Abstract
Introduction
We found isolated or clustered trophoblasts in the chorionic connective tissue of the extraplacental membranes, and defined this novel histologic feature as the “trophoblast islands of the chorionic connective tissue” (TICCT). This study was conducted to determine the clinical significance of TICCT.
Methods
Immunohistochemistry for cytokeratin-7 was performed on the chorioamniotic membranes (N=2155) obtained from singleton pregnancies of 1199 uncomplicated term and 956 preterm deliveries. The study groups comprised 1236 African-American and 919 Hispanic women. Gestational age ranged from 24+0 weeks to 41+6 weeks. Multiple logistic regression analysis was performed to investigate the magnitude of association between patient characteristics and the presence of TICCT.
Results
The likelihood of TICCT was significantly associated with advancing gestational age both in term (OR: 1.29, 95% CI: 1.16–1.45, p<0.001) and preterm deliveries (OR: 1.19, 95% CI: 1.07–1.32, p=0.001). Hispanic women were less likely than African-American women to have TICCT across gestation in term (OR: 0.23, 95% CI: 0.18–0.31, p<0.001) and preterm pregnancies (OR: 0.41, 95% CI: 0.29–0.58, p<0.001). Women with a female fetus were significantly more likely to have TICCT than women with a male fetus, in both term (OR: 1.64, 95% CI: 1.28–2.11, p<0.001) and preterm gestations (OR: 2.04, 95% CI: 1.46–2.85, p<0.001). TICCT was 40% less frequent in the presence of chronic placental inflammation [term (OR: 0.60, 95% CI: 0.45–0.81, p=0.001) and preterm gestations (OR: 0.58, 95% CI: 0.40–0.84, p=0.003)] and in parous women at term (OR: 0.60, 95% CI: 0.44–0.81, p=0.001).
Conclusions
Our findings suggest that the duration of pregnancy, fetal sex, and parity may influence the behavior of extravillous trophoblast and placental mesenchymal cells.
Keywords: Chorioamniotic membranes, Pregnancy, Fetal sex, Cytokeratin-7
1. Introduction
The chorioamniotic membranes play an important role in the maintenance of pregnancy and parturition [1–6]. The chorion laeve is composed of a trophoblast layer harboring stratified extravillous trophoblasts and a connective tissue layer containing myofibroblasts and macrophages [7–9]. Chorionic trophoblasts serve as a fetal immune barrier against maternal immunocytes and predominantly express and produce non-classical, non-polymorphic HLA-G, but do not express classical HLA class I and II molecules [10–12]. The trophoblasts of the chorion laeve have also been implicated in the regulation of prostaglandin metabolism and, therefore, in the regulation of myometrial contractility [13–16]. These cells express high levels of 15-hydroxy-prostaglandin dehydrogenase, which can inactivate prostaglandins by oxidation of these compounds to inactive 15-keto metabolites [17,18]. In addition to the conventional roles, phagocytic activity in the chorionic trophoblasts has been reported when chorioamnionitis is diagnosed, an observation that suggests another important role of the trophoblast in the chorion laeve for the innate immune response [19].
Integrins and CD9 (surface molecules) have been implicated in the regulation of extravillous trophoblast invasion [20,21]. Chorionic trophoblasts normally display a unidirectional invasion toward the decidua where they intermingle with decidual cells of maternal origin [22,23]. Therefore, the chorionic connective tissue, which is surrounded by the basal lamina from the chorionic trophoblast layer, is not expected to contain any trophoblasts [2]. Yet, we found some oval to polygonal cells in the chorionic connective tissue layer of the chorioamniotic membranes. These cells had glassy, eosinophilic or amphophilic cytoplasm, and were morphologically different from myofibroblasts or macrophages. Some of these cells had cytological features indistinguishable from those of chorionic trophoblasts. Subsequent immunostaining for cytokeratin-7 and HLA-G demonstrated co-localization of strong immunoreactivity in these cells. Based on their characteristic morphological and immunohistochemical features, we termed this novel histological finding of unknown significance as the “trophoblast islands of the chorionic connective tissue” (TICCT).
The aim of this study was to determine the clinical significance of TICCT by analyzing the chorioamniotic membranes obtained from women who presented with different obstetrical conditions such as normal delivery at term, preterm delivery, and preeclampsia. We conducted an immunohistochemical screening of a large number of cases across gestation for the detection of TICCT in two different study populations. The relationship between the frequency of TICCT and various placental pathologies was also assessed.
2. Materials and methods
2.1. Tissue materials
To evaluate for TICCT, one chorioamniotic membrane roll section from each pregnant woman was examined. Paraffin blocks of the membrane rolls were retrieved from the Bank of Biological Materials of Wayne State University, which has been assembled in collaboration with the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). The study groups included 1236 African-American women who delivered at Hutzel Women’s Hospital, Detroit, Michigan, USA, and 919 Hispanic women who delivered at the Sótero del Río Hospital, Santiago, Chile. All women provided written informed consent, and the Institutional Review Boards of the participating institutions approved the collection and use of biological samples and clinical data for research purposes.
2.2. Immunofluorescence staining/immunohistochemistry
For double immunofluorescence staining, 5-µm-thick frozen tissue sections of the chorioamniotic membranes were used. The frozen sections were ethanol-fixed for 5 min, and used for immunofluorescence staining. A mouse monoclonal anti-HLA-G antibody (MEM-G1, 1:50 dilution; Abcam, Cambridge, MA, USA) and a rabbit polyclonal anti-cytokeratin 7 antibody (1:50 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used as primary antibodies. Alexa Fluor® 488 donkey anti-rabbit IgG and Alexa Fluor® 594 donkey anti-mouse IgG were used as secondary antibodies (1:1000 dilution for both; Invitrogen, Carlsbad, CA, USA), and the slides were mounted in ProLong Gold antifade reagent with DAPI (Invitrogen). The immunofluorescence stained sections were evaluated with a Leica TCS SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany).
For immunohistochemistry, formalin-fixed, paraffin-embedded, 5-µm-thick tissue sections of the chorioamniotic membranes were obtained on silanized slides. Deparaffinization, antigen retrieval, and immunostaining were done using a Ventana Discovery automatic staining system (Ventana Medical Systems, Tucson, AZ, USA). A mouse monoclonal anti-cytokeratin-7 antibody (OV-TL, 1:2000 dilution; Dako, Carpinteria, CA, USA) was a primary antibody, and the Discovery® DAB Map™ Kit (Ventana Medical Systems) was used for chromogen reaction.
2.3. Definition and grading of trophoblast islands of the chorionic connective tissue (TICCT)
We defined TICCT as the presence of cytokeratin-7-positive cells or cell clusters with cytological characteristics of trophoblasts in the chorionic connective tissue layer of the reflected chorion (rather than the placental amnion). The extent of TICCT was graded as follows: grade 1, when TICCT was observed in three or less isolated high-power fields (HPFs, X400); grade 2, when TICCT was observed in four to 10 isolated HPFs; grade 3, when TICCT was multifocal in more than 10 isolated HPFs; and grade 0, when none of these were observed. All cases (n = 2155) were reviewed by one pathologist (CJK) who was blinded to the clinical information while reviewing the immunostaining results. To determine intra- and inter-observer reliability in the diagnosis of TICCT, 398 cases which consisted of grade 0 (n=199), grade 1 (n=119), grade 2 (n=50), and grade 3 (n=30), selected by another author (JSH), were re-examined independently by two pathologists (CJK, JSK) who were blinded to the original results.
2.4. Transmission electron microscopy
The chorioamniotic membranes in the paraffin blocks were deparaffinized using xylene and rehydrated through serial incubations in graded ethanol. After rinsing with distilled water, the samples were incubated with 0.1 mol/L phosphate buffer (pH 7.4). Deparaffinized samples were stained with 1% OsO4 for 1 h and dehydrated with graded ethanol. Semi-thin sections of epoxy resin–embedded tissues were evaluated to locate the areas with trophoblasts in TICCT, and ultra-thin sections were examined using a JEM-1200EX II transmission electron microscope (JEOL Ltd., Tokyo, Japan).
