Abstract
Patients with acute myeloid leukemia (AML) harboring ≥3 acquired chromosome aberrations in the absence of prognostically favorable t(8;21)(q22;q22), inv(16)(p13q22)/t(16;16)(p13;q22) and t(15;17)(q22;q21) form a separate category - AML with a complex karyotype. They constitute 10–12% of all AML patents, with the incidence of complex karyotypes increasing with the more advanced age. Recent studies using molecular-cytogenetic techniques (spectral karyotyping, M-FISH) and array comparative genomic hybridization considerably improved characterization of previously unidentified, partially identified or cryptic chromosome aberrations, and allowed precise delineation of genomic imbalances. The emerging nonrandom pattern of abnormalities includes relative paucity, but not absence, of balanced rearrangements (translocations, insertions or inversions), predominance of aberrations leading to loss of chromosome material (monosomies, deletions and unbalanced translocations) that involve, in decreasing order, chromosome arms 5q, 17p, 7q, 18q, 16q, 17q, 12p, 20q, 18p and 3p, and the presence of recurrent, albeit less frequent and often hidden (in marker chromosomes and unbalanced translocations) aberrations leading to overrepresentation of segments from 8q, 11q, 21q, 22q, 1p, 9p, and 13q. Several candidate genes have been identified as targets of genomic losses, e.g., TP53, CTNNA1, NF1, ETV6 and TCF4, and amplifications, e.g., ERG, ETS2, APP, ETS1, FLI1, MLL, DDX6, GAB2, MYC, TRIB1 and CDX2. Treatment outcomes of complex karyotype patients receiving chemotherapy are very poor; they can be improved to some extent by allogeneic stem-cell transplantation in younger patients. It is hoped that better understanding of genomic alterations will result in identification of novel therapeutic targets and improved prognosis in patients with complex karyotypes.
INTRODUCTION
Cytogenetic findings at the time of diagnosis are among the most important independent prognostic factors in patients with acute myeloid leukemia (AML), both adults and children.1–14 While t(15;17)(q22;q12-21), t(8;21)(q22;q22) and inv(16)(p13q22)/t(16;16)(p13;q22) are associated with a relatively favorable clinical outcome,15–17 several recurrent abnormalities, e.g., inv(3)(q21q26)/t(3;3)(q21;q26) or t(6;9)(p23;q34) bestow very poor prognosis.4,6,9 In addition to specific chromosome rearrangements that confer adverse prognosis, a subset of patients whose treatment outcomes have been consistently very poor presents with a complex karyotype defined as the presence of ≥3 (in some studies ≥5) chromosome abnormalities.2,5–8,11–14 Until recently, this group of patients was not well characterized cytogenetically or molecularly because in addition to recognizable chromosome abnormalities, such as deletion of the long arm of chromosome 5, monosomy 7 or trisomy 8, these complex karyotypes contain, sometimes numerous, aberrations that could only be partially or not at all recognized using standard cytogenetic analysis with G- or R-banding.18 Such aberrations include marker chromosomes, i.e., abnormal chromosomes in which no part can be identified, ring chromosomes of unknown origin, homogeneously staining regions, double minutes and unbalanced translocations, in which the origin of translocated chromosome material cannot be established (usually designated as “add”). The application of molecular-cytogenetic techniques, such as multicolor spectral karyotyping (SKY),19–29 multiplex fluorescence in situ hybridization (M-FISH)28–34 and cross-species color banding,35,36 that allow simultaneous display of all human chromosomes in different colors, as well as comparative genomic hybridization (CGH)37,38 and array CGH (a-CGH)39 enabled more precise characterization of complex karyotypes. This has also led to identification of several genes whose expression is altered as a result of genomic imbalances in patients in this cytogenetic group.40–45 Although these advancements have not yet been translated into improvements of the patients’ outcome, there are promising reports of patients with a complex karyotype responding to treatment that contains inhibitors of histone deacetylase and/or DNA methyltransferase.46 This article will briefly review recent advances in delineating cytogenetic, molecular and clinical features of AML patients with a complex karyotype.
DEFINITION AND INCIDENCE
Technically, any karyotype with at least 3 chromosome aberrations, regardless of their type and the individual chromosomes involved, can be referred to as “complex”. Thus, approximately 20% of patients with t(8;21) and around 10% of patients with inv(16)/t(16;16) and of those with t(15;17) would be considered to have a complex karyotype because they harbor ≥2 secondary aberrations in addition to their respective primary translocation or inversion.16,47 However, since several studies have shown that patients with the aforementioned abnormalities constitute separate biological and clinical entities, and that increased karyotype complexity in these cytogenetic groups does not affect adversely clinical outcome in a manner comparable to other patients with ≥3 (or ≥5) abnormalities,2,6,15,16,47 the category “complex karyotype” excludes patients with t(8;21), inv(16)/t(16;16) and t(15;17). This definition was adopted by studies analyzing the impact of cytogenetics on clinical outcome in AML2,5,48 and those focused on improved characterization of genetic rearrangements using multicolor FISH.24,34 Some other studies also do not include in the complex karyotype category patients harboring t(9;11)(p22;q23),6,12 any balanced rearrangement involving band 11q23,11,39 or any “primary balanced abnormality”.31
In large series of AML patients, those with a complex karyotype defined as ≥3 aberrations consistently constituted 10–12% of all patients analyzed,3,6,48 whereas patients with ≥5 aberrations comprised 8–9%.2,3,5,6 Moreover, it has become clear that the incidence of complex karyotypes increases with age. Whereas complex karyotypes with ≥5 aberrations were detected in 6% of AML patients younger than 55 years (including children), they were found in 13% of patients 55 years old and older in the MRC study.5 Similarly, in a German series, patients with complex karyotypes with ≥3 aberrations constituted 8% of patients <60 years of age but 18% of those aged 60 years and above.48 Two recent large studies restricted to patients 60 years of age or older12 and patients older than 60 years11 reported similar percentages of complex karyotypes with ≥3 abnormalities, 19% and 17%, respectively, although the third study of AML patients aged ≥60 years reported a lower incidence - 12%.14 In a population-based study, Schoch et al.43 estimated that the incidence of karyotypically complex AML with ≥3 aberrations in the German population was 0.05 per 100,000 people aged 21 to 30 years, but it was almost 25 times higher—1.15 per 100,000 people—in the 61 to 70 years age group.
Compared with de novo AML, complex karyotypes are approximately twice as common in secondary AML, both therapy-related and evolving from antecedent hematologic malignancy.5,43
MAJOR CYTOGENETIC FEATURES
Even though 3 is the lowest number of chromosome aberrations necessary to consider a karyotype as complex, the vast majority of patients harbor a higher number of aberrations, which in rare cases can reach as many as 30!24,30,31 In three large series of AML patients with a complex karyotype analyzed using multicolor FISH, >90 percent of patients had at least 5 abnormalities, with the median number of aberrations being, respectively, 6,30 824 and 10.31 An example of such a complex karyotype with 8 chromosome abnormalities is depicted in Figure 1.
