Abstract
Diverse developmental and degenerative single-gene disorders such as polycystic kidney disease, nephronophthisis, retinitis pigmentosa, the Bardet–Biedl syndrome, the Joubert syndrome, and the Meckel syndrome may be categorized as ciliopathies — a recent concept that describes diseases characterized by dysfunction of a hairlike cellular organelle called the cilium. Most of the proteins that are altered in these single-gene disorders function at the level of the cilium–centrosome complex, which represents nature’s universal system for cellular detection and management of external signals. Cilia are microtubule-based structures found on almost all vertebrate cells. They originate from a basal body, a modified centrosome, which is the organelle that forms the spindle poles during mitosis. The important role that the cilium–centrosome complex plays in the normal function of most tissues appears to account for the involvement of multiple organ systems in ciliopathies. In this review, we consider the role of the cilium in disease.
STRUCTURE AND FUNCTION OF THE CILIUM– CENTROSOME COMPLEX
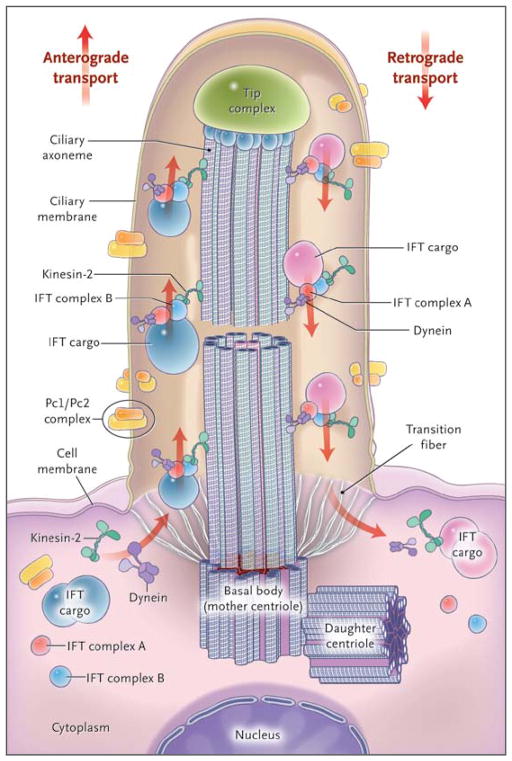
Primary cilia consist of a microtubule-based ciliary axoneme, assembled from a basal body, which represents one of the two centrioles of the centrosome (Fig. 1). Primary cilia are surrounded by a membrane lipid bilayer that maintains a lipid and protein content distinct from that of the plasma membrane. Cilia are classified as 9+2 or 9+0, depending on whether the axoneme includes an additional central pair of microtubules.5 The boundary between the ciliary and other cell compartments is demarcated by the transition zone (Fig. 1). Motor proteins transport cargo proteins along the ciliary axoneme, a process known as intraflagellar transport.
Figure 1. Structure of the Cilium and Intraflagellar Transport.
The cilium is a hairlike structure on the cell surface that consists of a microtubule-based axoneme covered by a specialized plasma membrane, which is assembled from the basal body, or mother centriole. Transition fibers act as a filter for molecules passing into or out of the cilium. Nephrocystin-1 is localized at the transition zone of epithelial cells (not shown).1 Axonemal and membrane components are transported in raft macromolecular particles (complexes A and B) by means of intraflagellar transport (IFT) along the axonemal doublet microtubules2 toward the tip complex by heterotrimeric kinesin-2. Mutations of Kif3a cause renal cysts and aplasia of the cerebellar vermis in mice.3 Retrograde transport occurs by means of the motor protein cytoplasmic dynein. (Adapted from Bisgrove and Yost.4)
Cilia are highly conserved throughout evolution. Thus, studies in the green alga Chlamydomonas reinhardtii have identified evolutionarily conserved intraflagellar-transport proteins and have enhanced our understanding of cilia biology.6 Virtually all vertebrate tissues or cell types can produce primary cilia, also termed sensory cilia, which transmit signals to the interior of cells (Fig. 1). Cilia sense a wide variety of extracellular signals and transduce them into decisions regarding proliferation, polarity, nerve growth, differentiation, or tissue maintenance. A broad range of signals can be received by specific ciliary receptors, including photosensation, mechanosensation, osmosensation, thermosensation, hormone sensation, and olfactory sensation. Another type of cilia, termed motile cilia, is structurally similar to primary cilia. Genetic defects of motile cilia cause primary ciliary dyskinesia, which characterizes a group of diseases, such as Kartagener’s syndrome, that are beyond the scope of this review.
