium prior to growth factor treatment (Hudson et al, 2007). Growth factors were obtained from Sigma (St. Louis, MO) and included EGF, TGF-α, KGF, IGF-1, and bFGF. For experiments involving ectopic Slug expression, subconfluent SCC 12F cells plated in a 12-well multiwell plate were incubated with AdSlug, AdGFP, or with virus-free medium (Chandler et al., 2007) as follows: Adenovirus (AdGFP or AdSlug, 10μl, viral titer of 108 pfu/mL) was incubated with 2.5 μl Lipofectamine (Invitrogen) for 15min at room temperature then added to 300 ul serum and antibiotic free DME:F12 medium. Cells were then incubated for 4h at 37°C, the medium replaced with 500 μl DME:F12 containing 5% FBS and further incubated for 48h. A preliminary study titrating the virus concentration against the level of transgene expression at 48 h indicated a transduction efficiency of 65% to 80% at this concentration (data not shown). After 48 h cells were moved to complete medium containing 5% FBS with or without 1 μM AG1478. A cell-free area was introduced into the now confluent cell cultures by scraping the monolayer with a sterile pipette tip and washing extensively to remove cellular debris [Savagner et al., 2005].. In vitro reepithelialization was monitored by migration of cells into the cleared area after 24h.
Skin Explants
Skin explants from wild type, heterozygous and homozygous Slug-lacZ mice were grown exactly as described by Mazzalupo et al. [Mazzalupo et al., 2002; Savagner et al., 2005]. For specific studies, EGF was eliminated from the medium or supplemented to levels indicated in figure legends. To measure outgrowth area, the central skin core was removed and the remaining rim of epithelial cells was fixed briefly in 10% neutral-buffered formalin, permeabilized for 5 minutes in 70% ethanol and stained for 5 minutes with a 1:10 dilution of Mayer’s hematoxylin. Images were captured digitally, adjusted in Adobe Photoshop to enhance contrast and the area representing outgrowth from the skin plug was quantitated using NIH ImageJ. Beta-galactosidase staining of explants was performed as previously described [Parent et al., 2004].
RNA Isolation and Quantitative mRNA Measurement
Total RNA from cultured cells and explant outgrowths was isolated using Trizol as directed by the manufacturer (Invitrogen, Carlsbad, CA). DNA was removed using the DNA-free kit (Ambion, Austin, TX) as directed, and cDNA was produced from 250–500 ng of RNA using SuperScript II reverse transcriptase and oligo(dT)primers as recommended by the enzyme supplier (Invitrogen, Carlsbad, CA). For explants, the central tissue cores were removed before RNA isolation. Each explant sample analyzed consisted of a pool of RNA from 4 explants, representing 2 samples from each of 2 mice.
Quantitative polymerase chain reaction (Q-PCR) was performed using the Brillant SYBR Green QPCR mix (Stratagene, LaJolla, CA) as directed and 100 nM of each primer. Primer sets included those for human Slug (5′-CCCTGAAGATGCATATTCGGAC-3′; 5′-CTTCTCCCCCGTGTGAGTTCTA-3′), human Snail (5′-CGGAAGCCTAACTACAGCGA-3′; 5′-GGACAGAGTCCCAGATGAGC-3′), human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (5′-GCCGTGGAATTTGCCGT-3′; 5′-GCCATCAATGACCCCAT-3′), mouse Slug (5′-GATGTGCCCTCAGGTTTGAT-3′; 5′-ACACATTGCCTTGTGTCTGC-3′), mouse Snail (5′-TGTCCAGAGGCTACACCTCA-3′; 5′-CTCACTGCCAGGACTCCTTC-3′), and mouse GAPDH (5′-ACCCAGAAGACTGTGGATGG-3′; 5′-CACATTGGGGGTAGGAACAC-3′). Amplifications were carried out on a Stratagene (LaJolla, CA) MX3000P Real-Time PCR System. Forty-five cycles of 94° C (30 seconds), 60° C (30 seconds), and 72° C (30 seconds) were performed. Electrophoresis of the products revealed single bands of the appropriate size (data not shown). RNA concentrations were calculated using the LinReg PCR program [Ramakers et al., 2003] and normalized to GAPDH values.
Immunoblot Analysis of Slug
Slug/Snai2 antibody for western blot assay was a kind gift from Dr. Pascale Leroy. 25 μg of protein were loaded on 15% SDS-polyacrylamide gel, transferred to nitrocellulose membrane and blocked in 5% powdered milk in TBST (50 mM Tris, pH 7.5, 150 mM NaCl, 0.01% Tween 20). The membrane was incubated with Slug primary antibodies (1:5000) overnight at 4 °C in 5% powdered milk in TBST and then was washed extensively with TBST, and incubated with 1:5000 anti-chicken secondary antibodies (1:8000, Santa Cruz, CA). Proteins were visualized with the ECL detection kit (Amersham Pharmacia Biotech). Equivalent loading of proteins in each well was confirmed by loading controls using antibodies to GAPDH (1:2000)
Statistical Analysis
Results for different treatment groups were compared by the Student t test and the value for statistical significance was considered to be p<0.05.
