Summary
Cells lining the respiratory tract are equipped with mechanisms that dampen the effects of oxidative stress. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a mediator involved in regulating oxidative stress. Recent data indicate Nrf2 also controls expression of secretory leukocyte protease inhibitor (SLPI). Sulforaphane (SFN), an isothiocyanate found in cruciferous vegetables, enhances Nrf2 activity. Therefore, we hypothesized that SFN supplementation induces SLPI secretion in the nasal mucosa in an Nrf2 dependent manner. Healthy nonsmoking adults ingested SFN-containing broccoli shake homogenate (BSH) for 3 consecutive days. Nasal lavage fluid (NLF) was collected before and after BSH ingestion and analyzed for SLPI protein levels. In follow up in vitro experiments, differentiated primary nasal epithelial cells were used to evaluate the relationship between SFN, Nrf2, and SLPI. Epithelial cells were transduced with Nrf2-specific shRNA to examine the regulatory role of Nrf2 on SLPI expression. Supplementation with BSH significantly increased SLPI levels in NLF. SFN supplementation in vitro significantly enhanced SLPI secretion and these effects were significantly decreased in cells transduced with Nrf2-specific shRNA.
Keywords: SLPI, Sulforaphane, Nasal mucosa
To combat and alleviate exogenous stressors, cells lining the respiratory tract have evolved sophisticated sensing and signaling pathways to dampen the effects of cellular damage. The transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) plays a critical role in maintaining respiratory homeostasis through the regulation of detoxifying enzymes and antioxidants.1 Nrf2 has been shown to protect against infection and ameliorates the development of respiratory disorders such as COPD and asthma.2,3
Recent data indicate that Nrf2 also regulates expression of an antiprotease, secretory leukocyte protease inhibitor (SLPI), by binding to the antioxidant response element (ARE) regions [GenBank AF205374] in the 1.2 kb promoter region of the SLPI gene.3,4 SLPI is an 11.7 kDa heavily disulfide bonded, positively charged protein found highly expressed in epithelial cells at mucosal surfaces as well as in inflammatory cells such as neutrophils and macrophages and contributes to host defense by regulating unchecked inflammation.5 Reduced levels of SLPI have been associated with several different respiratory diseases, including emphysema, COPD, and cystic fibrosis.5
Agents known to increase Nrf2 activation and Nrf2-dependent gene expression include sulforaphane (SFN), an isothiocyanate found in cruciferous vegetables. In the context of the respiratory epithelium, SFN induces Nrf2 levels and exhibits protective effects in airway epithelial cells via enhancing the expression of phase II enzymes.6 However, despite SFN induction of Nrf2-dependent genes, its effect on the production and release of SLPI is not known. As such, we hypothesize that ingestion of SFN-containing foods has therapeutic potential to augment SLPI expression and secretion in an Nrf2 dependent manner. To test this hypothesis, we conducted an in vivo nutritional supplementation study in a small cohort of healthy volunteers as well as in vitro experiments to determine if SFN or SFN-containing foods induce SLPI secretion in the nasal mucosa.