2.5. Placental pathology
To determine whether the frequency of TICCT was associated with specific pathological changes of the placenta, we examined the relationship between this finding and the presence or absence of four categories of placental lesions: 1) amniotic fluid infection (acute chorioamnionitis); 2) maternal vascular underperfusion; 3) fetal vascular thrombo-occlusive disease; and 4) chronic placental inflammation (villitis of unknown etiology/chronic chorioamnionitis/chronic deciduitis). These samples were retrieved from the files maintained at Wayne State University and the Detroit Medical Center in collaboration with the Perinatology Research Branch, NICHD/NIH/DHHS.
2.6. Statistical analysis
Pearson’s chi-square test for categorical variables and the t-test for continuous variables were performed to determine differences among maternal characteristics according to the TICCT results. Pearson’s chi-square test and the linear by linear association were used to assess the magnitude of association between TICCT and gestational age at delivery. The association between TICCT and ethnicity across gestation was tested using the Mantel-Haenszel (M-H) test. Multiple logistic regression analysis was performed to evaluate the magnitude of association between selected patient characteristics and TICCT, adjusting for potentially confounding factors selected based on clinical knowledge. The association between high-grade TICCT (grade 2 or 3) and the related factors was tested by multinomial regression. To determine intra- and inter-observer reliability in the diagnosis of TICCT, quadratic weighted κ (κw) statistics [24] were calculated using R (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org). All statistical analyses except κw were conducted using SPSS version 18.0 software (SPSS, Inc., Chicago, IL, USA). A 5% threshold was used to determine statistical significance.
3. Results
3.1. Patterns of trophoblast islands of the chorionic connective tissue (TICCT)
To confirm the extravillous trophoblast phenotype of the cells, double immunofluorescence staining was performed for cytokeratin-7 and HLA-G in frozen chorioamniotic membrane tissues of four cases of African-American origin with prominent TICCT. These cells showed variable degrees of HLA-G immunoreactivity along with strong cytoplasmic cytokeratin-7 immunofluorescent signals (Fig. 1a–d). Cytokeratin-7-positive TICCT was observed as an isolated single cell or clusters of cells with variable sizes (Fig. 2a–h). Some TICCT clusters featured a direct extension from the basal portion of the chorionic trophoblast layer with interruption of the basal lamina, which was consistent with the features of migration of the chorionic trophoblasts into the connective tissue layer (Fig. 2a–d). On the other hand, many TICCT clusters were noted as isolated trophoblasts in the middle of the chorionic connective tissue without clear continuity with the chorionic trophoblast layer (Fig. 2e,f). Small cells with little but clearly cytokeratin-positive cytoplasm were commonly detected at the junction of the chorionic trophoblast and connective tissue layers (Fig. 2g,h).
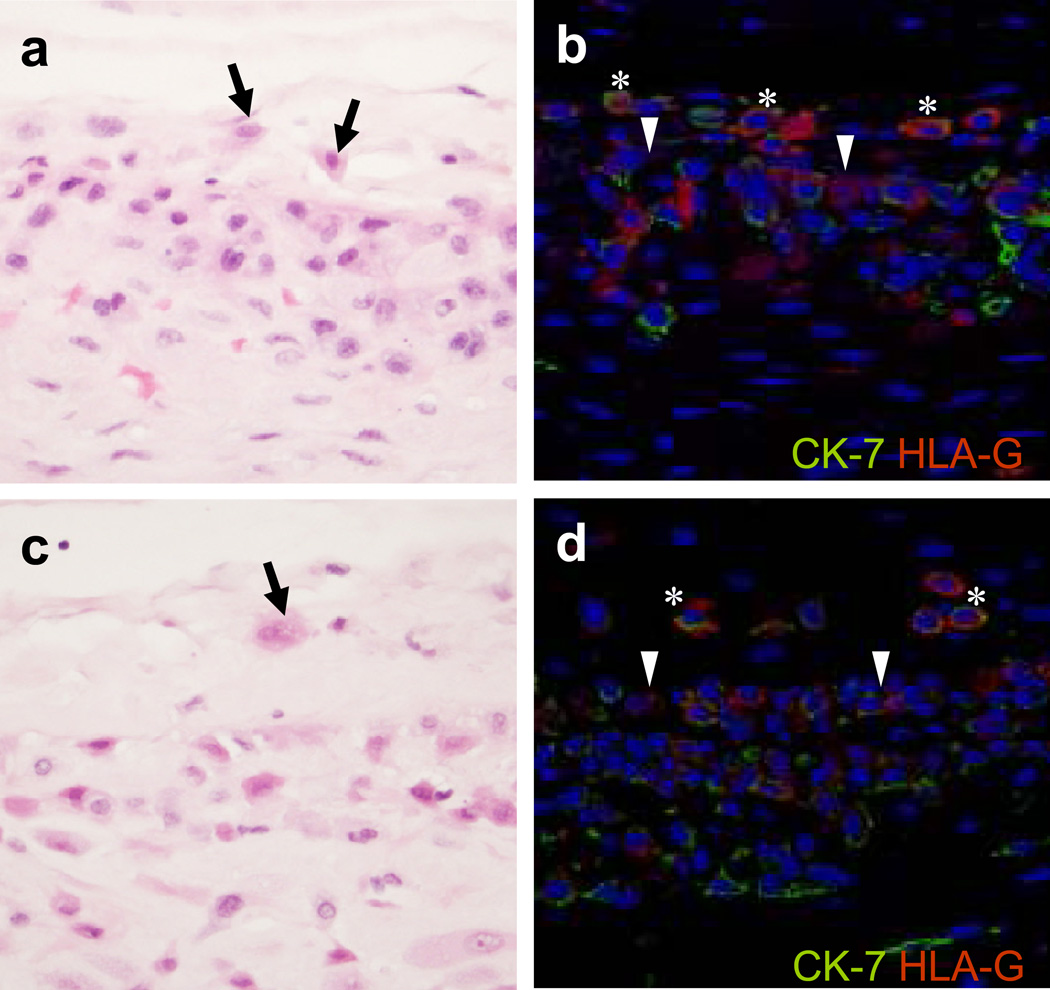
Figure 1. Histologic and immunophenotypic characteristics of ‘trophoblast islands of the chorionic connective tissue’ (TICCT).
a, c: Scattered cells with amphophilic cytoplasm in the chorionic connective tissue layer (arrows) in normal term delivery cases at the gestational ages of 41.9 and 40.0 weeks, respectively. b, d: Double immunofluorescence staining showing cytokeratin-7 (green; Alexa Fluor® 488) and HLA-G (red; Alex Fluor® 594) positive extravillous trophoblasts in the chorionic connective tissue layer (*). The border between the chorionic trophoblast layer and the connective layer is indicated by arrowheads.
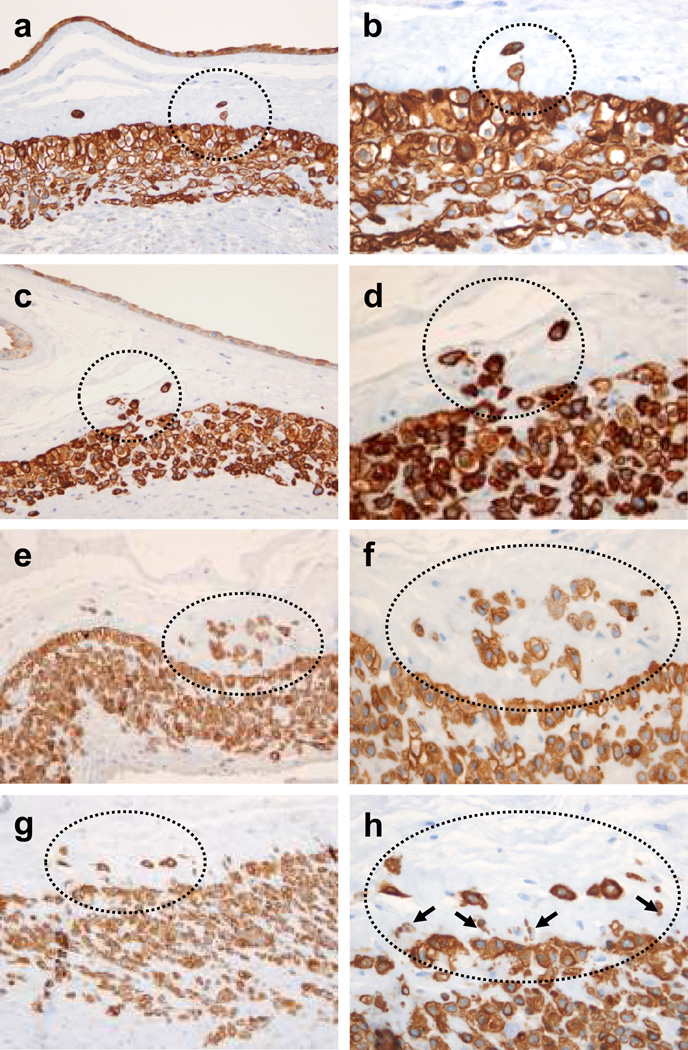
Figure 2. Histological spectrum of TICCT confirmed by immunostaining against cytokeratin-7.
