Abstract
Across taxa, both neural growth and cognitive function show considerable developmental plasticity. Data from studies of decision-making, learning and discrimination demonstrate that early life conditions have an impact on subsequent neural growth, maintenance and cognition, with important ecological and evolutionary implications. We provide a synthesis of the evidence that spatial and vocal learning are condition-dependent, addressing what is known about their physiological control and the functional explanations. Neural investment is predicted to be affected by environmental conditions, but the shape of the response should depend on the fitness benefits of the cognitive traits under control. From an evolutionary perspective, traits promoting resistance to environmental perturbations should be favoured when the cognitive trait is a crucial determinant of fitness.
Condition-dependent cognitive development
Many crucial tasks, such as the ability to find food, locate a mate or make decisions about how to reproduce effectively are dependent on essential cognitive functions. Animal cognition here, refers to the neural processes used to interpret the external environment and it includes perception, learning, memory and decision-making [1]. Variation among taxonomic groups in cognitive ability has inspired a vast literature on phylogenetic relationships in brain size, inferring selection on cognition per se as a determinant of fitness [1]. In the fields of physiology, psychology and medicine it is well accepted that, within the boundaries of certain physiological states, the ability to develop and maintain effective cognitive skills is dependent on individual physiological condition (Supplementary Table 1). Depending on their exact nature, environmental conditions can have either enhancing or detrimental effects on a range of cognitive traits, mediated by changes in neural development, structure and function (Fig. 1). In this article we summarise the evidence over the last decade testing the condition-dependence of cognition and the brain, and the important ecological and evolutionary implications for such dependence. We explore the potential for selection to act on the heritable portion of the phenotypic variation in cognitive ability, produced as a result of variation in environmental stress. We address the evolutionary implications of individual variation in the ability of the brain to withstand the effects of environmental challenges, as individuals seek to maximise their reproduction and survival. Environment-dependent variation in different cognitive traits may not have equal fitness consequences in all species and/or populations, hence it may be expected that the strength of selection on the mechanisms maintaining developmental stability in particular cognitive traits would depend on the degree of their relevance for fitness. Evolutionary ecologists familiar with the concept of canalisation understand that strongly fitness-related traits should show higher levels of developmental stability in the face of environmental perturbations. Finally, we investigate whether neuroplasticity is a product of selection on cognitive abilities, determining variation among species in their ability to compensate for developmental challenges.
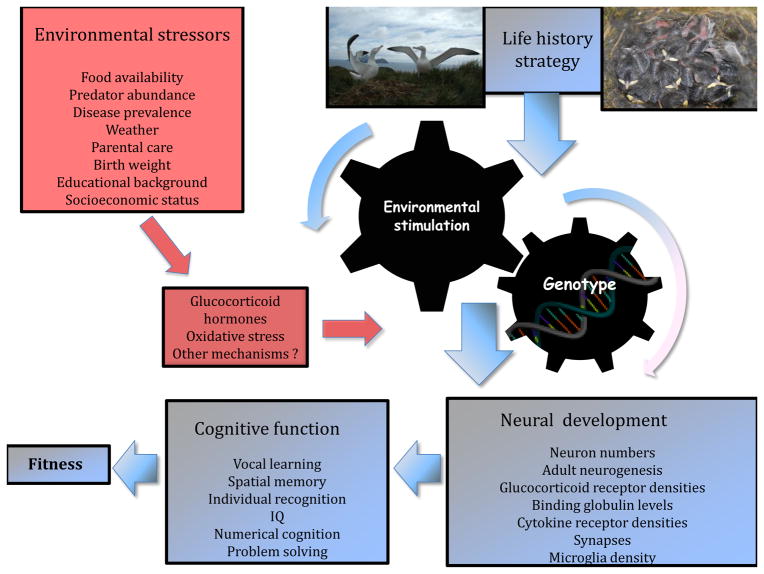
Figure 1.
Diagrammatic representation of how environmental stress is likely to affect cognition. Neural development and cognitive function (phenotype) will be determined by a combination of genotype in interaction with environmental stimulation opportunities, such as social learning, territorial interactions, song tutoring, determining key cognitive abilities which ultimately impact on fitness. Glucocorticoid hormone level, as well as their receptor placement, number and sensitivity, oxidative stress levels and other unidentified mechanisms mediate the effects of environmental stress on cognitive function, by affecting neural development. The life history strategy of the animal is central in determining not only neural investment and opportunities to learn, but also the susceptibility of the brain to stress.
In this article we focus on the detrimental impacts of environmental challenges on two key cognitive traits: spatial memory and vocal learning (Box 1), as the best comparative evidence for such effects lies with experimental tests of the effects of environmental stress on these traits. The best examples come from studies of birds, which have been used as a model for understanding not only spatial learning and memory, but also vocal learning. However, many of the concepts discussed here have direct relevance to other taxa.
Box 1. Comparative evidence for condition-dependent cognition: spatial memory and vocal learning.