To test the impact of SFN-containing foods on SLPI secretion in vivo, we enrolled 12 healthy volunteers (Age: 24.8 ± 3.5; Sex: 6 Male, 6 Female; Race: 7 Caucasian, 4 African American, 1 Asian; BMI: 25.8 ± 4.8) in a pilot clinical nutritional supplementation study using a SFN-rich broccoshake homogenate (BSH). Commercially available Broccosprouts® (Brassica Protection Products LLC) were used in the study and BSH were prepared similar to a previously described formula and supplementation protocol, resulting in enhanced phase II enzyme expression in the nasal mucosa.7 Briefly, 200 g Broccosprouts® were homogenized with 4.8 oz water and frozen at −20 °C until the morning of the ingestion protocol. Subjects were asked to consume the entire BSH and to keep a food diary. To assure that the SFN content did not change over time in storage, we determined free SFN levels in BSH as previously described and found that BSH samples prepared on two different days, separated by three months, revealed similar free SFN levels, indicating batch to batch compatibility (May SFN level: 95.9 µmol/L; Aug SFN level: 118 µmol/L).8
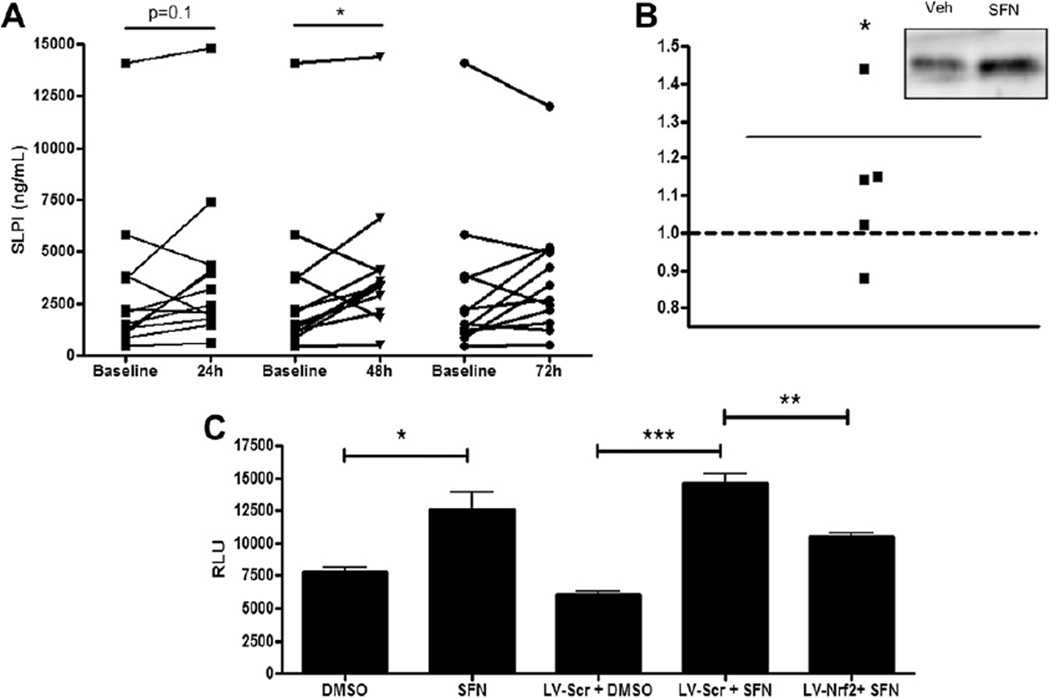
The BSH supplementation study took place over four consecutive days. Subjects were asked to avoid ingestion of cruciferous vegetables for one week prior to and during the study. Informed consent was obtained from all subjects and the protocol was approved by the UNC Biomedical Institutional Review Board. Baseline nasal lavage fluid (NLF) was taken before the first of three daily ingestions of BSH. Additional NLF samples were collected two hours post ingestion of the second and third BSH dose as well as 24 h after the last ingestion. We chose to collect NLF since it is a non-invasive, easily accessible procedure used in previous BSH supplementation studies.7 All NLF samples were obtained and processed as previously described.9 Cell-free NLF was analyzed for SLPI protein levels using commercially available ELISA kits (R&D, Minneapolis, MN). Supplementation of human volunteers with SFN-containing BSH enhanced SLPI levels in NLF within 24 h and was statistically significant after 48 h BSH ingestion (Fig. 1A). Additionally we analyzed serum SLPI levels taken at baseline and 24 h post last BSH ingestion, to test if SLPI levels in the NLF were from exudate or transudate. We found a slight non-statistically significant induction of SLPI serum levels (data not shown). However, since serum was collected 24 h post last BSH ingestion and considering the short half-life of SFN,7 we are not able to determine if SLPI levels in the NLF are from exudate or transudate.
Figure 1.
SFN supplementation induces SLPI secretion in vivo and in vitro and is mediated by Nrf2. (A) Nasal lavage fluid obtained from 12 healthy volunteers on days 1–3 after daily BSH ingestion was analyzed for SLPI protein levels. (B) Densitometric analysis of SLPI expression in apical washes of NEC treated with SFN and normalized to levels in vehicle treated cells from n = 5 different subjects. Representative Western blot of SLPI levels in apical washes from NEC treated with vehicle (Veh) or SFN for 24 h (insert). (C) BEAS-2B cells were transfected with SLPI-promoter reporter plasmid, exposed to 10 µM for 5 h, and evaluated for SLPI gene transcription. SLPI-promoter reporter dependent luciferase activity was normalized to Renilla levels and expressed as relative luciferase units (RLU). In some experiments, BEAS-2B were transduced with lentiviral vectors encoding Nrf2-specific shRNA (LV-Nrf2) or scrambled vectors (LV-Scr) 48 h prior to transfection with SLPI-reporter plasmid and exposed to 10 µM for 5 h to evaluate SLPI-promoter reporter dependent luciferase. *p < 0.05, **p < 0.01, ***p < 0.001.
To confirm and expand these in vivo observations, we examined whether supplementation of nasal epithelial cells (NEC) with SFN enhances production of SLPI. Human differentiated EC were treated with 10 mM SFN from the basolateral side and analyzed for intracellular and secreted SLPI levels 24 h post supplementation. Analysis of whole cell lysates using Western blot indicated that SFN exposure did not enhance intracellular SLPI levels (data not shown). However, secreted SLPI protein levels measured in the apical washes of differentiated NEC were enhanced by SFN supplementation (Fig. 1B), corroborating the in vivo data, which showed enhanced SLPI levels in the NLF.
