Abstract
The immunoaffinity isolation of protein complexes is an essential technique for the purification and concentration of protein complexes from cells and tissues. In this chapter we present the methodologies for the purification of proteins and protein complexes from Xenopus laev is and Xenopus tropical is. Specific to this protocol are the techniques for the cryolysis of Xenopus cells and tissues, a procedure that limits contamination from yolk proteins while preserving endogenous protein complexes, the methodologies for immunoaffinity purification of proteins using magnetic beads, and the protocols for western blot analysis. In addition, the procedures in this chapter can be extended to use with proteomic analysis of protein complexes as presented in the following chapter.
Keywords: Xenopus, Immunoaffinity purification, Immunoprecipitation, Immunoisolation, Cryolysis, Tissue lysis, Protein extraction, Protein complex, Protein interactions, Western blot analysis
1. Introduction
It is becoming increasingly clear that many forms of human disease are associated with defects in genes that are required for early steps in embryonic development. Moreover, the molecular and cellular pathways through which these genes function can be elucidated using established model systems such as the African clawed frog, Xenopus. Xenopus has numerous advantages as a model system in which to identify and characterize cellular and developmental processes particularly in regards to proteomic-based approaches. Most critically, unlike the mouse, the Xenopus embryo develops externally and the embryo is relatively large and is amenable to surgical manipulations, allowing defined regions to be excised and cultured in simple salt solutions. These classical approaches are complemented by molecular techniques that allow the ectopic expression, overexpression, of knock-down of specific gene transcripts in the early embryo and transgenic technologies.
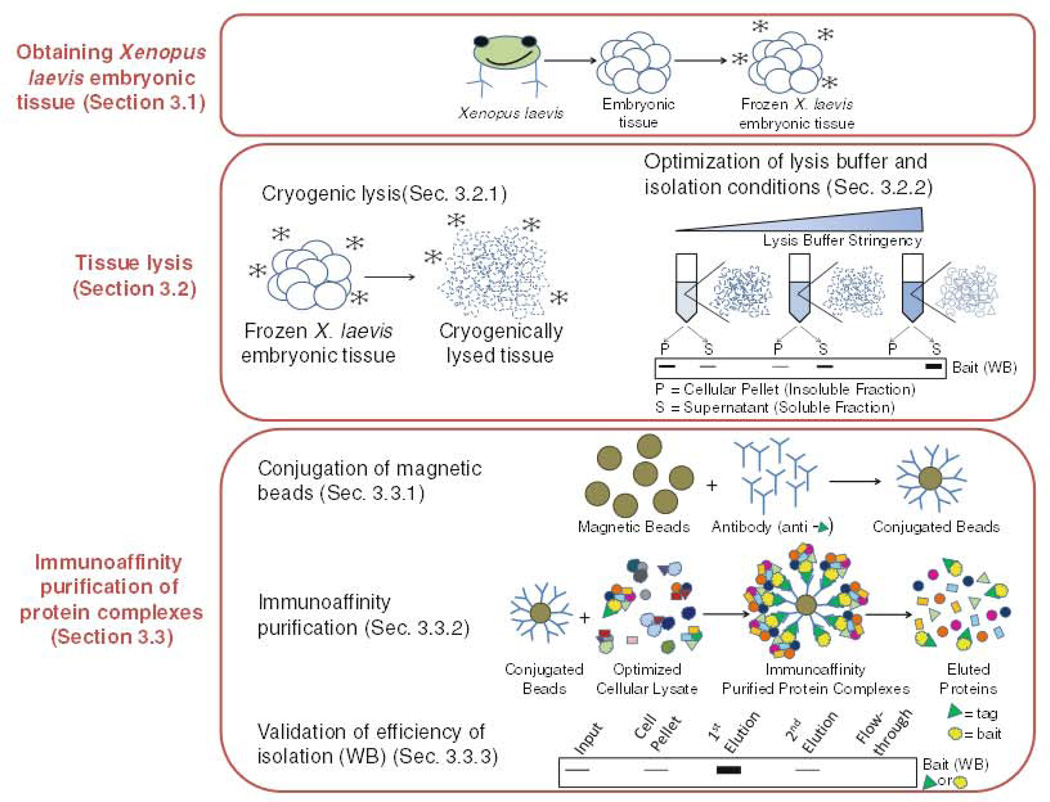
Complementary to these approaches are emerging biochemical approaches. In this regard, Xenopus offers a unique model system for the identification and characterization of protein complexes in vivo. However, the use of these approaches has been limited due to the lack of optimized protocols for isolation of early stage Xenopus tissues and the large abundance of yolk proteins. As shown in Fig. 1, this chapter describes methods for conducting immunoprecipitation of endogenous protein complexes in Xenopus laevis and Xenopus tropicalis which combines the cryogenic lysis of tissues with immunoisolation on magnetic beads. An overview of the approach is shown in Fig. 1. Collectively, these approaches function to preserve endogenous protein complexes, limit problems associated with yolk platelets, and provide a specific isolation of a given protein.
Fig. 1.
Immunoisolation of protein complexes from Xenopus laevis.
2. Methods and Equipment
2.1. Obtaining Xenopus laevis Embryonic Tissue
Fine watchmaker’s forceps such as Dumont number 5 forceps.
X. laevis embryos cultured to desired stage of development (1)
10× Modified Barth’s Saline (MBS), pH 7.8: 880 mM NaCl, 10 mM KCl, 10mM MgSO4, 50 mM HEPES pH 7.8, 25 mM NaHCO3. 1× MBS is made by mixing 100 mL of 10× stock solution with 700 mL 1 M CaCl2, and adjusting the volume to 1 L with dH2O. Store at room temperature.
1% agarose plates for dissections: Weigh 1 g agarose and transfer to 250 mL Erlenmeyer flask containing 100 mL dH2O. Heat flask in microwave until agarose has completely dissolved. Cool molten agarose until cool enough to hold flask. Pour a layer of agarose into small plastic Petri dishes (5 cm). Allow agarose to set. Store plates at 4°C.
Plastic transfer pipettes.
Liquid nitrogen.
Syringe needle (19G11/2).
50 ml, conical tubes.
A dissecting microscope (e.g., Leica MZ6).
2.2. Tissue Lysis and Protein Extraction
Retsch MM 301 Mixer Mill with 2 × 25 mL jars and 2 × 20 mm (tungsten carbide or stainless steel) grinding balls (Retsch, Newtown, PA).
Liquid nitrogen, Styrofoam container, and a pair of long forceps.
Windex.
Methanol.
50 mL conical tubes.
Ultrapure water.