Spatial learning is an important cognitive trait that most likely has a direct bearing on fitness in many animals [40]. Food-caching animals rely on spatial learning to retrieve food caches, which may be crucial for winter survival [41]. In polygynous territorial animals, such as meadow voles (Microtus pennsylvanicus), males rely on spatial learning to find females [28] and males with better spatial learning abilities are preferred by females [28]. Spatial learning is often used for multiple tasks, such as orientation and navigation, or for locating shelters, mates or nests.
Conditions during development have been clearly shown to affect spatial learning of adults [42–44]: In mammals, malnutrition [45], maternal separation, social stress, as well as stressors experienced by mothers during pregnancy cause impaired spatial learning in adulthood [42, 46], whereas in birds, malnutrition during early post-hatching development has been linked to impaired spatial learning performance [43]. Developmental stress appears to exert especially strong negative effects on spatial learning, while non-spatial learning abilities, appear to be unaffected [43, 47].
Although vocal learning is often not classically referred to as a cognitive trait [1], it involves perception, learning and memory controlled by well-defined neural structures in parrots, hummingbirds, songbirds and humans [3]. These structures have definable developmental timelines, are plastic in structure and relate to vocal capacity and complexity. In songbirds this network of nuclei includes the HVC (used as proper name), which is thought to be under strong sexual selection in species with complex songs. Several independent studies have shown that song production is affected by developmental conditions [48] [49–51], mediated in part through structural changes to key brain centres [52–54]. Some studies have failed to find an effect of early developmental conditions on brain morphology [55] or song complexity [56], suggesting that the nature or timing of the environmental stress is important. In the songbird brain, the HVC, which relates to song complexity appears to be selectively susceptible to stress [24, 57], presumably through the indirect or direct action of the glucocorticoid hormone corticosterone on neurogenesis or the maintenance of existing neurons [58]. There are, therefore, striking parallels in the response of the avian song system and the hippocampus to environmental change, with both showing a reduction in nucleus volume and the number of neurons in response to detrimental conditions and evidence that glucocorticoid hormones may mediate these effects.
Seasonal effects and plasticity
Both the costs and the benefits of protecting cognitive function seem likely to vary seasonally. Seasonal variation in patterns of food storing and cache retrieval, mean that the fitness benefits of effective spatial memory may vary throughout the year. Dispersal, when new spatial information is vital to determining survival, may also be an important stage at which to protect spatial memory. It remains to be addressed whether there is seasonal variation in stress-susceptibility of the hippocampus through, for example modulation of the glucocorticoid receptor densities, which would alter the downstream transcription effects resulting from changes in glucocorticoid production. Mechanistically, one might simplistically predict that a reduction in receptor numbers could occur facultatively in food caching species during the autumn or winter months, when memory formation and retrieval may determine survival. But, interestingly, moderate corticosterone elevations have been shown to improve spatial memory in animals including food-caching birds [2]. Considering that there are two main types of glucocorticoid receptors in the brain (see Glossary), it may be expected that the density of mineralocorticoid receptors (MR) with high affinity may increase in the winter, while the density of glucocorticoid receptors (GR) with lower affinity may decrease, to improve memory performance at moderate corticosterone elevations, preventing potential damage associated with high corticosterone elevations. Equally, seasonal modulation in circulating antioxidant levels might be predicted, as oxidative stress is known to have detrimental effects on neuron numbers and neurogenesis rates (Fig. 1). We know of no studies testing whether these physiological mechanisms might exist as a result of selection for cognitive resilience (Box 2).
Box 2. Selection for cognitive resilience?
Selection for cognitive resilience should involve selection for physiological or behavioural strategies for protecting the brain against the detrimental effects of stress. Physiological mechanisms could involve changes to glucocorticoid receptor numbers, placement or sensitivity, or enhancing neurological resistance, e.g. reduced susceptibility of neurons to oxidative stress [11]. Behavioural adaptations can also effectively reduce stress exposure, e.g. timing of breeding, microhabitat choice, dispersal strategy. Different vertebrate taxa show enrichment of glucocortioid immunoreactive neurons (GRir) within the brain in largely homologous regions e.g. [58], suggesting that across species certain traits, for example spatial and vocal learning, are more susceptible to environmental stress. In birds the vocal control system, the hippocampus and the optic tectum are particularly enriched [58], suggesting that glucocorticoids play an important role in mediating function within these regions. It also seems highly possible, although is as yet untested, that different regions of the brain differ in their susceptibility to oxidative stress with consequences for cognition.
