Abstract
Vascular disease was once considered the principal cause of aging-related dementia. More recently, however, research emphasis has shifted to studies of progressive neurodegenerative disease processes such as those giving rise to neuritic plaques, neurofibrillary tangles, and Lewy bodies. While these studies have led to critical insights and potential therapeutic strategies, interest in the role of systemic and cerebrovascular disease mechanisms waned and has received relatively less attention and research support. Recent studies suggest that vascular disease mechanisms play an important role in the risk for aging-related cognitive decline and disorders. Vascular disease frequently coexists with cognitive decline in aging individuals, shares many risk factors with dementias considered to be of the “Alzheimer-type,” and is observed more frequently than expected in postmortem material from individuals manifesting “specific” disease stigmata such as abundant plaques and tangles. Considerable difficulties have emerged in attempting to classify dementias as being related to vascular vs. neurodegenerative causes, and several systems of criteria have been used. Despite multiple attempts, a lack of consensus remains regarding the optimal means of incorporating vascular disease into clinical diagnostic, neurocognitive, or neuropathologic classification schemes for dementias.
We propose here an integrative, rather than a strictly taxonomic approach to the study and elucidation of how vascular disease mechanisms contribute to the development of dementias. We argue that, instead of discriminating between, e.g., “Alzheimer’s disease,” “vascular dementia,” and other diseases, there is a greater need to focus clinical and research efforts on elucidating specific pathophysiologic mechanisms that contribute to dementia phenotypes and neuropathologic outcomes. We outline a multi-tiered strategy, beginning with clinical and public health interventions that can be implemented immediately; enhancements to ongoing longitudinal studies to increase their informative value; and new initiatives to capitalize on recent advances in systems biology and network medicine. This strategy will require funding from multiple public and private sources to support collaborative and interdisciplinary research efforts in order to take full advantage of these opportunities and realize their societal benefits.
1. INTRODUCTION
Improvements in public health and medical care during the 20th century led to substantial increases in life expectancy [1]. As a result, the principal causes of death have undergone a substantial shift from predominantly infectious diseases to cardiovascular disease, cancers, and increasingly, progressive neurodegenerative dementias [2]. With this increased longevity in industrialized societies, it has become clear that while some limited decline in certain cognitive functions with the aging process is almost universal, as many as half of all individuals living into their 80s and 90s undergo more severe cognitive and functional deterioration warranting a clinical syndrome diagnosis of dementia.
As the post World War II “baby boom” generation now enters the vulnerable ages for dementia, increasing attention has been drawn to the medical and economic impact of dementias[3]. During this time, advances in scientific methodology and technological capabilities (brought about to a considerable extent by members of this same birth cohort) have greatly enhanced our ability to characterize the clinical and neuropathologic features of aging-associated cognitive disorders. In particular, the inventions of laboratory-based immunochemistry, molecular biology, and advanced microscopy as well as clinic-based magnetic resonance, nuclear medicine and digital tomographic neuroimaging imaging have revolutionized our understanding and diagnosis of brain diseases.
The principal disease processes leading to dementia in older adults can be broadly thought of as falling into 2 groups: 1) neurodegenerative processes occurring within brain tissue itself; and 2) vascular disease processes resulting in brain injury and dysfunction. The past half-century has seen dramatic growth in our understanding of neurodegenerative disease processes, most notably the processes underlying the formation of amyloid plaques and neurofibrillary tangles eponymously associated with Alzheimer. On the other hand, our view of the role of vascular disease processes in dementias has undergone several swings in emphasis over this interval, during which vascular mechanisms became less intensively studied. This shift in emphasis is evident even from the titles of some review articles on “vascular dementia” alluding to this condition as an “enigma” [4] and asking if we are on a “dead end road” [5].
In this paper, we will present a strategic overview of this extensive literature, highlighting some of the key findings that changed prevailing views regarding the role of vascular mechanisms in dementias. We will also consider the sources of research funding for studies conducted to date, and identify areas where gaps in funding priorities currently exist. We will then propose an agenda for future research in this area, including recommended modifications to the current paradigms by which vascular contributions to dementia syndromes are conceptualized.
As the historical aspects and evolution of concepts leading up to the present analysis have been well covered in several recent review articles and book chapters [6-9], we will only briefly summarize key points and will not recapitulate this material here except where necessary to illustrate salient concepts.
2. HISTORICAL CONTEXT
The notion that vascular factors mediate age-related cognitive decline was the prevailing view of “senile dementia” until the 1960s [4,10]. Thus, the terms “arteriosclerotic dementia” and its lay counterpart, “hardening of the arteries in the brain” was in common use. Although this concept is often associated with the work of Binswanger first published in 1894 [6,11], many of the pathologic features can be traced back to the work of the French neurologist, Max Durand-Fardel in the 1840s (cited in ref. [12]). Until the last part of the 20th century, the occurrence of neuritic plaques and neurofibrillary tangles, the hallmark lesions described by Alzheimer in his seminal paper of 1907 [13], were considered to be rare findings in patients with dementia, and more closely associated with early-onset or “presenile” dementias.
The emphasis in dementia research shifted dramatically starting in the 1970s and 80s, based on several sets of observations. These include: 1) the discovery that plaques and tangles were more common in postmortem material from individuals with dementing illnesses than had previously been thought, and more frequently than in those dying without dementia [14,15]; 2) the findings of neurodegeneration and associated deficits in the cholinergic system in the brains of patients with dementia [16], coupled soon thereafter with the discovery that anticholinesterase drugs had some therapeutic benefit in dementias[17] (see also subsequent meta-analysis in ref. [18]); and 3) the discovery of the principal molecular composition of plaques (i.e., abnormally-folded amyloid peptides[19]) and tangles (i.e., hyperphosphorylated tau proteins[20,21]). With the recognition that cholinergic-enhancing drugs have at best a limited impact on the underlying neurodegenerative processes[22], and the emergent pre-eminence of the amyloid cascade hypothesis[23], treatment development efforts have been intensely focused on interrupting the mechanisms of amyloid plaque formation. Several agents designed to affect amyloid production or clearance have been and are being tested in clinical trials[24]; however, none of these trials has yet progressed to the stage of applying for approval by FDA or other regulatory agencies.
3. CURRENT STATE OF KNOWLEDGE
3.1 What is “Vascular Dementia”?
Vascular dementia is often said to be the second most common form of dementia after Alzheimer disease[4,25]; some authors have even suggested it is the most common form[26]. However, such statements, and the data on which they are based, are predicated on having a commonly-accepted definition of this condition. In the case of dementia attributable to vascular causes, this is far from the case. In fact, considerable variability exists in the literature with regard to the estimated prevalence of “vascular dementia,” with estimates as low as 0.03% to as high as 98% (see Jellinger’s review in ref. [4]). Most of the estimates range between 11% and 18% for ‘“vascular dementia,” and between 22% and 34% for “mixed dementia,” which typically reflects features of both Alzheimer disease and vascular disease. Estimates are somewhat higher in series from Japan than in those from the United States and Europe.
This variability in the estimated frequency of “vascular dementia” is due to at least 3 major factors: 1) there is no standard consensus definition of what constitutes “vascular dementia,” 2) most studies have relied on clinical or autopsy samples, which are likely to be biased with regard to population estimates; and 3) an implicit assumption is made that “vascular dementia” can be defined as a unique entity, existing apart from other conditions or diseases.
“Vascular dementia” is commonly understood as a condition in which cognitive impairment results from deleterious effects of vascular disease processes on brain structure and function. However, the kinds of vascular disease processes that can give rise to cognitive decline are highly varied (Table 2). These can include: 1) cerebrovascular diseases involving either larger or smaller cerebral arteries, often (but not always) resulting in clinical stroke syndromes; and 2) cerebrovascular injury resulting from vascular events occurring outside the cerebral vasculature, such as emboli arising from the heart or large vessels proximal to the intracerebral circulation.
Table 2. Vascular diseases and conditions associated with cognitive impairment and decline*.
| Post-stroke dementia (heterogeneous pathobiology) |
| Vascular dementias |
| Cortical infarct dementia (multi-infarct dementia) |
| Subcortical ischemic vascular dementia (Binswanger’s disease and lacunar state) |
| Strategic infarct dementia |
| Hypoperfusion dementia |
| Dementia caused by intracerebral hemorrhage |
| Dementia as a result of specific arteriopathies |
| Mixed dementia (vascular dementia and Alzheimer’s disease) |
| Vascular mild cognitive impairment (does not meet formal criteria for dementia) |
From: Bradley, principles and practices of neurology Table 70-15. Reprinted from the Lancet Neurology Vol 2, O’Brien et al. Vascular cognitive impairment. pgs 89-98, 2003, with permission from Elsevier.
In contrast to Alzheimer’s disease, which has come to be defined as the progressive dementing syndrome associated with the accumulation of neuritic plaques and neurofibrillary tangles, it has been more difficult to define coherent syndromes of vascular disease processes leading to progressive cognitive decline and dementia. As a result, considerable debate has occurred about not only how to define “vascular dementia,” but even about what to call it. Thus, numerous terms have been used to describe dementia or cognitive impairment attributed to vascular disease involving the central nervous system (see Table 1 in ref. [4]).
3.2 Clinical Diagnostic Criteria for Dementias Attributed to Vascular Diseases: Different Criteria Sets in Use and Limitations of Existing Systems
One of the first attempts to develop standard diagnostic criteria for vascular dementia was included in the third edition of American Psychiatric Association’s Diagnostic and Statistical Manual for psychiatric disorders (DSM-III)[27], published in 1980. Three additional sets of criteria were published in the early 1990s. One of these was developed by the California Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC)[28] and published in 1992; another was part of the International Classification of Diseases version 10 (ICD-10)[29], and the third, the result of a cooperative effort between the National Institute of Neurological Disorders and Stroke (NINDS) and the European Association Internationale pour la Recherche et l’Enseignement en Neurosciences (AIREN), published in 1993[30]. Shortly thereafter, the DSM-IV[31] was published, with a revised set of criteria from previous versions1.