2.3. Immunoaffinity Purification of Protein Complexes
2.3.1. Conjugation of Magnetic Beads
Unless otherwise stated all solutions can be stored at room temperature
Dynabeads M-270 Epoxy (Invitrogen). Store at 4°C.
Affinity purified antibodies against a protein of interest or tag (e.g., anti-GFP antibodies as shown below for the isolation of GFP-tagged proteins) or Immunoglobulin G (for isolation of Protein A-tagged proteins). Store at −80°C.
0.1 M Sodium Phosphate buffer, pH 7.4: Prepare as 19 mM NaH2PO4, 81 mM Na2HPO4 in water and adjust pH to 7.4, if necessary. Filter sterilize (0.2 mm filter (Millipore)). Store at 4°C.
3 M Ammonium Sulfate: Prepare in 0.1 M Sodium Phosphate buffer, pH 7.4. Filter sterilize (0.2 mm filter (Millipore)).
100 mM Glycine-HCl, pH 2.5: Prepare in water. Adjust pH to 2.5 with HCl. Filter sterilize (0.2 mm filter (Millipore)). Store at 4°C.
10 mM Tris, pH 8.8: Prepare in water. Adjust pH to 8.8 with HCl. Filter sterilize (0.2 mm filter (Millipore)).
100 mM Triethylamine: Prepare fresh in water, CAUTION: toxic and extremely flammable. Must handle in a chemical hood and dispose of appropriately.
DPBS, pH 7.4 (Dulbecco’s Phosphate-Buffered Saline (1×), liquid), (Invitrogen): Store at 4°C.
0.5% Triton X-100: Prepare in DPBS. Store at 4°C.
0.02% Sodium azide (NaN3): Prepare in DPBS. Store at 4°C. CAUTION: NaN3 is a toxic solid compound. Must handle in a chemical hood and dispose of appropriately.
Rotator (at 30°C).
Magnetic separation tube rack (Invitrogen).
Tube shaker (Tomy shaker).
Round bottom 2 mL Safe-Lock tubes (Eppendorf).
Ultrapure water (e.g., from a Milli-Q Integral Water Purification System).
2.3.2. Immunoaffinity Purification
Frozen tissue powder (see Subheading 3.1). Store at −80°C
Optimized lysis buffer (see Subheading 3.2) prepared fresh prior to each experiment. Store on ice.
Magnetic beads conjugated with antibodies (see Subheading 3.3). Store at 4°C.
50 mL conical tubes.
Polytron for tissue homogenization (e.g., PT 10–35 Polytron from Kinematica).
Centrifuge and rotor, compatible with 50 mL conical tubes and capable of 8,000× g at 4°C.
Tube rotator at 4°C.
Ultrapure dH2O.
Round bottom eppendorf tubes (Fisher).
Axygen Maxymum Recovery microcentrifuge tubes, 1.5 mL (VWR).
Bar magnets (for conical tubes) and magnetic separation rack (for eppendorf tubes) (Invitrogen).
Ammonium hydroxide, 14.8 M (Sigma). Store at 4°C.
Base elution buffer: Mix 4.826 mL of ultrapure H2O, 5 mL of 0.5 M EDTA, pH 8.0, and 169 mL of ammonium hydroxide. Prepare fresh before use.
4× LDS elution buffer: Dissolve 0.666 g of Tris-HCl, 0.682 g of Tris-Base, 0.8 g of LDS, and 0.006 g of EDTA (free acid) in ultrapure dH2O to a final volume of 10 mL. Aliquot and store at −20°C.
10× Reducing Agent (Invitrogen). Store at 4°C.
1 M iodoacetamide (IAA) (Sigma): Dissolve 0.185 g of iodoacetamide in 1 mL HPLC grade water. Dispense into 50 × 20 mL aliquots and store at −20°C.
Heat block at 70°C.
2.3.3. Assessment of immunoaffinity Purification
Sample Preparation
- Reserved fractions (from Subheading 3.3.2).
- Cell pellet (step 7).
- Input supernatant (step 7).
- Flow-through (step 11).
- Primary eluate (step 21).
- Secondary eluate (step 19).
Ultrapure dH2O.
Acetone (−20°C).
1.7 mL eppendorf tubes (Fisher).
Microcentrifuge.
NuPAGE 4–12% Bis-Tris precast SDS-PAGE gel, 10 well (Invitrogen).
Xcell Sure Lock Mini-Cell electrophoresis system (Invitrogen).
20× NuPAGE MOPS SDS Running Buffer (Invitrogen).
4× NuPAGE LDS Sample Buffer (Invitrogen).
10× Reducing Agent (Invitrogen). Store at 4°C
Heat block at 70°C.
SDS-PAGE and Western Blot Analysis
- Prepared fractions (from Subheading 3.3.3).
- Cell pellet (step 1).
- Input supernatant (step 2).
- Flow-through (step 3).
- Secondary eluate (step 4).
- Primary eluate (step 5).
NuPAGE 4–12% Bis-Tris gel, 10 well (Invitrogen).
Xcell Sure Lock Mini-Cell electrophoresis system (Invitrogen).
20× NuPAGE MOPS SDS Running Buffer (Invitrogen).
1× Running Buffer: Dilute 20× NuPAGE MOPSSDS Running Buffer in 700 mL of ultrapure water.
Precision Plus Protein Dual Color Molecular Weight Standards (BioRad).
4× NuPAGE LDS Sample Buffer (Invitrogen).
10× Reducing Reagent (Invitrogen). Store at 4°C.
PVDF membrane (BioRad).
Methanol.
Transfer apparatus (e.g., Mini Trans-blot cell from BioRad).
10× Transfer Buffer: Dissolve 144 g glycine and 30.3 g Tris base in final volume of 1 L dH2O. Prepare 1 L of l× Transfer Buffer containing 20% methanol. Chill at 4°C for 30 min before use.
2× Whatman filter paper and 2× sponges for transfer.
20× TBST: 200 mM Tris–HCl pH 8, 3 M NaCl, 2% Tween-20 in dH2O. Dilute to 1× with dH2O for use.
Blocking Buffer: 5% nonfat dry milk powder in l× TBST. Appropriate primary and secondary antibodies, diluted in Blocking Buffer.
Autoradiography cassette (FisherBiotech Gat# FBCA 57).
ECL chemiluminescent substrate kit (Thermo Scientific).
Autoradiography film (Kodak).
3. Methods and Procedures
3.1. Obtaining Xenopus laevis Embryonic Tissue
Of all the proteins in X. laevis embryonic tissue, yolk proteins are among the most abundant, especially at earlier developmental stages when the embryo is still dependent on yolk for nutrients. The abundance of yolk proteins can be problematic when performing immunoaffinity purifications, as these proteins can nonspecifically react with antibodies and mask a less abundant interaction, For this reason, it is desirable to remove as much of the yolk from the embryo as possible.