SINGLE-GENE CILIOPATHY SYNDROMES
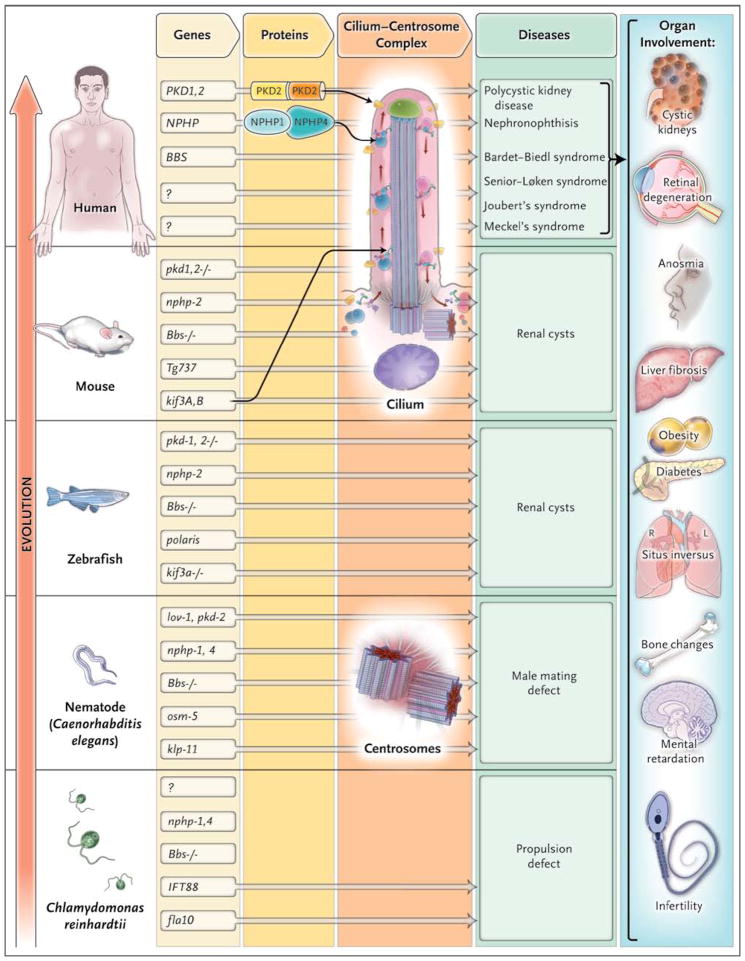
Genes causing ciliopathies are highly conserved. Many different genes are involved in the maintenance of cilia, and their encoded proteins interact dynamically within multimeric protein complexes that are expressed at the cilium, basal body, centrosome, and mitotic spindle in a cell-cycle–dependent manner. Since cilia are widespread, mutations in these genes affect a variety of tissues and organ systems in which the functions of the cilium–centrosome complex are critical. Figure 2 summarizes the strong evolutionary conservation of ciliopathy-related genes and the finding that functional convergence at cilia and centrosomes may underlie the multiorgan involvement in ciliopathies.
Figure 2. Evolutionary Conservation of Ciliopathy Genes and Organ Involvement.
The cilium–centrosome complex (CCC) has been conserved throughout evolution and across organ systems. The flow of genetic information is shown from left to right, from genes to proteins and their expression at the CCC and then to disease phenotypes. The vertical axis represents evolutionary time. There is strong evolutionary conservation of “ciliopathy genes.” Many ciliopathy proteins directly interact. The pathogeneses of ciliopathies converge at cilia and centrosomes. Many of the orthologues of genes in vertebrate cystic kidney disease (e.g., lov-1 or nph-4) are expressed in ciliated neurons of the head and tail of the nematode Caenorhabditis elegans, where they cause a phenotype of a male mating defect if knocked down. Many proteins altered in the Bardet–Biedl syndrome are conserved as basal body components of motile cilia of Chlamydomonas reinhardtii, in which mutations lead to a phenotype of defective intraflagellar transport or propulsion. Sensory cilia and centrosomes are key to ciliopathies because they serve distinct functions in different tissues, causing a broad range of organ involvement. (Adapted from Hildebrandt and Zhou.7)
THE CILIUM–CENTROSOME COMPLEX IN CYSTIC KIDNEY DISEASES
The possibility that dysfunction of nonmotile cilia might play a role in human disease was first considered after the orthologous protein of human polycystin-1, the gene for which is mutated in autosomal dominant polycystic kidney disease (ADPKD) type 1, was shown to be expressed in ciliated neurons of the nematode Caenorhabditis elegans.6 Subsequently, protein products of the mutated genes in cystic kidney diseases were found to localize in primary cilia, best exemplified by the intraflagellar-transport protein IFT88/polaris.8 It was shown that polycystin-2, the second gene mutated in ADPKD, is located in kidney cilia, as are many other proteins.9–12 In addition, positional cloning of NPHP2/inversin and NPHP313 — genes involved in the degenerative cystic kidney disease nephronophthisis — and the demonstration that the encoded proteins of these genes localize to primary cilia12,13 further supported the pathogenic role of ciliary proteins in cystic kidney disease.11–14 Similar observations made in the Bardet–Biedl syndrome,15 a disorder characterized by variable combinations of kidney disease, blindness, mental retardation, polydactyly, obesity, and diabetes, offered additional evidence to support a major role of ciliary proteins in cystogenesis and indicated that ciliary dysfunction can lead to pleiotropic clinical effects; these observations, in turn, led to the hypothesis that a group of diseases can be classified as ciliopathies.12–16 Figure 2 summarizes the pathogenic basis for the concept of ciliopathies, in which mutated genes and their products cause cystic kidney diseases in humans, mice, and zebrafish and are expressed in the primary cilia or centrosomes of renal epithelial cells.11–13
We now consider the most prominent single-gene ciliopathies, their clinical features, and their pathogenic relationship to the function of the cilium–centrosome complex (Fig. 2). Thereafter, we describe the signaling mechanisms downstream to this complex and the mechanisms of multiorgan involvement.