These criteria are summarized in Table 1. They have been compared directly in several studies (see refs. [33] and[34] for a comprehensive review of the findings). In general, although acceptable interrater reliability could be achieved among clinicians at a single institution for locally-developed criteria[35], agreement between criteria is relatively low, both with each other and with clinical diagnoses of vascular dementia[36,37]. This is not entirely unexpected, given the substantial differences between criteria sets in terms of required elements, resulting in different subgroups of patients being captured. For example, the only element that is common to all these criteria sets is evidence of cerebrovascular disease (CVD), although the definition of what constitutes CVD varies. Only DSM-III and NINDS-AIREN require a stepwise deterioration in function; only DSM-III and ICD-10 require a “patchy” distribution of cognitive deficits; and the criteria vary in their requirement of a temporal relationship between cerebrovascular events and cognitive decline. In addition, clinical judgment is required by many of the criteria, e.g., of an “etiologic” relationship between cerebrovascular events and cognitive impairment.
Table 1. Comparison of Diagnostic Criteria Used for Vascular Dementia Syndromes.
Note: Numbers in table refer to footnotes listed below
| DSM-III 1980 |
ADDTC (“California”) 1992 |
ICD-10 1992 |
NINDS- AIREN 1993 |
DSM-IV 1994 |
|
|---|---|---|---|---|---|
| Stepwise deterioration | + | − | − | 1 | − |
| “Patchy” (unequal) distribution of cognitive deficits |
+ | − | + | − | − |
| Focal neurological signs | + | 2 | + | + | 4 |
| Focal neurological symptoms | + | − | − | − | 4 |
| 2 Ischemic strokes | − | 2 | − | 3 | − |
| Evidence of significant CVD | + | 2 | + | 3 | + |
| Etiologic relation to the disturbance | + | − | + | + | + |
| Temporal relationship between stroke and dementia |
− | 2 | − | 1 | − |
+ indicates obligatory; −, not needed
1: either onset of dementia within 3 mo after a recognized stroke and/or abrupt deterioration in cognitive functions, or fluctuating, stepwise progression of cognitive deficits
2: evidence of ≥2 ischemic strokes by history, or neurological signs, and/or neuroimaging studies, or occurrence of a single stroke with a clearly documented temporal relationship to the onset of dementia and evidence of >=1 infarct outside the cerebellum by CT or T1-weighted MRI
3: either multiple strokes or a single strategically placed infarct
4: focal neurological signs and symptoms or laboratory evidence indicative of CVD and judged to be etiologically related to the disturbance.
Reprinted from Stroke, Vol 31, Pohjasvaara et al. Comparison of Different Clinical Criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the Diagnosis of Vascular Dementia. pgs 2952-7, 2000, with permission from Wolters Kluwer Health.
3.2.1 Biases in Definitions of “Dementia” and “Cognitive Impairment”
One important difficulty with the study of vascular mechanisms in dementia is that until relatively recently, the definitions of “dementia” included memory dysfunction as an obligatory feature. Dementias associated with vascular disease processes do not necessarily present with memory dysfunction, often manifesting initially by alterations in behaviors and executive functions [38]. Thus, the definitions of “dementia” and “mild cognitive impairment” needed to be broadened in order to encompass syndromes in which memory dysfunction is not prominent, but other cognitive domains are substantively impaired. Subsyndromal distinctions within this broader category (“mild cognitive impairment”) can thus be made, depending on the specific cognitive domain(s) affected. Importantly, assessment batteries used in neurocognitive evaluation of dementia need to be modified in order to include more detailed assessment of domains of executive function, such as attention, working memory, planning, and set-shifting, as well as speed of processing. Standard neurocognitive testing protocols of varying lengths ranging from 5 – 60 minutes have been proposed [39].
The notion of mild cognitive impairment (MCI) involving domains exclusive of memory was also recognized by subdividing MCI into amnestic and non-amnestic subtypes [40]. Recent studies suggest that vascular disease processes are important contributors to non-amnestic MCI phenotypes[41,42]. In addition, the DSM-V Work Group has proposed criteria for cognitive impairment that will not require memory dysfunction[32]. Nevertheless, the bias towards memory impairment as an “entry criterion” for vascular dementia or cognitive impairment in prior diagnostic schemes must still be taken into account in the evaluation of studies conducted with those criteria.
Finally, there are sociocultural and nosologic trends within medicine arguing for a shift in terminology and classifications of dementias. As the technical term “dementia” has gained wide public usage, it has taken on pejorative and potentially stigmatizing connotations[43]. This has led to current moves to eliminate the term from the medical lexicon. Some authors have adopted the term “Vascular Cognitive Impairment”[7,44]; however, this is variously used to refer either to frank dementia syndromes or all cognitive impairment attributed to vascular causes[45]. While still controversial, the DSM-V Work Group has adopted the term “major neurocognitive disorder” for dementia and “minor neurocognitive disorder” for MCI, which are then subclassified according to suspected etiology (e.g., AD, vascular neurocognitive disorder, Lewy body disease, “mixed”). Increasingly, the term “Vascular Cognitive Impairment” is being used, with the notion that this encompasses both dementia and MCI due to vascular brain injury [7,39,45-48]. Such efforts are critical to improve specificity and acceptable use of diagnosis as knowledge evolves but do present challenges for comparative interpretation.
3.3 Neuroimaging Findings: Relationship to Vascular and Brain Lesions
As neuroimaging methodologies became more widely available and were applied to dementia research, patients with dementia and cerebrovascular disease were often found to have areas of altered appearance in white matter areas. These were initially observed on X-ray computed tomography (CT) as areas of hypodensity, leading to the introduction of the term “leukoaraiosis” by Hachinski and colleagues[49]. As the use of magnetic resonance imaging (MRI) became more widespread, areas of increased signal intensity on T2-weighted scans, now termed “white matter hyperintensities” (WMH) were observed in similar patterns. Subsequently, these areas were more clearly shown in some cases using sequences such as fluid-attenuated inversion recovery (FLAIR; to attenuate the CSF signal and thus better delineate periventricular hyperintensities) and proton density imaging.
These white matter alterations are typically considered in terms of their location, i.e. a distinction is made between those observed in periventricular regions and those seen in other subcortical regions[50]. The number and size of these lesions were found to increase with age[51] and were more prevalent in those with dementia than those without; among those with dementia, WMH were more frequent in those diagnosed with vascular than Alzheimer-type dementias[52].
Although WMH show a strong association with cerebrovascular disease [53], they are also increased in mood disorders such as bipolar disorder [54], and in migraine [55]. Whether these indicate cerebrovascular disease and if so, what type of cerebrovascular disease and injury, are not known, as WMH due to different underlying mechanisms may be indistinguishable using standard neuroimaging approaches. Such findings may complicate the interpretation of WMH observed in patients with co-occurring conditions.
3.3.1 Lack of Standardization of Neuropathologic Criteria for Vascular Disease in Brain
A major handicap to diagnosis and research on vascular dementia is the lack of a widely-accepted, standardized, neuropathological approach to the identification, categorization, localization, and in particular, quantitation of cerebrovascular disease and vascular brain injury. Common approaches used by major research groups have consisted of characterizing CVD according to the presence or absence of large or small vessel infarcts and lacunes evident on gross inspection of coronal sections, the presence and severity of cerebral amyloid angiopathy, and the presence and severity of hippocampal sclerosis. Less consistently applied have been notations of atheromatous disease in the circle of Willis, microinfarcts, micorhemorrhages, perivascular expansions, lipohyalinosis, microaneurysms, laminar necrosis or granular atrophy. Indeed, the National Alzheimer’s Coordinating Center Neuropathology Diagnosis Guidebook [56] includes in its assessment items “meant to indicate the presence of vascular pathology, but not the absolute burden, volume, or severity of change.” (p. 5)
Of particular importance for current concepts of vascular dementia is the absence of any standardized neuropathological approach for classifying and measuring deep white matter and periventricular disease and injury. Furthermore, there have been no widely used, systematic classification schema that incorporate lesion location. Clearly, a small volume of vascular infarction in the medial thalami will likely have very different and potentially more significant effects on cognitive function than similar or even much larger injuries in posterior white matter.
Arterial CVD encompasses a broad range of pathological processes (e.g., atheromas, hyalinosis, arteritides, aneurysm) that occur in large extracranial and intracranial blood vessels, small arteries and arterioles, and microvasculature including capillaries. Cerebral thrombosis, embolism, and hemorrhage at any of these levels of the cerebral arterial vasculature are the principal proximal causes of ischemic brain injury. In addition, an under-investigated pathology in the realm of aging that is unique to the brain is breakdown of the blood brain barrier. This may be particularly relevant to the promotion of neurodegeneration independently, additively, or synergistically with AD and other common neurodegenerative disease processes.
3.4 High Rate of Co-occurrence of Alzheimer-Like and Vascular Disease-Related Neuropathologic Features on Postmortem Examination of Individuals with Dementia
Cerebrovascular disease is common in the population; its risk increases with advancing age as does that for AD and other neurodegenerative dementias. Neuropathologic evidence of chronic vascular disease can take various forms, reflecting the diverse ways in which the brain can be affected by vascular disease processes. These include large vessel infarcts with regional or lobar encephalomalacia, small vessel macroscopic cystic/lacunar infarcts (typically < 1cm in white matter or deep cerebral nuclei), microinfarcts in gray or white matter (observable only microscopically), leukoencephalopathy (microscopically-observable rarefaction of white matter), cribriform changes (perivascular space with ischemic gliosis) and cerebral amyloid angiopathy.
While physicians and scientists would prefer to apply a singular etiologic diagnosis for dementia, it is readily apparent that multiple pathologies in the brain are the rule rather than the exception. Large-scale epidemiologic clinicopathologic programs around the world have convincingly demonstrated that many, or even the majority of people with dementia have mixed pathologies, chiefly AD with infarcts and to a lesser degree, Lewy bodies [57-67]. However, it is difficult to glean from the extant literature just how common pathological concurrence is given widely varying methods for identifying, classifying and reporting Alzheimer’s and vascular disease frequencies in the same patient populations.