Fill a 1% agarose plate with cold l× MBS. Transfer X. laevis embryos to MBS in agarose plate.
Using fine forceps and a dissecting microscope, remove as much of the yolk as possible from the rest of the embryo. Using a plastic transfer pipette, transfer the embryo to a new agarose plate containing fresh l× MBS. Keep tissue on ice until all dissections are completed. Collect appropriate number of embryos for each immunopurification to be performed (see Note 1).
Using a syringe needle, poke four holes in the cap of a 50 mL conical tube. Remove cap and secure tube into a rack in a styrofoam cooler. Fill cooler and tube with liquid nitrogen.
Using a plastic transfer pipette, drop embryos one by one into liquid nitrogen in conical tube. When finished, replace the cap and screw on tightly. Remove the tube from the cooler (using a paper towel for protection) and invert to remove the liquid nitrogen. Store frozen tissue at −80°C.
3.2. Tissue Lysis and Protein Extraction
Tissue lysis can be carried out utilizing several approaches, including homogenization in a detergent-containing lysis buffer, passage through a needle (different needle gauges can be tested for efficiency of lysis), and cryogenic tissue disruption using traditional mortar and pestle or a Mixer Mill. While the procedures described below for immunoaffinity purification of protein complexes utilize as starting material tissue disrupted cryogenically using a Mixer Mill, the other types of tissue lysis can also be incorporated. We prefer the type of cryogenic disruption described below as it leads to an increased efficiency of extraction (i.e., isolation of the targeted protein) and decreased level of nonspecific associations. This method has provided us with a reliable and effective means of cell lysis for isolating varied protein complexes (2–7). In circumstances that require a mild tissue lysis, such as the maintenance of intact organelles or large structures, e.g., postsynaptic densities (8), cryogenic disruption may not be the method of choice.
3.2.1. Cryogenic Tissue Disruption
Clean one spatula, the Retsch Mixer Mill jars, and the grinding balls sequentially with ultrapure dH2O, Windex, ultrapure dH2O, and 100% methanol. Allow all parts to dry completely in a chemical hood.
Cool the jars and balls in liquid nitrogen (e.g., using a Styrofoam container filled with liquid nitrogen). Once cooled (i.e., liquid nitrogen no longer appears to be bubbling) remove them from the liquid nitrogen container using a pair of long forceps and place the frozen tissue into the jar. The tissue can fill up to a maximum of one-third of the total volume of the jar for optimal cryogenic grinding (e.g., ~7 g frozen tissue pellets per 25 mL jar). Add the chilled ball on top of the tissue (use one-ball per jar), close the jar, and place it back into the liquid nitrogen container to cool.
Place the filled jars in the Retsch Mixer Mill holders. If only one jar contains frozen tissue for grinding, then use the other empty jar (without a ball) as a balance. Grind the tissue using 20 cycles of 2 min 30 s each at a frequency of 30 Hz. Place the jars in liquid nitrogen in between cycles to cool and ensure that the jars are still tightly closed.
Open the jar and use a chilled spatula to transfer the frozen tissue powder to a 50 mL conical tube kept on dry ice. Work as quickly as possible to avoid thawing of the ground sample. Periodically chill the spatula in liquid nitrogen. Store the powder at −80°C until immunopurification is to be performed.
3.2.2. Optimization of Lysis Buffer and isolation Conditions
Successful isolation of a protein of interest and its interacting partners is dependent on several criteria including protein abundance and subcellular localization, sample amount, affinity of the antibody used for immunoaffinity purification, efficiency of bead conjugation, and lysis buffer conditions for immunoaffinity purification. During the cell lysis and protein isolation steps it is crucial to extract and preserve the targeted protein with its interactions in a soluble fraction. Therefore, the lysis buffer conditions utilized prior and during the affinity purification have to be optimized for each protein of interest before proceeding with larger scale immunoaffinity purifications for proteomics studies. This can be done by performing small-scale experiments (i.e., 20 embryos per immunopurification) that use western blotting to assess (1) the efficiency of protein solubilization (see procedure below) and (2) efficiency of isolation (see Subheading 3.3.3). It is recommended to compare at least three lysis buffer conditions with varied levels of stringency. Generally, the stringency of a lysis buffer is determined by the concentrations and combinations of detergents and salts. Table 1 provides examples of frequently used detergents, and Table 2 lists several lysis buffers that differ slightly in their compositions and were successfully utilized in immunoaffinity purifications of protein complexes from varied species.
Split cryogenically ground tissue into equal small aliquots (e.g., 0.1 g) (see Note 2). Ensure that the tissue powder does not thaw during the weighing.
Place the small aliquots on ice (4°C) and add a different lysis buffer (5 mL buffer per 1 g cells) to each sample.
Homogenize the tissue powder in the buffer by vortexing for 1 min with intermittent cooling. This step is different than the usual homogenization for immunoaffinity purifications (see Subheading 3.3.2), which uses a polytron and a larger volume for the starting material.
Separate the soluble and insoluble fractions by centrifugation at 8,000 × g at 4°C for 10 min. Recover soluble fraction and label “supernatant.”
Wash the pellet in water and discard supernatant. Extract pellet by sequential sonication then boiling at 95°C for 5 min in 50 mM Tris–HCl, pH 7.4, containing 2% SDS. Centrifuge at 20,000× g for 10 min. Recover supernatant and label “Pellet.”
Assess the levels of bait protein in the supernatant and pellet fractions (5–10% aliquots) using western blotting and antibodies against either the affinity tag (if present) or the endogenous protein.
To proceed with a larger scale immunoaffinity purification for proteomics analyses, select the lysis buffer condition that provides the highest proportion of bait protein in the soluble fraction, at the lowest necessary stringency. This will allow for a balance between an efficient extraction of the protein of interest and maintenance of interacting partners (see Note 3).
Table 1.