a, b: Isolated trophoblasts in the chorionic connective tissue in a case of normal term delivery at 37.4 weeks of gestation. Fine cytoplasmic connection is evident in one trophoblast of TICCT. c, d: A cluster of TICCT, which also features direct extension from the chorionic trophoblasts (normal term delivery, 38.7 weeks). e, f: A cluster of TICCT, showing many trophoblasts of variable sizes (normal term delivery, 38.6 weeks). Some trophoblasts have very little cytokeratin-7-positive cytoplasm. g, h: In this image of TICCT, small cells with little cytoplasm (arrows) extend from the junction of the chorionic trophoblast layer and the basal lamina of the connective tissue (normal term delivery, 37.4 weeks).
To further determine whether TICCT clusters are largely derived from direct extensions from the chorionic trophoblast layer, we performed immunohistochemical screening of 20 serial cytokeratin-7-immunostained sections from seven cases with TICCT. Serial sections demonstrated the direct extension of some TICCT cells from the chorionic trophoblast layer, but many of the TICCT cells did not show connections with the underlying chorionic trophoblast layer in the serial sections (Fig. 3). We then examined the nature of TICCT cells using electron microscopy in a case with TICCT. The TICCT cells had euchromatic nuclei with prominent nucleoli, abundant intermediate filaments, a fair number of rough endoplasmic reticula and mitochondria, and short microvilli on the cell surface (Fig. 4). These ultrastructural features suggested a unique differentiation in the phenotype of these trophoblastic cells in the TICCT.
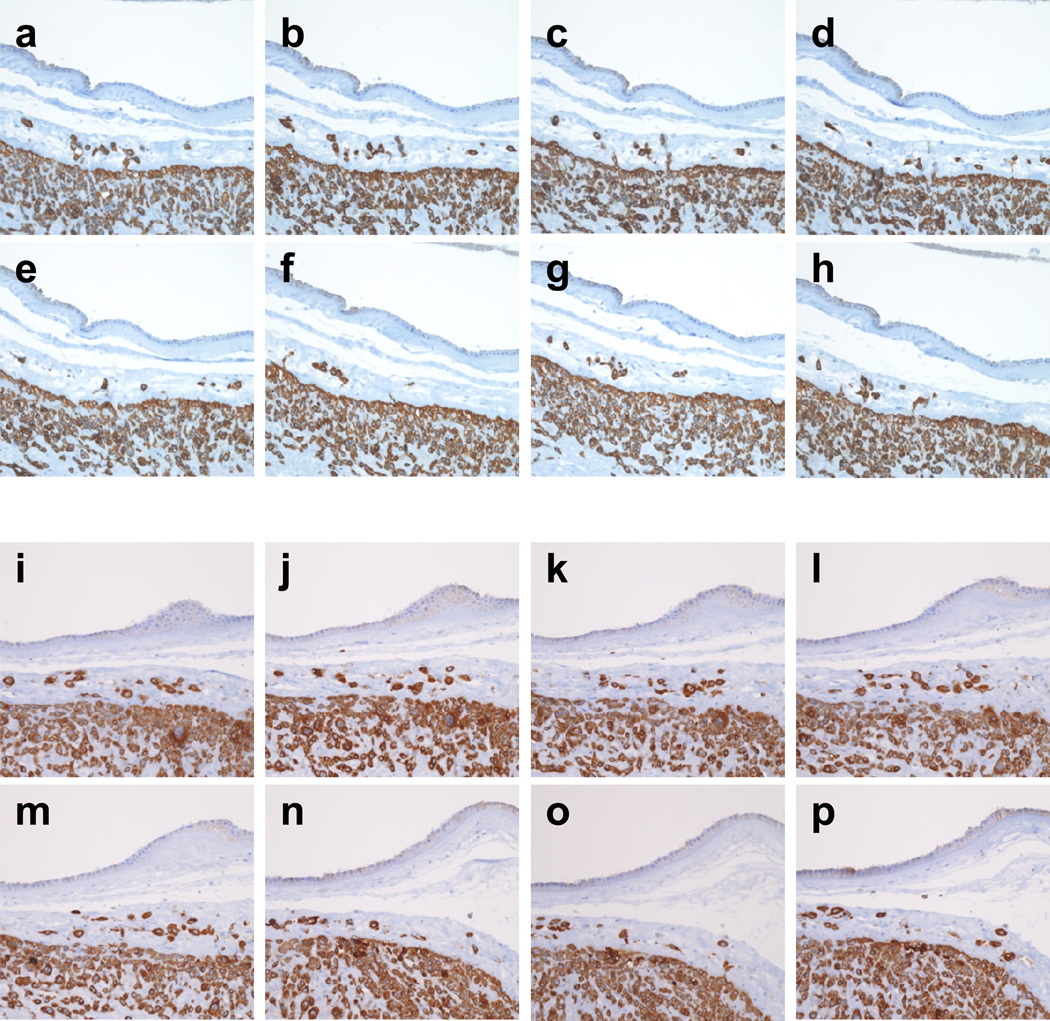
Figure 3. Tracing of TICCT cells by serial sectioning.
The cytokeratin-7 immunostained images of serial sections were taken from two representative cases (a–h, i–p). Both are normal term-in-labor cases without specific placental pathological findings (41.7 weeks and 41.9 weeks, respectively). (a–h) Direct extension of some TICCT cells from the chorionic trophoblast layer is evident (c). (i–p) Many TICCT cells are isolated from the chorionic trophoblast layer throughout the serial section planes.
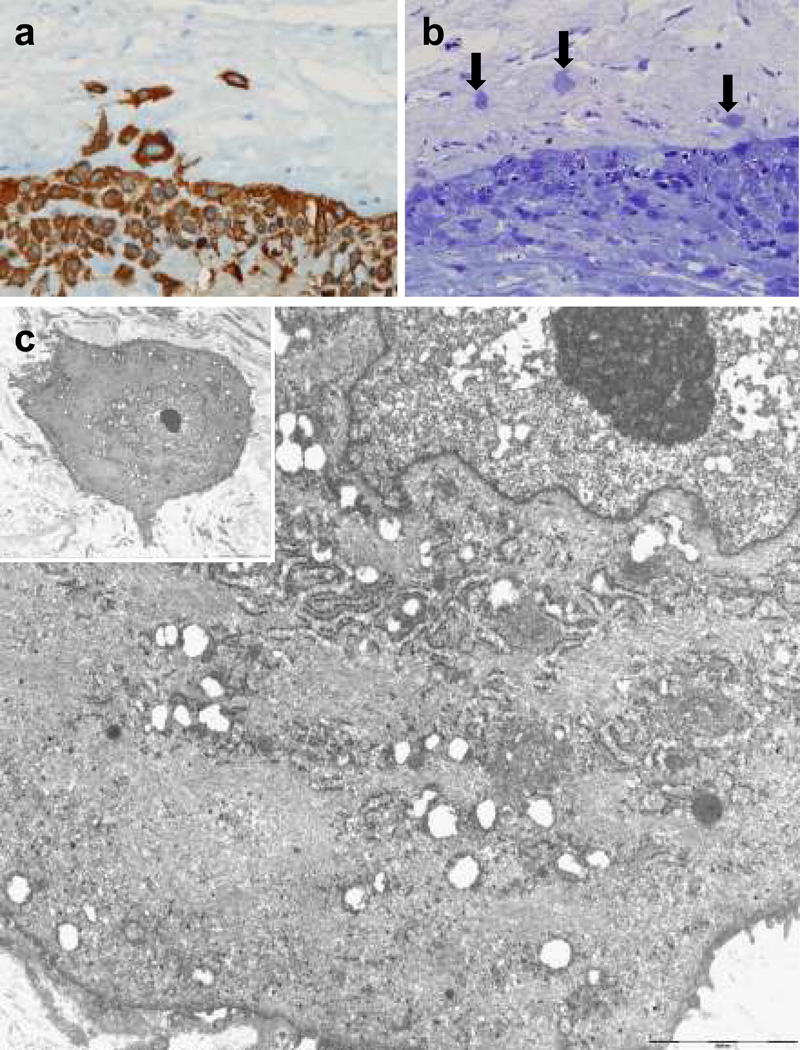
Figure 4. Ultrastructural features of trophoblasts in TICCT found in a case of normal term delivery after labor (41.4 weeks).