The frequent co-occurrence of “Alzheimer-like” features and stigmata of vascular disease can make it difficult or impossible to attribute cognitive decline to one process or the other. However, if headway can be made in determining the degree to which vascular pathology contributes to cognitive impairment, systematic accounting of this pathology along the dimensions of neuropathological type, size, location and perhaps chronicity will need to be incorporated into standardized postmortem assessment.
While postmortem examination of the brain is the most definitive means of identifying structural changes in dementias, and has provided invaluable information regarding these changes, its intrinsic limitations must also be kept in mind. Postmortem examination is inherently a static, post-hoc and correlative measure that necessarily reflects the end result of pathologic processes that typically have progressed over many years. On the other hand, we know that the brain shows considerable plasticity throughout adult life[68,69] and thus has the ability to compensate for the effects of a wide range of disease processes[70]. As a result, pathologic examination alone does not provide information regarding the degree to which the abnormalities observed are responsible for the clinical features observed during life. Thus, neuropathologic evidence of vascular disease may not have been associated with clinical evidence of cognitive impairment; conversely, cognitive impairments may be observed during life that do not have a clear counterpart on postmortem brain examination[71]. This difficulty in extrapolating between clinical dysfunction and neuropathologic stigmata is reflected in the recent proposed revision of diagnostic criteria for Alzheimer-type dementia[72], in which clinical and neuropathologic diagnoses are made independently. Longitudinal studies including both in-life measures and postmortem evaluation are critical in order to better define these relationships. Furthermore, both clinical and neuropathologic criteria for vascular disease need to be standardized by a consensus process so that results will be comparable across institutions and over time.
3.5 Overlap in Risk Factors for “Vascular” and “Alzheimer-Type” Dementias
We are increasingly recognizing that risk for “Alzheimer-type dementias” (AD) is increased by factors traditionally associated with cardiovascular diseases. Even the major single gene associated with increased AD risk, namely apolipoprotein E (apoE), was earlier identified as a risk gene for cardiovascular diseases[73]. The modifiable risk factors for which the best evidence exists include obesity during mid-life, type 2 diabetes, and cigarette smoking[74] (Table 3). Of these, the co-occurrence of obesity (in particular, visceral or intra-abdominal obesity) together with insulin resistance or glucose intolerance, subclinical degrees of elevated blood pressure, and elevated total or LDL-cholesterol is associated with a comparable increase in risk for adverse cardiovascular outcomes as a threshold-level degree of any one of these factors[75] and has been called the “metabolic syndrome.”
Table 3. Risk Factors for Alzheimer-type Dementias.
| Risk factor | Modifiability | Level of evidence |
Risk ratio- mean |
Risk ratio- LL95CI |
Risk ratio- UL95CI |
|---|---|---|---|---|---|
| ApoE4 carrier (vs. E3) | Non- modifiable |
Moderate | 3.68 | 3.31 | 4.11 |
| Traumatic brain injury (males) |
? | Low | 2.29 | 1.47 | 3.58 |
| Depression | Modifiable | Low | 1.90 | 1.55 | 2.23 |
| Obesity (mid-life) | Modifiable | Low | 1.80 | 1.00 | 3.29 |
| Current smoking vs. never smoked |
Modifiable | Low | 1.79 | 1.43 | 2.23 |
| Current vs. former smoking | Modifiable | Low | 1.70 | 1.25 | 2.31 |
| Diabetes mellitus | Modifiable | Low | 1.39 | 1.17 | 1.66 |
Adapted from Williams JW et al, Preventing Alzheimer’s Disease and Cognitive Decline. Evidence Report/Technology Assessment #193. Ref.: Williams et al., 2010
Intensive study of this syndrome over the past 10-15 years has shown that these various components mutually interact with each other and with other factors such as inflammatory responses, alterations in vascular endothelial function, platelet activation, and engagement of hemostatic and antifibrinolytic mechanisms to contribute to the progression of atherosclerotic and atherothrombotic disease leading to adverse cardiac outcomes such as myocardial infarction and death[75]. Although there is ongoing debate as to the core components of this syndrome, the precise mechanisms involved, and the implications of the overall syndrome for cardiovascular risk apart from those of its constituent risk factors[76-78], there is general agreement that these risk factors are frequently associated and can exert mutually reinforcing influences on each other.
These observations draw attention to the potential role of vascular disease mechanisms in Alzheimer s disease, traditionally conceptualized as a dementia characterized exclusively by the neuropathologic stigmata of plaques and tangles. A major question is whether dementias related to vascular disease processes and those related to amyloid and tau-related processes are fundamentally different, producing additive effects on cognitive function, or whether the co-occurrence of both disease processes has synergistic effects on cognitive failure. Such questions require careful longitudinal assessment of both vascular and neurodegenerative disease measures in conjunction with a broad sampling of neurocognitive functions, imaging and biochemical biomarkers, and measures of functional status, with postmortem assessment of both the cerebral vasculature and brain parenchyma for correlation with in-life measures. Animal and cell-based models are also needed to test specific mechanistic hypotheses of interactive effects.
4. PARADIGM SHIFT FOR FUTURE RESEARCH: FOCUS ON RISK FACTORS, MULTIPLE AND INTERACTING MECHANISMS, AND DISEASE MODIFICATION
It should be clear from the above discussion that 1) there is considerable overlap in the risk factors for what is now termed “Alzheimer’s disease” and “Vascular dementia” or “Vascular cognitive impairment”; 2) there is a lack of consensus regarding clinical criteria for cognitive impairment related to vascular disease, including even what to call it; and 3) postmortem examination of individuals with dementia commonly shows features of both “AD” (i.e., plaques and tangles) and “VaD” (i.e., cerebrovascular disease). We propose here that one major obstacle that impedes future research is the focus on defining and distinguishing these entities as discrete and independent diseases. We may not precisely understand the relative contributions each “lesion” (however it is defined) makes to cognitive decline, but we can appreciate how much they overlap, and thus presume they are intimately interactive. As a result, we propose an alternative approach of distinguishing between risk factors, pathophysiologic processes, clinical syndromes, and neuropathologic findings observed at autopsy. We believe that such an approach permits the development of a useful blueprint for future research on the causes, treatment, and ultimately prevention of these diseases.
4.1 Risk factors - General Comments
Risk factors are defined as characteristics of individuals that are associated with statistically valid predictions about the likelihood of a specific outcome (usually a disease or event such as cancer, stroke, myocardial infarction or death) at some future time[79]. The complementary, albeit less frequently used term “protective factor” denotes characteristics associated with a reduced likelihood of a future outcome.
It is important to bear in mind that risk and protective factors, being largely identified through observational studies, are correlational and do not directly identify a mechanism of disease; however, they can provide important clues about underlying mechanisms that can be tested by interventional studies designed to correct the risk factor.
As indicated in the Introduction, cerebrovascular diseases have long been implicated as risk factors for dementias, and at one time were considered their primary causes. Cerebrovascular diseases are now considered as part of the broader framework of atherothrombotic vascular disease (ATVD), which also includes coronary artery disease (CAD) and peripheral vascular disease (PVD). In turn, the most common modifiable risk factors for ATVD include systemic hypertension, Type II diabetes mellitus, and dyslipidemia[80]. We now discuss these specific conditions with regard to their potential roles as risk factors for dementia and cognitive decline.
4.2 Cardiovascular Risk Factors
4.2.1 Stroke and Cerebrovascular Disease
The occurrence of a stroke is associated with a doubling of the risk of developing dementia[81]. A single stroke can cause dementia if it causes sufficient damage to brain areas or pathways important for cognitive functions (see review in ref. [81]); risk estimates vary across studies and are difficult to compare because of different patient sampling methodologies, follow-up intervals, and criteria used for diagnosis of dementia. The risk of dementia appears to increase with recurrent strokes and with the volume of brain tissue sustaining lasting injury, although there is considerable heterogeneity within this general framework. Small strokes in areas critical for cognitive functions can result in greater impairment than expected; this is often termed “strategic infarct dementia.” Most of the literature on dementia associated with “strategic” strokes is published in the form of single case reports or small case series (see ref. [81] for review).
The development of cognitive decline in association with the occurrence of multiple small strokes or lacunar infarcts was termed “multi-infarct dementia” by Hachinski[82]. Typically, patients manifesting these features have longstanding hypertension and/or diabetes, which results in damage to smaller arteries in the cerebrovascular system, and associated damage to subcortical brain structures and white matter tracts. These lacunar infarcts appear to correspond closely to white matter abnormalities intensities observed with contemporary neuroimaging methods[49].
4.2.2 “Silent strokes” and Microbleeds
Postmortem examination of the brain frequently reveals evidence of small infarcts[83] and hemorrhages[84] that can occur in the absence of macroscopic strokes, and that were not clearly associated with acute or chronic stroke symptoms during life. While neuroimaging methods are becoming increasingly sensitive to such lesions, many remain beyond the resolution of current technology[85]. There is some evidence that these lesions may be associated with cognitive impairment[83], although the nature and extent of their direct contribution to cognitive impairment has not been elucidated.
4.2.3 Hypertension
Systemic hypertension is one of the best-known risk factors for cerebrovascular disease and stroke[86]. The risk of stroke increases by about 30% for each 10 mmHg increase in systolic blood pressure above 115 mmHg, and for each 5 mmHg increase in diastolic blood pressure above 75 mmHg; a concomitant decrease in stroke risk is seen for lowering of blood pressure with treatment[86]. Hypertension, especially during mid-life, has also been shown to be an important risk factor for cognitive impairment and dementia[87]. The etiology of most forms of hypertension is not known with certainty. Family history and racial/ethnic background are known non-modifiable risk factors. Some single-gene variants are beginning to be identified based on genome-wide association studies, although the effects of any given risk allele tend to be small[88]. Modifiable risk factors include salt intake in some cases, and other lifestyle factors such as physical activity. In many cases, dietary and lifestyle modifications are not sufficiently effective in lowering blood pressure, and medications must be used. Numerous classes of medications are now available to treat hypertension, and algorithms have been developed to guide the use of these medications (see, e.g., ref. [89]).
