Abstract
Background
Patients with asthma engage in less physical activity than peers without asthma. Protocols are needed to prudently increase physical activity in asthma patients. We evaluated whether an educational intervention enhanced with positive affect induction and self-affirmation was more effective than the educational protocol alone in increasing physical activity in asthma patients.
Methods
We conducted a randomized trial in New York City from September 28, 2004, through July 5, 2007, of 258 asthma patients, 252 completed the trial. At enrollment, control subjects completed a survey measuring energy expenditure, made a contract to increase physical activity, received a pedometer and an asthma workbook, and then underwent bimonthly follow-up telephone calls. Intervention patients received this protocol plus small gifts and instructions in fostering positive affect and self-affirmation. The main outcome was the within-patient change in energy expenditure in kilocalories per week from enrollment to 12 months with an intent-to-treat analysis.
Results
Mean (SD) energy expenditure at enrollment was 1767 (1686) kcal/wk among controls and 1860 (1633) kcal/wk among intervention patients (p=.65) and increased by 415 (95% CI, 76–754; p=.02) and 398 (95% CI, 145–652; p=.002) kcal/wk, respectively, with no difference between groups (p=.94). For both groups, energy expenditure was sustained through 12 months. No adverse events were attributed to the trial. In multivariate analysis, increased energy expenditure was associated with less social support, decreased depressive symptoms, more follow-up calls, use of the pedometer, fulfillment of the contract, and the intervention among patients who required urgent asthma care (all p<.10, 2-sided test).
Conclusions
A multiple-component protocol was effective in increasing physical activity in asthma patients, but an intervention to increase positive affect and self-affirmation was not effective within this protocol. The intervention may have had some benefit, however, in the subgroup of patients who required urgent asthma care during the trial.
Patients with asthma engage in less physical activity than age-matched peers, thereby increasing risks for cardiovascular and other diseases.1 Although most asthma patients have some exercise-induced bronchospasm,2 the ability to participate in prudent exercise is recommended by the National Asthma Expert Panel.3 Fortunately, there now is evidence that lifestyle activity, such as daily walking, can be effective.4,5
Increasing daily physical activity, however, requires conscious behavior change.6 According to well-established psychosocial models, behavior change occurs by increasing knowledge of why the change is worthwhile,7 by making a contract to do the behavior,8 and by having social support that facilitates the behavior.6,9 Another component is increasing confidence, or self-efficacy, by acknowledging prior successes and having physiological feedback showing the behavior is effective.7,9 Novel psychological techniques that may foster behavior change are increasing positive affect and self-affirmation. Inducing positive affect, such as from thinking uplifting thoughts, increases connections between effort and outcome and increases persistence.10,11 Reaffirmation by recalling proud achievements strengthens core values.12 Thus, it is hypothesized that positive affect and self-affirmation foster adaptability and willingness to accept behavior change.13,14
The primary goal of this trial was to increase walking and exercise in asthma patients in the course of 12 months. Patients randomized to the control group received a multiple-component educational protocol consisting of making a contract to participate in a specific physical activity, increasing knowledge of exercise and asthma, and increasing self-efficacy through physiological feedback from a pedometer. Patients randomized to the intervention group received these same maneuvers plus were taught techniques to increase positive affect and self-affirmation.