DOMINANT DISORDERS
Autosomal Dominant Polycystic Kidney Disease
In the United States and Europe, ADPKD is the most common potentially lethal autosomal dominant disease, afflicting about 1 in 1000 persons.17 The two mutated genes in ADPKD, PKD1 (in the majority of cases) and PKD2, encode polycystin-1 and polycystin-2, respectively, and both these proteins are important in renal tubular cell differentiation and maintenance.17,18 End-stage renal disease (ESRD) generally develops by 55 to 75 years of age. At an earlier age, the manifestations of ADPKD include hypertension, abdominal pain, a palpable abdominal mass, hematuria, urinary tract infections, cerebral aneurysms, and intestinal diverticulosis. ADPKD types 1 and 2 exhibit autosomal dominant segregation within families. However, the cellular defect that leads to cyst formation is probably the result of rare spontaneous somatic mutations of the second allele in a few cells within the kidney and other organs (the second-hit hypothesis).19 Thus, the pathogenic effect of loss of function of PKD1 or PKD2 is genetically recessive (i.e., the loss of both alleles appears to be required). Studies of a conditional knockout mouse model for Pkd120 have confirmed the central role of polycystin-1 in renal tubular morphogenesis, as well as in tissue maintenance and repair.18
Von Hippel–Lindau Disease
An autosomal dominant disorder, von Hippel–Lindau disease is caused by heterozygous germ-line inactivation of the VHL tumor-suppressor gene, which resides on chromosome 3p25.21 It is characterized by the development of multiple hemangioblastomas in the central nervous system and retina, clear-cell carcinoma of the kidney, and pheochromocytoma. The relationship between von Hippel–Lindau disease and ciliary function is discussed in the Supplementary Appendix, available with the full text of this article at NEJM.org.
RECESSIVE DISORDERS
Autosomal Recessive Polycystic Kidney Disease
Autosomal recessive polycystic kidney disease (ARPKD) is characterized by bilateral renal cystic enlargement that may become evident in utero. ESRD may develop in the neonatal period, in infancy, in childhood, in adulthood, or not at all, depending on the severity of the two recessive mutations of the causative gene in polycystic kidney and hepatic disease type 1, PKHD1.22 Intrahepatic bile-duct dysplasia causes chronic liver fibrosis in this rare disorder. The PKHD1 gene was identified by positional cloning and by the demonstration that mutations in the orthologous rat gene (pck) cause polycystic kidney disease in the rat model.23,24 PKHD1 encodes the membrane-associated receptor-like protein fibrocystin (also known as polyductin), which plays a role in terminal differentiation of the collecting-duct and biliary systems. PKHD1 is found in the primary cilia of renal epithelial cells, where it colocalizes with polycystin-2.25
Nephronophthisis
Nephronophthisis is the most frequent genetic cause of ESRD during the first three decades of life (median age, 13 years).7,26 In contrast to polycystic kidney disease, nephronophthisis is characterized by cysts that are restricted for the most part to the corticomedullary junction, and kidney size is normal or reduced. Mutations in 11 different recessive genes (NPHP1 to NPHP11) have been identified as causing nephronophthisis.7,27 Mutations in NPHP1 cause juvenile nephronophthisis type 1.28,29 NPHP1 encodes the protein nephrocystin-1 (NPHP1),1 and NPHP1 interacts with the products of other genes associated with nephronophthisis, such as NPHP2 (also known as inversin),14 NPHP3,13 and NPHP4,30–32 as well as with other signaling proteins33,34 (Fig. 3). Whereas mutations of the inversin gene (INVS) cause infantile nephronophthisis (NPHP2), either with or without situs inversus or cardiac ventricular septal defect, missense mutations in NPHP3 are associated with disease of adolescent onset.13 Demonstration of the interaction of NPHP2 with β-tubulin, the major component of the ciliary axoneme, and localization of NPHP2 and NPHP3 expression in primary cilia extended the discovery of ciliary expression to the nephronophthisis group of cystic kidney diseases (Fig. 3).11,12,14,27,35 Mutations in NPHP4 were identified in patients who had nephronophthisis with or without retinal degeneration.30,31 Interestingly, NPHP4 is conserved in C. elegans and is expressed in a group of ciliated neurons in the heads and tails of this nematode,36,37 where the mutated genes in the Bardet–Biedl syndrome15 and in ADPKD38 are also expressed (Fig. 2). Knockout of nphp-1 and nphp-4 function in C. elegans led to male mating defects36,37 that are similar to those described in association with pkd1 and pkd2 loss of function38 (Fig. 2). These mating difficulties were attributed to defects of osmosensor ciliated neurons in the nematode.39
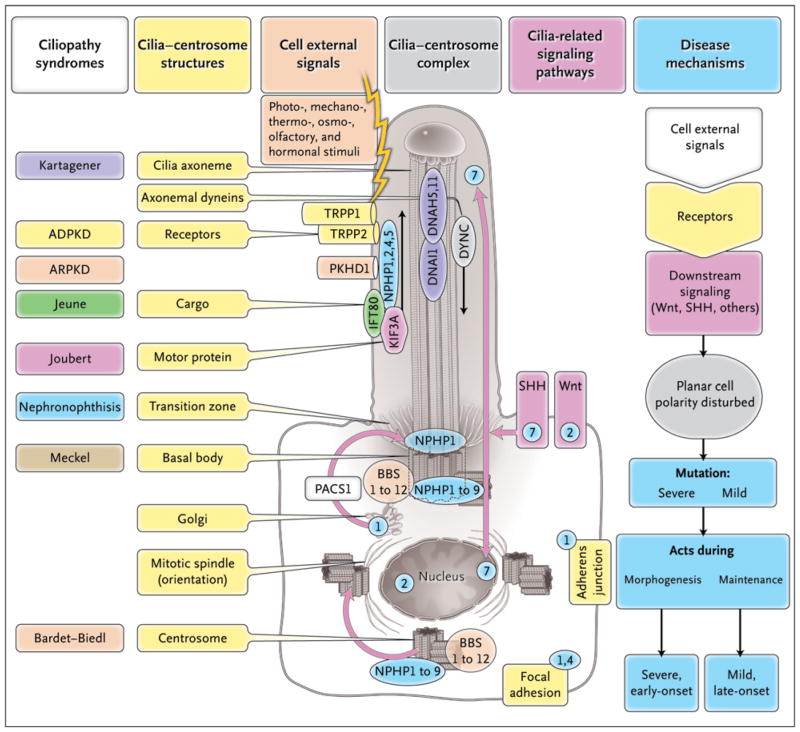
Figure 3. Ciliopathy Proteins and Their Relationships to the Cilium–Centrosome Complex (CCC).