Examples of detergents commonly used for cell lysis and their properties
| Detergent | Properties | Notes |
|---|---|---|
| Triton X-100 | Nonionic detergent | Depending on the utilized concentration, Triton is considered a relatively mild, nondenaturing detergent. Many enzymes remain active in 0.1–0.5% Triton X-100 solution (e.g., Proteinase K is still active in 1% solution) |
| pH 6.0–8.0 (5% aqueous solution) | ||
| Critical micelle concentration (CMC): 0.22–24 mM | ||
| Soluble at 25°C in all proportions; soluble in water, benzene, toluene, xylene, trichloroethylene, ethylene glycol, ethyl ether, ethanol, isopropanol, ethylene dichloride | Can be used to preserve protein–lipid interactions | |
| Sodium deoxycholate (DOC) | Anionic detergent | Common component of RIPA lysis buffer |
| pH 5.0–9.0 (1% aqueous solution) | ||
| CMC: 2–6 mM (0.083–0.249%, w/v) Micelle molecular weight: 2,000 g (average), at concentrations above 2 mM | Suitable for isolating membrane-associated proteins and liposome preparation. Disrupts protein–lipid interactions | |
| Soluble at 20°C; soluble in water in less than 5% solution | ||
| Digitonin | Nonionic detergent | Suitable for analyzing membrane-bound proteins and solubilizing lipids |
| pH: data not available | ||
| CMC: <0.5 mM, at 20–25°C. Micelle molecular weight: 70,000 g (average) | ||
| Soluble in water at ~5% (w/v); must be heated to 95–98°C first, then cooled to room temperature. Soluble in ethanol at 10 mg/mL | ||
| Octyl-beta-glucoside | Nonionic detergent | Suitable for studying membrane-associated proteins |
| pH: data not available | ||
| CMC: 23–25 mM (0.6716–0.7300%, w/v); Micelle Molecular Weight: 8,000 g | Readily integrated with mass spectrometry studies (i.e.., does not interfere as much as other detergents with ionization in MALDI MS experiments) | |
| Soluble in water | ||
| Nonidet P-40 | Nonionic detergent pH 5.0–8.0 (5% aqueous solution) CMC: 0.059 mM (20–25 C) Soluble in water | Milder alternative to Triton X-100; depending on the concentration it may not penetrate nuclear membranes |
Table 2.
Examples of lysis buffers used for immunoaffinity purification of protein complexes
| Bait (GFP-tagged) |
Description and localization |
Species and sample type |
Optimized lysis buffer | References |
|---|---|---|---|---|
| Apl1 | beta-adaptin of AP-2 complex; cellular membrane | S. cerevisiae cells | 20 mM K-HEPES, pH 7.4, 110 mM KOAc, 2 mM MgCl2, 0.1% Tween, 0.4% Triton, 200 mM NaCl, 1/100 (v/v) protease inhibitor mixture (20 mg/mL PMSF + 0.4 mg/mL pepstatin A), and 1/200 (v/v) protease inhibitor cocktail | (2) |
| Nup37 | Member of nuclear pore complex; subunit of Nup107–160 subcomplex | Homo sapiens cells | 20 mM K-HEPES, pH 7.4, 110 mM KOAc, 2 mM MgCl2, 0.1% TWeen, 0.5% Triton, 200 mM NaCl, 1/100 (v/v) protease inhibitor mixture (20 mg/mL PMSF + 0.4 mg/mL pepstatin A), and 1/200 (v/v) protease inhibitor cocktail | (2) |
| HDAC5 | Histone deacetylase 5; nucleus and cytoplasm | Homo sapiens cells | 20 mM HEPES-KOH, pH 7.4, 0.1 M potassium acetate, 2 mM MgCl2, 0.l% Tween-20, 1 mM ZnCl2, 1 mM CaCl2, 0.5% Triton X-100, 250 mM NaCl, 4 mg/ml DNase, 1/100 (v/v) protease, and phosphatase inhibitor cocktails | (7) |
| PAP I | poly (A) polymerase I; cytoplasm and inner membrane | Escherichia coli cells | 20 mM HEPES, pH 7.4, 0.11 M KOAc, 2 mM MgCl2, 0.1% Tween-20 (v/v), 1 mM ZnCl2 1 mM CaCl2, 1% Triton X-100, 0.5% Deoxycholate, 150 mM NaCl, 1/100 protease inhibitor cocktail, 1/200 phenylmethylsulphonyl fluoride | (6) |
| H3 | Histone 3 isoforms; nucleus | Mus musculus ES cells | 20 mM K-HFPES, pH 7.4, 110 mM K-acetate, 0.1% Tween 20, 0.5% Triton, 300 mM NaCl, and 1/100 (v/v) protease inhibitor cocktail | (5) |
| nsP3 | Sindbis nonstructural protein 3; cytoplasm | Sindbis-infected Rattus Norvegicus cells | 20 mM K-HEPES, pH 7.4, 110 mM KOAc, 2 mm MgCl2, 0.1% Tween 20, 1% Triton, 0.5% deoxycholate, 500 mM NaCl, 25 units/mL DNase, 1/100 (v/v) protease inhibitor mixture | (3) |
| PSD (via VGluRd2) | postsynaptic: densities; cerebellar excitatory synapses | Mus musculus tissue | 10 mM HEPES, pH 7.4, 2 mM CaCl2, 132 mM NaCl, 3 mM KCl, 2 mM MgSO4, 1.2 mM NaH2PO4, 0.5% Triton X-100, 1/100 (v/v/) protease inhibitor cocktail | (8) |
3.3. Immunoaffinity Purification of Protein Complexes
3.3.1. Conjugation of Magnetic Beads
This protocol has been optimized for conjugation of Dynabeads M-270 Epoxy; however, it can also be applied for conjugation of other types of magnetic beads with larger or smaller diameters. In such cases, it is important to adjust the amount of antibody used for conjugation, depending on the bead capacity of binding. This protocol can be utilized for conjugating beads with high-affinity purified in-house developed antibodies as well as commercially available ones, provided their storage buffer doesn’t hinder covalent conjugation to Epoxy.
It is best to start this protocol in the late afternoon and perform all washing steps (steps 7–9) in the morning of the following day. Unless otherwise stated all steps should be performed at room temperature. Do not allow the beads to dry out (i.e., do not keep the beads without a washing; solution in between the steps).
- Weigh out the necessary amount of magnetic Dynabeads in a round-bottom tube.
- Round-bottom tubes are preferred to avoid the trapping of beads in conical-shape tubes during the conjugation.
- The necessary amount of beads is dependent on the purpose of the experiment and the abundance of the protein that will be immunoaffinity purified. An approximate guidance: 1–2 mg beads are appropriate for small-scale optimization experiments (as described in Subheading 3.2), 5–7 mg beads are usually sufficient for performing single immunoaffinity purifications, and 10–20 mg beads are suitable for highly abundant proteins.
Add 1 mL Sodium Phosphate buffer pH 7.4 to the beads; mix by vortexing for 30 s, followed by 15 min on a tube shaker (vigorous setting).
Place the tube on a magnetic rack. After all the beads settle towards the magnetic side, discard the buffer.