(a) Cytokeratin-7 immunopositive TICCT. (b) Semi-thin sections disclosing isolated trophoblasts of TICCT (arrows). (c) Electron microscopic features of TICCT embedded in an abundant collagen matrix (inset). The cell has euchromatic nuclei with prominent nucleoli, a fair number of rough endoplasmic reticula and mitochondria, and abundant intermediate filaments that show its unique differentiation characteristics. Original magnification: ×4000 (inset), ×10,000.
3.2. TICCT in the chorioamniotic membranes of uncomplicated term pregnancies
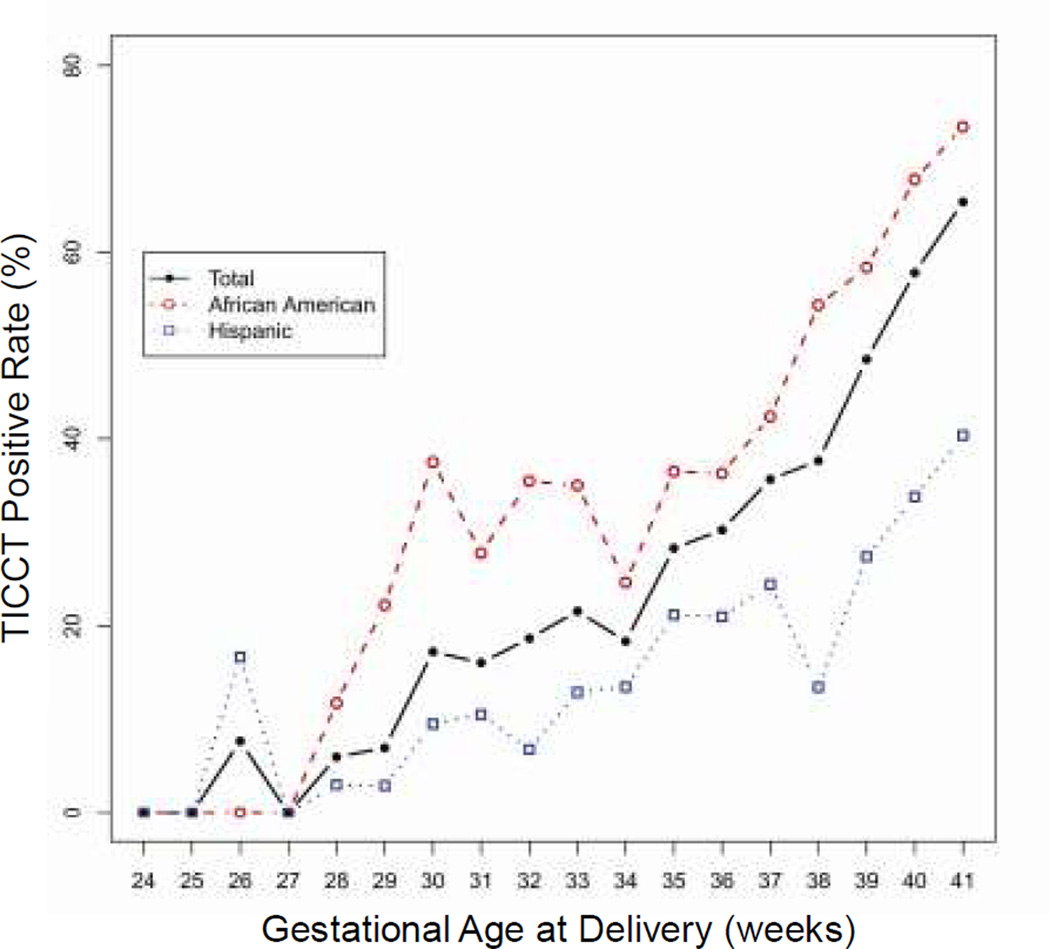
The histologic diagnosis of TICCT was reproducible. The κw value for intra-observer reliability in the diagnosis of TICCT was 0.807 (95% CI: 0.764–0.850), and the κw value for inter-observer reliability was 0.816 (95% CI: 0.777–0.856), both of which are considered excellent. TICCT was observed in 49.0% (587/1199) of placentas from uncomplicated deliveries at term (African-American and Hispanic women). Specific regional distribution patterns of TICCT in a given membrane roll were not observed. TICCT detection rates at gestational weeks 37, 38, 39, 40, and 41 were 35.7%, 37.6%, 48.5%, 57.8%, and 65.4%, respectively (Fig. 5). The rate of TICCT and gestational age at delivery were significantly correlated (p<0.001 by linear-by-linear association). The rate of TICCT in the placentas of African-American women was significantly higher than in those of Hispanic women from 37 weeks to 41 weeks of gestation (p<0.001 by M-H test) (Fig. 5).
Figure 5. The changes in the positive rate of TICCT across gestation in two study populations (African-American vs. Hispanic).
There is a robust association between the positive rate and advancing gestational age. The TICCT positive rate is also significantly higher in African-American women who delivered at Hutzel Women’s Hospital, Detroit, Michigan, USA, than in Hispanic women who delivered at Sótero del Río Hospital, Santiago, Chile.
The relationship between clinical parameters and TICCT in patients who delivered at term is summarized in Table 1. The presence of TICCT was associated with significantly higher maternal age and gestational age at delivery, higher birth weight, African-American ethnicity, nulliparity, female fetal sex, and a lower rate of chronic inflammation of the placenta. However, spontaneous labor status (presence versus absence) and other placental pathologies were not associated with the occurrence of TICCT.
Table 1.
Clinical characteristics of uncomplicated term deliveries and a TICCT positive rate.
| TICCT (−) | TICCT (+) | p value | adjusted p valuea | |
|---|---|---|---|---|
| Cases: number [n (%)] | 612 (51.0%) | 587 (49.0%) | ||
| Ethnicity [n (%)] | <0.001 | <0.001 | ||
| African-American | 321 (52.5%) | 484 (82.5%) | ||
| Hispanic | 291 (47.5%) | 103 (17.5%) | ||
| Maternal age (years, mean ± s.d.) | 25.0 (± 5.7) | 23.7 (± 5.5) | <0.001 | 0.903 |
| Parity [n (%)] | <0.001 | 0.001 | ||
| Nulliparity | 175(28.6%) | 240 (40.9%) | ||
| Multiparity | 437 (71.4%) | 347 (59.1%) | ||
| GAD (weeks, mean ± s.d.) | 39.1(± 1.3) | 39.7(± 1.4) | <0.001 | <0.001 |
| Fetal sex [n (%)] | <0.001 | <0.001 | ||
| Male | 355(58.0%) | 278 (47.4%) | ||
| Female | 257 (42.0%) | 309 (52.6%) | ||
| Birth weight (grams, mean ± s.d.) | 3263 (± 345) | 3339 (± 319) | <0.001 | <0.001b |
| Spontaneous labor [n (%)] | 0.745 | |||
| No | 236 (38.6%) | 221 (37.6%) | ||
| Yes | 376 (61.4%) | 366 (62.4%) | ||
| ACA [n (%)] | 0.095 | |||
| No | 505 (82.5%) | 462 (78.7%) | ||
| Yes | 107 (17.5%) | 125 (21.3%) | ||
| MVU [n (%)] | 0.357 | |||
| No | 586 (95.8%) | 568 (96.8%) | ||
| Yes | 26 (4.2%) | 19 (3.2%) | ||
| FVTOD [n (%)] | 0.961 | |||
| No | 573 (93.6%) | 550 (93.7%) | ||
| Yes | 39 (6.4%) | 37 (6.3%) | ||
| CPI [n (%)] | <0.001 | 0.001 | ||
| No | 419 (68.5%) | 465 (79.2%) | ||
| Yes | 193 (31.5%) | 122 (20.8%) |
GAD: Gestational age at delivery, ACA: acute chorioamnionitis, MVU: maternal vascular underperfusion, FVTOD: fetal vascular thrombo-occlusive disease, CPI: chronic placental inflammation.