4.3 Metabolic Risk Factors
4.3.1 Type II Diabetes Mellitus
Type II diabetes mellitus (T2DM), an important risk factor for cerebrovascular disease, is associated with a 1.5 – 2-fold increase in the risk of dementia[90] and this is independent of stroke[90]. The incidence of T2DM is increasing in developed countries[91]. This trend has been attributed in part to the dramatic increase in the number of overweight and obese individuals, as weight gain is a key risk factor for the development of T2DM. The diagnosis of T2DM is based on fasting blood glucose (FBG) levels of >126 mg/dL; individuals with FBG > 110 are considered to be “at risk.” However, it is recognized that elevated fasting glucose most often results from increasing degrees of insulin resistance developing over many years, leading to alterations in glucose homeostasis and energy metabolism, and culminating in a failure of pancreatic insulin secretion to maintain blood glucose within the normal range. Accordingly, the development of fasting hyperglycemia is preceded by elevated postprandial blood glucose or glucose intolerance. The progression of glucose intolerance to fasting hyperglycemia can be prevented in some cases by medications or, perhaps even more effectively, by lifestyle interventions[92].
Diabetes is associated with pathologic changes in both large (macrovascular) and small (microvascular) blood vessels. Some of the pathologic consequences of T2DM are attributed to the effects of sustained hyperglycemia, while others are related to the preceding phase of hyperinsulinemia and its adverse effects. Still other morbidities have been proposed to be related to episodes of hypoglycemia that can occur as a consequence of treating T2DM or its prodromal phases with hypoglycemic agents and/or insulin.
4.3.2 Metabolic Syndrome: Effects of Interactions Among Multiple Risk Factors
Epidemiologic data had suggested specific thresholds for the effects of the major risk factors on coronary artery disease risk[75] [e.g., total and low-density lipoprotein (cholesterol (TC and LDL-C), blood pressure, fasting and post-prandial glucose] In the course of analyzing these data, it became apparent that subthreshold levels of multiple risk factors jointly conferred similar levels of risk to those from threshold levels of any one risk factor. These findings led to the concept of the “metabolic syndrome,”[75] in which multiple pathophysiologic mechanisms interact to produce adverse outcomes. This concept continues to evolve[76-78], and its components and relevance to the development of cognitive impairment due to vascular disease will be discussed further below.
4.4 Vascular Pathophysiologic Processes Contributing to Dementia and Cognitive Decline
The extensive research on mechanisms underlying the development and progression of atherosclerotic and atherothrombotic vascular diseases offers a number of potential mechanisms likely to contribute to the impact of such diseases on cognitive function during the aging process. We highlight here some of the key findings from this research. In doing so, we recognize that many of these processes exert mutual influences on one another, while other processes may be involved that are not delineated here; thus, treating them separately may understate the degree of interactive and dynamic effects that may be operating in vivo. It should also be noted that this is a rapidly-advancing field, and it is critical for future research on vascular mechanisms in dementia to integrate and apply these findings to inform our understanding and to identify and test new targets for prevention and treatment.
4.4.1 Aging-associated biomechanical changes
Aging is associated with increased stiffness of arteries as a result of fragmentation and depletion of elastin and increased deposition of collagen in the vessel wall[93,94]. These changes primarily affect larger arteries, and reduce the ability of these arteries to respond to regulatory influences. In addition, the increased stiffness reduces the ability of larger arteries to buffer the pulse wave that occurs with cardiac contraction, and can thus result in greater mechanical strain on smaller arteries. Finally, many of the risk factors for vascular disease, such as hypertension, widened pulse pressure, diabetes and metabolic syndrome, and inflammatory processes and oxidative stress associated with atherosclerosis, can exacerbate the effects of aging on vascular stiffness[95].
4.4.2 Atherosclerotic plaque formation and progression
Atherosclerosis is a disease process that develops over many years, beginning with the formation of fatty streaks in large arteries during adolescence[96]. This process can be conceptualized as occurring in stages. The initial stages in atheroma formation involve uptake of low-density lipoprotein (LDL) particles into the intimal layer of arteries. These LDL particles become modified by oxidation and glycation, inducing local inflammatory responses. The inflammatory foci attract macrophages through secretion of chemokines and chemoattractant factors, leading to a cyclic process of oxidative stress and release of inflammatory mediators. The next major step in this process involves entry of smooth muscle cells into the intima, enlarging the plaque and leading to fibrosis and the formation of a fibrous cap. This plaque can be stable for extended periods, until an event occurs to cause rupture of the plaque, releasing its contents into the circulation and resulting in occlusion of one or more downstream blood vessels. In the cerebral vasculature, this will precipitate a stroke or other cerebrovascular event if the occluded blood vessels are sufficiently large to cause compromise of brain function. Vascular occlusions involving smaller territories or “silent” areas of brain may not cause acute symptoms.
4.4.3 Insulin resistance and related components of the “metabolic syndrome”
As indicated above, the occurrence of subthreshold levels of several risk factors for atherosclerotic cardiovascular disease (chiefly coronary artery disease) had been found to confer a degree of risk equivalent to that of any one risk factor present at threshold levels[75]. The key elements of risk identified in this analysis were: 1) excessive visceral adiposity, as assessed by measurement of waist circumference; 2) an atherogenic pattern of alterations in serum lipid measures (i.e., elevated total and LDL-cholesterol, elevated triglycerides, and decreased HDL-cholesterol); 3) elevated blood pressure; 4) insulin resistance, with or without glucose intolerance; and 5) evidence of a prothrombotic and proinflammatory state.
The pathophysiologic basis underlying the associations between these factors is an area of active investigation and continued controversy[76-78,97]. The most prevalent view is that visceral obesity leads to systemic insulin resistance, which in turn results in a number of deleterious consequences that promote atherogenesis, progression of atherosclerotic lesions, and eventually increases the risk of acute ischemic events due to compromise of blood flow and oxygenation to susceptible tissues such as the heart and brain[98,99].
4.4.4 Relationship between (visceral) obesity and insulin resistance
Obesity, particularly that involving intra-abdominal regions, is associated with an increased risk of insulin resistance[100,101]. In large studies of apparently healthy individuals, body mass accounts for approximately 25% of the variability in insulin sensitivity [102]. A number of potential mechanisms have been implicated in this effect. These include alterations in lipid metabolism[103]; increased production of proinflammatory cytokines[104]; and increased arterial blood pressure[105].
Some authors have questioned whether the driving force for insulin resistance is visceral obesity, or whether obesity in general is sufficient[77]. Thus, there is evidence that intra-abdominal adipose tissue can be regulated differently than that in subcutaneous regions[106], shows differential expression of proinflammatory cytokines[106] and chemotactic factors[107,108], and in obese subjects shows increased infiltration of macrophages and other inflammatory cells[109,110]. Free fatty acids released by lipolysis in visceral adipose tissue are delivered directly to the liver via the hepatic portal system[111], which can inappropriately increase hepatic glucose production[112] and may contribute to non-alcoholic hepatic steatosis[113]. However, in a recent study, surgical removal of omental fat did not improve insulin resistance or other metabolic parameters[114]. Further research is needed to better define the role of different fat depots in the mechanism of insulin resistance associated with weight gain. Nevertheless, there is strong reason to believe that the dramatic global increase in the number of overweight and obese individuals over the past several decades will substantially increase the long-term burden of cognitive impairment in addition to the metabolic and vascular diseases attributable to insulin resistance. Further understanding of the underlying mechanisms will aid in identifying the individuals most at risk for such consequences and more effective targeting of interventions to mitigate these risks.
4.4.5 Intrinsic Brain Insulin Resistance in Alzheimer’s Disease
There is also emerging evidence that neuronal insulin resistance may be an important feature of Alzheimer’s disease dementia, independent of systemic T2DM [115]. A number of postmortem and clinical biomarker studies have now documented abnormalities in insulin and insulin receptor levels in the brain as well as major changes in intraneuronal insulin signaling pathways that together demonstrate a state of intrinsic insulin resistance in the brain in Alzheimer’s disease. Some have even proposed that this may be called a “Type 3” diabetes mellitus, although it is not clear that deficient neuronal insulin signaling is directly associated with alterations in glucose metabolism as would be implied by this designation[116].
4.4.6 Alterations in circulating lipids and lipoproteins
Elevated blood levels of cholesterol were recognized as a major risk factor for atherosclerotic diseases in the original Framingham studies[117]. While LDL-C has been the principal target of preventive efforts through the widespread use of statins[118], other lipoprotein fractions such as high-density lipoproteins are also important. Obesity in general is associated with increased serum triglycerides and decreased high density lipoprotein cholesterol (HDL-C)[103]; the presence of insulin resistance is associated with an increase in this ratio[119]. The blood level of HDL-C is an important protective factor for ASCVD; thus, higher HDL-C is associated with a reduced risk of cardiovascular death, myocardial infarction, and stroke[120]. However, measures of HDL function may be better indices of cardiovascular risk than the common laboratory measure of the amount of cholesterol present in the HDL fraction[121]. The protective value of HDL may result from their ability to transport cholesterol from peripheral sites back to the liver, so-called “reverse cholesterol transport” or “cholesterol scavenging” [122]. This function is particularly important at sites of inflammation, where excess cholesterol and other lipid molecules accumulate in macrophages[122]. Many factors can affect HDL function, and other mechanisms may contribute to their atheroprotective effects[123,124]; improvement in cholesterol scavenging by HDL is an active area for drug development[125,126]
Alterations in lipoproteins have been implicated as risk factors for Alzheimer-type dementias in some recent studies, although conflicting results have been reported. Thus, in one study, higher levels of HDL-C were associated with as much as a 50-60% reduction in the risk of Alzheimer-type dementias[127], while an opposite relationship was found in another study[128]. These apparent discrepancies may be attributable to (i.e., neuropathologic vs. clinical), differences in subject populations (nursing home residents vs. community-dwelling individuals), or other factors. Further research is needed to clarify these apparent discrepancies.