Remove the tube from the rack. Add 1 mL Sodium Phosphate buffer pH 7.4. Mix by vortexing for 30 s and remove the buffer in the same manner as above.
- Remove the tube from the rack. Add, in this order, the necessary amount of antibodies. Sodium Phosphate buffer, and Ammonium Sulfate solution.
- The optimal total volume during the beads conjugation (that includes the antibodies, Sodium Phosphate buffer, and Ammonium Sulfate solution) is ~20 mL/mg beads
- As a guideline of amounts of antibodies or IgG that we routinely use: 8–10 mg Ab/mg beads for commercially available antibodies and IgG, and 3–5 mg Ab/mg beads for purified high-affinity antibodies (e.g., in-house developed anti-GFP antibodies).
- The 3 M Ammonium Sulfate solution is added last and will be one-third of total volume to give a final concentration of 1 M.
- For example, to conjugate 18 mg beads, use a total volume of 360 mL. For this, add 54 mg antibody to beads (if using 3 mg Ab/mg beads), then add 0.1 M Sodium Phosphate Buffer (volume 0.1 M Sodium Phosphate Buffer = 360 mL – volume of antibody used – 120 mL 3 M Ammonium Sulfate), then add 120 mL of 3 M Ammonium Sulfate.
Secure the tube with parafilm and rotate bead slurry overnight on rotator at 30°C.
The next morning, place the tube with bead slurry on a magnetic rack. Remove and reserve the supernatant to assess the efficiency of bead conjugation by SDS-PAGE (see Note 4).
- Wash the beads sequentially with the following buffers. For each wash, gently resuspend the beads in 1 mL of the buffer and then place the tube on the magnet and remove buffer. “FAST” indicates that buffer should not be in contact with beads for longer than it takes to resuspend them:
- 1 mL of Sodium Phosphate buffer.
- 1 mL 100 mM Glycine-HCl, pH 2.5 (FAST).
- 1 mL 10 mM Tris-HCl pH 8.8.
- 1 mL 100 mM Triethylamine solution (FAST).
- 4× 1 mL DPBS.
- 1 mL DPBS containing 0.5% Triton X-100. Leave the tube on a Tomy shaker (gentle setting) for 15 min.
- 1 mL DPBS.
Beads can be used immediately or stored at 4°C in DPBS containing 0.02% NaN3. For beads stored for future use, measure the final volume of the bead slurry (the bead size will contribute to the final volume) and make a note of the volume required for 1 mg beads as this will permit known aliquots of beads to be removed for multiple immunoaffinity purifications. Beads should be used within 2 weeks of conjugation. After 1 month of storage, their efficiency for isolation decreases by approximately 40%.
3.3.2. Immunoaffinity Purification
Basic Elution of Immunoisolates
It is important to prepare all necessary reagents beforehand. Carry out all procedures on ice unless otherwise noted. At several steps during the protocol aliquots of samples (indicated with RESERVE) are taken to assess bait protein extraction and isolation efficiency (see Subheading 3.3.3).
- Day 1
-
1Prepare appropriate volume of optimized lysis buffer as determined in Subheading 3.2.2. Precool to 4°C. Add protease inhibitors just before use. Prepare 10 mL of wash buffer per sample (used in steps 6, 12–15), which is usually identical in composition to the optimized lysis buffer, except protease and phosphatase inhibitors cocktails are not included.
-
2Incubate the frozen tissue powder on ice for 1–2 min, but do not thaw. Proceed immediately to step 3.
-
3Resuspend the frozen tissue powder in appropriate volume of lysis buffer by first adding a small amount of lysis buffer and swirling homogenate to solubilize pellet. Continue to add lysis buffer and gently mixing by hand until tissue powder has been completely solubilized (sec Note 5).
-
4Run Polytron 10 s in ultrapure dH2O to wash. Ensure that the tissue homogenate occupies 1/3 of the conical tube volume. Subject tissue lysates to Polytron homogenization for 2× 15 s (speed = 22.5k), resting the sample on ice for a few minutes between homogenizations. If processing additional samples, rinse and run Polytron in ultrapure dH2O to wash out excess lysate. When finished, perform a final rinse with methanol.
-
5Centrifuge the lysate at 8,000× g at 4°C for 10 min.
-
6During centrifugation, place tube containing antibody-conjugated magnetic beads on a magnetic rack for 30–60 s. Discard the storage buffer and wash with 3× 1 mL wash buffer by gently pipetting up and down to resuspend the beads. Do not vortex antibody-conjugated beads. Suspend beads in 100–200 mL of wash buffer.
-
7Carefully pour the clarified lysates (supernatant) into new 50 mL conical tubes (see Note 6). RESERVE (i) the cell pellet and (ii) 40 mL of the input supernatant (see Subheading 3.3.3 for analysis).
-
8Gently flick tube of antibody-conjugated beads to mix beads in solution. Pipette the appropriate amount of beads into the clarified lysates.
-
9Rotate the lysates with beads on a rotator at 4°C for 1 h. Do not use longer incubation times as this promotes the accumulation of nonspecific binding and loss of weak interacting partners.
-
10During incubation, prepare base elution buffer and l× LDS elution buffer (see Note 7).
-
11Attach a bar magnet to the lysates/bead suspension tube using a rubber band. Incubate on ice for 5 min. RESERVE the flow-through (unbound) fraction by pouring the supernatant into a clean conical tube (see Subheading 3.3.3 for analysis).
-
12Resuspend the beads in 1 mL of wash buffer and transfer the bead slurry to a round-bottom eppendorf tube.
-
13Place on a magnetic rack for 30 s to pellet the beads and discard wash buffer. Perform this procedure between all subsequent wash steps.
-
14Wash the beads 3 × 1 mL wash buffer. On the third wash, transfer the bead slurry to a clean round-bottom eppendorf tube, then pellet beads and discard wash buffer.
-
15Wash the beads 2 × 1 mL with wash buffer.
-
16Add 1 mL DPBS to beads and transfer slurry to clean round-bottom eppendorf tube. Repeat wash once with 1 mL of DPBS to remove residual detergent. Quantitatively remove DPES wash.
-
17Add 750 mL of base elution buffer. Incubate at RT for 20 min while shaking.
-
18Place the tube on the magnetic rack and transfer the eluate to an Axygen microcentrifuge tube. Freeze primary eluate in liquid nitrogen and evaporate to dryness overnight by vacuum centrifugation.