Single-gene ciliopathies are shown, with colors matching the respective gene products located at the CCC machinery. Subcellular components of the CCC can be seen within a ciliated epithelial cell and include polycystin-1 (TRPP1), polycystin-2 (TRPP2), fibrocystin-polyductin (PKHD1), intraflagellar-transport (IFT) cargo, kinesin anterograde motor components (KIF3A), and cytoplasmic dynein (DYNC). Receptors on cilia perceive cell external signals and process them through the Wnt, sonic hedgehog, and focal adhesion signaling pathways. These pathways play a role in planar cell polarity, which is mediated partially through the orientation of centrosomes and the mitotic spindle poles. Depending on the severity of mutations within the same gene (e.g., in nephronophthisis type 6 [NPHP6]), they may act either during morphogenesis to cause a severe, early-onset, developmental disease phenotype (e.g., Meckel’s syndrome) or during tissue maintenance and repair to cause a mild, late-onset, degenerative disease phenotype (e.g., the Senior-Løken syndrome). The numbers in blue circles denote subcellular sites of different nephrocystins (NPHP1, 2, 4, 5, and 7).
Retinal–Renal Syndromes
Nephronophthisis is often accompanied by extrarenal symptoms. Nephronophthisis in association with retinal degeneration is known as the Senior–Løken syndrome. Although retinal degeneration is not present in all forms of nephronophthisis, it is invariably present when there are mutations in NPHP5.40 Nephrocystin-5 interacts with the GTPase regulator in retinitis pigmentosa (RPGR), which, if mutated, causes X-linked retinitis pigmentosa.41 Nephrocystin-5 and RPGR are both localized in the connecting cilia of photoreceptors and in the primary cilia of renal epithelial cells.40 The fact that these two types of cilia are structural equivalents probably explains why both the eye and the kidney are affected in persons with the Senior–Løken syndrome (Fig. 2).
Joubert’s Syndrome
Another disorder frequently associated with nephronophthisis is Joubert’s syndrome, which is characterized by mental retardation and ataxia due to hypoplasia of the cerebellar vermis in association with retinal coloboma, and by an irregular breathing pattern during the neonatal period.42,43 Joubert’s syndrome is characterized by a peculiar malformation of the midbrain–hindbrain junction, which appears radiologically as the “molar-tooth sign” and consists of hypoplasia or aplasia of the cerebellar vermis, thick and maloriented superior cerebellar peduncles, and abnormally deep interpeduncular fossae. Recessive mutations in NPHP3,44 NPHP6/CEP290,45,46 NPHP8/RPGRIP1L,47–49 AHI1,50,51 MKS3,52 ARL13B,53 INPP5E,54 and TMEM21655 and NPHP1 deletions56 can cause Joubert’s syndrome. NPHP6/CEP290 serves as a good example of two specific characteristics of ciliopathies. First, ciliopathy proteins localize to the cilium–centrosome complex in a cell-cycle–dependent manner, as indicated by the finding that NPHP6 (or CEP290) is part of the centrosomal proteome57 but is also expressed at the mitotic spindle45 (Fig. 3). Second, the type of the two recessive mutations can determine the severity of the disease phenotype, in that the presence of two “strong” (protein-truncating) mutations in NPHP6/CEP290 causes a severe, early-onset developmental disorder, with a broad range of organ involvement (as in Meckel’s syndrome),52 whereas the presence of at least one “weak” (missense) mutation leads to a mild, late-onset, degenerative disorder with limited organ involvement (as in NPHP).41,58,59
Meckel’s Syndrome
Meckel’s syndrome is an autosomal recessive disease that leads to perinatal death as a result of dysplasia and malformation of multiple organs. It is characterized by occipital meningoencephalocele, microphthalmia, lung hypoplasia, polycystic kidneys or renal hypodysplasia or dysplasia, bile-duct dilatation, postaxial polydactyly, and situs inversus. As stated above, it now appears that different recessive mutations in each of many different ciliopathy genes may cause a wide spectrum of organ involvement, depending on the severity of the mutated allele involved. This effect of multiple allelism has been described for the genes that cause Meckel’s syndrome — MKS1,60–62 MKS3,52 NPHP3,44 NPHP6/CEP290,63 NPHP8/RPGRIP1L,47,49 TMEM216,55 and CC2D2A64,65 — and has led to the realization that different combinations of recessive mutations may cause a wide range of disorders or syndromes.