Acknowledgments
Financial support: This study was funded by a Department of Health and Human Services grant from the U.S. National Institutes of Health R01 GM079381, R01 AR42989 and RO1 CA89216 (DFK). Support was also provided by the UNM Cancer Research and Treatment Center P30 CA118100 and the UNM NIEHS Center P30 ES-012072.
Abbreviations Used
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transformation
- FBS
fetal bovine serum
- PCR
polymerase chain reaction
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Arnoux V, Come C, Kusewitt D, Hudson L, Savagner P. Cutaneous Wound Reepithelializaton: A partial and reversible EMT. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition. Springer; Berlin: 2005. pp. 111–134. [Google Scholar]
- Bhora FY, Dunkin BJ, Batzri S, Aly HM, Bass BL, Sidawy AN, Harmon JW. Effect of growth factors on cell proliferation and epithelialization in human skin. Journal of Surgical Research. 1995;59:236–244. doi: 10.1006/jsre.1995.1160. [DOI] [PubMed] [Google Scholar]
- Chandler HL, Colitz CM, Lu P, Saville WJ, Kusewitt DF. The role of the slug transcription factor in cell migration during corneal re-epithelialization in the dog. Exp Eye Res. 2007;84:400–411. doi: 10.1016/j.exer.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A. Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–857. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Comm. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116:633–640. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- Hudson LG, McCawley LJ. Contributions of the epidermal growth factor receptor to keratinocyte motility. Microsc Res Tech. 1998;43:444–455. doi: 10.1002/(SICI)1097-0029(19981201)43:5<444::AID-JEMT10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hudson LG, Choi C, Newkirk KM, Parkhani J, Cooper KL, Lu P, Kusewitt DF. Ultraviolet radiation stimulates expression of Snail family transcription factors in keratinocytes. Mol Carcinog. 2007;46:257–268. doi: 10.1002/mc.20257. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Kawajiri K. Zinc finger transcription factor Slug is a novel target gene of aryl hydrocarbon receptor. Exp Cell Res. 2006;312:3585–3594. doi: 10.1016/j.yexcr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol. 2006;126:2096–2105. doi: 10.1038/sj.jid.5700350. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol. 1998;198:277–285. [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Mazzalupo S, Wawersik MJ, Coulombe PA. An ex vivo assay to assess the potential of skin keratinocytes for wound epithelialization. J Invest Dermatol. 2002;118:866–870. doi: 10.1046/j.1523-1747.2002.01736.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Cubillo E, Sarrio D, Peinado H, Rodriguez-Pinilla SM, Villa S, Bolos V, Jorda M, Fabra A, Portillo F, Palacios J, Cano A. Genetic profiling of epithelial cells expressing e-cadherin repressors reveals a distinct role for snail, slug, and e47 factors in epithelial-mesenchymal transition. Cancer Res. 2006;66:9543–9556. doi: 10.1158/0008-5472.CAN-06-0479. [DOI] [PubMed] [Google Scholar]
- Myers SR, Leigh IM, Navsaria H. Epidermal repair results from activation of follicular and epidermal progenitor keratinocytes mediated by a growth factor cascade. Wound Repair Regen. 2007;15:693–701. doi: 10.1111/j.1524-475X.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- Nanney LB, Paulsen S, Davidson MK, Cardwell NL, Whitsitt JS, Davidson JM. Boosting epidermal growth factor receptor expression by gene gun transfection stimulates epidermal growth in vivo. Wound Repair Regen. 2000;8:117–127. doi: 10.1046/j.1524-475x.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Parent AE, Choi C, Caudy K, Gridley T, Kusewitt DF. The developmental transcription factor slug is widely expressed in tissues of adult mice. J Histochem Cytochem. 2004;52:959–965. doi: 10.1369/jhc.4A6277.2004. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. J Cell Physiol. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Schmidt CR, Gi YJ, Patel TA, Coffey RJ, Beauchamp RD, Pearson AS. E-cadherin is regulated by the transcriptional repressor SLUG during Ras-mediated transformation of intestinal epithelial cells. Surgery. 2005;138:306–312. doi: 10.1016/j.surg.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation In skin organ culture. J Clin Invest. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck CM, Nanney LB, King LE., Jr Quantitative determination of EGF-R during epidermal wound healing. J Invest Dermatol. 1992;99:645–649. doi: 10.1111/1523-1747.ep12668143. [DOI] [PubMed] [Google Scholar]
- Stramer B, Martin P. Cell biology: master regulators of sealing and healing. Curr Biol. 2005;15:R425–427. doi: 10.1016/j.cub.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Tokumaru S, Higashiyama S, Endo T, Nakagawa T, Miyagawa JI, Yamamori K, Hanakawa Y, Ohmoto H, Yoshino K, Shirakata Y, Matsuzawa Y, Hashimoto K, Taniguchi N. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenczak BA, Lynch JB, Nanney LB. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. Journal of Clinical Investigation. 1992;90:2392–2401. doi: 10.1172/JCI116130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]