Figure 1.
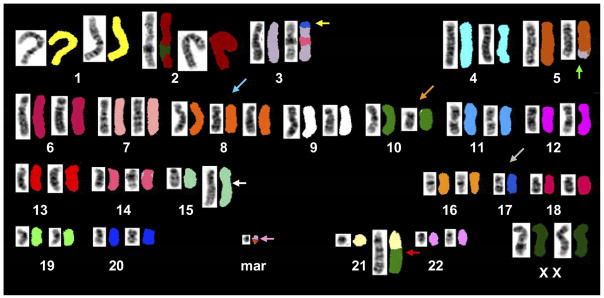
Complex karyotype containing 8 chromosome abnormalities detected in a patient with acute myeloid leukemia, analyzed using spectral karyotyping (SKY). Each chromosome is represented twice, by G-banding-like inverted and contrast-enhanced DAPI-stained image on the left and SKY image shown in classification colors on the right. This karyotype contains several chromosome abnormalities relatively common in AML with a complex karyotype: an unbalanced translocation between chromosomes 3 and 17 leading to loss of material from 3p and chromosome 17 (yellow arrow), an unbalanced translocation between chromosomes 3 and 5 resulting in partial loss of 5q (green arrow), trisomy of chromosome 8 (blue arrow), loss of one copy of chromosome 17 (grey arrow) and a complex rearrangement between chromosome 10 and 21 leading to gain of material from 21q (red arrow). Also present are: a complex rearrangement of chromosome 15 resulting in amplification of 15q material (white arrow), abnormal chromosome 10 (orange arrow) and a small marker chromosome whose origin could not be established reliably by SKY technique (pink arrow).
The modal chromosome number is hypodiploid, i.e., ≤45 chromosomes, in the majority, between 55% and 75%, of the patients. Approximately one-fourth of patients have a hyperdiploid karyotype, i.e., comprising ≥47 chromosomes, whereas the karyotype of the remaining patients is pseudodiploid, i.e., the chromosome number is equal to 46 but the karyotype contains clonal chromosome abnormalities.24,30,31 This pattern of ploidy is in part a reflection of the fact that whole chromosome losses are more frequent than whole chromosome gains in this group of patients.
Interestingly, whole chromosome losses appear to be more frequent that they really are if only results of standard cytogenetic analysis using G- or R-banding are considered. In such analyses, the most frequently identified as lost are chromosomes no. 7, 18, 20, 21, 5, 12 and 17.24 However, studies using SKY and M-FISH revealed that only a fraction, approximately 30% in one study,24 of chromosomes deemed lost in G-banded karyotypes were confirmed to be truly missing. The remaining chromosomes were not lost entirely but their parts were found in marker and/or ring chromosomes, or in unidentified material attached to a chromosome. Some chromosomes were more likely than others to be structurally rearranged instead of being totally lost. This is particularly true for chromosome 5, true monosomy of which was shown to be very rare, occurring in only about 3% of all patients with a complex karyotype, and also for chromosomes 21, 20 and 12.24,30,31,49 On the other hand, monosomy 7 detected in G-banded preparations was confirmed by molecular-cytogenetic techniques in most, and monosomy 18 in one-half of the patients. Importantly, when segments from the apparently missing chromosomes 5, 20, 17, 7, and 18 are found to be hidden in structurally altered chromosomes, the net result of these structural rearrangements is still loss of chromosomal material, mainly from, respectively, chromosome arms 5q, 20q, 17p, 7q, and 18q.24 In contrast, most patients with a supposed monosomy of chromosome 21 harbor cryptic structural rearrangements resulting in overrepresentation of material from the 21q arm, which in some instances amounts to high level amplification.
Consequently, the true pattern of whole chromosome losses in patients with a complex karyotype revealed by SKY and M-FISH analyses includes −7 as the most frequent monosomy, occurring in 20–30% of patients, followed by −18 (20%) and −17 (10–15%).24,30,31 With regard to the whole-chromosome gains, which in contrast to chromosome losses can be reliably detected by G-banding alone, trisomy 8 is by far the most frequent - it is present in 10–25% of patients. Other recurrent trisomies observed in 5–10% of patients include +9, +10, +11, +13, +21 and +22.24,30,31
The aforementioned numerical aberrations notwithstanding, the vast majority of aberrations detected in patients with complex karyotypes are structural. Although balanced aberrations such as reciprocal translocations, inversions or insertions can be detected, sometimes only after employing SKY or M-FISH, they are relatively rare.24,30,31 Unbalanced abnormalities predominate, and most of them result in net loss of chromosome material. Interestingly, SKY and M-FISH analyses revealed that many abnormalities considered to be described accurately as deletions in G-banded preparations were in fact unbalanced translocations or, less frequently, reciprocal translocations or insertions. In our SKY study of 29 AML patients with a complex karyotype,24 11 (34%) of 32 deletions in G-banded karyotypes turned out to be such cryptic translocations or insertions, including three of four deletions of chromosome 3, two of three del(7q), and two of 12 deletions of 5q. On the other hand, partial gains of chromosome material caused by structural rearrangements recognized by G-banding are usually confirmed by SKY or M-FISH. These techniques, however, allow uncovering additional cryptic gains of chromosome segments hidden in homogenously staining regions, double minutes and/or marker and ring chromosomes, with chromosomes 21, 11, 22, 13 and 15 being the most frequently involved.
Table 1 presents the distribution of the most frequent genomic losses and gains (seen in at least 10% of patients) that affect specific chromosome arms as a result of both structural and numerical chromosome abnormalities in AML patients with complex karyotypes. The data come from the three largest studies that used multicolor FISH24,30,31 and one large study that used a-CGH.39 Of note, the assay used in the latter study was not capable of detecting imbalances of sex chromosomes X and Y. However, even though aberrations affecting chromosomes X and Y do occur in complex karyotypes,24,30,31,50 they are infrequent enough to allow combining results from analyses performed using multicolor FISH with results obtained using a-CGH. The most common by far is loss of material from 5q, seen in 80% of all patients with a complex karyotype. Only 2 other chromosome losses, of 17p and 7q, occur in approximately 50% of cases. Importantly, these abnormalities often occur together, in the same patient, and approximately 85% of all patients harbor loss of at least one of the aforementioned chromosome arms.43 Other imbalances listed in Table 1, both losses and gains, are each seen in less than 30% of the patients.
Table 1.