-
19Perform a second elution from the beads by suspending the beads in 40 mL of 1× LDS elution buffer containing 50 mM DTT, incubating at 70°C for 10 min, and then at RT for 10 min while shaking. Place the tube on a magnetic rack and transfer the eluate to a clean microcentrifuge tube. RESERVE 10% (4 mL) of the secondary eluate in a clean microcentrifuge tube (see Subheading 3.3.3 for analysis). Freeze remaining 90% of secondary eluate in liquid nitrogen and store at −20°C.
-
20Proceed to Subheading 3.3.3 to prepare reserved samples for Western blot analysis. Continue with step 21 the following day.
-
1
- Day 2
-
21Remove dried eluate from the Speedvac.
- It performing SDS-PAGE-in-gel digestion (see Note 8), suspend dried eluate in 40 mL of l× NuPAGE sample buffer containing l× reducing agent and heat at 70°C for 10 min. RESERVE 10% (4 mL) of the eluate in a clean microcentrifuge tube. Add 4 mL of 1 M iodoacetamide to remaining 90% of primary eluate and incubate 30 min at RT protected from light. Freeze in liquid nitrogen and store at −20°C or proceed immediately to proteomic analysis (see Chapter 22, Subheading 2.1).
- If in-solution digestion is to be performed (see Note 8), suspend dried eluate in 40 mL of l× LDS elution buffer containing 50 mM DTT and heat at 70°C for 10 min. RESERVE 10% (4 mL) of the eluate in a clean microcentrifuge tube. Freeze remaining 90% of primary eluate in liquid nitrogen and store at −20°C or proceed immediately to proteomic analysis (sec Chapter 22, Subheading 2.2).
-
21
Alternate Procedure (Detergent Elution of Immunoisolates; See Note 7)
- Day 1
- Perform steps 1–16 as described earlier, except preparation of base elution buffer can be omitted (step 10).
- Add 40 mL of l× LDS elution buffer to beads. Incubate 10 min at 70°C, then 10 mm at RT while shaking.
- Pellet beads on magnetic rack and transfer primary eluate to an Axygen microcentrifuge tube.
- Repeat steps 2 and 3, transferring secondary eluate to an Axygen microcentrifuge tube.
- Add 2.0 mL of 1 M DTT to primary and secondary eluates. Heat at 70°C for 10 min. RESERVE 10% (4 mL) of eluates in clean microcentrifuge tubes.
- Freeze remaining 90% of eluates in liquid nitrogen and store at −20°C or proceed immediately to proteomic analysis (see Chapter 22, Subheadings 2.1 or 2.2).
3.3.3. Assessment of Immunoaffinity Purification
Sample Preparation
Reserved sample aliquots from Subheading 3.3.2 are prepared for SDS-PAGE and Western blot analysis. The recommended amount of each aliquot analyzed is provided as a starting point, and may need further optimization for differences in input material and bait protein abundance.
- Cell pellet
- Wash the cell pellet with 1.0 mL dH2O. Homogenize washed cell pellet in 1.0 mL of 2% SDS, transfer to microcentrifuge tube, and heat at 70°C for 10 min. Centrifuge at maximum speed for 5 min at RT (see Note 9).
- Remove aliquot of SDS-soluble pellet fraction that corresponds to an identical percent of the input supernatant. For example, assuming a 40 mL aliquot of input supernatant was reserved from a total lysis volume of 10 mL, a 4 mL aliquot of the pellet sample should be removed.
- Dilute aliquot of SDS-soluble pellet fraction to a final volume of 60 mL containing l× NuPAGE LDS Sample Buffer/l× Reducing Agent.
- Input supernatant
- Dilute 40 mL of reserved input supernatant to a final volume of 60 mL containing l× NuPAGE LDS Sample Buffer/l× Reducing Agent.
- Flow-through (see Note 10)
- Transfer 10% of flow-through to clean tube. Slowly add 4 volumes of −20°C acetone. Vortex briefly. Incubate at −20°C for at least 1 h.
- Centrifuge at 3,000× g at 4°C for 10 min. Pour off supernatant.
- Briefly wash pellet with 4 volumes of 80% acetone/20% dH2O and discard.
- Air dry pellet for 5 min, then partially solubilize in 40 mL of l× NuPAGE LDS Sampler Buffer/l× Reducing Agent by gentle agitation.
- Secondary eluate
- Dilute 4 mL (10%) of the secondary eluate into a final volume of 40 mL l× NuPAGE LDS Sample Buffer/l× Reducing Agent.
- Primary Eluate (prepared the following day, see Note 11)
- Dilute 4 mL (10%) of the primary eluate into a final volume of 40 mL l× NuPAGE LDS Sample Buffer/l× Reducing Agent.
Heat all samples at 70°C for 10 min. Freeze the samples at −20°C until ready to proceed with SDS-PAGE and Western Blot Analysis.