Bardet–Biedl, Orofaciodigital, and Jeune Syndromes
The Bardet–Biedl syndrome is a multisystem disorder characterized by retinal degeneration, cystic kidney disease or urinary tract malformation, cognitive impairment, diabetes mellitus, obesity, infertility, and postaxial polydactyly. Mutations in 16 genes (BBS1 to BBS12, MKS1, NPHP6/CEP290, SDCCAG8, and SEPT7 [septin 7]) can cause the Bardet–Biedl syndrome phenotype. The relationships between ciliary function and the Bardet–Biedl, orofaciodigital, and Jeune syndromes are described in the Supplementary Appendix.
SIGNALING DEFECTS AS A CAUSE OF CILIOPATHIES
Cilia transmit signals to the interior of the cell. Many receptors are expressed at the ciliary membrane and are required for the cell to perceive physical stimuli (e.g., mechanical strain), light, the binding of hormones, chemokines and growth factors (e.g., somatostatin, stromal-cell–derived factor 1 [SDF-1], and platelet-derived growth factor), or modulation of signaling pathways through morphogens (e.g., sonic hedgehog [SHH] or Wnt). Although it is now clear that mutations of many genes lead to ciliopathies, much less is known about specific ciliary signaling pathways and the pathogenic principles that ultimately result in the disease phenotypes at the tissue and organ levels. Multiple signaling mechanisms act in concert with primary cilia, including the Wnt signaling–planar cell polarity pathway, signaling at focal adhesions and at adherens junctions, hedgehog signaling, notch signaling, and the JAK–STAT (Janus-associated kinase–signal transducers and activators of transcription) pathway (Fig. 3). All of them play a direct or indirect role in mechanisms of planar cell polarity that are central to the ciliopathies described above.
NONCANONICAL WNT SIGNALING AND PLANAR CELL POLARITY
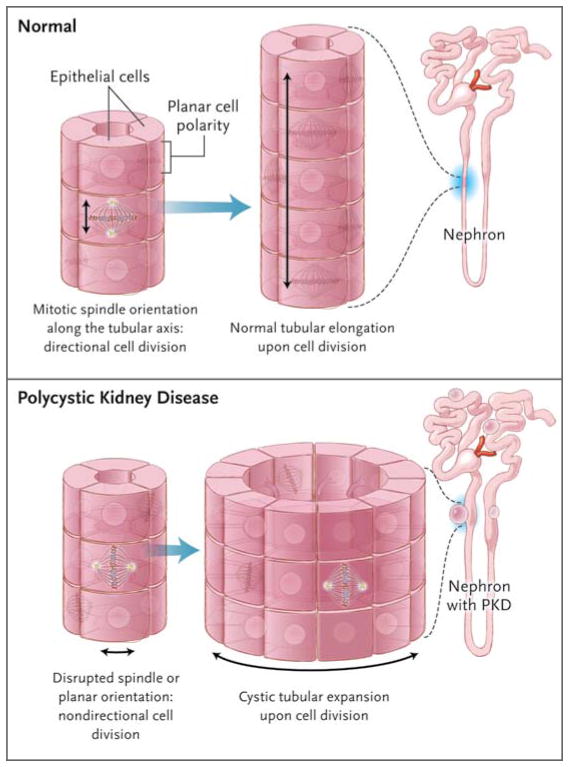
Planar cell polarity refers to a conserved signaling pathway for the coordinated polarization of cells within the plane of an epithelial cell layer. It is also required during organ morphogenesis for polarized cellular rearrangements, known as convergent extension. During such signaling, core planar cell polarity proteins are sorted asymmetrically along the polarization axis. This sorting is thought to direct coordinated downstream morphogenetic changes across the entire tissue. Jones et al. found that the protein ift88/polaris, the gene for which is mutated in cystic kidney disease in mice, is required for establishing epithelial planar cell polarity.66 Proper positioning of ciliary basal bodies and the formation of polarized cellular structures are disrupted in mice with mutant ciliary proteins.67–69 This finding points to a distinct requirement for ciliary genes in basal-body positioning and morphologic polarization during the regulation of planar cell polarity (Fig. 4).
Figure 4. Defects in the Noncanonical Wnt/PCP Pathway Leading to Renal Cysts.
Correct orientation of the mitotic spindle and centrosomes with respect to the longitudinal axis of the tubule is critical for proper planar cell polarity (i.e., the orientation of an epithelial cell layer in three-dimensional space). Noncanonical Wnt/PCP signaling is involved in the regulation of planar cell polarity during renal tubular morphogenesis, when in 2-week-old rodents the tubules still elongate. In this model, if the mitotic spindle is maloriented, such as at an oblique angle to (or, to cite an extreme example, perpendicular to) the longitudinal orientation of tubule growth, the resulting structure would be a dilated tubule or cyst.