Frequencies of the most common genomic imbalances (losses and gains) affecting specific chromosome arms in AML patients with complex karyotypes revealed by studies using multicolor FISH and array CGH techniques
| Chromosome arm | Study (no. of patients) | Total (n=250)a | |||
|---|---|---|---|---|---|
| Mrózek et al.24 (n=29) | Van Limbergen et al.30 (n=36)b | Schoch et al.31 (n=125) | Rücker et al.39 (n=60) | ||
| Abnormalities resulting in loss of chromosome material | |||||
| 5q | 76% | 81% | 82% | 77% | 80% |
| 17p | 62% | 25% | 53% | 55% | 50% |
| 7q | 59% | 44% | 46% | 45% | 47% |
| 18q | 41% | 11% | 30% | 30% | 29% |
| 16q | 24% | 25% | NR | 32% | 28% |
| 17q | 24% | 36% | NR | 22% | 27% |
| 12p | 41% | 17% | 27% | 18% | 25% |
| 20q | 38% | 14% | NR | 18% | 22% |
| 18p | 41% | 11% | NR | 15% | 20% |
| 3p | 24% | 8% | NR | 20% | 18% |
| 12q | 14% | 6% | NR | 20% | 14% |
| 13q | 10% | 17% | NR | 12% | 13% |
| 11q | 14% | 6% | NR | 12% | 10% |
| Abnormalities resulting in gain of chromosome material | |||||
| 8q | 14% | 14% | >30% | 38% | >28% |
| 11q | 24% | 22% | 22% | 40% | 27% |
| 21q | 28% | 25% | 22% | 25% | 24% |
| 22q | 24% | 19% | NR | 17% | 19% |
| 1p | 7% | 8% | NR | 25% | 16% |
| 9p | 3% | 14% | NR | 18% | 14% |
| 13q | 14% | 6% | NR | 15% | 12% |
Abbreviations: NR, not reported
Percentages for particular abnormalities calculated using only those studies that provided relevant data.
This series includes 23 patients diagnosed with AML and 13 with myelodysplastic syndromes.
MOLECULAR GENETIC ALTERATIONS RESULTING FROM CHROMOSOME REARRANGEMENTS
In general, molecular consequences of the majority of segmental losses and gains of specific chromosomes observed recurrently in AML patients with complex karyotypes are not well characterized. However, there has been some progress in identifying genes that likely play a role in leukemogenesis in this subset of AML patients.
The first association between aberrations leading to the loss of chromosome material from 17p and mutations in the TP53 gene has been reported in the early 1990s,51 and confirmed by later studies.32,52,53 It has been demonstrated that loss of p53 protein function due to TP53 mutations and/or loss of this gene result in genetic instability and increased cell survival.54 Thus, it is likely that TP53 loss and/or mutations, which occur in most, but not all, patients with a complex karyotype, represent one of the molecular pathways responsible for marked genomic instability, which is manifested by the simultaneous presence of multiple related clones, creation of complex abnormal chromosomes that contain material from 3 or even more separate chromosomes and/or abnormal “sandwich-like” chromosomes that are comprised from several small interchanging segments from two different chromosomes (Figure 2).20,24,32
Figure 2.
Metaphase cells from an AML patient with a complex karyotype that contains an abnormal “sandwich-like” derivative chromosome comprised from several interchanging segments from chromosomes 7 and 21, analyzed using SKY (A) and whole-chromosome-painting probes for chromosomes 7 (B) and 21 (C). Inverted and contrast-enhanced DAPI images are shown on the left. SKY in display colors (A) and FISH images (B and C) are shown on the right. Long arrows point to the centromeric area of the abnormal “sandwich-like” derivative chromosome, short arrows denote normal chromosomes 7 (A and B) and 21 (A and C). A: Full metaphase cell analyzed using SKY. B. Partial metaphase hybridized with the whole-chromosome-painting probe for chromosome 7. C. Partial metaphase hybridized with the whole-chromosome-painting probe for chromosome 21.
However, the intensive search for other tumor suppressor genes, especially those located on chromosome arms 7q and 5q, whose one allele would be lost due to a deletion or unbalanced translocation and the other mutated, has been largely unsuccessful. It has been proposed that it is haploinsufficiency, i.e., lower gene expression because of the presence of only one functional allele as a result of deletion of the second allele, affecting many genes mapped to the relatively large segments lost from 5q and 7q, that plays a role in leukemogenesis in patients with a complex karyotype.43 Indeed, two gene-expression studies that compared, respectively, expression of genes located on 5q between complex karyotype patients with loss of 5q material and those with a normal karyotype,43 and expression of genes located on 5q, 7q and 17p between complex karyotype patients with and those without loss of chromosomal material from these chromosome arms,39 revealed that the average expression levels for many genes located in 5q, 7q and 17p were significantly lower in patients harboring rearrangements of these chromosome arms.
The concept of haploinsufficiency does not necessarily exclude, however, the possibility that some of the deleted and/or underexpressed genes are more important than others, and that the elusive 5q- and 7q- tumor suppressor genes may still be identified. A candidate for such a tumor suppressor gene that is mapped to 5q31, the gene encoding α-catenin (CTNNA1), has been recently identified.55 CTNNA1 expression was much lower in leukemia-initiating stem cells from AML or MDS patients with del(5q) than in AML or MDS patients without this deletion or in normal hematopoietic stem cells. Importantly, the promoter of the retained CTNNA1 allele was epigenetically suppressed by methylation and histone deacetylation. When CTNNA1 expression was restored in vitro, cell proliferation was reduced and the cells underwent apoptosis.55 This observation is important because it suggests that treatment that includes methyltransferase and histone deacetylase inhibitors capable of re-establishing CTNNA1 expression might be especially beneficial in patients with complex karyotypes,55 80% of whom harbor loss of 5q material (Table 1).
Another potential target of recurrent genomic loss in complex karyotype AML, identified recently by Rücker et al.,39 is the NF1 gene, encoding a negative regulator of RAS signaling. NF1 is located at 17q11, in the smallest region lost in patients with rearrangements of 17q. Interestingly, another study that confirmed the recurrent occurrence of NF1 deletions in AML patients with a complex karyotype, found NF1 loss also in patients with inv(16).56 Additional candidate genes located within commonly deleted regions in complex karyotype AML are listed in Table 2.
Table 2.