The best comparative data investigating the processes underlying the detrimental effects of stress on neural development are from the hippocampus [43, 45, 59, 60]. The mechanisms underlying this effect are still unclear. The hippocampus is one of the brain regions that continues its development after nutritional independence from the parents and also exhibits constant experience-dependent changes in structure and processes (neurogenesis, dendrites, synapses, etc) throughout adult life. Even though it is unclear whether most detrimental effects occur prior to independence, the hippocampus appears to be vulnerable to both current stressful experiences and to developmental conditions [44]. Such vulnerability of spatial learning and the hippocampus to various developmental stressors presents an important evolutionary dilemma – if spatial learning and the hippocampus are critical for fitness, selection should favour their developmental stability promoting cognitive resilience, yet these traits exhibit constant experience-dependent changes in structure and processes (neurogenesis, dendrites, synapses, etc) throughout adult life. Developmental instability may therefore be a constraint imposed by selection for enhanced neuroplasticity (26), in which case selection should act on both parental and offspring behaviour that may minimize potential early developmental stress exposure. Comparative work is needed to test if susceptibility to developmental stress covaries with the degree of neuroplasticity and if behavioural adaptations to minimise stress are more developed in species with pronounced neuroplasticity.
From the vocal learning perspective, some songbirds show seasonal regression and regrowth of their song system [3], suggesting a good reason to expect seasonal modulation of the susceptibility of the brain to stress. Oscine songbirds can be divided into open and closed ended learners (see Glossary) [3]. Although only a small number of species have been investigated, open-ended learners are presumed to show greater seasonal neuroplasticity. As a result of the seasonal regrowth of their song brain system and changes in both numbers and distributions of endocrine receptors within the brain [4], such open ended learners potentially have a greater ability to compensate for any detrimental early developmental stress in later life. This suggests that the strength of selection for cognitive resilience may vary among songbird species, depending on the opportunity to compensate at a later date for stress exposure. Similarly to the hippocampus, we would predict seasonal variation in GR or MR, or antioxidant levels within the song system, but to date this remains untested. Seasonal effects in the protection of neural development may also be seen in territorial species, which adapt their song structures to match their neighbours [3] during dispersal, but this remains untested. Future work also needs to focus on interspecific variation in the susceptibility of the brain to environmental stress. For example, protection of the song system against the detrimental effects of stress would be expected to be more pronounced in species with more complex songs, as these species may experience a higher fitness cost from song simplification.
Cognitive resilience and life history
The fitness benefits of cognition seem likely to vary as a function of life history strategy, however, there has been surprisingly little consideration of the costs and benefits of cognition from a life history perspective [5]. The most obvious costs associated with complex cognitive abilities are the development and maintenance of the underlying enlarged and complex neural structures [5, 6]. These costs may mediate trade-offs with lifespan [7], immune function (Box 3), competitive ability [8], onset of reproductive maturity [9] and the length of the developmental period, which may result in reduced lifetime reproductive output [5, 10]. If cognition is costly, then cognitive abilities should only be favoured when individuals accrue some fitness benefit from the ability to learn [5]. Longer lived animals are predicted to benefit most from complex cognitive traits and are, therefore, most likely to have evolved mechanisms to protect the brain from potentially harmful environmental perturbations. Protecting the brain against the detrimental effects of stress could involve physiological (e.g. endocrine receptor numbers, sensitivity or feedback) or neurological resistance (e.g. reduced susceptibility of neurons to oxidative stress associated with free radicals [11]) to developmental stress, or behavioural adaptations (e.g. microhabitat choice, dispersal) that would minimize stress exposure.
Box 3. Trade-offs between cognition and immune function.
Cognitive function may represent the output of an ongoing trade-off between the immune and nervous systems. Infection with viruses, bacteria, or parasites and experimentally simulated infections induced by injections of viral coat proteins or bacterial endotoxin have been documented to impair memory in rodents (reviewed in [61]). The observed deleterious effects on learning and memory are thought to be mediated by pro-inflammatory cytokines, particularly IL-1β [61]. IL-1β specifically impairs spatial memory [62], through impacts on the retention, rather than acquisition, of spatial memories [63].
Current evidence indicates that the immune system positively regulates learning, memory, neural plasticity, and neurogenesis under homeostatic conditions and during learning, the nervous system activates brain immune cells [61]. However, when the immune system is strongly activated either due to an infection or injury, the overall impact of the immune response on the brain is to suppress learning, memory, neural plasticity, and neurogenesis [61]. Glucocorticoids seem to have a similar effect on the brain. Strong stimulation of the hypothalamic-pituitary-adrenal (HPA) axis disrupts memory consolidation [64]. In contrast at low levels, glucocorticoids can enhance spatial memory, neural plasticity and neurogenesis [61]. Cross-talk between the nervous and immune systems has also been demonstrated in invertebrates and signalling between the two systems has been documented across phyla [65]. Honeybees (Apis mellifera) injected with lipopolysaccharide exhibit reduced associative learning [66]. It is unclear whether this effect is mediated by a trade-off between the nervous and immune systems for limiting substrates, such as protein availability, or if the effect is mediated by direct communication between the immune and nervous systems [66]. Eicosanoids are the most likely candidate to mediate communication between the nervous and immune systems in invertebrates [67].