4.4.7 Inflammation
Obesity is associated with increased circulating levels of a number of cytokines known to reflect and promote inflammatory processes, such as tumor necrosis factor-α (TNF-α and interleukin-6 (IL-6)[129,130]. Some of these cytokines, such as interleukin-6 (IL-6), are produced by adipocytes; as much as 30% of circulating IL-6 can be accounted for by fat mass[131,132]. The expression and secretion of these proinflammatory cytokines[133] reflects in part their common, albeit remote, evolutionary origin with macrophages[104], with which they are heavily commingled in abdominal adipose tissue as indicated above[109,110]. Recent research has shown that adipocytes produce a wide and complex array of mediators influencing inflammatory processes, insulin resistance, and energy metabolism[104,110,133], many of which have been implicated in the increased risk of atherosclerotic vascular diseases associated with the metabolic syndrome[134]. The relative roles of these mediators in cardiovascular outcomes, however, is far from clear as yet, and remains an active area of investigation. Nevertheless, there is general agreement that low-grade systemic inflammation is an important pathophysiologic component of the accelerated atherosclerotic processes associated with the metabolic syndrome[112,130,135,136].
4.4.8 Platelet Activation and Altered Hemostatic Functions
The vascular endothelial injury resulting from the atherosclerotic process results in activation of platelet and hemostatic functions in several ways[137]. Intact function of the hemostatic system has a number of beneficial effects, including prevention of excessive bleeding and extravasation of vascular contents into tissue spaces, and vascular repair[138]; however, the cascade of events following sustained or unchecked activation of this system can have deleterious consequences[96,137,139]. In addition to the critical role of the vascular endothelium in actively maintaining a smooth surface to support laminar blood flow as described above, the major components of the hemostatic system include platelets, the coagulation pathways, and fibrinolytic mechanisms[140]. All of these components have been implicated in the pathophysiology of atherosclerosis and thrombotic events[137,141,142] and in metabolic syndrome[143,144]. The critical role of platelet aggregation in acute coronary syndromes and cerebrovascular events forms the basis for the use of antiplatelet agents such as aspirin and clopidogrel in the prevention of such events, particularly in secondary prevention[145].
Two recent population-based longitudinal studies have shown an association between biomarkers of prothrombotic and/or antifibrinolytic activity and cognitive and functional decline[146] or the risk of developing vascular dementia by NINDS-AIREN criteria[147]. Although many of the same biomarkers were assessed in these two studies, differences in outcomes makes direct comparison difficult. Nevertheless, these findings provide support for the notion that a prothrombotic and/or antifibrinolytic state increases the risk of cognitive decline with aging.
4.4.9 Cerebral Amyloid Angiopathy: Relationship to Intracerebral Vascular Compromise and Dementias
Cerebral amyloid angiopathy (CAA) refers to the deposition of Congo-red staining material in or around cerebral blood vessels and/or leptomeninges on pathologic examination [148-152]. There are hereditary forms of CAA associated with single-gene mutations resulting in production or overproduction of amyloidogenic proteins[150]; these include the hereditary forms of Alzheimer disease due to mutations in the amyloid precursor protein (APP) gene, although mutations in other amyloidogenic proteins have been described[152].
The most common forms of CAA are sporadic; these are associated with normal aging and are more common in individuals with dementias than in those without[151,152]. In a recent population-based neuropathologic study, moderate-to-severe CAA was found in 17/183 (10%) of autopsies from individuals without dementia and 81/243 (34%) of those with dementia[59]. In contrast to hereditary forms, in which several amyloidogenic proteins may be involved, the sporadic forms of CAA are almost always associated with deposition of amyloid precursor protein products, primarily Abeta-40[151,152]. It has been reported that as many as 80 – 100% of patients with Alzheimer disease examined at autopsy show evidence of CAA[151,152].
CAA is linked to the occurrence of intracerebral hemorrhages, including both lobar hemorrhages and cerebral microbleeds observed at autopsy [84]. This linkage is likely related to accumulation of Abeta-40 in perivascular spaces, resulting in obstruction of the clearance of Abeta peptides from the CNS[84]. ApoE genotypes apoE4 and apoE2 are associated with an increased presence and severity of CAA lesions and cerebrovascular hemorrhages[152].
4.5 Potential Mechanisms by which Vascular and Metabolic Disease Processes Convergently Result in Cognitive Impairment
The human brain is heavily dependent on the integrity of its vascular supply due to its high rate of oxidative metabolism, resulting in disproportionately high rates of glucose utilization and oxygen consumption for its relatively low mass [153]. In addition, the central nervous system lacks intrinsic stores of energy substrates, and is thus exquisitely dependent on the systemic circulation for continuous delivery of energy substrates [154]. The extensive tissue damage and functional impairment resulting from acute compromise of perfusion during an acute stroke is perhaps the clearest example of this intimate interdependency between local or regional blood flow and neuronal function. However, more subtle and insidious effects can occur over protracted time periods due to progressive structural changes in cerebral blood vessels [155] and impairment in the normally closely-regulated mutual coupling of blood flow and oxygen consumption [156]. It has been pointed out that lesser degrees of vascular compromise integrated over an extended time period may cause similar impairments in neuronal integrity and function to those caused by more abrupt and clinically observable ischemic events [157].
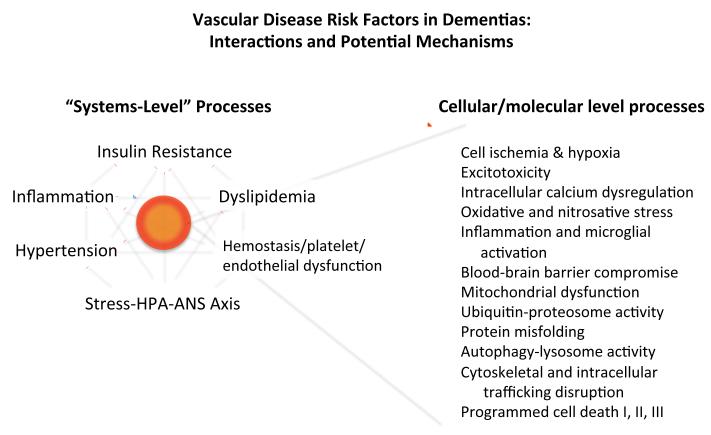
In Figure 1 we depict the mutually interacting metabolic and vascular factors that may contribute to dementia risk at a systemic level, as well as cellular and molecular mechanisms that have been proposed to underlie the structural damage to brain parenchyma that ultimately leads to cognitive and functional impairment. These cellular and molecular mechanisms are likely to include excitotoxicity due to excessive release of glutamate (albeit on a more localized fashion than during acute stroke); alterations in intracellular calcium concentrations due to failure of intracellular calcium buffering mechanisms [158]; blood-brain barrier compromise [159]; and activation of microglia and release of proinflammatory mediators [160]. Angiogenic factors are also released [161], although effective revascularization may be limited [162]. Hypoxia due to insufficient perfusion of and oxygen delivery to neuronal cells triggers a series of intracellular and intercellular responses, including activation of hypoxia inducible factor-1 (HIF-1), mitochondrial dysfunction, and oxidative and nitrosative stress [163-165]. Ultimately, neuronal degeneration occurs through necrosis, apoptosis, and/or autophagy. Damage to oligodendrocytes and myelin also develops as a consequence of vascular compromise and the resulting cellular events [149]. Recent research suggests that vascular compromise interacts with the amyloid pathway in such a way as to exacerbate Alzheimer-type pathologic changes [159,163]; it has been proposed that cerebral microvessels are the initial nidus for the formation of amyloid lesions [166]. The reader is referred to several recent reviews that describe these events in greater detail [148,156,159,162,163,167,168]. Many questions remain, however, regarding the precise sequence of and interactions among these events; their relative importance in contributing to cognitive and functional impairment; whether and how these events can be detected through the use of biomarkers; and the most promising targets for interventions to prevent cognitive decline.
Figure 1.
Systems-level risk factors and cellular/molecular mechanisms of dementias related to vascular disease processes. Left side of figure shows major vascular, endocrine, and metabolic disorders and processes that have been shown to increase the risk of dementias, including those of the Alzheimer-type. Note that these mechanisms exhibit clinically important interactive effects that have yet to be fully characterized. Right side of figure lists some candidate mechanisms by which the risk factors identified on left side may exert their effects on cognitive function and neurodegenerative processes.
Abbreviations: HPA, hypothalamic-pituitary-adrenal; ANS, autonomic nervous system.
5. RECOMMENDATIONS FOR FUTURE RESEARCH: TOWARDS AN INTEGRATED APPROACH TO THE DIAGNOSIS, TREATMENT, AND PREVENTION OF DEMENTIAS
We believe that several clear messages emerge from the above review of current research on the role of vascular diseases in the risk, course, and conceptualization of disorders characterized by aging-related cognitive impairment and decline.
5.1 Most Dementias Are Associated With Combinations of Risk Factors
The difficulties in categorizing dementias as Alzheimer disease, vascular dementia, “mixed dementia,” and so on, are based on the assumption that these are distinguishable diseases. However, the available research shows that most cases of dementia are characterized by a “mixed” phenotype, both during life and at postmortem examination. The most common co-occurring conditions are those associated with “Alzheimer-type” and “vascular” dementias. In addition, many of the same risk factors predispose not only to dementias associated with plaques and tangles on postmortem brain examination, but also to those associated with evidence of vascular disease. Thus, it would seem more pertinent to focus research efforts on elucidating the mechanisms by which vascular and neurodegenerative mechanisms jointly contribute to the development and progression of aging-related cognitive disorders than to consider them as separate diseases. In some respects, this integrative conceptual paradigm is analogous to the situation with the metabolic syndrome, in which multiple pathophysiologic mechanisms mutually interact to yield the clinical and pathologic outcomes of acute coronary syndromes, cerebrovascular events, and mortality.