Candidate genes located in recurrently lost or gained and/or amplified chromosome regions in AML patients with a complex karyotype
| Candidate Genes | Chromosome location | Reference no. | |
|---|---|---|---|
| Symbol | Name | ||
| Loss | |||
| MLH1 | mutL homolog 1, colon cancer, nonpolyposis type 2 (E. coli) | 3p22.3 | 39 |
| CTNNA1 | catenin (cadherin-associated protein), alpha 1, 102kDa | 5q31.2 | 55 |
| ETV6 | ets variant gene 6 (TEL oncogene) | 12p13 | 30,39 |
| CDKN1B | cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 12p13.1-p12 | 39 |
| TRADD | TNFRSF1A-associated via death domain | 16q22 | 39 |
| CBFB | core-binding factor, beta subunit | 16q22.1 | 39 |
| TP53 | tumor protein p53 (Li-Fraumeni syndrome) | 17p13.1 | 31,39,51–53 |
| NF1 | neurofibromin 1 (neurofibromatosis, von Recklinghausen disease, Watson disease) | 17q11.2 | 39,56 |
| TCF4 | transcription factor 4 | 18q21.1 | 39 |
| Gain and/or amplification | |||
| TNF | tumor necrosis factor (TNF superfamily, member 2) | 6p21.3 | 39 |
| PBX2 | pre-B-cell leukemia homeobox 2 | 6p21.32 | 39 |
| TRIB1 | tribbles homolog 1 (Drosophila) | 8q24.1 | 39,44 |
| MYC | v-myc myelocytomatosis viral oncogene homolog (avian) | 8q24 | 22,25,39,79 |
| NSMCE2 | non-SMC element 2, MMS21 homolog (S. cerevisiae) | 8q24.13 | 39 |
| JAK2 | Janus kinase 2 (a protein tyrosine kinase) | 9p24 | 39 |
| GAB2 | GRB2-associated binding protein 2 | 11q13.4-q13.5 | 45 |
| MLL | myeloid/lymphoid or mixed-lineage leukemia (trithorax homolog, Drosophila) | 11q23.3 | 39,41,80–83 |
| DDX6 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 6 | 11q23.3 | 39,41 |
| ETS1 | v-ets erythroblastosis virus E26 oncogene homolog 1 (avian) | 11q23.3 | 25,39,41 |
| FLI1 | Friend leukemia virus integration 1 | 11q24.1-q24.3 | 39,41 |
| FGF6 | fibroblast growth factor 6 | 12p13 | 39 |
| CCND2 | cyclin D2 | 12p13 | 39 |
| FLT3 | fms-related tyrosine kinase 3 | 13q12 | 39 |
| PAN3 | PAN3 polyA specific ribonuclease subunit homolog (S. cerevisiae) | 13q12.2 | 39 |
| CDX2 | caudal type homeobox 2 | 13q12.2 | 39,61 |
| ID1 | inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | 20q11 | 39 |
| BCL2L1 | BCL2-like 1 | 20q11.21 | 39 |
| APP | amyloid beta (A4) precursor protein (peptidase nexin-II, Alzheimer disease) | 21q21.2 | 40 |
| ERG | v-ets erythroblastosis virus E26 oncogene homolog (avian) | 21q22.3 | 39,40 |
| ETS2 | v-ets erythroblastosis virus E26 oncogene homolog 2 (avian) | 21q22.3 | 39,40 |
| BCR | breakpoint cluster region | 22q11.2 | 39 |
| CHEK2 | CHK2 checkpoint homolog (S. pombe) | 22q12 | 39 |
| NF2 | neurofibromin 2 (merlin) | 22q12.2 | 39 |
Although in general recurrent gains of chromosome material and high level amplification are less common than segmental losses (Table 1), they too have led to identification of gene alterations that seem to play a role in leukemogenesis of not only cytogenetically complex AML (Table 2), but also other cytogenetic subsets of AML. Following our discovery of a recurrent, mostly cryptic, overrepresentation of 21q material in cytogenetically complex AML patients with an apparent monosomy 21,24,57 shown by FISH not to involve amplification of the RUNX1 gene,24 we applied high-resolution bacterial artificial chromosome-based a-CGH and gene-expression profiling to search for overexpressed genes within the amplified 21q regions.40 This study disclosed two regions of 21q amplification: the first containing the most overexpressed gene, APP, which encodes a glycoprotein of unknown function previously implicated in Alzheimer’s disease, but not in AML, and the second region, in which the most overexpressed were the members of the ETS gene family, ERG and ETS2, encoding transcription factors critical for the control of proliferation, differentiation, and apoptosis.40 Importantly, high expression of ERG has also been found in a subset of patients with cytogenetically normal AML (CN-AML), and it was demonstrated to confer significantly worse clinical outcome compared with that of CN-AML patients with low ERG expression.58,59 The mechanism through which ERG is upregulated in CN-AML without 21q amplification is unknown.
The important role of ETS gene family overexpression in karyotypically complex AML is underscored by analyses of another recurrently amplified region – 11q23-24. One of the two distinct amplicon clusters identified in this region, located at 11q23.3-q24.1,39,41 encompasses the ETS1 and FLI1 genes, the latter of which was shown to be significantly up-regulated in cases with increased 11q23 copy numbers.41 The second amplified region at 11q23.3 contains the DDX6 and MLL genes that were found by Poppe et al.41 to be the most differentially expressed genes among samples with and without 11q23 copy gain or amplification. Overexpression of MLL was associated with increased expression of one of its physiologic downstream targets, HOXA9.41
The third amplicon identified in patients with gains of 11q is located more proximally, at 11q13.5. It was found in one study to coexist with the amplicon containing the MLL gene in the same abnormal chromosome 11 in 60% of the patients,45 although the frequency of such co-amplification was much smaller in another study.39 The amplicon at 11q13.5 contains the GAB2 gene, which encodes an adaptor molecule shown to play a role in cytokine receptor signaling in hematopoietic cells.45 GAB2 amplification and overexpression was also found in breast cancer, and it has been suggested that it contributes to mammary carcinogenesis by cooperating with activated HER2 through activating the SHP2-ERK pathway.60 Zatkova et al.45 demonstrated using real-time RT-PCR a significant transcriptional up-regulation of GAB2 in AML patients with concurrent amplification of MLL, and proposed that GAB2 is a novel target of 11q amplification in AML and MDS.
Gains of 8q, detected in 30% of complex karyotype patients, are caused to a large extent by the presence of trisomy 8. Hence, it is not surprising that gene-expression profiling revealed significantly higher average expression levels across many genes located in chromosome 8q.39 However, some complex karyotype cases exhibit high-level amplifications of 8q material, which are often manifested as double minutes containing material from 8q24.24,25,39,44 The genomic segment commonly amplified in these double minutes contains five known genes that include TRIB1 (also known as C8FW), NSMCE2 (FLJ32440), and MYC.39,44 The MYC gene was long considered to be main target of 8q24 amplification, and was fund overexpressed in a recent study.39 However, Storlazzi et al.44 assessed expression of MYC by the use of northern blot analysis and, against expectations, did not observe MYC overexpression in any of nine cases studied. Instead, the TRIB1 gene was overexpressed, but only in a subset of cases. Thus, another target or targets of high level amplification of 8q24 in AML with complex karyotype remains to be discovered.
Rücker et al.39 reported rare but recurring high level amplification of 13q12 that encompassed the FLT3, FLT1, PAN3, and CDX2 genes. The latter gene encodes an upstream regulator of several HOX family members whose up-regulation has been associated with leukemogenesis. A recent study from the same group61 revealed that aberrant expression of CDX2 is not restricted to cytogenetically complex AML or AML with a rare t(12;13)(p13;q12), in which it was originally discovered,62 but could be detected in 90% of 170 AML patients who belonged to several cytogenetic subsets. The highest expression levels of the CDX2 gene were found in patients with t(9;11)(p22;q23), followed by patients with a normal karyotype, and those with t(15;17), t(8;21), inv(16), a complex karyotype, and other chromosome aberrations.61 Aside from a few patients with a complex karyotype that contained 13q12 amplification, which correlated well with high CDX2 expression, the mechanism underlying aberrant expression of CDX2 in complex karyotype patients without 13q12 amplification or in patients with a non-complex karyotype has not been established.61 Likewise, it is currently unknown whether the level of CDX2 expression has an impact on the AML patients’ prognosis.