To further investigate the link between SFN supplementation, Nrf2 activation, and nasal SLPI expression, we used an in vitro model similar to previous studies.2 Based on these previous studies, we determined that a SFN concentration of 10 µM is sufficient to distinguish differences in Nrf2 dependent gene expression without causing cytotoxicity. 2 Specifically, to examine the effects of SFN supplementation on the transcriptional regulation of SLPI, BEAS-2B cells, a human bronchial epithelial cell line, were transfected with a SLPI promoter-reporter construct containing 1385 base pairs of the 5′ regulatory region of the SLPI gene. Fig. 1C demonstrates that SFN supplementation significantly increased SLPI gene transcriptional activation. To evaluate if SFN induction of SLPI gene expression was dependent on Nrf2, we transduced BEAS-2B cells with lentiviral vectors that expressed either Nrf2-specific shRNA (LV-Nrf2) or scrambled vectors expressing a nonspecific sequence for use as a control (LV-Scr) as previously described.2 Transduced BEAS-2B cells were subsequently transfected with the SLPI promoter-reporter and exposed to 10 µM SFN for 5 h, demonstrating that reducing Nrf2 expression results in decreased SFN-induced SLPI expression (Fig. 1C), thus mechanistically linking SFN, Nrf2, and SLPI expression.
Our study provides novel in vivo and in vitro evidence linking nutritional SFN supplementation, Nrf2 activation, and enhanced SLPI secretion in the nasal mucosa. SLPI inhibits proteases such as cathespin G and neutrophil elastase, which are both associated with tissue damage in the context of chronic respiratory conditions like COPD and asthma. In addition, we have recently demonstrated that SLPI protects against influenza A virus (IAV) infection in nasal epithelial cells and that supplementation with SFN protects against IAV entry.2,10 Since susceptibility to respiratory virus infection, including IAV, is enhanced in subjects with COPD and asthma, our data suggest a potential role for nutritional supplementation to improve respiratory defense and homeostasis in these subpopulations via changes in the protease/antiprotease balance, which is an ongoing focus of our research.
Acknowledgments
We thank M. Brighton, P. Murphy, and M. Herbst for expert technical assistance. We thank the NC TraCS Institute CTRC Nutrition Research and Biometabolism Team for preparing the BSH. This study was supported by grants from the National Heart, Lung, and Blood Institute (R01HL095163), the National Institute of Health (R01CA122906), and the National Institute for Environmental Health Sciences (R01ES013611, P30 ES00210). This article has been funded in part by the U.S. Environmental Protection Agency through cooperative agreement CR83346301 with the Center for Environmental Medicine, Asthma, and Lung Biology at The University of North Carolina at Chapel Hill, but does not reflect the official views of the agency and has not been subjected to the agency’s required peer and policy review. No official endorsement should be inferred.
Footnotes
Our data support a relationship between nutritional supplementation, Nrf2 activation, and SLPI secretion. Therefore, ingestion of SFN-containing foods has therapeutic potential to augment SLPI expression in the nasal mucosa.
Conflict of interest statement
None.
References
- 1.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Kesic MJ, Simmons SO, Bauer R, Jaspers I. Nrf2 expression modifies influenza a entry and replication in nasal epithelial cells. Free Radic Biol Med. 2011;51:444–453. doi: 10.1016/j.freeradbiomed.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, et al. Nrf2-deficient mice are highly susceptible to cigarette smokeinduced emphysema. Genes Cells. 2005;10:1113–1125. doi: 10.1111/j.1365-2443.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, et al. Transcription factor nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 5.Scott A, Weldon S, Taggart CC. Slpi and elafin: multifunctional antiproteases of the wfdc family. Biochem Soc Trans. 2011;39:1437–1440. doi: 10.1042/BST0391437. [DOI] [PubMed] [Google Scholar]
- 6.Ritz SA, Wan J, Diaz-Sanchez D. Sulforaphane-stimulated phase ii enzyme induction inhibits cytokine production by airway epithelial cells stimulated with diesel extract. Am J Physiol Lung Cell Mol Physiol. 2007;292:L33–L39. doi: 10.1152/ajplung.00170.2006. [DOI] [PubMed] [Google Scholar]
- 7.Riedl MA, Saxon A, Diaz-Sanchez D. Oral sulforaphane increases phase ii antioxidant enzymes in the human upper airway. Clin Immunol. 2009;130:244–251. doi: 10.1016/j.clim.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JD, Riedl K, Bella D, Schwartz SJ, Stevens JF, Ho E. Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. J Agric Food Chem. 2011;59:10955–10963. doi: 10.1021/jf202887c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect. 2011;119:78–83. doi: 10.1289/ehp.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesic MJ, Meyer M, Bauer R, Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza a infection. PLoS One. 2012;7:e35108. doi: 10.1371/journal.pone.0035108. [DOI] [PMC free article] [PubMed] [Google Scholar]