Thus, there is some evidence from both vertebrates and invertebrates that cognition is condition-dependent and that the immune system plays an important role in mediating this effect. However, at least in vertebrates, cognition may not be condition-dependent solely as a result of a resource-based trade-off [68]. Instead, cognition is likely linked to condition through the immune system because of bidirectional communication between the nervous and immune systems [68]. From an evolutionary perspective, future work needs to assess whether selection for cognitive resilience impacts on immune function, or whether both traits are a function of individual condition.
Life history trade-offs resulting from selection for cognitive resilience can be demonstrated by examining populations that experience different levels of severity of environmental perturbations. Evidence for this idea has been found by comparing learning, memory and neophobia, as well as hippocampal morphology in populations of black-capped chickadees (Poecile atricapillus) at the northern and southern extremes of the species range [12, 13]. In a common garden experiment, chickadees originating from the northern population(harsher conditions), exhibited faster problem-solving, a lower incidence of neophobic behaviours, and better spatial memory associated with a larger number of hippocampal neurons and higher hippocampal neurogenesis rates than chickadees originating from the southern population. That these differences were observed in birds raised and maintained in uniform laboratory conditions together with the data showing that the number of hippocampal neurons and neurogenesis rates were similar in lab-reared and in wild birds strongly suggests the heritable nature for these traits [12]. Similar differences were also found between mountain chickadees (P. gambeli) from different elevations, with birds from higher elevations exhibiting better spatial memory, enlarged hippocampus, higher total number of hippocampal neurons and increased neurogenesis rates [14]. Remarkably, these differences do not appear to be a direct response to harsh winter environment, as they were evident in juvenile birds prior to winter. Environments with high temporal variability may also favour phenotypically plastic enhanced cognitive abilities or cognitive flexibility in resident species. Partial support for this hypothesis comes from a study of cichlid fish (Simochromis pleurospilus). Fish raised on a variable amount of food, had higher performance in a learning task later in life, than fish raised on a constant quantity of food (either low or high quantity) [15]. In this case, it was the predictability of the diet, not quantity per se which was the relevant cue.
Invertebrates also show evidence for life history trade-offs involving cognition. In fruit flies (Drosophila melanogaster), artificial selection for enhanced ability to learn simple associations has been shown to result in reduced lifespan [7, 16]. The direct cost of memory formation per se has been elegantly demonstrated by the same group by exposing fruit flies to training paradigms that either generate memories or do not [17], demonstrating an association between long term memory formation and reduced lifespan. In cabbage white butterflies (Pieris rapae), the ability to learn to search for a rare host colour has a genetic basis and is associated with degree of neural investment [18]. Butterflies with the highest performance in a test of rare host colour finding ability have been shown to have less well-developed ovaries at the time of emergence than butterflies with reduced performance in the task [18]. Thus in this system, learning is associated with a constitutive fitness trade-off, in the form of reproductive delays.
Phenotypic plasticity and GxE effects
Phenotypic plasticity, the ability to alter phenotype in relation to environmental conditions, has been hypothesized to alter the rate of evolutionary change either by accelerating it, slowing it down or in some cases having no effect on the rate of evolutionary change [19]. While variation in cognition is, at least in part heritable, it may also be produced by phenotypic plasticity: environmental challenges experienced during key developmental windows impair cognition through impacts on neural development and at the other end of the environmental continuum, stimulation through tutoring or social learning increase neural proliferation and development. The key questions are: is this plasticity adaptive and is there a genetic basis for it? Just as for any phenotypic trait, cognitive ability is affected by the genotype (G) and the environment (E), as well as differences among genotypes in their response to the environment (GxE) [20]. If certain genotypes are more likely to experience, or are more susceptible to environmental challenges, then these animals are likely to be disproportionately affected by poor conditions. Similarly, due to genetic predispositions to learn, some individuals may benefit disproportionately from the same amount of tutoring/exposure to social learning opportunities. Unfortunately, many neurological studies to date rely on small sample sizes without the necessary experimental manipulations to separate genetic and environmental effects. We are unaware of any comparative study that coherently addresses the important question: is there a genetic basis for the reaction norms of cognitive traits in response to environmental challenges [21]?
The relationship between brain morphology and cognitive capacity is highly contentious [22], but there are data that allude to the strength of selection on the brain. Direct selection experiments on brain size in guppies (Poecilia reticulata) have shown that brain size is linked to cognitive ability, at least in females with a realized mean heritability of 0.465 [10]. Heritability values for components of the avian song system are moderate, although there is a considerable range (h2 = 0.18 – 0.72) [23], although are likely to be overinflated, due to the confounding effects of shared environments [24]. A review of human twin studies, which addressed genetic and environmental effects separately, estimating the heritability of brain volumes using brain imaging concluded that hippocampal volume shows substantially lower heritability (0.40–0.69) than the heritability of other brain areas, such as frontal lobe volumes (> 0.90) [25]. This may indicate a strong environmental influence on hippocampal development and consequently spatial ability (although comparing heritability values across studies is notoriously problematic). Studies of chickadees suggest that the number of neurons, but not hippocampal volume is likely to have a heritable basis [13]. Hippocampal volume, but not the total number of neurons, is very sensitive to immediate environmental conditions [26], so ultimately both environmental and genetic influences are likely to be involved in species/population variation in spatial memory.