Recent studies discovered several gene alterations that are relatively frequent in specific cytogenetic groups, and whose presence or absence influences the patients’ prognosis.63–72 These include internal tandem duplication of the FLT3 gene (FLT3-ITD)63–66 and mutations in the NPM1 gene67,68 in patients with CN-AML, and mutations in the KIT gene69,70 in patients with core-binding factor AML with t(8;21) or inv(16)/t(16;16). Importantly, AML patients with complex karyotypes very rarely harbor FLT3-ITD,64–66 or mutations in the NPM167,68 and KIT70 genes. Also rare in these patients are mutations in the NRAS and KRAS genes,73 which were shown to sensitize AML patients to high doses of cytarabine.74 Consequently, the impact of the aforementioned mutations on response to treatment and clinical outcome of patients with a complex karyotype appears to be negligible.
CLINICAL OUTCOME
Prognosis of patients with a complex karyotype is in general very poor (Table 3), especially in patients treated with conventional induction and consolidation chemotherapy. Among older adults above the age of 60 years, who constitute the majority of patients with a complex karyotype,43 only 10% to 44% of those who harbor ≥3 abnormalities achieve a complete remission (CR).11,12,14,48 The CR rates for patients with ≥5 abnormalities are even lower, 7% to 26%.5,11,12 Resistant disease is the main reason for failure to achieve a CR, and it is more than twice as common as early death, which nevertheless affects 18% to 28% of elderly patients with a complex karyotype.5,11,12,14,48 Almost all patients who achieve a CR relapse. CR durations are short, with medians of 6 to 8 months, and the probabilities or remaining in CR at 3 years are between 0 and 11%, and at 5 years between 0 and 9% (Table 3).
Table 3.
Clinical outcome of AML patients with complex karyotypes
| Referencea | Definition | No. of patients | Age range (median) | CR rate (95% CI) | RD | ED/HD | Complete remission | Relapse rate | Survival | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median duration (mo) | Probabilityb | Median duration (mo) | Probabilityb | ||||||||
| OLDER ADULTS | |||||||||||
| Farag (2006)12 | ≥3 | 122 | 60–86 (68)c | 25% (17–32) | 50% | 25% | — | 3% (5y) | 90% | — | 2% (5y) |
| Fröhling (2006)11 | ≥3 | 61 | 61–84 (67)c | 10% (5–20) | 62% | 28% | — | 0% (2y) | 100% | 3.1 | 4% (3y) |
| Schoch (2001)48 | ≥3 | 45 | 60–81 (68) | 44% | 38% | 18% | 6 | 11% (3y) | 90% | 8 | 6% (3y) |
| van der Holt (2006)14 | ≥3 | 36 | 60–78 (67) | 39% (23–57) | — | — | 7 | — | 79% | 5 | 6% (3y) 3% (5y) |
| Grimwade (2001)5 | ≥5 | 145 | 44–91 (66)c | 26% | 56% | 19% | — | 9% (5y) | — | 3.7 | 2% (5y) |
| Farag (2006)12 | ≥5 | 94 | 60–86 (68)c | 23% | 49% | 28% | 5.9 | 0% (5y) | 95% | 2.6 | 0% (5y) |
| Fröhling (2006)11 | ≥5 | 44 | 61–84 (67)c | 7% (2–18) | 68% | 25% | — | 0% (2y) | 100% | 2.7 | 3% (3y) |
| YOUNGER ADULTS | |||||||||||
| Schoch (2001)48 | ≥3 | 45 | 19–59 (47) | 47% | 44% | 9% | 8 | 15% (3y) | 71% | 7 | 12% (3y) |
| Slovak (2000)3 | ≥3 with -5/5q-/-7/7q- | 33 | 17–54 (39)d | 37% (19–54) | — | — | — | — | — | — | 3% (2y) |
| Slovak (2000)3 | ≥3 without -5/5q-/-7/7q- | 30 | 17–54 (39)d | 50% (31–69) | — | — | — | — | — | — | 20% (2y) |
| ADULTS | |||||||||||
| Haferlach (2004)75 | ≥3 | 99 | 16–81 (58)c | 35% | 48% | 17% | 5 | — | — | 4 | — |
| Visani (2001)76 | ≥4 | 106 | 15–83c | 35% | — | — | — | — | — | — | — |
| Byrd (2002)6 | 3–4 | 36 | 15–86 (51)e | 47% | — | — | — | 12% (5y) | — | 10 | 8% (5y) |
| Byrd (2002)6 | ≥5 | 99 | 15–86 p (64)e | 30% | — | — | — | 3% (5y) | — | 4 | 2% (5y) |
| YOUNGER ADULTS AND CHILDREN | |||||||||||
| Grimwade (1998)2 | ≥5 | 95 | 0–55 (34)e | 67% | 20% | 13% | — | 32% (5y) | — | — | 21% (5y) |
| CHILDREN | |||||||||||
| Stark (2004)8 | ≥3f | 9 | 0–18 (6) | 78% | — | — | — | 67% (4y)g | — | — | 36% (4y) |
| Betts (2007)13 | ≥3 | 8 | 1–14 (3) | 75% | — | — | 11h | — | 83%h | 16h | 19% (3y)h |
Abbreviations: CR, complete remission; ED/HD, early death or hypoplastic death; —, not reported.
The references column lists the first author and year of publication (in parentheses), followed by the superscript reference number.
The number in parentheses indicates a time point (in years) at which the probability was determined.
Age range and median reported for all patients included in this study.
Age range and median reported for patients comprising the prognostically unfavorable category that also included patients with non-complex karyotype.
Median age pertains to patients with a complex karyotype, but the age range is for all patients included in this study.
Category defined as “miscellaneous ≥3 abnormalities”.
4-year event-free survival rate.
CR rates of karyotypically complex patients are slightly higher in studies restricted to younger adults,3,48 and in those that included adults both younger and older than 60 years.6,75,76 The highest rates of CR, 75 to 78%, were reported in children, although the outcome data on pediatric AML patients with a complex karyotype are limited.8,13 A comparatively high CR rate of 67% was also observed in the MRC study, in which the cytogenetically complex group included 20% of children aged ≤14 years, and the remaining 80% of adult patients were younger than 56 years.2 Nevertheless, the long term treatment outcome of younger patients with a complex karyotype, both children and adults, is still very poor (Table 3).