5.2 Types of Studies Most Likely to Improve Public Health and Advance Knowledge
Based on our analysis of the current state of knowledge regarding vascular disease mechanisms in the risk, pathophysiology, and clinical manifestations of dementias and cognitive decline, we propose here a blueprint for achieving maximum benefits to health and society in addressing the urgent need to mitigate the impact of vascular disease on cognitive function in susceptible populations. We have organized this overall plan into: 1) Steps that can (and should) be taken immediately based on our existing evidence base; 2) strategies for enhancing the informational value of currently-active longitudinal research projects for identifying key predictors of cognitive decline related to vascular disease mechanisms; 3) recommendations for new research initiatives to extend our knowledge base regarding interactions among metabolic pathways and their ability to predict future cognitive impairment and responses to treatment; and 4) more long-range research strategies to make maximum use of available data and suggest molecular and systemic targets for the development of novel therapeutic agents.
1) Translational and Implementation Research
Research findings regarding the effectiveness of a given treatment do not always result in rapid changes in clinical practice[169]. The basis for this gap comprises multiple separable factors[170] that require distinct strategies. The need to address these factors in the case of dementias is abundantly evident from a recent analysis[171] estimating that the incidence of Alzheimer-type dementias could be dramatically reduced by a 10% reduction in the prevalence of major modifiable risk factors, including several of those cited in the present paper (i.e., diabetes, hypertension, and obesity).
The emerging field of implementation science, also considered as a type of translational medicine[172] deals with the barriers and obstacles that limit or delay the process of modifying clinical care in response to the availability of scientific evidence. Research, demonstration projects, and provider and consumer education are urgently needed to translate current evidence about vascular disease prevention and treatment into interventions that can mitigate risk or delay onset of disease. Importantly, such interventions would have a broad impact on disease morbidity and mortality in the population, due to their high prevalence and the multitude of adverse health consequences with which they are associated.
2) Enhance informational value of existing studies
There are a number of ongoing longitudinal studies of cognitive function that are designed to include neuropathologic examination, in which information value can be substantially enhanced by incorporating cost-effective measures of cardiovascular disease and risk. Examples of such studies include the Alzheimer Disease Neuroimaging Initiative (ADNI); the Rush Memory and Aging Project; the Cognitive Function and Aging Study (CFAS) of the U.K. Medical Research Council; and the Australian Imaging Biomarkers and Lifestyle (AIBL).
Conversely, incorporating measures of neurocognitive function into ongoing longitudinal studies of cardiovascular diseases can provide great value to the elucidation on the time course of processes leading to cognitive impairment and functional decline. The addition of such information may have a particularly strategic impact due to the large sample sizes needed for such studies in order to obtain sufficient statistical power to detect the endpoints typically used (e.g., acute coronary or cerebrovascular events and mortality).
3) Design new studies of mid-life individuals to examine the effects of earlier interventions on biomarkers of adverse cognitive outcomes
There is typically a considerable lag time between the inception of a longitudinal study such as those currently in progress, and the initiation of such studies, and an even longer latency for the emergence of data on key long-term outcomes. Much new information is often accrued during this time interval, which often cannot be readily obtained or added once the study is in progress. As a result, there is a need to design new studies incorporating current evidence in order to take advantage of such advances. As an example, information on multiple pathophysiologic domains associated with insulin resistance, and perhaps some of the controversies and uncertainties that currently exist regarding their interrelationship, may be feasibly addressed by the initiation of prospective studies assessing biomarkers representing multiple domains contemporaneously over time. Some biomarkers (e.g., those relating to platelet and hemostatic system function) require specific sample collection or other preanalytic methods that may not have been employed in prior studies of other kinds of biomarkers. Prospectively-designed studies would allow consideration of such factors prior to study initiation, maximizing the quality of the data obtained.
4) Apply emerging principles, methods, and findings of systems biology and network medicine[173]
to make optimal use of existing knowledge and generate models of disease processes that will lead to testable hypotheses about the impact of interventions for which sufficient evidence does not yet exist. Given the degree of interrelatedness among the systems implicated in and influencing metabolic and vascular dysfunction, a mathematical biologic approach can substantially inform the elucidation of the relevant pathophysiologic processes that contribute to the progression of the disease process over time. For example, hypertension and type 2 diabetes are both identified above as risk factors for vascular disease-mediated cognitive decline; however, hypertension is associated with insulin resistance, which in turn is a risk factor for type 2 diabetes as well as for other forms of vascular disease that may independently predispose to cognitive impairment. Similarly, obesity is a risk factor for both insulin resistance (and hence type 2 diabetes) and hypertension. A mathematical biologic approach to quantifying the net impact of risk factors and their component pathophysiologic mechanisms over time would integrate known data using statistical models of the dynamic and interactive processes involved, as well as identifying areas where data are incomplete and further research is needed.
Conceptualizing the disease process as a result of a loss of the ability to compensate for the cumulative burden of internal and external challenges faced over a lifetime [174] provides an opportunity to formulate key questions about the normal interregulatory relationships among these systems, i.e. what relationships keep them from breaking down. Such a conceptual framework can help identify what parameters need to be measured to better define not only the critical nodal points influencing to the expression of clinically and functionally-significant cognitive impairment, but also to form testable hypotheses about where to target preventive and therapeutic interventions in order to achieve the maximum benefit to the individual.
6. SUMMARY AND CONCLUSIONS
Our understanding of the role of vascular disease processes in the development of aging-associated cognitive disorders has undergone several shifts over the past half-century. To some extent, the perceived importance of vascular disease to dementia risk was overshadowed for some time by the explosion of research on the underlying basis of the brain lesions identified by Alzheimer. This research has led to the identification of potential molecular targets for treatment and prevention, some of which are currently being tested. Nevertheless, it is critical to recognize that Alzheimer-type neuropathology usually does not occur in isolation, and vascular disease mechanisms are likely to play crucial roles in the evolution of the brain injury that eventually results in cognitive impairment and functional decline. We believe that a new paradigm is called for in order to advance research and mitigate the societal burden of these devastating diseases, one that is inclusive of both vascular and neuropathologic mechanisms. We outline a series of steps for applying this paradigm to current clinical practices and ongoing and future research.
ACKNOWLEDGMENTS
This work was supported in part by funding from the Alzheimer’s Disease Coordinating Center (NIH P30 AG010124), and by a grant from the Allen H. and Selma W. Berkman Charitable Trust.
Abbreviations used frequently in text
- AD
Alzheimer disease or Alzheimer-type dementia
- IVD
ischemic vascular disease
- CT
computed (X-ray) tomography
- CVD
cerebrovascular disease
- DSM
Diagnostic and Statistical Manual of the American Psychiatric Association
- IVD
ischemic vascular dementia
- MCI
mild cognitive impairment
- MRI
magnetic resonance imaging
- T2DM
Type 2 diabetes mellitus
- VaD
vascular dementia
- VCD
vascular cognitive disorder
- VCI
vascular cognitive impairment
- VCIND
vascular cognitive impairment, no dementia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
At the time of this writing, the APA has released a draft online version of DSM-V for public comment (ref. 32. American Psychiatric Association. Home | APA DSM-V. Washington, DC: American Psychiatric Association,; 2011 [cited 2011 5/29/2011]; Available from: http://www.dsm5.org/Pages/Default.aspx.), and is currently conducting field trials of the criteria in preparation for its planned publication in 2013.