Adjustment for ethnicity, maternal age, parity, gestational age at delivery, fetal sex, and chronic placental inflammation was based on a multiple logistic regression model.
Without adjustment for gestational age at delivery.
Multiple logistic regression analysis was performed to control for clinical parameters, such as ethnicity, maternal age, parity, gestational age at delivery, fetal sex, birth weight, and chronic inflammation of the placenta, that were significantly different according to the presence or absence of TICCT (Table 1). TICCT was 80% less likely to be detected in the membranes of Hispanic women than in those of African-American women (OR: 0.23, 95% CI: 0.18–0.31, p<0.001); similarly, TICCT was 40% less likely to be detected in the membranes of multiparas compared to nulliparas (OR: 0.60, 95% CI: 0.44–0.81, p=0.001). Interestingly, TICCT was 70% more prevalent in the membranes of female rather than male fetuses (OR: 1.64, 95% CI: 1.28–2.11, p<0.001). Detectable TICCT was 40% less likely to occur in placentas with lesions of chronic inflammation (OR: 0.60, 95% CI: 0.45–0.81, p=0.001).
The detection of TICCT increased as a function of gestational age. The likelihood of TICCT increased by 30% with each week of gestation (OR: 1.29, 95% CI: 1.16–1.45, p<0.001). Maternal age was not significantly associated with TICCT (OR: 0.998, 95% CI: 0.97–1.03, p=0.903.
The extent of TICCT was diagnosed as grade 1 in 28.3% (339/1199), grade 2 in 14.3% (171/1199), and grade 3 in 6.4% (77/1199) in uncomplicated term deliveries among African-American and Hispanic women. Controlling for potentially confounding factors (ethnicity, parity, fetal sex, and chronic inflammation of the placenta), high-grade TICCT (grade 2 or 3) was significantly associated with: 1) African-American ethnicity (OR: 6.76, 95% CI: 4.39–10.42, p<0.001); 2) gestational age at delivery (OR: 1.47, 95% CI: 1.30–1.66, p<0.001); and 3) a female fetus (OR: 1.74, 95% CI: 1.26–2.41, p=0.001). Alternatively, high-grade TICCT was significantly less common in multiparous women (OR: 0.45, 95% CI: 0.33–0.63, p<0.001) and those with chronic inflammation of the placenta (OR: 0.48, 95% CI: 0.32–0.71, p<0.001), adjusted for potential confounders.
3.3. TICCT in the chorioamniotic membranes of preterm deliveries
Given the magnitude of the association between TICCT and gestational age at delivery and other clinical variables in term pregnancies, findings from the placentas of patients who delivered preterm were examined in detail. Demographic characteristics of patients with preterm deliveries according to the presence or absence of TICCT are summarized in Table 2. Results are presented stratified by gestational age at delivery (Group 1: 35–36 weeks, Group 2: 32–34 weeks, Group 3: 29–31 weeks, Group 4: 24–28 weeks).
Table 2.
Clinical characteristics of preterm delivery cases and a TICCT positive rate
| TICCT (−) | TICCT (+) | p value | adjusted p valuea | |
|---|---|---|---|---|
| Cases: number [n (%)] | 758 (79.3%) | 198 (20.7%) | ||
| Ethnicity [n (%)] | <0.001 | <0.001 | ||
| African-American | 302 (39.8%) | 129 (65.2%) | ||
| Hispanic | 456 (60.2%) | 69 (34.8%) | ||
| Maternal age (years, mean ± s.d.) | 26.0 (± 7.1) | 25.4 (± 6.6) | 0.259 | |
| Parity [n (%)] | 0.356 | |||
| Nulliparity | 299 (39.4%) | 71 (35.9%) | ||
| Multiparity | 459 (60.6%) | 127 (64.1%) | ||
| GAD (weeks, mean ± s.d.) | 33.0 (± 3.1) | 34.5 (± 2.1) | <0.001 | 0.001 |
| Fetal sex [n (%)] | <0.001 | <0.001 | ||
| Male | 453 (59.8%) | 81 (40.9%) | ||
| Female | 305 (40.2%) | 117 (59.1%) | ||
| Birth weight (grams, mean ± s.d.) | 2026 (± 677) | 2271 (± 619) | <0.001 | <0.001b |
| Diagnosis [n (%)] | 0.879 | |||
| Preterm labor | 324 (42.7%) | 89 (44.9%) | ||
| pPROM | 240 (31.7%) | 52 (26.3%) | ||
| Preeclampsia | 194 (25.6%) | 57 (28.8%) | ||
| Spontaneous labor [n (%)] | 0.058 | |||
| No | 344 (45.4%) | 75 (37.9%) | ||
| Yes | 414 (54.6%) | 123 (62.1%) | ||
| ACA [n (%)] | 0.911 | |||
| No | 633 (83.5%) | 166 (83.8%) | ||
| Yes | 125 (16.5%) | 32 (16.2%) | ||
| MVU [n (%)] | 0.857 | |||
| No | 613(80.9%) | 159 (80.3%) | ||
| Yes | 145(19.1%) | 39 (19.7%) | ||
| FVTOD [n (%)] | 0.109 | |||
| No | 710 (93.7%) | 179 (90.4%) | ||
| Yes | 48 (6.3%) | 19 (9.6%) | ||
| CPI [n (%)] | <0.001 | 0.003 | ||
| No | 456 (60.2%) | 147 (74.2%) | ||
| Yes | 302 (39.8%) | 51 (25.8%) |
GAD: Gestational age at delivery, pPROM: preterm prelabor rupture of membranes, ACA: acute chorioamnionitis, MVU: maternal vascular underperfusion, FVTOD: fetal vascular thrombo-occlusive disease, CPI: chronic placental inflammation.
Adjustment for ethnicity, gestational age at delivery, fetal sex, and chronic inflammation of the placenta was based on a multiple logistic regression model.
Without adjustment for gestational age at delivery.
There was a strong linear association between TICCT and gestational age at delivery (range 28–36 weeks; p<0.001, by linear-by-linear association). The detection rate of TICCT was 29.4% (107/364), 19.4% (65/335), 14.0% (22/157), and 4.0% (4/100) at 35–36 weeks, 32–34 weeks, 29–31 weeks, and 24–28 weeks, respectively. TICCT was significantly more frequent in African-American than in Hispanic women with preterm deliveries (range 28–36 weeks; p<0.001 by M-H test) (Fig. 5). The presence of TICCT was associated with a higher birth weight, higher gestational age at delivery, African-American ethnicity, female fetal sex, and a lower rate of chronic inflammation of the placenta (Table 2, p<0.001 for each). There were no differences in maternal age, etiology of preterm birth, parity, and other placental pathologies according to the presence or absence of TICCT.
After adjustment for potential confounders (ethnicity, fetal sex, gestational age at delivery, birth weight, and chronic inflammation of the placenta) (Table 2), Hispanic women were 60% less likely to have TICCT than African-American women (OR: 0.41, 95% CI: 0.29–0.58, p<0.001). TICCT was two-fold more likely in women carrying a female fetus than in those with a male fetus (OR: 2.04, 95% CI: 1.46–2.85, p<0.001). The likelihood of TICCT increased by 20% with each progressive week of gestational age at delivery (OR: 1.19, 95% CI: 1.07–1.32, p=0.001). TICCT was significantly less prevalent in the presence of chronic inflammation of the placenta (OR: 0.58, 95% CI: 0.40–0.84, p=0.003).
The extent of TICCT was diagnosed as grade 1 in 15.6% (149/956), grade 2 in 4.4% (42/956), and grade 3 in 0.7% (7/956) in the preterm deliveries of African-American and Hispanic women. Controlling for potential confounders (ethnicity, fetal sex, and chronic inflammation of the placenta), high-grade TICCT (grade 2 or 3) was significantly more common in African-American women (OR: 2.68, 95% CI: 1.40–5.11, p=0.003), those with advancing gestational age (OR: 1.32, 95% CI: 1.14–1.54, p<0.001), and those who delivered a female fetus (OR: 3.89, 95% CI: 2.00–7.55, p<0.001). High-grade TICCT was less common in placentas with chronic inflammatory lesions (OR: 0.33, 95% CI: 0.15–0.73, p=0.006).