It has been shown that clinical outcomes of younger patients with a complex karyotype or those classified in an unfavorable cytogenetic prognostic category, which includes complex karyotypes, can be improved by allogeneic hematopoietic stem-cell transplantation (HSCT).77,78 Schmid et al.77 reported that for complex karyotype patients who underwent allogeneic HSCT following regimen of chemotherapy, reduced-intensity conditioning for HSCT, and prophylactic donor lymphocyte transfusion, the 2-year leukemia-free and overall survival rates were, respectively, 44% and 47%. In another study,78 for patients in unfavorable risk group who underwent matched unrelated donor HSCT in first CR, the 5-year disease-free and overall survival rates were, respectively, 27% and 30%, and for patients who were transplanted in second CR, the respective rates were 38% and 36%. However, treatment-related mortality was high.78
Interestingly, a recent phase I study evaluating decitabine, a DNA demethylating agent, alone or in combination with valproic acid, a histone deacetylase inhibitor, reported that all four AML patients who achieved a morphologic and cytogenetic CR had a complex karyotype with ≥5 abnormalities and all were older than 60 years.46 Although the CR durations were relatively short for three of the four patients, this encouraging observation warrants further study with a prospect of developing regimens that would be effective in older adults with a complex karyotype who are not eligible for allogeneic HSCT.
Thus far limited data suggest that prognosis of patients with a complex karyotype may also depend on the kind of detected abnormalities. Slovak et al.3 compared the outcome of patients whose complex karyotype contained abnormalities of chromosomes 5 and/or 7 (-5/5q- and/or -7/7q-) with that of patients whose complex karyotype did not include abnormalities of these chromosomes, and observed that the CR rate was higher in the latter group (50% vs. 37%) as was the probability of surviving 2 years (20% vs. 3%).3 Likewise, a significantly longer survival of patients with “atypical” complex karyotype compared with survival of patients with “typical” complex karyotype (median, 10.2 vs. 5.4 months) was found in another study.43 In this study,43 a complex karyotype with ≥3 abnormalities was classified as “typical” if it contained loss of material from at least one of the 3 chromosome arms, 5q, 7q or 17p, as well as at least one additional loss from either 18q21q22, 12p13, or 16q22q24 or gain of a chromosomal segment containing 11q23q25, 1p33p36, 8q22q24, or 21q11q22. Patients with “atypical” complex karyotype constituted a minority of cases, 12–18%, were slightly older and had more often hyperdiploid chromosome number than “typical” cases (54% vs. 19%). In a multivariable analysis, overall survival was associated with age and there was a trend for the association of survival with the type of complex karyotype (“typical” vs. “atypical”). Future studies confirming these results are necessary.
CONCLUSION
Recent studies using multicolor FISH, FISH with region-specific probes, a-CGH and molecular genetic techniques have begun unraveling the complexity of genomic alterations occurring in AML patients with multiple chromosome aberrations. It is hoped that these and future studies will not only lead to better understanding biologic basis of leukemic transformation in these patients but will also result in identification of cytogenetic and molecular subsets of patients with a complex karyotype that may respond differently to therapy because of the presence or absence of specific chromosome and/or gene alterations. Further characterization of such genetic alterations will likely allow development of targeted therapeutic approaches that will improve the currently very poor prognosis of AML patients with a complex karyotype.
Acknowledgments
The author gratefully acknowledges Clara D. Bloomfield, M.D., for her constant help and encouragement, and Amy S. Ruppert, M.S., for her assistance with data analysis.
Supported in part by National Cancer Institute, Bethesda, MD grants CA77658, CA101140, and CA16058, and The Coleman Leukemia Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mrózek K, Heinonen K, de la Chapelle A, et al. Clinical significance of cytogenetics in acute myeloid leukemia. Semin Oncol. 1997;24:17–31. [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: Analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 3.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 4.Mrózek K, Heinonen K, Bloomfield CD. Prognostic value of cytogenetic findings in adults with acute myeloid leukemia. Int J Hematol. 2000;72:261–271. [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 7.Schoch C, Kern W, Schnittger S, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): An analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 8.Stark B, Jeison M, Gabay LG, et al. Classical and molecular cytogenetic abnormalities and outcome of childhood acute myeloid leukaemia: Report from a referral centre in Israel. Br J Haematol. 2004;126:320–337. doi: 10.1111/j.1365-2141.2004.05038.x. [DOI] [PubMed] [Google Scholar]
- 9.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 10.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 11.Fröhling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: Results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–3288. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 12.Farag SS, Archer KJ, Mrózek K, et al. Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: Results from Cancer and Leukemia Group B 8461. Blood. 2006;108:63–73. doi: 10.1182/blood-2005-11-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betts DR, Ammann RA, Hirt A, et al. The prognostic significance of cytogenetic aberrations in childhood acute myeloid leukaemia. A study of the Swiss Paediatric Oncology Group (SPOG) Eur J Haematol. 2007;78:468–476. doi: 10.1111/j.1600-0609.2007.00854.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Holt B, Breems DA, Beverloo HB, et al. Various distinctive cytogenetic abnormalities in patients with acute myeloid leukaemia aged 60 years and older express adverse prognostic value: Results from a prospective clinical trial. Br J Haematol. 2007;136:96–105. doi: 10.1111/j.1365-2141.2006.06403.x. [DOI] [PubMed] [Google Scholar]
- 15.Schlenk RF, Benner A, Krauter J, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: A survey of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2004;22:3741–3750. doi: 10.1200/JCO.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Marcucci G, Mrózek K, Ruppert AS, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–5717. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 17.Frankfurt O, Tallman MS. Strategies for the treatment of acute promyelocytic leukemia. J Natl Compr Canc Netw. 2006;4:37–50. doi: 10.6004/jnccn.2006.0005. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins JM, Bain B, Mehta AB, et al. Complex hypodiploidy in acute myeloid leukaemia: A United Kingdom Cancer Cytogenetics Group study. Leuk Res. 1995;19:905–913. doi: 10.1016/0145-2126(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohr B, Bornhäuser M, Thiede C, et al. Comparison of spectral karyotyping and conventional cytogenetics in 39 patients with acute myeloid leukemia and myelodysplastic syndrome. Leukemia. 2000;14:1031–1038. doi: 10.1038/sj.leu.2401775. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall C, Nordenskjöld M, Porwit A, et al. Molecular cytogenetic characterization of acute myeloid leukemia and myelodysplastic syndromes with multiple chromosome rearrangements. Haematologica. 2001;86:1158–1164. [PubMed] [Google Scholar]