REFERENCES
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196–208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Ault AB. The fourth stage of the epidemiologic transition: the age of delayed degenerative diseases. Milbank Q. 1986;64:355–91. [PubMed] [Google Scholar]
- 3.Wimo A, Winblad B, Jonsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6:98–103. doi: 10.1016/j.jalz.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol. 2007;113:349–88. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri M, Enzinger C, Petrovic K, Pluta-Fuerst A, Homayoon N, Schmidt H, et al. Vascular dementia and Alzheimer’s disease - are we in a dead-end road? Neurodegener Dis. 2010;7:122–6. doi: 10.1159/000285521. [DOI] [PubMed] [Google Scholar]
- 6.Forstl H, Howard R, Levy R. Binswanger’s clinical and neuropathological criteria for “Binswanger’s disease”. J Neurol Neurosurg Psychiatry. 1991;54:1122–3. doi: 10.1136/jnnp.54.12.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libon DJ, Price CC, Swenson RA, Penney D, Haake B, Pennisi A. Vascular cognitive impairment. In: Festa JR, Lazar R,M, editors. Neurovascular Neuropsychology. Springer Science+Business Media; 2009. pp. 75–86. [Google Scholar]
- 8.Roman GC. A historical review of the concept of vascular dementia: lessons from the past for the future. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S4–8. doi: 10.1097/00002093-199912001-00002. [DOI] [PubMed] [Google Scholar]
- 9.Roman GC. Historical evolution of the concept of vascular dementia. In: Bowler JV, Hachinski V, editors. Vascular Cognitive Impairment: Preventable Dementia. Oxford University Press; New York: 2003. pp. 12–20. [Google Scholar]
- 10.McMenemey WH. The dementias and progressive diseases of the basal ganglia. In: GreenWeld JG, editor. Neuropathology. 3rd edition E. Arnold; London: 1961. pp. 475–521. [Google Scholar]
- 11.Blass JP, Hoyer S, Nitsch R. A translation of Otto Binswanger’s article, ‘The delineation of the generalized progressive paralyses’. 1894. Archives of neurology. 1991;48:961–72. doi: 10.1001/archneur.1991.00530210089029. [DOI] [PubMed] [Google Scholar]
- 12.Hauw JJ. The history of lacunes. In: Donnan GA, Norrving JM, Bamford JM, Bogousslavsky J, editors. Lacunar and other subcortical infarctions. Oxford University Press; New York, NY: 1995. pp. 3–15. [Google Scholar]
- 13.Alzheimer A, Stelzmann RA, Schnitzlein HN, Murtagh FR. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat. 1995;8:429–31. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J Neurol Sci. 1968;7:331–56. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–42. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 16.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 17.Summers WK, Majovski LV, Marsh GM, Tachiki K, Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N Engl J Med. 1986;315:1241–5. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]
- 18.Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. Cmaj. 2003;169:557–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 20.Kosik KS. Tau protein and Alzheimer’s disease. Curr Opin Cell Biol. 1990;2:101–4. doi: 10.1016/s0955-0674(05)80038-9. [DOI] [PubMed] [Google Scholar]
- 21.Trojanowski JQ, Schmidt ML, Shin RW, Bramblett GT, Rao D, Lee VM. Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer’s disease and Lewy body dementias. Brain Pathol. 1993;3:45–54. doi: 10.1111/j.1750-3639.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 22.Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer’s disease. Annu Rev Med. 2006;57:513–33. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 23.Selkoe DJ. Amyloid beta protein precursor and the pathogenesis of Alzheimer’s disease. Cell. 1989;58:611–2. doi: 10.1016/0092-8674(89)90093-7. [DOI] [PubMed] [Google Scholar]
- 24.Rafii MS, Aisen PS. Recent developments in Alzheimer’s disease therapeutics. BMC Med. 2009;7:7. doi: 10.1186/1741-7015-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganguli M. Epidemiology of dementia. In: Abou-Saleh MT, Katona CKE, Kumar A, editors. Principles and Practice of Geriatric Psychiatry. 3rd edition Wiley-Blackwell; New York: 2011. pp. 207–212. [Google Scholar]
- 26.Roman GC. Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci. 2002;203-204:7–10. doi: 10.1016/s0022-510x(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Diseases, Version III. 3rd edition American Psychiatric Association; Washington, DC: 1980. [Google Scholar]
- 28.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–80. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. 10ed World Health Organization; Geneva: 1992. [Google Scholar]
- 30.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Report of the NINDS-AIREN International Workshop Vascular dementia: diagnostic criteria for research studies. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 31.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition. 4th ed American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- 32.American Psychiatric Association . Home | APA DSM-V. American Psychiatric Association; Washington, DC: 2011. [cited 2011 5/29/2011]; Available from: http://www.dsm5.org/Pages/Default.aspx. [Google Scholar]
- 33.Wiederkehr S, Simard M, Fortin C, van Reekum R. Comparability of the clinical diagnostic criteria for vascular dementia: a critical review. Part I. J Neuropsychiatry Clin Neurosci. 2008;20:150–61. doi: 10.1176/jnp.2008.20.2.150. [DOI] [PubMed] [Google Scholar]
- 34.Wiederkehr S, Simard M, Fortin C, van Reekum R. Validity of the clinical diagnostic criteria for vascular dementia: a critical review. Part II. J Neuropsychiatry Clin Neurosci. 2008;20:162–77. doi: 10.1176/jnp.2008.20.2.162. [DOI] [PubMed] [Google Scholar]
- 35.Wentzel C, Darvesh S, MacKnight C, Shea C, Rockwood K. Inter-rater reliability of the diagnosis of vascular cognitive impairment at a memory clinic. Neuroepidemiology. 2000;19:186–93. doi: 10.1159/000026254. [DOI] [PubMed] [Google Scholar]
- 36.Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, et al. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch Neurol. 2000;57:191–6. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- 37.Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000;31:2952–7. doi: 10.1161/01.str.31.12.2952. [DOI] [PubMed] [Google Scholar]
- 38.Lamar M, Price CC, Giovannetti T, Swenson R, Libon DJ. The dysexecutive syndrome associated with ischaemic vascular disease and related subcortical neuropathology: a Boston process approach. Behav Neurol. 2009;22:53–62. doi: 10.3233/BEN-2009-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 41.Roberts RO, Geda YE, Knopman DS, Cha RH, Boeve BF, Ivnik RJ, et al. Metabolic syndrome, inflammation, and nonamnestic mild cognitive impairment in older persons: a population-based study. Alzheimer disease and associated disorders. 2010;24:11–8. doi: 10.1097/WAD.0b013e3181a4485c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2010;31:1894–902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trachtenberg DI, Trojanowski JQ. Dementia: a word to be forgotten. Arch Neurol. 2008;65:593–5. doi: 10.1001/archneur.65.5.593. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 45.Black SE. Vascular cognitive impairment: epidemiology, subtypes, diagnosis and management. J R Coll Physicians Edinb. 2011;41:49–56. doi: 10.4997/JRCPE.2011.121. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–33. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 47.Pantoni L, Gorelick P. Advances in vascular cognitive impairment 2010. Stroke. 2011;42:291–3. doi: 10.1161/STROKEAHA.110.605097. [DOI] [PubMed] [Google Scholar]
- 48.Rockwood K, Black SE, Song X, Hogan DB, Gauthier S, MacKnight C, et al. Clinical and radiographic subtypes of vascular cognitive impairment in a clinic-based cohort study. J Neurol Sci. 2006;240:7–14. doi: 10.1016/j.jns.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–3. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 50.Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin Neuropsychol. 2007;21:73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- 51.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, et al. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry. 1999;67:66–72. doi: 10.1136/jnnp.67.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–52. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 54.Beyer J, Young R, Kuchibhatla M, Krishnan KRR. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. International review of psychiatry. 2009;21:394–409. doi: 10.1080/09540260902962198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porter A, Gladstone J, Dodick D. Migraine and white matter hyperintensities. Current Pain and Headache Reports. 2005;9:289–293. doi: 10.1007/s11916-005-0039-y. [DOI] [PubMed] [Google Scholar]
- 56.ADC Neuropathology Core Leaders’ Steering Committee . NACC Neuropathology (NP) Diagnosis Coding Guidebook. National Alzheimer’s Coordinating Center; St. Louis, MO: 2008. Portable Document Format. [updated 2008; cited 2011 8/4/2011]; 9.1:[Coding guideline for neuropathologic diagnosis, Alzheimer’s Disease Centers]. Available from: https://http://www.alz.washington.edu/NONMEMBER/NP/npguide9.pdf. [Google Scholar]
- 57.Jellinger KA, Attems J. Is there pure vascular dementia in old age? J Neurol Sci. 2010;299:150–4. doi: 10.1016/j.jns.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 58.Lim A, Tsuang D, Kukull W, Nochlin D, Leverenz J, McCormick W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–9. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 59.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 61.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wharton SB, Brayne C, Savva GM, Matthews FE, Forster G, Simpson J, et al. Epidemiological Neuropathology: The MRC Cognitive Function and Aging Study Experience. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-091402. [DOI] [PubMed] [Google Scholar]
- 63.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 64.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sonnen JA, Larson EB, Haneuse S, Woltjer R, Li G, Crane PK, et al. Neuropathology in the adult changes in thought study: a review. J Alzheimers Dis. 2009;18:703–11. doi: 10.3233/JAD-2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strozyk D, Dickson DW, Lipton RB, Katz M, Derby CA, Lee S, et al. Contribution of vascular pathology to the clinical expression of dementia. Neurobiol Aging. 2010;31:1710–20. doi: 10.1016/j.neurobiolaging.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29:1587–90. doi: 10.1016/j.neurobiolaging.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nudo RJ. Neural bases of recovery after brain injury. J Commun Disord. 2011 doi: 10.1016/j.jcomdis.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–51. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- 70.Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–95. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE. Cognition and Neuropathology in Aging: Multidimensional Perspectives from the Rush Religious Orders Study and Rush Memory and Aging Project. Curr Alzheimer Res. 2011 doi: 10.2174/156720511795745302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montine TJ, Hyman BT, Beach TG, Bigio EH, Cairns NJ, Dickson D, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease [Draft document for public comment] 2011 [cited 2011 8/14/11]; Available from: http://www.alz.org/research/diagnostic_criteria/ [Google Scholar]
- 73.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–95. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 74.Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Evidence Report/Technology Assessment. Agency for Healthcare Research and Quality; Rockville, MD: 2010. Preventing Alzheimer’s Disease and Cognitive Decline. April 2010. Report No.: 193 Contract No.: Publication No. 10-E005. [PMC free article] [PubMed] [Google Scholar]
- 75.National Cholesterol Education Program Adult Treatment Panel III Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 76.Unwin N. The metabolic syndrome. J R Soc Med. 2006;99:457–62. doi: 10.1258/jrsm.99.9.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reaven GM. The metabolic syndrome: time to get off the merry-go-round? J Intern Med. 2011;269:127–36. doi: 10.1111/j.1365-2796.2010.02325.x. [DOI] [PubMed] [Google Scholar]
- 78.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Annals of internal medicine. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 80.Libby P, Ridker PM. Risk Markers for Atherothrombotic Disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease - A Textbook of Cardiovascular Medicine. 9th ed. 9th edition. Elsevier: W.B. Saunders; Philadelphia: 2011. pp. 914–934. (online version) ed. [Google Scholar]
- 81.Leys D, Henon H, Mackowiak-Cordoliani MA, Pasquier F. Poststroke dementia. Lancet Neurol. 2005;4:752–9. doi: 10.1016/S1474-4422(05)70221-0. [DOI] [PubMed] [Google Scholar]