4. Discussion
We report a novel histological finding in the chorioamniotic membranes, which we define as “trophoblast islands of the chorionic connective tissue” (TICCT). The primary findings of this study are that: 1) TICCT is significantly more likely to occur with advancing gestational age at delivery; 2) TICCT was more frequent across gestation in African-American than Hispanic women included in this study; 3) female fetal sex is associated with the presence of TICCT; 4) TICCT was significantly less common in the presence of chronic placental inflammation; and 5) maternal age was not associated with TICCT. To the best of our knowledge, this is the first demonstration showing that placentas of differing fetal sex have histological differences.
The placenta displays features of growth and differentiation as shown in the development and maturation of the chorionic villi across gestation [25]. Other histological findings, such as calcifications [26] and squamous metaplasia of the amnion [23], have been shown to be more frequent with advancing gestational age. However, TICCT is unique in that its presence is more likely to occur with advancing gestational age in both preterm and term cases. We were able to quantitate the increase in the likelihood of TICCT with each advancing week of gestation. One interpretation is that TICCT may be a phenomenon associated with placental aging, senescence, or maturation. We are aware of the proposal that there is a “placental clock” determining the duration of pregnancy, and that such a clock would operate through the corticotropin-releasing hormone (CRH) [27–29]. The relationship between TICCT and the control of parturition requires further study.
An unexpected finding of this study was the difference in the frequency of TICCT between African-American and Hispanic women. Future studies are required for replication and to determine whether there is variability in the frequency of TICCT among other ethnic groups. For example, is it biologically plausible that there could be a difference in the frequency of TICCT among ethnic groups, due to the variability of the length of gestation among such groups? Similarly, the frequency of preterm birth varies according to race and ethnicity [30–36]. Patel et al. reported that after studying a cohort of 122,415 nulliparous women, the median gestational age at delivery was shorter in African-Americans and Asians (39 weeks) than in white Europeans (40 weeks). The authors also found an increase in the odds of preterm delivery in African-Americans and Asians compared to white Europeans, adjusted for body mass index and social deprivation [30]. Variation in the length of normal gestation has also been found in animals. Murray et al. demonstrated differences in the length of gestation in 15 inbred mice strains of as much as two full days, and proposed that the maternal genome may account for much of this variation [37]. The possible relationship among TICCT, fetal maturation or age, and parturition requires further study.
The association of increased frequency of TICCT and the placentas of female fetuses was also unexpected. There is some evidence that fetal sex may influence the outcome of pregnancy [38–40]. Pregnant women with a male fetus have a higher risk of preterm delivery [39,41,42], gestational diabetes mellitus [40], macrosomia [40], prolonged pregnancy [43], and abruptio placentae [44]. On the other hand, those with a female fetus have a higher rate of hyperemesis gravidarum [45], hypertension-related growth restriction [46], preeclampsia among preterm births [47], and placenta accreta [48]. The serum concentrations of free beta human chorionic gonadotropin are higher and those of alpha-fetoprotein are lower in women with a female fetus than in those with a male fetus [49,50]. Other studies have also shown evidence of sexual dimorphism in the biology of the placenta. Yeganegi et al. have demonstrated that the key cytokine response of placental trophoblasts to lipopolysaccharide or endotoxin was greater in male fetuses [51], and that up-regulation of colony-stimulating factor 3 (CSF3) in trophoblasts by Lactobacillus rhamnosus GR-1 was detected only in trophoblasts obtained from placentas of female fetuses [52]. Murphy et al. have shown that placental 11β-hydroxysteroid dehydrogenase activity differs between male and female fetuses in humans [53]. Furthermore, the mean gestational age at delivery of male fetuses was significantly higher by one day compared to that of female fetuses in humans [43]. The difference in the positive rate of TICCT, therefore, is additional and novel histological evidence that there is indeed a biological difference between pregnancies with male and female fetuses.
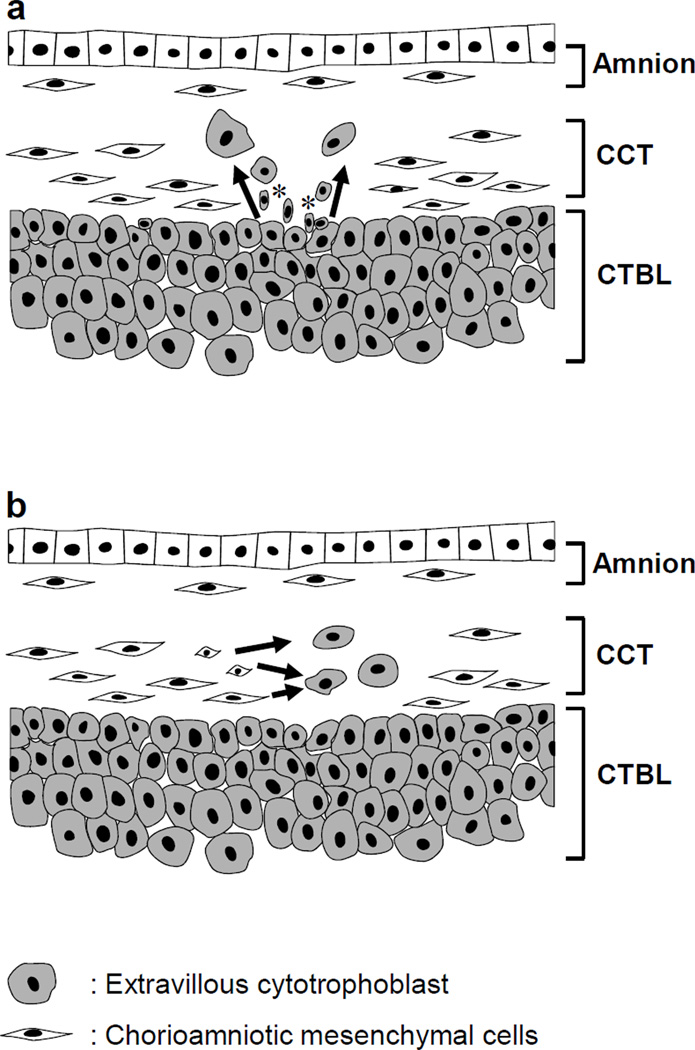
What is the origin of TICCT? Potential histogenetic explanations for TICCT include: 1) proliferation/migration/differentiation of chorionic extravillous cytotrophoblasts resting on the basal lamina into the chorionic connective tissue; or 2) de novo transdifferentiation of the mesenchymal cells of the chorionic connective tissue into trophoblasts (Fig. 6). The development of TICCT by the first mechanism would be consistent with trophoblast heterotopia, while the second mechanism would be more akin to trophoblastic metaplasia. Cases of TICCT showing direct extension of the chorionic trophoblasts into the connective tissue layer could be explained by the first mechanism. Those cases with isolated trophoblasts in the midst of the connective tissue layer could be either a consequence of direct extension or migration/differentiation of extravillous cytotrophoblasts which have lost a connection with the chorionic trophoblast layer, or a consequence of transdifferentiation of the mesenchymal cells of the connective tissue. It was intriguing to find the cells with small nuclei and little but clearly cytokeratin-positive cytoplasm at the border between the chorionic connective tissue and the trophoblast layer as shown in Fig. 2.
Figure 6. Schematic illustrations of the potential histogenesis (arrows) of TICCT.
a. Extravillous cytotrophoblasts sitting on the basal lamina migrate into the chorionic connective tissues. These small cytokeratin-positive trophoblasts (*) would be functionally similar to the stem cells described in other types of epithelium such as skin and esophagus. b. Transdifferentiation of chorionic mesenchymal cells into the trophoblasts. It is uncertain, however, whether chorionic mesenchymal cells have stemness to undergo trophoblast differentiation. CCT: chorionic connective tissue, CTBL: chorionic trophoblast layer.