- 21.Kerndrup GB, Kjeldsen E. Acute leukemia cytogenetics: An evaluation of combining G-band karyotyping with multi-color spectral karyotyping. Cancer Genet Cytogenet. 2001;124:7–11. doi: 10.1016/s0165-4608(99)00223-x. [DOI] [PubMed] [Google Scholar]
- 22.Hilgenfeld E, Padilla-Nash H, McNeil N, et al. Spectral karyotyping and fluorescence in situ hybridization detect novel chromosomal aberrations, a recurring involvement of chromosome 21 and amplification of the MYC oncogene in acute myeloid leukaemia M2. Br J Haematol. 2001;113:305–317. doi: 10.1046/j.1365-2141.2001.02723.x. [DOI] [PubMed] [Google Scholar]
- 23.Odero MD, Carlson KM, Calasanz MJ, et al. Further characterization of complex chromosomal rearrangements in myeloid malignancies: Spectral karyotyping adds precision in defining abnormalities associated with poor prognosis. Leukemia. 2001;15:1133–1136. doi: 10.1038/sj.leu.2402158. [DOI] [PubMed] [Google Scholar]
- 24.Mrózek K, Heinonen K, Theil KS, et al. Spectral karyotyping in patients with acute myeloid leukemia and a complex karyotype shows hidden aberrations, including recurrent overrepresentation of 21q, 11q, and 22q. Genes Chromosomes Cancer. 2002;34:137–153. doi: 10.1002/gcc.10027. [DOI] [PubMed] [Google Scholar]
- 25.Sait SNJ, Qadir MU, Conroy JM, et al. Double minute chromosomes in acute myeloid leukemia and myelodysplastic syndrome: Identification of new amplification regions by fluorescence in situ hybridization and spectral karyotyping. Genes Chromosomes Cancer. 2002;34:42–47. doi: 10.1002/gcc.10038. [DOI] [PubMed] [Google Scholar]
- 26.Cigudosa JC, Odero MD, Calasanz MJ, et al. De novo erythroleukemia chromosome features include multiple rearrangements, with special involvement of chromosomes 11 and 19. Genes Chromosomes Cancer. 2003;36:406–412. doi: 10.1002/gcc.10180. [DOI] [PubMed] [Google Scholar]
- 27.Mrózek K, Tanner SM, Heinonen K, et al. Molecular cytogenetic characterization of the KG-1 and KG-1a acute myeloid leukemia cell lines by use of spectral karyotyping and fluorescence in situ hybridization. Genes Chromosomes Cancer. 2003;38:249–252. doi: 10.1002/gcc.10274. [DOI] [PubMed] [Google Scholar]
- 28.Tchinda J, Volpert S, McNeil N, et al. Multicolor karyotyping in acute myeloid leukemia. Leuk Lymphoma. 2003;44:1843–1853. doi: 10.1080/10428190310001603605. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez S, Cigudosa JC. Gains, losses and complex karyotypes in myeloid disorders: A light at the end of the tunnel. Hematol Oncol. 2005;23:18–25. doi: 10.1002/hon.744. [DOI] [PubMed] [Google Scholar]
- 30.Van Limbergen H, Poppe B, Michaux L, et al. Identification of cytogenetic subclasses and recurring chromosomal aberrations in AML and MDS with complex karyotypes using M-FISH. Genes Chromosomes Cancer. 2002;33:60–72. doi: 10.1002/gcc.1212. [DOI] [PubMed] [Google Scholar]
- 31.Schoch C, Haferlach T, Bursch S, et al. Loss of genetic material is more common than gain in acute myeloid leukemia with complex aberrant karyotype: A detailed analysis of 125 cases using conventional chromosome analysis and fluorescence in situ hybridization including 24-color FISH. Genes Chromosomes Cancer. 2002;35:20–29. doi: 10.1002/gcc.10088. [DOI] [PubMed] [Google Scholar]
- 32.Andersen MK, Christiansen DH, Pedersen-Bjergaard J. Centromeric breakage and highly rearranged chromosome derivatives associated with mutations of TP53 are common in therapy-related MDS and AML after therapy with alkylating agents: An M-FISH study. Genes Chromosomes Cancer. 2005;42:358–371. doi: 10.1002/gcc.20145. [DOI] [PubMed] [Google Scholar]
- 33.Shali W, Hélias C, Fohrer C, et al. Cytogenetic studies of a series of 43 consecutive secondary myelodysplastic syndromes/acute myeloid leukemias: Conventional cytogenetics, FISH, and multiplex FISH. Cancer Genet Cytogenet. 2006;168:133–145. doi: 10.1016/j.cancergencyto.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Babicka L, Ransdorfova S, Brezinova J, et al. Analysis of complex chromosomal rearrangements in adult patients with MDS and AML by multicolor FISH. Leuk Res. 2007;31:39–47. doi: 10.1016/j.leukres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira MR, Micci F, Dietrich CU, et al. Cross-species color banding characterization of chromosomal rearrangements in leukemias with incomplete G-band karyotypes. Genes Chromosomes Cancer. 1999;26:13–19. [PubMed] [Google Scholar]
- 36.Harrison CJ, Yang F, Butler T, et al. Cross-species color banding in ten cases of myeloid malignancies with complex karyotypes. Genes Chromosomes Cancer. 2001;30:15–24. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1061>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Verdorfer I, Brecevic L, Saul W, et al. Comparative genomic hybridization-aided unraveling of complex karyotypes in human hematopoietic neoplasias. Cancer Genet Cytogenet. 2001;124:1–6. doi: 10.1016/s0165-4608(00)00287-9. [DOI] [PubMed] [Google Scholar]
- 38.Casas S, Aventín A, Fuentes F, et al. Genetic diagnosis by comparative genomic hybridization in adult de novo acute myelocytic leukemia. Cancer Genet Cytogenet. 2004;153:16–25. doi: 10.1016/j.cancergencyto.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Rücker FG, Bullinger L, Schwaenen C, et al. Disclosure of candidate genes in acute myeloid leukemia with complex karyotypes using microarray-based molecular characterization. J Clin Oncol. 2006;24:3887–3894. doi: 10.1200/JCO.2005.04.5450. [DOI] [PubMed] [Google Scholar]
- 40.Baldus CD, Liyanarachchi S, Mrózek K, et al. Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: Amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci USA. 2004;101:3915–3920. doi: 10.1073/pnas.0400272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poppe B, Vandesompele J, Schoch C, et al. Expression analyses identify MLL as a prominent target of 11q23 amplification and support an etiologic role for MLL gain of function in myeloid malignancies. Blood. 2004;103:229–235. doi: 10.1182/blood-2003-06-2163. [DOI] [PubMed] [Google Scholar]
- 42.Lindvall C, Furge K, Björkholm M, et al. Combined genetic and transcriptional profiling of acute myeloid leukemia with normal and complex karyotypes. Haematologica. 2004;89:1072–1081. [PubMed] [Google Scholar]
- 43.Schoch C, Kern W, Kohlmann A, et al. Acute myeloid leukemia with a complex aberrant karyotype is a distinct biological entity characterized by genomic imbalances and a specific gene expression profile. Genes Chromosomes Cancer. 2005;43:227–238. doi: 10.1002/gcc.20193. [DOI] [PubMed] [Google Scholar]
- 44.Storlazzi CT, Fioretos T, Surace C, et al. MYC-containing double minutes in hematologic malignancies: Evidence in favor of the episome model and exclusion of MYC as the target gene. Hum Mol Genet. 2006;15:933–942. doi: 10.1093/hmg/ddl010. [DOI] [PubMed] [Google Scholar]
- 45.Zatkova A, Schoch C, Speleman F, et al. GAB2 is a novel target of 11q amplification in AML/MDS. Genes Chromosomes Cancer. 2006;45:798–807. doi: 10.1002/gcc.20344. [DOI] [PubMed] [Google Scholar]