Cognition, signalling and mate choice
Cognition is likely to play an important role in determining offspring provisioning and appears vital for mate choice [27]. This logic has led to the suggestion that courtship feeding, foraging performance or even ornaments may be mate choice criteria used by females because they represent the ability to secure access to resources. There are many plausible examples of choice for individuals with traits linked to enhanced cognition e.g. [28], although direct mate choice for individual cognitive skills is yet to be demonstrated. In zebra finches (Taeniopygia guttata), male song complexity is positively associated with learning ability in a novel foraging task [29], although it is notoriously difficult to separate learning ability from motivation to participate. The condition-dependent carotenoid-based ornamentation of male siskins (Carduelis spinus) is positively associated with ability to solve a foraging task [30]. In starlings (Sturnus vulgaris) song bout length is correlated with spatial learning ability in a group of experimentally stressed birds [31]. In great bowerbirds (Ptilonrhynchus nuchalis), males employ forced perspective in construction of their bower in order to manipulate the female’s perception of the bower contents [32], whilst in satin bowerbirds (Ptilonorhynchus violaceus), the ability to problem solve is correlated with mating success [33]. Both traits may represent mate choice for enhanced complex cognitive abilities, although these purely behavioural results should be interpreted with caution. It is currently unclear how ornamentation and cognition are functionally linked, or whether these correlations actually represent a choice for a meaningful aspect of cognitive ability. In addition as mentioned above, differences in condition may potentially lead to differences in motivation to participate, without any real change in learning ability. In these cases, experimental manipulations of condition are required to show that cognitive function is condition-dependent. Furthermore, none of the studies above has examined the brain, which is necessary if we are to understand the mechanisms underlying how sexual selection influences mating behaviours.
Of course the inverse of this question, is whether female cognitive ability is crucial for accurate mate choice assessment and discrimination. Recent attempts to quantify the effects of developmental stress on female song preferences have concluded that developmental stress affects preference strength for unfamiliar songs, but not preference for a familiar song model [34], or that stress either has little or no effect on female preferences [35, 36], or that females prefer to mate assortatively, according to developmental background [37, 38]. If female preference strength is altered by developmental stress, due to changes in perception, discrimination or decision making then we would expect relaxed selection on the relevant male traits. Variation in female cognitive abilities, due to their own experiences of developmental stress impacting on their ability to discriminate, may also partly explain why many studies find that females do not agree on male attractiveness rankings. Future work is needed to assess the developmental susceptibility of the mechanisms underlying female mate choice, as we know of few studies investigating the effects of stress on decision making in a mate choice context [36]. In oscine songbirds, the same areas of the brain are thought to be involved in song discrimination in females, as for song learning and production in males and so neural function in females may be equally susceptible to the effects of adverse environmental conditions. Future work should test the effects of environmental conditions on song perception in female birds, to confirm whether there is strong pleiotropic selection on both male and females for cognitive resilience in species where cognitive ability is important for reproductive success.
Conclusions
Although the mechanisms are unclear, there is good evidence, across diverse taxonomic groups, for condition-dependent neural development and cognition. We have focused here on spatial memory and vocal learning, due to the current bias in the published literature. Future work should examine the effects of environmental stress on decision making, perception and memory, from a comparative angle. From a life history perspective, selection for cognitive resilience against environmental stress should occur at life stages or during seasonal changes when specific regions within the brain are particularly crucial for determining reproductive success and survival. This paves the way for clear predictions about how and when the brain should protect itself. Future work should focus on variation in the susceptibility of the brain to environmental change, in relation to seasonal changes, population-level differentiation, and life history stage, which may show different selective outcomes predicted from the cost/benefit trade-off of complex cognition. For example, seasonal profiles of hormone receptor distributions or antioxidant levels in the brain would be useful. The brain may also show enhanced levels of plasticity at times when protection is particularly valuable.
Whilst the cognitive abilities of invertebrates differ considerably from those of vertebrates, invertebrates offer some invaluable opportunities to test, for example the heritability of cognitive processes and the metabolic costs of cognitive tasks such as memory formation [39]. Vertebrates which show dedicated brain space for specific cognitive traits, offer opportunities to address the costs of developing learned tasks, such as spatial memory and complex vocal communication, which may ultimately explain their evolution. Although it may be practically challenging, future studies need to quantify the fitness benefits of cognitive ability in order to understand selection on the traits. Key to this are good estimates of the genetic component to cognitive ability. It would be illuminating to test the co-variation of a range of cognitive abilities between individual animals. If our predictions are correct and the effects of stress on the brain are generally mediated, then we predict that individuals with poor spatial memory should also show reduced problem solving, vocal learning or associative learning capacity, (although if there is a selective trade-off in the value of these abilities, we might not see such co-variation). Finally, the concept that stress-susceptibility may be a constraint of selection for neuroplasticity generates a number of predictions, which can be addressed both at the individual level and comparatively to demonstrate how selection acts on the brain.