HEDGEHOG SIGNALING
The connection between hedgehog (HH) signaling and cilia emerged from a mouse mutagenesis screen that identified mutations in IFT genes as a cause for hedgehog mutant phenotypes.70,71 HH is a secreted protein that regulates development through binding to its transmembrane receptor patched homologue 1 (PTCH1) on the ciliary membrane.72 Moreover, mutations in NPHP7/GLIS2, encoding the transcription factor Gli-similar protein 2 (GLIS2), have been identified as the cause of nephronophthisis.73 Glis2-mutant mice have severe renal atrophy and fibrosis resembling human nephronophthisis.73 Because GLIS2 is related to the Gli transcription factor, it will be interesting to investigate a possible connection between GLIS2 and the HH pathway.
CELL-CYCLE CONTROL
Recent studies suggest that cilia signaling has an important role in the control of cell division. As described above, the ciliary axoneme emanates from the basal body, the mother centriole of the centrosome, which directs assembly of the bipolar spindle during mitosis. Disassembly of the primary cilium and liberation of its captive centriole are essential for cell division. The signaling pathways that control cilia resorption involve the mitotic kinase aurora A and HEF1, a scaffolding protein, which interact and activate HDAC6, a tubulin deacetylase, resulting in disassembly of the primary cilium.74 Moreover, mutations in NPHP9/ NEK8 (never in mitosis kinase 8) cause nephronophthisis type 975–77 through defects in ciliary and centrosomal localization.78 Since NEK8 plays a major role in cell-cycle regulation, these data provide another link between proteins that are defective in ciliopathies and the control of the cell cycle. In this context, it is interesting that polycystin-1 and polycystin-2 signaling is linked to the regulation of cell growth. Polycystin-1 expression activates the JAK–STAT pathway, thereby up-regulating p21(WAF1) and inducing cell-cycle arrest in G0/G1.19 Involvement of cell-cycle regulation in renal cystic disease was confirmed in a study in which two mouse models of polycystic kidney disease (jck and cpk) were effectively treated with the cyclin-dependent kinase inhibitor roscovitine.79
MECHANISMS OF GENOT YPE–PHENOT YPE CORREL ATION
FOUR GENETIC MECHANISMS TO DETERMINE CILIOPATHY PHENOTYPE
Nephronophthisis-like ciliopathies are recessive disorders (i.e., the two recessive mutations in a single gene are sufficient to cause disease). However, for patients with nephronophthisis-like ciliopathies, the extent and severity of organ involvement are determined by four independent genetic mechanisms. The first is heterogeneity of the genetic locus: mutations in specific genes determine disease severity. For example, homozygous deletions of NPHP1 primarily cause nephronophthisis, whereas two truncating mutations of NPHP6/ CEP290 cause a severe Meckel’s syndrome–like phenotype.59 The second mechanism is multiple allelism: two truncating mutations of NPHP3,44 NPHP6,59 NPHP8,47 or NPHP11/MKS380 cause Meckel’s syndrome, but the presence of at least one missense mutation may favor the milder phenotype of Joubert’s syndrome. The third mechanism concerns modifier genes: in patients with homozygous NPHP1 deletions, the presence of an additional heterozygous mutation in NPHP6 or NPHP8 causes additional eye or cerebellar involvement. The fourth mechanism concerns “true oligogenicity”: it has been proposed that the actions of two or more recessive genes with heterozygous mutations (which are not sufficient to result in a phenotype) may result in a phenotype only when the mutations act together. This last mechanism has been proposed in some instances of the Bardet–Biedl syndrome.81
DYSPLASTIC AND DEGENERATIVE CILIOPATHIES CAUSED BY THE SAME GENE
A surprising discovery is that a mutation of the same recessive gene may cause different nephronophthisis-like ciliopathy phenotypes.44,47,59,80 If a gene defect becomes manifest during organ development, dysplasia will result, whereas if NPHP defects become manifest in adult tissue, degeneration will develop in organs that had normal architecture at birth. A similar effect was first described for Pkd1, in which the occurrence of cysts in the kidney depends on developmental status.20 Studies of ciliopathy in the kinesin family member 3A (Kif3A) knockout mouse model have also shown this to be true.82 In addition, it has been suggested that sporadic kidney cysts that develop during tissue-repair processes in acute renal injury may be related to aberrant planar cell polarity and consecutive malorientation of mitotic spindles,82 although this concept has been challenged.83 A strikingly similar dependence of phenotypic severity on developmental status is seen in other organ systems involved in ciliopathies. In nephronophthisis-like ciliopathies, most organs appear to express one of two contrasting groups of possible disease phenotypes, depending on the severity of the gene mutation in NPHP3,44 NPHP6,59 NPHP8,47 or NPHP11.80 In one group, two truncating mutations that act during development cause a severe, early-onset, developmental phenotype that affects morphogenesis and leads to organ dysplasia or malformation. In the other, two mis-sense mutations that act during tissue maintenance and repair in adult tissue cause a mild, late-onset, mature tissue phenotype that affects tissue maintenance and repair and leads to organ degeneration, as is seen in nephronophthisis. The severe phenotype is found preferentially in diseases such as Meckel’s syndrome and ARPKD, whereas the mild phenotype is seen primarily in the Senior–Løken syndrome and nephronophthisis. The Joubert’s syndrome phenotype is characterized by moderate severity.