- 46.Blum W, Klisovic RB, Hackanson B, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 47.de Botton S, Chevret S, Sanz M, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: Results of APL 93 trial. Br J Haematol. 2000;111:801–806. doi: 10.1046/j.1365-2141.2000.02442.x. [DOI] [PubMed] [Google Scholar]
- 48.Schoch C, Haferlach T, Haase D, et al. Patients with de novo acute myeloid leukaemia and complex karyotype aberrations show a poor prognosis despite intensive treatment: A study of 90 patients. Br J Haematol. 2001;112:118–126. doi: 10.1046/j.1365-2141.2001.02511.x. [DOI] [PubMed] [Google Scholar]
- 49.Herry A, Douet-Guilbert N, Morel F, et al. Evaluation of chromosome 5 aberrations in complex karyotypes of patients with myeloid disorders reveals their contribution to dicentric and tricentric chromosomes, resulting in the loss of critical 5q regions. Cancer Genet Cytogenet. 2007;175:125–131. doi: 10.1016/j.cancergencyto.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 50.MacKinnon RN, Zordan A, Campbell LJ. Recurrent duplication of Xq27~qter in hematological malignancies revealed by multicolor fluorescence in situ hybridization and multicolor banding. Cancer Genet Cytogenet. 2005;161:125–129. doi: 10.1016/j.cancergencyto.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Fenaux P, Jonveaux P, Quiquandon I, et al. P53 gene mutations in acute myeloid leukemia with 17p monosomy. Blood. 1991;78:1652–1657. [PubMed] [Google Scholar]
- 52.Laï J-L, Preudhomme C, Zandecki M, et al. Myelodysplastic syndromes and acute myeloid leukemia with 17p deletion. An entity characterized by specific dysgranulopoiesis and a high incidence of P53 mutations. Leukemia. 1995;9:370–381. [PubMed] [Google Scholar]
- 53.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19:1405–1413. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- 54.Kirsch DG, Kastan MB. Tumor-suppressor p53: Implications for tumor development and prognosis. J Clin Oncol. 1998;16:3158–3168. doi: 10.1200/JCO.1998.16.9.3158. [DOI] [PubMed] [Google Scholar]
- 55.Liu TX, Becker MW, Jelinek J, et al. Chromosome 5q deletion and epigenetic suppression of the gene encoding α-catenin (CTNNA1) in myeloid cell transformation. Nat Med. 2007;13:78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- 56.Suela J, Largo C, Ferreira B, et al. Neurofibromatosis 1, and not TP53, seems to be the main target of chromosome 17 deletions in de novo acute myeloid leukemia. J Clin Oncol. 2007;25:1151–1152. doi: 10.1200/JCO.2006.09.3013. [DOI] [PubMed] [Google Scholar]
- 57.Mrózek K, Theil KS, Heinonen K, et al. Spectral karyotyping (SKY) in acute myeloid leukemia (AML): A study of 20 cases. Cytogenet Cell Genet. 1999;85:40. [Google Scholar]
- 58.Marcucci G, Baldus CD, Ruppert AS, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:9234–9242. doi: 10.1200/JCO.2005.03.6137. [DOI] [PubMed] [Google Scholar]
- 59.Marcucci G, Maharry K, Whitman SP, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2007;25:3337–3343. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 60.Bentires-Alj M, Gil SG, Chan R, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–121. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- 61.Scholl C, Bansal D, Döhner K, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117:1037–1048. doi: 10.1172/JCI30182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chase A, Reiter A, Burci L, et al. Fusion of ETV6 to the caudal-related homeobox gene CDX2 in acute myeloid leukemia with the t(12;13)(p13;q12) Blood. 1999;93:1025–1031. [PubMed] [Google Scholar]
- 63.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 64.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 65.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 66.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 67.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 68.Thiede C, Koch S, Creutzig E, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006;107:4011–4020. doi: 10.1182/blood-2005-08-3167. [DOI] [PubMed] [Google Scholar]
- 69.Paschka P, Marcucci G, Ruppert AS, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): A Cancer and Leukemia Group B study. J Clin Oncol. 2006;24:3904–3911. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 70.Schnittger S, Kohl TM, Haferlach T, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–1799. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 71.Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mrózek K, Bloomfield CD. Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2006:169–177. doi: 10.1182/asheducation-2006.1.169. [DOI] [PubMed] [Google Scholar]
- 73.Bowen DT, Frew ME, Hills R, et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood. 2005;106:2113–2119. doi: 10.1182/blood-2005-03-0867. [DOI] [PubMed] [Google Scholar]
- 74.Neubauer A, Maharry K, Mrózek K, et al. Patients with acute myeloid leukemia and RAS mutations benefit most from postremission treatment with high-dose cytarabine: A Cancer and Leukemia Group B study. J Clin Oncol. doi: 10.1200/JCO.2007.14.0418. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haferlach T, Kern W, Schoch C, et al. A new prognostic score for patients with acute myeloid leukemia based on cytogenetics and early blast clearance in trials of the German AML Cooperative Group. Haematologica. 2004;89:408–418. [PubMed] [Google Scholar]
- 76.Visani G, Bernasconi P, Boni M, et al. The prognostic value of cytogenetics is reinforced by the kind of induction/consolidation therapy in influencing the outcome of acute myeloid leukemia - Analysis of 848 patients. Leukemia. 2001;15:903–909. doi: 10.1038/sj.leu.2402142. [DOI] [PubMed] [Google Scholar]
- 77.Schmid C, Schleuning M, Ledderose G, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5675–5687. doi: 10.1200/JCO.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 78.Tallman MS, Dewald GW, Gandham S, et al. Impact of cytogenetics on outcome of matched unrelated donor hematopoietic stem cell transplantation for acute myeloid leukemia in first or second complete remission. Blood. 2007;110:409–417. doi: 10.1182/blood-2006-10-043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alitalo K, Saksela K, Winqvist R, et al. Acute myelogenous leukemia with c-myc amplification and double minute chromosomes. Lancet. 1985;2:1035–1039. doi: 10.1016/s0140-6736(85)90907-9. [DOI] [PubMed] [Google Scholar]
- 80.Avet-Loiseau H, Godon C, Li J-Y, et al. Amplification of the 11q23 region in acute myeloid leukemia. Genes Chromosomes Cancer. 1999;26:166–170. [PubMed] [Google Scholar]
- 81.Streubel B, Valent P, Jäger U, et al. Amplification of the MLL gene on double minutes, a homogeneously staining region, and ring chromosomes in five patients with acute myeloid leukemia or myelodysplastic syndrome. Genes Chromosomes Cancer. 2000;27:380–386. [PubMed] [Google Scholar]
- 82.Cuthbert G, Thompson K, McCullough S, et al. MLL amplification in acute leukaemia: A United Kingdom Cancer Cytogenetics Group (UKCCG) study. Leukemia. 2000;14:1885–1891. doi: 10.1038/sj.leu.2401919. [DOI] [PubMed] [Google Scholar]
- 83.Michaux L, Wlodarska I, Stul M, et al. MLL amplification in myeloid leukemias: A study of 14 cases with multiple copies of 11q23. Genes Chromosomes Cancer. 2000;29:40–47. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1003>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]