Supplementary Material
Acknowledgments
This paper was initially inspired by the NSF-funded RCN Network in Ecoimmunology. We would like to thank Scott MacDougall-Shackleton, Clive Catchpole, Stefan Leitner and Joe Woodgate and four anonymous reviewers for comments on earlier drafts. Vladimir Pravosudov has been supported by grants from NSF (IOB 0615021 and 0918268). Jennifer Grindstaff is supported by a grant from NIH (1R15HD066378-01)
Glossary
- Associative learning
a learning process that involves association of a response with a specific stimulus
- Closed learner
a songbird species which has a limited developmental period for learning song
- Cognitive resilience
the ability to maintain cognitive function despite external environmental challenges
- Condition
the ability to maintain optimal cellular processes despite exposure to environmental challenges such as nutritional deprivation, parasite infestation or thermal stress
- Condition dependence
development or expression of a trait depends on the ability of the individual to withstand environmental challenges
- Corticosterone
a steroid hormone produced by adrenal glands, which is responsive to a variety of detrimental environmental challenges. A common glucocorticoid hormone in amphibians, reptiles, birds and rodents
- Developmental stability
the ability of an organism to maintain stable development in variable environmental conditions
- Gene-environment (GxE) interaction
phenotypic changes produced by the interactions of genes with the environment
- Glucocorticoid hormones
steroid hormones, the excretion rates of which respond to various detrimental environmental challenges
- Glucocorticoid receptors
(GR) and mineralocorticoid receptors (MR) are both found in the cytoplasm of neurons. GR have low affinity, whilst MR has a much higher affinity for binding glucocorticoid hormones
- Hippocampus
a vertebrate brain region involved in spatial learning and memory
- Hippocampal neurogenesis
production and survival of new neurons in the hippocampus in adult animals
- Hypothalamus Pituitary Adrenal (HPA)axis
interactions among the hypothalamus, the pituitary gland and adrenal glands that are responsible for regulating the physiological stress response involving glucocorticoid hormones
- HVC
a nucleus within the brain of songbirds involved in avian song learning and the production of complex song. Individually and comparatively, volume has been shown to correlate with song complexity
- Neuroplasticity
the ability to produce neuronal and synaptic changes in response to environmental challenges and stimulation
- Nidopallium
an avian brain region thought to be involved in higher order cognitive tasks
- Open-ended learner
a songbird species which can adjust its song output throughout life
- Oscine songbirds
a suborder of passerine birds which includes species of songbirds
- Oxidative stress
oxidative damage caused by free radicals produced by the organism, as a result of cellular interactions with oxygen
- Phenotypic plasticity
the ability of organisms to alter their phenotype in response to different environments
- Stress
conditions where an environmental demand exceeds the natural regulatory capacity of an organism
- Stress hormones
glucocorticoid hormones such as cortisol and corticosterone, which are usually elevated in response to detrimental environmental challenges
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shettleworth SJ. Cognition, Evolution and Behavior. Oxford University Press; 2010. [Google Scholar]
- 2.Pravosudov VV. Long-term moderate elevation of corticosterone facilitates avian food-caching behaviour and enhances spatial memory. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:2599–2604. doi: 10.1098/rspb.2003.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catchpole C, Slater P. Bird Song: Biological themes and variations. Cambridge University Press; 2008. [Google Scholar]
- 4.Ball G, Balthazart J. The neuroendocrinology and neurochemistry of birdsong. In: Blaustein J, editor. Handbook of Neurochemistry and Molecular Neurobiology. Springer; 2007. pp. 419–459. [Google Scholar]
- 5.Ricklefs RE. The cognitive face of avian life histories - The 2003 Margaret Morse Nice Lecture. Wilson Bulletin. 2004;116:119–133. [Google Scholar]
- 6.Walker R, et al. Evolution of brain size and juvenile periods in primates. Journal of Human Evolution. 2006;51:480–489. doi: 10.1016/j.jhevol.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Burger JMS, et al. Learning ability and longevity: A symmetrical evolutionary trade-off in Drosophila. Evolution. 2008;62:1294–1304. doi: 10.1111/j.1558-5646.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 8.Mery F, Kawecki TJ. A fitness cost of learning ability in Drosophila melanogaster. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:2465–2469. doi: 10.1098/rspb.2003.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laughlin SB, et al. The metabolic cost of neural information. Nature Neuroscience. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- 10.Kotrschal A, et al. Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Current Biology. 2013;23:168–171. doi: 10.1016/j.cub.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikonomidou C, Kaindi A. Neuronal death and oxidative stress in the developing brain. Antioxidants & Redox Signalling. 2011;14:1535–1550. doi: 10.1089/ars.2010.3581. [DOI] [PubMed] [Google Scholar]