In addition, Pkd1 and Pkd2 mutations result primarily in cystic phenotypes, whereas most of the nephronophthisis genes result in a primarily fibrotic phenotype. This suggests that at least some of the ciliopathy proteins have nonoverlapping functions. Furthermore, the core ciliary gene Tg737 (polaris) has a function in a cell type that has no known cilia — that is, lymphocytes. As ciliary proteins localize to subcellular locations other than the cilium–centrosome complex, they presumably have additional functions at those locations. One of the major challenges ahead will be determining which cellular functions are associated with ciliary versus nonciliary localization of the various proteins.
SUMMARY
Ciliopathies may be framed as a genetically heterogeneous group of disorders that are caused by mutations in genes with products that localize to the cilium–centrosome complex. The phenotypes due to the altered proteins vary from cystic kidney disease and blindness to neurologic phenotypes, obesity, and diabetes. A common feature of monogenic ciliopathies such as polycystic kidney disease, nephronophthisis, Joubert’s syndrome, Meckel’s syndrome, and the Bardet–Biedl syndrome is that the disease-relevant gene products are expressed at primary cilia or centrosomes. Cilia are complex sensory organelles involved in the control of a variety of cellular signaling pathways, and although the complexity of these signaling pathways has been in part delineated, many essential questions remain.
Footnotes
Dr. Benzing reports receiving lecture fees from Novartis, Amgen, and Shire; and Dr. Katsanis, lecture fees from the Beijing Genomics Institute. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Fliegauf M, Horvath J, von Schnakenburg C, et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J Am Soc Nephrol. 2006;17:2424–33. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 2.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–23. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin F, Hiesberger T, Cordes K, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–91. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–43. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 6.Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans Nature. 1999;401:386–9. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–71. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 8.Pazour GJ, Baker SA, Deane JA, et al. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–13. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 10.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–16. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–98. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 12.Watnick T, Germino G. From cilia to cyst. Nat Genet. 2003;34:355–6. doi: 10.1038/ng0803-355. [DOI] [PubMed] [Google Scholar]
- 13.Olbrich H, Fliegauf M, Hoefele J, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapetoretinal degeneration and hepatic fibrosis. Nat Genet. 2003;34:455–9. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 14.Otto EA, Schermer B, Obara T, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–20. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansley SJ, Badano JL, Blacque OE, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 16.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–48. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 18.Kim E, Walz G. Sensitive cilia set up the kidney. Nat Med. 2007;13:1409–11. doi: 10.1038/nm1207-1409. [DOI] [PubMed] [Google Scholar]
- 19.Bhunia AK, Piontek K, Boletta A, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–68. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 20.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd. Nat Med. 2007;13:1490–5. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaelin WG., Jr The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol. 2003;14:2703–11. doi: 10.1097/01.asn.0000092803.69761.41. [DOI] [PubMed] [Google Scholar]
- 22.Adeva M, El-Youssef M, Rossetti S, et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD) Medicine (Baltimore) 2006;85:1–21. doi: 10.1097/01.md.0000200165.90373.9a. [DOI] [PubMed] [Google Scholar]
- 23.Ward CJ, Hogan MC, Rossetti S, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–69. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 24.Onuchic LF, Furu L, Nagasawa Y, et al. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–17. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I, Fu Y, Hui K, et al. Fibrocystin/ polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19:455–68. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanconi G, Hanhart E, von Albertini A, Uhlinger E, Dolivo G, Prader A. Die familiäre juvenile Nephronophthise. Helv Paediatr Acta. 1951;6:1–49. [PubMed] [Google Scholar]
- 27.Hildebrandt F, Otto EA. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–40. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrandt F, Otto E, Rensing C, et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet. 1997;17:149–53. doi: 10.1038/ng1097-149. [DOI] [PubMed] [Google Scholar]
- 29.Saunier S, Calado J, Heilig R, et al. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet. 1997;6:2317–23. doi: 10.1093/hmg/6.13.2317. [DOI] [PubMed] [Google Scholar]
- 30.Otto E, Hoefele J, Ruf R, et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71:1167–71. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mollet G, Salomon R, Gribouval O, et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet. 2002;32:300–5. doi: 10.1038/ng996. [DOI] [PubMed] [Google Scholar]
- 32.Mollet G, Silbermann F, Delous M, Salomon R, Antignac C, Saunier S. Characterization of the nephrocystin/nephrocystin-4 complex and subcellular localization of nephrocystin-4 to primary cilia and centrosomes. Hum Mol Genet. 2005;14:645–56. doi: 10.1093/hmg/ddi061. [DOI] [PubMed] [Google Scholar]
- 33.Benzing T, Gerke P, Höpker K, Hildebrandt F, Kim E, Walz G. Nephrocystin interacts with Pyk2, p130(Cas), and tensin and triggers phosphorylation of Pyk2. Proc Natl Acad Sci U S A. 2001;98:9784–9. doi: 10.1073/pnas.171269898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donaldson JC, Dise RS, Ritchie MD, Hanks SK. Nephrocystin-conserved domains involved in targeting to epithelial cell-cell junctions, interaction with filamins, and establishing cell polarity. J Biol Chem. 2002;277:29028–35. doi: 10.1074/jbc.M111697200. [DOI] [PubMed] [Google Scholar]
- 35.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 36.Wolf MT, Lee J, Panther F, Otto EA, Guan KL, Hildebrandt F. Expression and phenotype analysis of the nephrocystin-1 and nephrocystin-4 homologs in Caenorhabditis elegans. J Am Soc Nephrol. 2005;16:676–87. doi: 10.1681/ASN.2003121025. [DOI] [PubMed] [Google Scholar]