SDS-PAGE and Western Blot Analysis
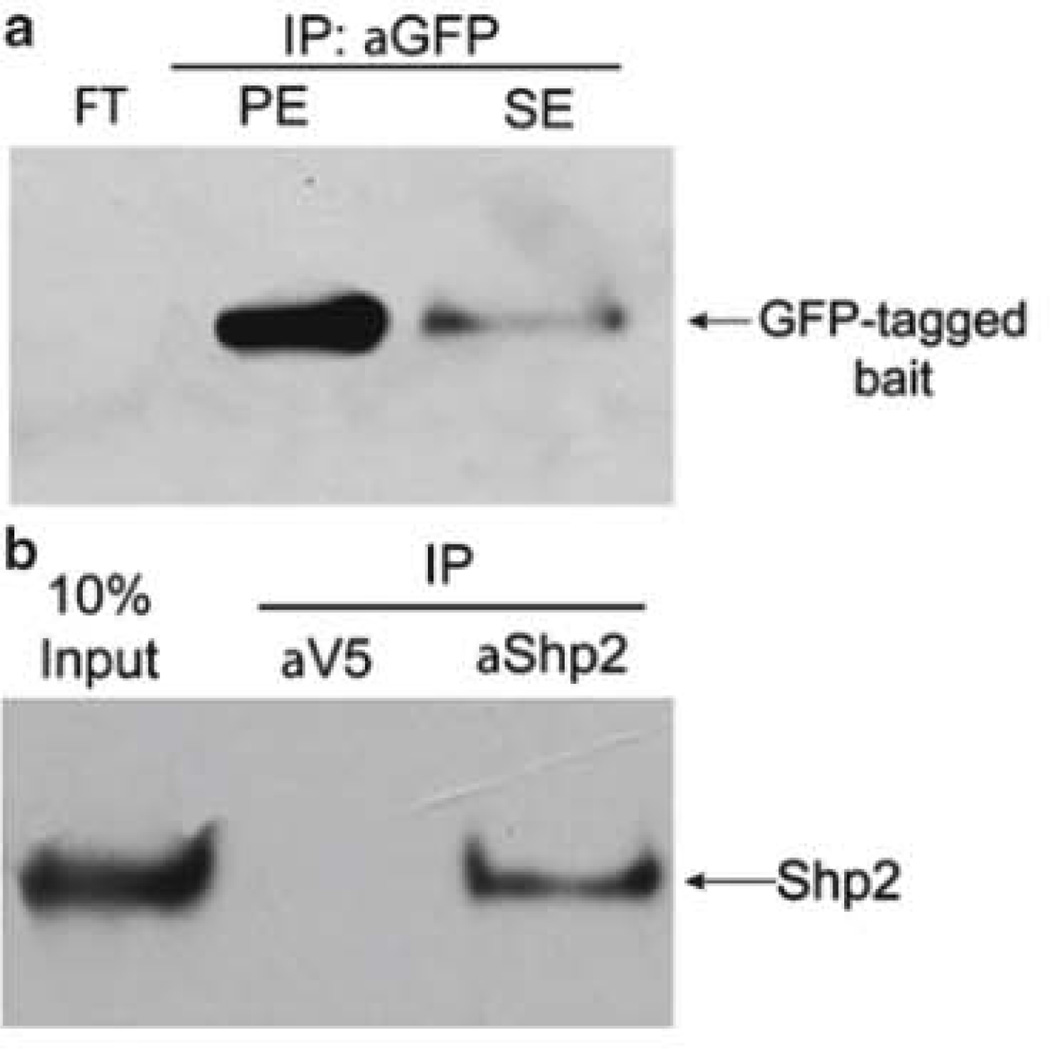
In this protocol the efficiency of immunoaffinity isolation of the bait protein is assessed by comparing five samples reserved at progressing stages of the immunoaffinity purification procedure: (1) cell pellet, (2) input supernatant, (3) flow-through, (4) secondary eluate, and (5) primary elute (see Subheading 3.3.2). While the amount of sample prepared (see Subheading “Sample Preparation”) is sufficient for duplicates analyses, the protocol below details a single experiment. A representative western blot is shown below (Fig. 2a) indicating efficient isolation (no protein remaining in the flow-through) and recovery of the intended GFP-tagged bait protein (a majority of the protein was in the primary eluate).
- Set up the Xcell SureLock Mini-Cell electrophoresis system:
- Remove the white strip and the upper comb from the NuPAGE 4–12% Bis-Tris precast SDS-PAGE gel and rinse wells with ultrapure dH2O. Place the gel in the apparatus, using a buffer dam for the opposing side, then lock the assembly in place.
- Fill the inner chamber with 200 mL and the outer chamber with 500 mL of l× Running Buffer.
Thaw fractions if previously frozen and load into wells as follows: Lane 2, 10 mL Molecular Weight Standards; Lane 3, 30 mL of input supernatant; Lane 4, 30 mL of cell pellet; Lane 5, 20 mL of primary eluate; Lane 6, 20 mL of secondary eluate; Lane 8,20 mL of flow-through; Empty lanes, 20 mL of 1× LDS Sample Buffer.
Eleetrophorese for 5 min at 100 V, then 45–50 min at 200 V, or until the dye front has migrated all the way down the gel.
While the gel is running, cut two pieces of Whatman filter paper and one PVDF membrane to the appropriate gel size. Always handle the membrane with tweezers.
Prewet PVDF in methanol and soak along with 2 transfer sponges, 2 filter papers, in precooled l× Transfer Buffer at least 15 min at 4°C.
Open gel cassette to expose gel and discard the wells. Working with wet gloves, transfer gel (by the thick ridge at the bottom) into a plastic tray containing l× Transfer buffer. Remove bottom ridge.
Assemble a WB “sandwich” in the tray with presoaked items. Layer them in a transfer cassette as follows, starting from the clear side of the cassette: sponge, filter paper, PVDF membrane, SDS-PAGE gel, filter paper (gently roll out any bubbles using a 15 mL conical tube), sponge, Close the sandwich and place into transfer apparatus with black side of cassette (gel side) facing black (anode) side transfer core. Place ice tray and stir bar into apparatus. Pour ice-cold l× Transfer Buffer into the apparatus until it covers fully the cassette.
Transfer at 100 V for 1.5 h at 4°C while stirring.
Open transfer cassette and discard the filter papers and gel. Verify the prestained Molecular Weight Standards transferred to the membrane.
Wash PVDF membrane with l× TBST, discard, then add Blocking Buffer and incubate for 1 h at RT on a rocking platform. Do not let the membrane dry.
Discard Blocking Buffer and add the primary antibody diluted in Blocking Buffer. Incubate for 1–2 h at RT or overnight at 4°C.
Wash the membrane 3 × 20 mL in l× TBST for min while rocking at RT.
Add the secondary horseradish peroxidase-conjugated antibody diluted in Blocking Buffer. Incubate for 1 h at RT.
Wash the membrane 4 × 20 mL in l× TBST for 5 min while rocking at RT.
Mix ECL chemiluminescence substrates in 1:1 ratio (a total volume of 1 mL is usually sufficient to cover the membrane). Using tweezers, place the membrane on a dry plastic surface and apply the ECL solution, incubating for 1 min. Blot off the excess substrate and place the membrane between a sheet protector or Saran-wrap and tape into a film cassette.
Carefully place a piece of autoradiography film on top of the membrane and close the cassette avoiding any film shifts as this will result in smeared bands. An initial exposure of 30 s will indicate whether subsequent exposures of longer or shorter duration are required.
Fig. 2.
Assessment of isolation efficiency and specificity of immunoaffinity purification. (a) A GFP-tagged bait protein was isolated (see Subheading 3.3.2) and eluted with the alternate detergent-based procedure. Ten percent of the following fractions are analyzed to assess tine efficiency of isolation of a GFP-tagged bait protein: FT flow-through; PE primary eluate; SE secondary eluate. The majority of bait protein was found in the primary eluate. (b) Immunoaffinity purification of endogenous Shp2 protein from 100. stage 40 Xenopus tropicals whole embryos. As a control for the specificity of the Shp2 antibody, a second immunopurification was performed in parallel using an anti-V5 antibody.
3.3.4. Appropriate Controls
To assess the specificity of immunoaffinity isolation it is critical to have a control sample analyzed in parallel, beginning from tissue lysis through the isolation of the target protein. The controls described below account for nonspecific binding of proteins to magnetic beads, antibody, and affinity tag (when present).