Typically, the mesenchymal cells of the chorionic connective tissue display either a myofibroblast or macrophage phenotype, and a myofibroblast can acquire a macrophage phenotype [7,8]. Chorionic mesenchymal stem cells can undergo osteogenic [54] and neuronal differentiation [55]; yet the stemness of chorionic mesenchymal cells is uncertain at this time. Immunofluorescence studies have not demonstrated SSEA-3 and SSEA-4 in these cells [56]. Therefore, migration and differentiation of extravillous cytotrophoblasts residing in the basal portion of the chorionic trophoblast layer are more likely to explain TICCT. The basal layer of extravillous cytotrophoblasts, in contact with the basal lamina of the connective tissue layer, incorporates 3H-thymidine uptake [57]. Kalabis et al. recently demonstrated in a mouse esophagus that basal cells of the esophageal epithelium are endowed with properties of stem cells with BrdU uptake [58], and small cytokeratin-positive cells at the basal portion of the trophoblast layer might be a functionally similar population of epidermal stem cells of the skin [59].
We previously proposed that there is a transdifferentiation program between myofibroblasts and macrophages in the chorionic connective tissue [8]. Questions that remain are: (1) what is the fate of the trophoblasts in TICCT; and (2) do extravillous cytotrophoblasts of the chorionic trophoblast layer replenish the reservoir of chorionic mesenchymal cells and macrophages? Although these questions are beyond the scope of the present study, they are interesting lines for future investigation. Epithelial-mesenchymal transition of trophoblasts is considered an important feature of placental development. Perturbation of such a process has been associated with preeclampsia and fetal growth restriction [60,61]. It is also noteworthy that macrophages and trophoblasts share several properties such as phagocytosis, syncytialization, and the capacity for invasion [62].
There are limitations in our study. Data on the frequency of TICCT in Caucasian and Asian women were unavailable. This information would be valuable to determine the extent of ethnic and racial variability. By its nature, this observational study cannot address the questions about the biological signals responsible for the migration of the trophoblast and the constitution of TICCT – this applies to the presence of this histologic feature, as well as the association with gestational age, fetal sex, or ethnic group. It is well-known that amniotic fluid concentrations of biologically active molecules (such as prostaglandins and cytokines/chemokines) change with the progression of gestation and also with fetal sex [63,64]. The potential relationship between clinical parameters associated with TICCT and various biological signals needs to be studied. Similarly, the lower rate of TICCT in placentas with chronic inflammatory lesions remains to be explained.
In conclusion, we describe herein a new feature in placental pathology – TICCT. The mechanisms responsible for the generation of this finding need to be explored in depth; however, this feature has the potential to enhance our understanding of the biology of cells comprising the human chorion.
Acknowledgements
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services. The authors are grateful to the patients who agreed to participate in our studies, the nurses, laboratory staff, and clinicians who made this work possible; and also to Maureen McGerty and Andrea Bernard (Wayne State University) for their critical readings of the manuscript.
References
- 1.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 2.Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19:1–11. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 3.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394, e1–e24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DC, Romero R, Kim JS, Yoo W, Lee J, Mittal P, et al. Evidence for a spatial and temporal regulation of prostaglandin-endoperoxide synthase 2 expression in human amnion in term and preterm parturition. J Clin Endocrinol Metab. 2010;95:E86–E91. doi: 10.1210/jc.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, et al. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol. 2010;202:462, e1–e41. doi: 10.1016/j.ajog.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HS, Romero R, Lee SM, Park CW, Jun JK, Yoon BH. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta. 2010;31:792–795. doi: 10.1016/j.placenta.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Schneider J. Fine structure of human chorionic membrane. Ultrastructural and histochemical examinations. Arch Gynecol. 1983;233:187–198. doi: 10.1007/BF02114599. [DOI] [PubMed] [Google Scholar]
- 8.Kim SS, Romero R, Kim JS, Abbas A, Espinoza J, Kusanovic JP, et al. Coexpression of myofibroblast and macrophage markers: novel evidence for an in vivo plasticity of chorioamniotic mesodermal cells of the human placenta. Lab Invest. 2008;88:365–374. doi: 10.1038/labinvest.3700749. [DOI] [PubMed] [Google Scholar]
- 9.McParland PC, Taylor DJ, Bell SC. Myofibroblast differentiation in the connective tissues of the amnion and chorion of term human fetal membranes-implications for fetal membrane rupture and labour. Placenta. 2000;21:44–53. doi: 10.1053/plac.1999.0439. [DOI] [PubMed] [Google Scholar]
- 10.Goldman-Wohl DS, Ariel I, Greenfield C, Hanoch J, Yagel S. HLA-G expression in extravillous trophoblasts is an intrinsic property of cell differentiation: a lesson learned from ectopic pregnancies. Mol Hum Reprod. 2000;6:535–540. doi: 10.1093/molehr/6.6.535. [DOI] [PubMed] [Google Scholar]
- 11.Morales PJ, Pace JL, Platt JS, Phillips TA, Morgan K, Fazleabas AT, et al. Placental cell expression of HLA-G2 isoforms is limited to the invasive trophoblast phenotype. J Immunol. 2003;171:6215–6224. doi: 10.4049/jimmunol.171.11.6215. [DOI] [PubMed] [Google Scholar]
- 12.Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, et al. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta. 2012;33:982–990. doi: 10.1016/j.placenta.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challis JR, Patel FA, Pomini F. Prostaglandin dehydrogenase and the initiation of labor. J Perinat Med. 1999;27:26–34. doi: 10.1515/JPM.1999.003. [DOI] [PubMed] [Google Scholar]
- 14.Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, et al. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- 15.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 16.Olson DM. The role of prostaglandins in the initiation of parturition. Best Pract Res Clin Obstet Gynaecol. 2003;17:717–730. doi: 10.1016/s1521-6934(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, He P, Sha J, Liu C, Dai L, Hui N, et al. Corticotropin-releasing hormone receptor type 1 and type 2 mediate differential effects on 15-hydroxy prostaglandin dehydrogenase expression in cultured human chorion trophoblasts. Endocrinology. 2007;148:3645–3654. doi: 10.1210/en.2006-1212. [DOI] [PubMed] [Google Scholar]
- 18.Okita RT, Okita JR. Prostaglandin-metabolizing enzymes during pregnancy: characterization of NAD(+)-dependent prostaglandin dehydrogenase, carbonyl reductase, and cytochrome P450-dependent prostaglandin omega-hydroxylase. Crit Rev Biochem Mol Biol. 1996;31:101–126. doi: 10.3109/10409239609106581. [DOI] [PubMed] [Google Scholar]
- 19.Matsubara S, Takizawa T, Yamada T, Minakami H, Sato I. Phagocytosis of chorion laeve trophoblasts in patients with chorioamnionitis-associated preterm delivery: ultrastructural and enzyme-histochemical observations. Placenta. 2000;21:273–279. doi: 10.1053/plac.1999.0463. [DOI] [PubMed] [Google Scholar]
- 20.Hirano T, Higuchi T, Ueda M, Inoue T, Kataoka N, Maeda M, et al. CD9 is expressed in extravillous trophoblasts in association with integrin alpha3 and integrin alpha5. Mol Hum Reprod. 1999;5:162–167. doi: 10.1093/molehr/5.2.162. [DOI] [PubMed] [Google Scholar]
- 21.Aplin JD. Expression of integrin alpha 6 beta 4 in human trophoblast and its loss from extravillous cells. Placenta. 1993;14:203–215. doi: 10.1016/s0143-4004(05)80261-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Kim KR, McKeon F, Yang A, Boyd TK, Crum CP, et al. A unifying concept of trophoblastic differentiation and malignancy defined by biomarker expression. Hum Pathol. 2007;38:1003–1013. doi: 10.1016/j.humpath.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Benirschke K, Kaufmann P, Baergen RN. Pathology of the human placenta. 5th ed. New York: Springer; 2006. [Google Scholar]