- 37.Jauregui AR, Barr MM. Functional characterization of the C. elegans nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp Cell Res. 2005;305:333–42. doi: 10.1016/j.yexcr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–6. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 39.Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–88. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto EA, Loeys B, Khanna H, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–8. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 41.Chang B, Khanna H, Hawes N, et al. In-frame deletion in a novel centrosomal/ ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–57. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valente EM, Marsh SE, Castori M, et al. Distinguishing the four genetic causes of Jouberts syndrome-related disorders. Ann Neurol. 2005;57:513–9. doi: 10.1002/ana.20422. [DOI] [PubMed] [Google Scholar]
- 43.Gleeson JG, Keeler LC, Parisi MA, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am J Med Genet A. 2004;125A:125–34. doi: 10.1002/ajmg.a.20437. [DOI] [PubMed] [Google Scholar]
- 44.Bergmann C, Fliegauf M, Brüchle NO, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–70. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayer JA, Otto EA, O’Toole JF, et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–81. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 46.Valente EM, Silhavy JL, Brancati F, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38:623–5. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 47.Delous M, Baala L, Salomon R, et al. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875–81. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 48.Arts HH, Doherty D, van Beersum SE, et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet. 2007;39:882–8. doi: 10.1038/ng2069. [DOI] [PubMed] [Google Scholar]
- 49.Wolf MT, Saunier S, O’Toole JF, et al. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72:1520–6. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- 50.Dixon-Salazar T, Silhavy JL, Marsh SE, et al. Mutations in the AHI1 gene, encoding Jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75:979–87. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferland RJ, Eyaid W, Collura RV, et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet. 2004;36:1008–13. doi: 10.1038/ng1419. [Erratum, Nat Genet 2004;36: 1126.] [DOI] [PubMed] [Google Scholar]
- 52.Baala L, Romano S, Khaddour R, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80:186–94. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cantagrel V, Silhavy JL, Bielas SL, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83:170–9. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bielas SL, Silhavy JL, Brancati F, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–6. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valente EM, Logan CV, Mougou-Zerelli S, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–25. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parisi MA, Bennett CL, Eckert ML, et al. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75:82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–4. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 58.den Hollander AI, Koenekoop RK, Yzer S, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 2006;79:556–61. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helou J, Otto EA, Attanasio M, et al. Mutation analysis of NPHP6/CEP290 in patients with Joubert syndrome and Senior-Løken syndrome. J Med Genet. 2007;44:657–63. doi: 10.1136/jmg.2007.052027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khaddour R, Smith U, Baala L, et al. Spectrum of MKS1 and MKS3 mutations in Meckel syndrome: a genotype-phenotype correlation: mutation in brief #960: online. Hum Mutat. 2007;28:523–4. doi: 10.1002/humu.9489. [DOI] [PubMed] [Google Scholar]
- 61.Consugar MB, Kubly VJ, Lager DJ, et al. Molecular diagnostics of Meckel-Gruber syndrome highlights phenotypic differences between MKS1 and MKS3. Hum Genet. 2007;121:591–9. doi: 10.1007/s00439-007-0341-3. [DOI] [PubMed] [Google Scholar]
- 62.Kyttälä M, Tallila J, Salonen R, et al. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38:155–7. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 63.Baala L, Audollent S, Martinovic J, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–9. doi: 10.1086/519494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tallila J, Jakkula E, Peltonen L, Salonen R, Kestilä M. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet. 2008;82:1361–7. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noor A, Windpassinger C, Patel M, et al. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet. 2008;82:1011–8. doi: 10.1016/j.ajhg.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones C, Roper VC, Foucher I, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 67.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–9. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gerdes JM, Liu Y, Zaghloul NA, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–60. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 69.Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet. 2006;38:1316–22. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- 70.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 71.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 72.Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol. 2007;9:1005–9. doi: 10.1038/ncb435. [DOI] [PubMed] [Google Scholar]
- 73.Attanasio M, Uhlenhaut NH, Sousa VH, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39:1018–24. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 74.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–63. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otto EA, Helou J, Allen SJ, et al. Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat. 2008;29:418–26. doi: 10.1002/humu.20669. [DOI] [PubMed] [Google Scholar]
- 76.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19:469–76. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu S, Lu W, Obara T, et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129:5839–46. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 78.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol. 2008;19:587–92. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–52. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 80.Otto EA, Tory K, Attanasio M, et al. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11) J Med Genet. 2009;46:663–70. doi: 10.1136/jmg.2009.066613. [DOI] [PubMed] [Google Scholar]
- 81.Leitch CC, Zaghloul NA, Davis EE, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40:443–8. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 82.Patel V, Li L, Cobo-Stark P, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–90. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishio S, Tian X, Gallagher AR, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21:295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]