- 82.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;2:207–10. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- 83.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011;42:722–7. doi: 10.1161/STROKEAHA.110.595082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. doi: 10.1016/j.jns.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 85.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–35. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 86.Lawes CM, Bennett DA, Feigin VL, Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35:776–85. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 87.Nagai M, Hoshide S, Kario K. Hypertension and dementia. Am J Hypertens. 2010;23:116–24. doi: 10.1038/ajh.2009.212. [DOI] [PubMed] [Google Scholar]
- 88.Arora P, Newton-Cheh C. Blood pressure and human genetic variation in the general population. Curr Opin Cardiol. 2010 doi: 10.1097/HCO.0b013e3283383e2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 90.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–14. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 91.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 92.Crandall JP, Knowler WC, Kahn SE, Marrero D, Florez JC, Bray GA, et al. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab. 2008;4:382–93. doi: 10.1038/ncpendmet0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Oxford University Press; Oxford: 2005. [Google Scholar]
- 94.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–4. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 95.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–62. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 96.Libby P. The vascular biology of atherosclerosis. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease - A Textbook of Cardiovascular Medicine, 9th ed. 9th edition. Elsevier: W.B. Saunders; Philadelphia: 2011. pp. 897–913. (online version) ed. [Google Scholar]
- 97.Lau DC. Metabolic syndrome: perception or reality? Curr Atheroscler Rep. 2009;11:264–71. doi: 10.1007/s11883-009-0041-7. [DOI] [PubMed] [Google Scholar]
- 98.Ferrer I. Cognitive impairment of vascular origin: neuropathology of cognitive impairment of vascular origin. J Neurol Sci. 2010;299:139–49. doi: 10.1016/j.jns.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 99.Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, et al. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2010;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- 100.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–8. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, et al. Insulin resistance and body fat distribution. Diabetes Care. 1996;19:287–91. doi: 10.2337/diacare.19.3.287. [DOI] [PubMed] [Google Scholar]
- 102.Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–47. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]
- 103.Reaven GM. Compensatory hyperinsulinemia and the development of an atherogenic lipoprotein profile: the price paid to maintain glucose homeostasis in insulin-resistant individuals. Endocrinol Metab Clin North Am. 2005;34:49–62. doi: 10.1016/j.ecl.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 105.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–93. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 106.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 107.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–83. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 108.O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–90. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. Bjog. 2006;113:1141–7. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 110.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 111.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med. 2006;38:52–63. doi: 10.1080/07853890500383895. [DOI] [PubMed] [Google Scholar]
- 112.Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med. 2010;77:511–23. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]
- 113.Jakobsen MU, Berentzen T, Sorensen TI, Overvad K. Abdominal obesity and fatty liver. Epidemiol Rev. 2007;29:77–87. doi: 10.1093/epirev/mxm002. [DOI] [PubMed] [Google Scholar]
- 114.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–55. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talbot K, Wang H-Y, Kazi H, Han L-Y, Bakshi KP, Stucky A, et al. Brain insulin resistance demonstrated in Alzheimer’s disease without diabetes is associated with dysregulated IRS-1 and cognitive decline. J Clin Invest. 2012 doi: 10.1172/JCI59903. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kannel WB, Dawber TR, Friedman GD, Glennon WE, McNamara PM. Risk Factors in Coronary Heart Disease. An Evaluation of Several Serum Lipids as Predictors of Coronary Heart Disease; the Framingham Study. Annals of internal medicine. 1964;61:888–99. doi: 10.7326/0003-4819-61-5-888. [DOI] [PubMed] [Google Scholar]
- 118.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 119.Abbasi F, Brown BW, Jr., Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–43. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 120.Pencina MJ, D’Agostino RB, Sr., Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078–84. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–55. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 123.Farmer JA, Liao J. Evolving concepts of the role of high-density lipoprotein in protection from atherosclerosis. Curr Atheroscler Rep. 2011;13:107–14. doi: 10.1007/s11883-011-0166-3. [DOI] [PubMed] [Google Scholar]
- 124.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 125.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011;8:266–77. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toth PP. Pharmacomodulation of high-density lipoprotein metabolism as a therapeutic intervention for atherosclerotic disease. Curr Cardiol Rep. 2010;12:481–7. doi: 10.1007/s11886-010-0136-3. [DOI] [PubMed] [Google Scholar]
- 127.Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Archives of neurology. 2010;67:1491–7. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lesser GT, Haroutunian V, Purohit DP, Schnaider Beeri M, Schmeidler J, Honkanen L, et al. Serum lipids are related to Alzheimer’s pathology in nursing home residents. Dement Geriatr Cogn Disord. 2009;27:42–9. doi: 10.1159/000189268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–36. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- 130.Espinola-Klein C, Gori T, Blankenberg S, Munzel T. Inflammatory markers and cardiovascular risk in the metabolic syndrome. Front Biosci. 2011;16:1663–74. doi: 10.2741/3812. [DOI] [PubMed] [Google Scholar]
- 131.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 132.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 133.Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10:156–64. doi: 10.1007/s11906-008-0029-7. [DOI] [PubMed] [Google Scholar]
- 134.Mehta S, Farmer JA. Obesity and inflammation: a new look at an old problem. Curr Atheroscler Rep. 2007;9:134–8. doi: 10.1007/s11883-007-0009-4. [DOI] [PubMed] [Google Scholar]
- 135.Elks CM, Francis J. Central adiposity, systemic inflammation, and the metabolic syndrome. Curr Hypertens Rep. 2010;12:99–104. doi: 10.1007/s11906-010-0096-4. [DOI] [PubMed] [Google Scholar]
- 136.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 137.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–60. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 138.Konkle BA. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Bleeding and Thrombosis. Harrison’s Principles of Internal Medicine. (17e ed) 2011 Chapter 59. Cited 2011 Cited]. Available from: http://www.accessmedicine.com/content.aspx?aID=2870098.
- 139.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 140.Weitz JI. Hemostasis, Thrombosis, Fibrinolysis, and Cardiovascular Disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, editors. Braunwald’s Heart Disease - A Textbook of Cardiovascular Medicine, 9th ed. 9th edition. Elsevier: W.B. Saunders; Philadelphia: 2011. pp. 1844–1867. (online version) ed. [Google Scholar]
- 141.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–94. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 142.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Alessi MC, Juhan-Vague I. Metabolic syndrome, haemostasis and thrombosis. Thromb Haemost. 2008;99:995–1000. doi: 10.1160/TH07-11-0682. [DOI] [PubMed] [Google Scholar]
- 144.Palomo I, Moore-Carrasco R, Alarcon M, Rojas A, Espana F, Andres V, et al. Pathophysiology of the proatherothrombotic state in the metabolic syndrome. Front Biosci (Schol Ed) 2010;2:194–208. doi: 10.2741/s57. [DOI] [PubMed] [Google Scholar]
- 145.Steg PG, Dorman SH, Amarenco P. Atherothrombosis and the role of antiplatelet therapy. J Thromb Haemost. 2011;9(Suppl 1):325–32. doi: 10.1111/j.1538-7836.2011.04277.x. [DOI] [PubMed] [Google Scholar]
- 146.Stott DJ, Robertson M, Rumley A, Welsh P, Sattar N, Packard CJ, et al. Activation of hemostasis and decline in cognitive function in older people. Arterioscler Thromb Vasc Biol. 2010;30:605–11. doi: 10.1161/ATVBAHA.109.199448. [DOI] [PubMed] [Google Scholar]
- 147.Gallacher J, Bayer A, Lowe G, Fish M, Pickering J, Pedro S, et al. Is sticky blood bad for the brain?: Hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler Thromb Vasc Biol. 2010;30:599–604. doi: 10.1161/ATVBAHA.109.197368. [DOI] [PubMed] [Google Scholar]
- 148.Altman R, Rutledge JC. The vascular contribution to Alzheimer’s disease. Clin Sci (Lond) 2010;119:407–21. doi: 10.1042/CS20100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta neuropathologica. 2010;119:277–290. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Revesz T, Holton JL, Lashley T, Plant G, Frangione B, Rostagno A, et al. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta neuropathologica. 2009;118:115–30. doi: 10.1007/s00401-009-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 152.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Magistretti PJ, Pellerin L, Martin J-L. Brain energy metabolism: an integrated cellular perspective. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology - 4th Generation of Progress. American College of Neuropsychopharmacollgy; Brentwood, TN: 2000. Cited 2000 Cited]. Available from: http://www.acnp.org/g4/GN401000064/Default.htm. [Google Scholar]
- 154.Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Prog Brain Res. 2006;153:129–40. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 155.Pase MP, Herbert A, Grima NA, Pipingas A, O’Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: A systematic review and meta-analysis. Intern Med J. 2011 doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 156.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 157.Khachaturian ZS. Calcium hypothesis of Alzheimer’s disease and brain aging. Annals of the New York Academy of Sciences. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- 158.Riascos D, de Leon D, Baker-Nigh A, Nicholas A, Yukhananov R, Bu J, et al. Age-related loss of calcium buffering and selective neuronal vulnerability in Alzheimer’s disease. Acta neuropathologica. 2011;122:565–76. doi: 10.1007/s00401-011-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–13. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–7. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 161.Grammas P, Tripathy D, Sanchez A, Yin X, Luo J. Brain microvasculature and hypoxia-related proteins in Alzheimer’s disease. Int J Clin Exp Pathol. 2011;4:616–27. [PMC free article] [PubMed] [Google Scholar]
- 162.Brown WR, Thore CR. Review: Cerebral microvascular pathology in aging and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–96. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ogunshola OO, Antoniou X. Contribution of hypoxia to Alzheimer’s disease: is HIF-1alpha a mediator of neurodegeneration? Cell Mol Life Sci. 2009;66:3555–63. doi: 10.1007/s00018-009-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. Faseb J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 167.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Annals of the New York Academy of Sciences. 2010;1207:46–9. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Institute of Medicine . Crossing the Quality Chasm: a new health system for the 21st century. Institute of Medicine of the U.S. National Academy of Science; Washington, DC: 2001. [Google Scholar]
- 170.Avorn J, Fischer M. ‘Bench to behavior’: translating comparative effectiveness research into improved clinical practice. Health Aff (Millwood) 2010;29:1891–900. doi: 10.1377/hlthaff.2010.0696. [DOI] [PubMed] [Google Scholar]
- 171.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thornicroft G, Lempp H, Tansella M. The place of implementation science in the translational medicine continuum. Psychol Med. 2011;41:2015–21. doi: 10.1017/S0033291711000109. [DOI] [PubMed] [Google Scholar]
- 173.Barabasi A-L, Gulbahce N, Loscalzo J. Network medicine: a network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]