- 12.Roth TC, et al. Learning capabilities enhanced in harsh environments: a common garden approach. Proceedings of the Royal Society B-Biological Sciences. 2010;277:3187–3193. doi: 10.1098/rspb.2010.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth T, II, et al. Variation in memory and the hippocampus across populations with different climates: a common garden approach. Proceedings of the Royal Society B-Biological Sciences. 2012;278:402–410. doi: 10.1098/rspb.2011.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freas C, et al. Elevation-related differences in memory and the hippocampus in mountain chickadees (Poecile gambeli) Animal Behaviour. 2012;84:121–127. [Google Scholar]
- 15.Kotrschal A, Taborsky B. Environmental Change Enhances Cognitive Abilities in Fish. Plos Biology. 2010;8:e1000351. doi: 10.1371/journal.pbio.1000351. 1000310.1001371/journal.pbio.1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mery F, Kawecki TJ. An operating cost of learning in Drosophila melanogaster. Animal Behaviour. 2004;68:589–598. [Google Scholar]
- 17.Mery F, Kawecki TJ. A cost of long-term memory in Drosophila. Science. 2005;308:1148–1148. doi: 10.1126/science.1111331. [DOI] [PubMed] [Google Scholar]
- 18.Snell-Rood EC, et al. Reproductive tradeoffs of learning in a butterfly. Behavioral Ecology. 2011;22:291–302. [Google Scholar]
- 19.Wund M. Assessing the impacts of phenotypic plasticity on evolution. Integrative and Comparative Biology. 2012;52:5–15. doi: 10.1093/icb/ics050. [DOI] [PubMed] [Google Scholar]
- 20.West-Eberhard M. Developmental plasticity and evolution. Oxford University Press; 2003. [Google Scholar]
- 21.Dukas R. Evolutionary Biology of Animal Cognition. Annu Rev Ecol Syst. 2004;35:347–374. [Google Scholar]
- 22.Healy S, Rowe C. A critique of comparative studies of brain size. Proc Roy Soc Lond B. 2007;274:453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Airey D, et al. Variation in the volume of zebra finch song controlnuclei is heritable: developmental and evolutionary implications. Proceedings of the Royal Society B-Biological Sciences. 2000;267:2099–2104. doi: 10.1098/rspb.2000.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan KL, et al. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peper J, et al. Genetic influences on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaDage L, et al. Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behavioral Neuroscience. 2009:123. doi: 10.1037/a0014817. [DOI] [PubMed] [Google Scholar]
- 27.Boogert NJ, et al. Mate choice for cognitive traits: a review of the evidence in nonhuman vertebrates. Behavioral Ecology. 2011;22:447–459. [Google Scholar]
- 28.Spritzer MD, et al. Female choice based on male spatial ability and aggressiveness among meadow voles. Animal Behaviour. 2005;69:1121–1130. [Google Scholar]
- 29.Boogert NJ, et al. Song complexity correlates with learning ability in zebra finch males. Animal Behaviour. 2008;76:1735–1741. [Google Scholar]
- 30.Mateos-Gonzalez F, et al. Sexy birds are superior at solving a foraging problem. Biology Letters. 2011;7:668–669. doi: 10.1098/rsbl.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell T, et al. Song bout length is indicative of spatial learning in European starlings. Behavioral Ecology. 2011;23:101–111. [Google Scholar]
- 32.Endler J, et al. Greater bowerbirds create their own theaters with forced perspective when seen by their audience. Current Biology. 2010;20:1679–1684. doi: 10.1016/j.cub.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Keagy J, et al. Male stain bowerbird problem-solving ability predicts mating success. Animal Behaviour. 2009;78:809–817. [Google Scholar]
- 34.Riebel K, et al. Experimental manipulation of the rearing environment influences adult female zebra finch song preferences. Animal Behaviour. 2009;78:1397–1404. [Google Scholar]
- 35.Woodgate JL, et al. Developmental stress and female mate choice behaviour in the zebra finch. Animal Behaviour. 2010;79:1381–1390. [Google Scholar]
- 36.Woodgate JL, et al. Developmental stressors that impair song learning in males do not appear to affect female preferences for song complexity in the zebra finch. Behavioral Ecology. 2011;22:566–573. [Google Scholar]
- 37.Holveck MJ, Riebel K. Low-quality females prefer low-quality males when choosing a mate. Proceedings of the Royal Society B-Biological Sciences. 2010;277:153–160. doi: 10.1098/rspb.2009.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holveck M-J, et al. An Experimental Test of Condition-Dependent Male and Female Mate Choice in Zebra Finches. Plos One. 2011;6:e23974. doi: 10.1371/journal.pone.0023974. 23910.21371/journal.pone.0023974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns J, et al. Costs of memory: lessons from ‘mini’ brains. Proceedings of the Royal Society B-Biological Sciences. 2011;278:923–929. doi: 10.1098/rspb.2010.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherry D. Neuroecology. Annu Rev Psychol. 2006;57:167–197. doi: 10.1146/annurev.psych.56.091103.070324. [DOI] [PubMed] [Google Scholar]