GFP Tagged
Often the bait protein is expressed as a GFP fusion protein. Here, parallel isolations of the GFP-tagged protein and GFP alone are highly desirable. If the GFP-tagged protein is stably overexpressed by mRNA injection into embryos, it is necessary to generate a GFP-only tissue/animal in the same manner as the GFP fusion protein, i.e. mRNA injection of a GFP-only expression construct. Moreover, all treatment conditions and experimental variables, such as lysis buffer composition, should be identical between the two isolations. Thus, both the tag alone and the fusion protein can be immunoaffinity purified using identical preparations of antibody-conjugated magnetic beads. If lysis buffer conditions or other isolation variables are altered, such as incubation time of sample with antibody, an additional control should be performed.
FLAG Tagged
If the bait protein is tagged with FLAG, then a more appropriate control (compared to the GFP tag) is the respective cell/tissue/animal but under wild-type conditions. For both FLAG-tagged and wild-type conditions, magnetic beads conjugated with an antibody against the FLAG tag should be utilized. Although this doesn’t control for proteins that bind nonspecifically to FLAG itself, nonspecificity due to the antibody and beads can be determined.
Endogenous, Nontagged Protein
In many experiments it is essential to immunoaffinity purify the bait protein at an endogenous level of expression (under its native promoter). Here, the control sample will use the identical cell/tissue/animal as for the experimental condition. In contrast to an affinity tag, the beads for the negative control are conjugated with either nonspecific IgG or an IgG that lacks reactivity towards the endogenous bait protein. The control protein isolation will reveal interactions that bind nonspecifically to the antibody and magnetic beads. A representative western blot from an immunoisolation of endogenous Shp2 from X. tropicalis embryos is shown in Fig. 2b.
Footnotes
The number of embryos needed for each immunopurification is dependent on a number of criteria including protein abundance and solubility as well as the affinity of the antibody used for immunoisolation. For this reason, the optimal number of embryos and resulting amount of tissue must be optimized individually for each protein studied. Generally, 20–50 embryos per immunoisolation is a good starting point for small-scale experiments and may be scaled up for larger immunoaffinity purifications for proteomics studies.
If starting directly with a small amount of tissue, the sample could be ground using round-bottom eppendorf tubes or used directly for incubation with the various lysis buffers. While performing the cryogenic grinding is preferred to mimic the conditions that will be utilized for protein isolation, direct resuspension in lysis buffer is an alternative that can adequately guide selection of an optimal buffer composition.
The balance between extraction efficiency and maintenance of interacting partners can be further optimized by increasing the stringency of the buffer used to wash the magnetic beads relative to the buffer used for tissue homogenization.
To test the efficiency of bead conjugation, prepare the following samples for SDS-PAGE analysis: (1) Dilute 1 mg neat antibody with 2.5 mL 10× Reducing Agent and 6.25 mL 4× NuPAGE LDS Sample Buffer. Bring the sample to 25 mL total volume with dH2O. (2) Calculate the amount of bead supernatant that is equivalent to the amount of neat antibody in the previous sample, assuming that no antibody successfully conjugated to the beads. Dilute this amount with 2.5 mL 10× Reducing Agent and 6.25 mL 4× NuPAGE LDS Sample Buffer. Bring sample to 25 mL with ultrapure H2O. Heat samples for 10 min at 70°C. Centrifuge the samples at 20,000× g for 3 min at room temperature and load onto an SDS-PAGE gel. Stain the gel with SimplyBlue SafeStain and look for a reduction in the amount of antibody in the lane containing the bead supernatant. Refer to Cristea et al. (2) for the expected amount of unbound antibody resulting from differing amounts of antibody used in the conjugation.
After suspending tissue powder in lysis buffer, the solution may be slightly turbid, but should be devoid of tissue “clumps.” Do not proceed to Polytron (step 4) until a homogenous suspension is observed. If necessary, additional rotation for 10–20 min at 4°C can be performed.
If insoluble particles are present in supernatant after centrifugation, a pipette can be used to selectively transfer supernatant to clean 50 mL conical tube.
The base elution buffer is preferred as significantly less background protein contamination is observed under these elution conditions. However, if low recovery of bait protein is observed, e.g., when a high affinity antibody is used, an “Alternate Procedure” can be followed that uses a harsher detergent-based elution buffer (see above).
The selection of an in-gel or in-solution digestion approach depends largely on the properties and nature of the proteins within the samples, e.g. pI, molecular weight, hydrophobicity, complexity, dynamic range, and total yield. In general, for high complexity and large yield and/or dynamic range of protein abundances, an in-gel approach is often desired. For further discussion, see Chapter 22, Subheading 1.
For viscous samples, brief sonication can be used to aid solubilization of cell pellet.
As the flow-through fraction often has higher protein concentration, the percent of material analyzed may need to be adjusted to prevent overloading of the SDS gel. For NuPAGE gels of 1.0 mm thickness, between 100 and 150 mg is recommended.
If base elution buffer is used, processing of the primary eluate and its reserved fraction is not performed until the day after the immunoisolation was started (see Subheading 3.3.2, step 20).
References
- 1.Nieuwkoop P, Faber J. Normal table of Xenopus laevis. Garland Publishing; New York: 1994. [Google Scholar]
- 2.Cristea IM, et al. Fluorescent proteins as proteomic probes. Mol Cell Proteomics. 2005;4(12):1933–1941. doi: 10.1074/mcp.M500227-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Cristea IM, et al. Tracking and elucidating alphavirus-host protein interactions. J Biol Chem. 2006;281(40):30269–30278. doi: 10.1074/jbc.M603980200. [DOI] [PubMed] [Google Scholar]
- 4.Moorman NJ, et al. A targeted spatial-temporal proteomics approach implicates multiple cellular trafficking pathways in human cytomegalovirus virion maturation. Mol Cell Proteomics. 2010;9(5):851–860. doi: 10.1074/mcp.M900485-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg AD, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell. 2010;140(5):678–691. doi: 10.1016/j.cell.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabetta VJ, Silhavy TJ, Cristea IM. The response regulator SprE (RssB) is required for maintaining poly(A) polymerase I-degradosome association during stationary phase. J Bacteriol. 2010;192(14):3713–3721. doi: 10.1128/JB.00300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greco TM, et al. Nuclear import of histone deacetylase 5 by requisite nuclear localization signal phosphorylation. Mol Cell Proteomics. 2011;10(2) doi: 10.1074/mcp.M110.004317. M110.004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selimi F, et al. Proteomic studies of a single CNS synapse type: the parallel fiber/purkinje cell synapse. PLoS Biol. 2009;7(4):e83. doi: 10.1371/journal.pbio.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]