- 24.Simmonds M, Jeffery H, Watson G, Russell P. Intraobserver and interobserver variability for the histologic diagnosis of chorioamnionitis. Am J Obstet Gynecol. 2004;190:152–155. doi: 10.1016/s0002-9378(03)00870-6. [DOI] [PubMed] [Google Scholar]
- 25.Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. 2008;32:172–177. doi: 10.1053/j.semperi.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Poggi SH, Bostrom KI, Demer LL, Skinner HC, Koos BJ. Placental calcification: a metastatic process? Placenta. 2001;22:591–596. doi: 10.1053/plac.2001.0688. [DOI] [PubMed] [Google Scholar]
- 27.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 28.Smith R, Smith JI, Shen X, Engel PJ, Bowman ME, McGrath SA, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- 29.Smith R, Nicholson RC. Corticotrophin releasing hormone and the timing of birth. Front Biosci. 2007;12:912–918. doi: 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- 30.Patel RR, Steer P, Doyle P, Little MP, Elliott P. Does gestation vary by ethnic group? A London-based study of over 122,000 pregnancies with spontaneous onset of labour. Int J Epidemiol. 2004;33:107–113. doi: 10.1093/ije/dyg238. [DOI] [PubMed] [Google Scholar]
- 31.Henderson M, Kay J. Differences in duration of pregnancy. Negro and white women of low socioeconomic class. Arch Environ Health. 1967;14:904–911. doi: 10.1080/00039896.1967.10664859. [DOI] [PubMed] [Google Scholar]
- 32.Omigbodun AO, Adewuyi A. Duration of human singleton pregnancies in Ibadan, Nigeria. J Natl Med Assoc. 1997;89:617–621. [PMC free article] [PubMed] [Google Scholar]
- 33.Papiernik E, Alexander GR, Paneth N. Racial differences in pregnancy duration and its implications for perinatal care. Med Hypotheses. 1990;33:181–186. doi: 10.1016/0306-9877(90)90173-c. [DOI] [PubMed] [Google Scholar]
- 34.Migone A, Emanuel I, Mueller B, Daling J, Little RE. Gestational duration and birthweight in white, black and mixed-race babies. Paediatr Perinat Epidemiol. 1991;5:378–391. doi: 10.1111/j.1365-3016.1991.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 35.Shiono PH, Klebanoff MA. Ethnic differences in preterm and very preterm delivery. Am J Public Health. 1986;76:1317–1321. doi: 10.2105/ajph.76.11.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anum EA, Springel EH, Shriver MD, Strauss JF., 3rd Genetic contributions to disparities in preterm birth. Pediatr Res. 2009;65:1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, et al. Mouse gestation length is genetically determined. PLoS One. 2010;5:e12418. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 39.McGregor JA, Leff M, Orleans M, Baron A. Fetal gender differences in preterm birth: findings in a North American cohort. Am J Perinatol. 1992;9:43–48. doi: 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- 40.Sheiner E, Levy A, Katz M, Hershkovitz R, Leron E, Mazor M. Gender does matter in perinatal medicine. Fetal Diagn Ther. 2004;19:366–369. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 41.James WH. Why are boys more likely to be preterm than girls? Plus other related conundrums in human reproduction. Hum Reprod. 2000;15:2108–2111. doi: 10.1093/humrep/15.10.2108. [DOI] [PubMed] [Google Scholar]
- 42.Cooperstock M, Campbell J. Excess males in preterm birth: interactions with gestational age, race, and multiple birth. Obstet Gynecol. 1996;88:189–193. doi: 10.1016/0029-7844(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 43.Divon MY, Ferber A, Nisell H, Westgren M. Male gender predisposes to prolongation of pregnancy. Am J Obstet Gynecol. 2002;187:1081–1083. doi: 10.1067/mob.2002.126645. [DOI] [PubMed] [Google Scholar]
- 44.James WH. Offspring sex ratios and the causes of placental pathology: the case of placental abruption. Hum Reprod. 2001;16:2031. doi: 10.1093/humrep/16.9.2031. [DOI] [PubMed] [Google Scholar]
- 45.Nageotte MP, Briggs GG, Towers CV, Asrat T. Droperidol and diphenhydramine in the management of hyperemesis gravidarum. Am J Obstet Gynecol. 1996;174:1801–1805. doi: 10.1016/s0002-9378(96)70213-2. discussion 05-6. [DOI] [PubMed] [Google Scholar]
- 46.Spinillo A, Capuzzo E, Nicola S, Colonna L, Iasci A, Zara C. Interaction between fetal gender and risk factors for fetal growth retardation. Am J Obstet Gynecol. 1994;171:1273–1277. doi: 10.1016/0002-9378(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 47.Brettell R, Yeh PS, Impey LW. Examination of the association between male gender and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2008;141:123–126. doi: 10.1016/j.ejogrb.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 48.James WH. Sex ratios of offspring and the causes of placental pathology. Hum.Reprod. 1995;10:1403–1406. doi: 10.1093/humrep/10.6.1403. [DOI] [PubMed] [Google Scholar]
- 49.Leporrier N, Herrou M, Leymarie P. Shift of the fetal sex ratio in hCG selected pregnancies at risk for Down syndrome. Prenat Diagn. 1992;12:703–704. doi: 10.1002/pd.1970120813. [DOI] [PubMed] [Google Scholar]
- 50.Bazzett LB, Yaron Y, O'Brien JE, Critchfield G, Kramer RL, Ayoub M, et al. Fetal gender impact on multiple-marker screening results. Am J Med Genet. 1998;76:369–371. [PubMed] [Google Scholar]
- 51.Yeganegi M, Watson CS, Martins A, Kim SO, Reid G, Challis JR, et al. Effect of Lactobacillus rhamnosus GR-1 supernatant and fetal sex on lipopolysaccharide-induced cytokine and prostaglandin-regulating enzymes in human placental trophoblast cells: implications for treatment of bacterial vaginosis and prevention of preterm labor. Am J Obstet Gynecol. 2009;200:532, e1–e8. doi: 10.1016/j.ajog.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 52.Yeganegi M, Leung CG, Martins A, Kim SO, Reid G, Challis JR, et al. Lactobacillus rhamnosus GR-1 stimulates colony-stimulating factor 3 (granulocyte) (CSF3) output in placental trophoblast cells in a fetal sex-dependent manner. Biol Reprod. 2011;84:18–25. doi: 10.1095/biolreprod.110.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, et al. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 54.Mohr S, Portmann-Lanz CB, Schoeberlein A, Sager R, Surbek DV. Generation of an osteogenic graft from human placenta and placenta-derived mesenchymal stem cells. Reprod Sci. 2010;17:1006–1015. doi: 10.1177/1933719110377471. [DOI] [PubMed] [Google Scholar]
- 55.Portmann-Lanz CB, Schoeberlein A, Portmann R, Mohr S, Rollini P, Sager R, et al. Turning placenta into brain: placental mesenchymal stem cells differentiate into neurons and oligodendrocytes. Am J Obstet Gynecol. 2010;202:294 e1–94 e11. doi: 10.1016/j.ajog.2009.10.893. [DOI] [PubMed] [Google Scholar]
- 56.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 57.Kaltenbach FJ, Sachs W. [The uptake of tritiated thymidine in human fetal membranes during the last third of pregnancy (author's transl)] Z Geburtshilfe Perinatol. 1979;183:285–295. [PubMed] [Google Scholar]
- 58.Kalabis J, Oyama K, Okawa T, Nakagawa H, Michaylira CZ, Stairs DB, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–3869. doi: 10.1172/JCI35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31:747–755. doi: 10.1016/j.placenta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Blechschmidt K, Mylonas I, Mayr D, Schiessl B, Schulze S, Becker KF, et al. Expression of E-cadherin and its repressor snail in placental tissue of normal, preeclamptic and HELLP pregnancies. Virchows Arch. 2007;450:195–202. doi: 10.1007/s00428-006-0343-x. [DOI] [PubMed] [Google Scholar]
- 62.Guilbert L, Robertson SA, Wegmann TG. The trophoblast as an integral component of a macrophage-cytokine network. Immunol Cell Biol. 1993;71(Pt 1):49–57. doi: 10.1038/icb.1993.5. [DOI] [PubMed] [Google Scholar]
- 63.Poggi SH, Spong CY, Ghidini A, Ossandon M. Gender differences in amniotic fluid cytokine levels. J Matern Fetal Neonatal Med. 2004;15:367–371. doi: 10.1080/14767050410001727396. [DOI] [PubMed] [Google Scholar]
- 64.Bry K, Lappalainen U, Waffarn F, Teramo K, Hallman M. Influence of fetal gender on the concentration of interleukin-1 receptor antagonist in amniotic fluid and in newborn urine. Pediatr Res. 1994;35:130–134. doi: 10.1203/00006450-199401000-00029. [DOI] [PubMed] [Google Scholar]