- 41.Pravosudov VV, Smulders TV. Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:859–867. doi: 10.1098/rstb.2009.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fumagalli F, et al. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Progress in Neurobiology. 2007;81:197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Pravosudov VV, et al. Nutritional deficits during early development affect hippocampal structure and spatial memory later in life. Behavioral Neuroscience. 2005;119:1368–1374. doi: 10.1037/0735-7044.119.5.1368. [DOI] [PubMed] [Google Scholar]
- 44.Pravosudov V. Development of spatial memory and the hippocampus under nutritional stress: adaptive priorities or developmental constraints in brain development? In: Ratcliffe RDaJM., editor. Cognitive Ecology II. University of Chicago Press; 2009. pp. 88–110. [Google Scholar]
- 45.Bedi KS. Nutritional effects on neuron numbers. Nutritional Neuroscience. 2003;6:141–152. doi: 10.1080/1028415031000098549. [DOI] [PubMed] [Google Scholar]
- 46.Hedges DW, Woon FL. Early-life stress and cognitive outcome. Psychopharmacology. 214:121–130. doi: 10.1007/s00213-010-2090-6. [DOI] [PubMed] [Google Scholar]
- 47.Sterlemann V, et al. Chronic stress during adolescence induces cognitive impairments in aged mice. Hippocampus. 2010;20:540–549. doi: 10.1002/hipo.20655. [DOI] [PubMed] [Google Scholar]
- 48.Nowicki S, et al. Song learning, early nutrition and sexual selection in songbirds. American Zoologist. 1998;38:179–190. [Google Scholar]
- 49.Spencer KA, et al. Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata) Hormones and Behavior. 2003;44:132–139. doi: 10.1016/s0018-506x(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 50.Spencer KA, et al. Parasites affect song complexity and neural development in a songbird. Proceedings of the Royal Society B-Biological Sciences. 2005;272:2037–2043. doi: 10.1098/rspb.2005.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchanan KL, et al. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:1149–1156. doi: 10.1098/rspb.2003.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDonald I, et al. Early nutritional stress impairs deveopment of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc Roy Soc Lond B-Biological Sciences. 2006;273:2559–2564. doi: 10.1098/rspb.2006.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchanan K, et al. Developmental stress selectively affects the song control nucleus HVC in the zebra finch brain. Proc Roy Soc Lond B. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spencer K, MacDougall-Shackleton S. Indicators of development as sexually selected traits: the developmental stress hypothesis. Behavioral Ecology. 2011;22:1–9. [Google Scholar]
- 55.Gil D, et al. Early condition, song learning, and the volume of song brain nuclei in the zebra finch (Taeniopygia guttata) Journal of Neurobiology. 2006;66:1602–1612. doi: 10.1002/neu.20312. [DOI] [PubMed] [Google Scholar]
- 56.Muller W, et al. Testing the developmental stress hypothesis in canaries: consequences of nutritional stress on adult song phenotype and mate attractiveness. Behavioral Ecology and Sociobiology. 2010;64:1767–1777. [Google Scholar]
- 57.Newman AEM, et al. Corticosterone and Dehydroepiandrosterone Have Opposing Effects on Adult Neuroplasticity in the Avian Song Control System. Journal of Comparative Neurology. 2010;518:3662–3678. doi: 10.1002/cne.22395. [DOI] [PubMed] [Google Scholar]
- 58.Shahbazi M, et al. Distribution and subcellular localization of glucocorticoid receptor-immunoreactive neurons in the developing and adult male zebra finch brain. General and Comparative Endocrinology. 2011;174:354–361. doi: 10.1016/j.ygcen.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Oomen C, et al. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharamacology. 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lister J, et al. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 2005;15:393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- 61.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behavior and Immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Song C, Horrobin D. Omega-3 fatty acid ethyl -eicosapentaenoate, but not soybean oil, attenuates memory impairment induced by central IL-1 beta administration. Journal of Lipid Research. 2004;45:1112–1121. doi: 10.1194/jlr.M300526-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Oitzl MS, et al. Interleukin-1-beta, but not interleukin-6, impairs spatial navigation learning. Brain Research. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- 64.Schwabe L, et al. Memory formation under stress: Quantity and quality. Neuroscience and Biobehavioral Reviews. 2010;34:584–591. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Demas G, et al. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: implications for host defence. Functional Ecology 2011 [Google Scholar]
- 66.Mallon EB, et al. Immune response inhibits associative learning in insects. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:2471–2473. doi: 10.1098/rspb.2003.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley DW. Eicosanoids in invertebrate signal transduction. Princeton University Press; 2000. [Google Scholar]
- 68.Hill GE. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecology Letters. 2011;7:625–634. doi: 10.1111/j.1461-0248.2011.01622.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.