Methods
This study was approved by the institutional review board at the Weill Cornell Medical College-New York Presbyterian Hospital and patients provided written informed consent. This trial was in response to an initiative from the National Heart, Lung and Blood Institute to translate novel behavioral research to improve health behaviors in patients with cardiopulmonary disease and was conducted simultaneously with 2 other trials with patients with hypertension and coronary artery disease. All trials used the same protocols; detailed methods have been described previously. 15 In summary, for the asthma trial, adult primary care patients in New York City were screened by reviewing appointment schedules and medical records. Patients were eligible if they were at least 18 years of age, had mild to moderate persistent asthma, and spoke English. Patients were excluded if they had severe or mobility-limiting comorbidity, such as a musculoskeletal deficit precluding increased physical activity. At enrollment patients completed standard questionnaires measuring asthma severity16 and control,17 use of asthma medications, knowledge of asthma self-management,18 and asthma-related quality of life.19 A portable spirometer was used to measure pulmonary function. Additional questionnaires were used to assess positive and negative affect,20 depressive symptoms,21 stress,22 and social support.23 Physical activity was measured with the well-established Paffenbarger Physical Activity and Exercise Index measuring the following 3 domains of activity: number of blocks walked daily, number of flights of stairs climbed daily, and exercise/sports participation during the past week.24 Physical activity was then converted into energy expenditure in kilocalories per week (to convert energy expenditure to kilojoules, multiply by 4.186) using a standard compendium of metabolic equivalents.25
Patients received a pedometer calibrated for weight and stride length. They practiced using the pedometer by walking up and down a corridor and then reported number of steps recorded by their pedometer.26 Patients were encouraged to wear the pedometer during the trial and record their number of steps.
Patients then were randomized to the control or the intervention group. We did not include a traditional control group because usual care has been shown to be ineffective in promoting and sustaining increased physical activity.27–32 In the present trial, control subjects received a composite of several behavioral maneuvers that have been shown to improve diverse health behaviors such as physical activity and chronic disease self-management.31,33 First, patients received a pedometer to provide physiological feedback. Second, they received an educational asthma workbook, which we reviewed with them emphasizing sections on physical activity and self management.34–36 Third, patients volunteered for an activity and made a contract specifying how often and how much they would do. Walking was encouraged and contracts were approved by patients’ physicians. Patients rated their self-efficacy for fulfilling the contract.
Intervention patients received these maneuvers plus additional positive affect and self-affirmation components. To foster positive affect patients were asked what things made them feel uplifted just by thinking about them, such as a beautiful sunset, and they were encouraged to think of these things throughout the day. To increase self-affirmation, patients were asked what things they were proud of, such as completing a challenging work assignment, and they were encouraged to think of these achievements when carrying out the contract. Intervention patients also were given a supplement to the workbook summarizing these techniques. At each follow-up, patients were asked how well they applied these techniques. Intervention patients also were mailed small gifts (eg, an umbrella and a desk clock) every 2 months to increase positive affect.37
During the subsequent year all patients were telephoned every two months to complete the Paffenbarger Index, and to report current self-efficacy for the activity, asthma status, pedometer readings, and how well they fulfilled their contract. During these follow-up calls, patients were encouraged to continue the activity, pedometer, and workbook, and were asked whether they required any interval asthma care, hospitalizations, or had any serious adverse events.
For both groups closeout was the 12-month telephone contact, when patients again completed questionnaires about physical activity, asthma status, and psychosocial well-being and were asked global questions about using the pedometer and the workbook.
The primary outcome was the within-patient change in total energy expenditure from enrollment to 12 months measured in kilocalories per week using the Paffenbarger Index. A secondary outcome was the pattern of energy expenditure measured at each bimonthly follow-up call. We hypothesized that intervention patients would increase total energy expenditure more than controls.
The sample size was based on the primary outcome. According to prior longitudinal studies, SDs for weekly energy expenditure ranged from 200 to 350 kcal/wk,38 and an increase in walking 6 or more blocks daily (approximately 336 kcal/wk) corresponds to decreased cardiovascular risk.39 Conservatively using an SD of 450 kcal/wk, a goal to detect a difference between groups of 250 kcal/wk (approximately 4 blocks daily), an alpha value of .05, and a power of 90%, we required 68 patients per group. Based on prior studies showing 73%–90% of patients enrolled in physical activity trials maintained some increased activity at 3 to 6 months, we estimated that 80% would still be involved in the activity at 12 months.27,28 We also considered a possible rate of loss of 15%. Thus, 123 patients were required per group (246 total).
Randomization was performed in permuted blocks by the study statistician (M.T.W.) and was concealed and verified at the trial conclusion. Patients were not blinded to the randomization group, and study personnel administering the enrollment, follow-up, and closeout interviews were not blinded. Spot checks were performed throughout the trial to ensure uniformity in enrollment and follow-up protocols.
The primary analysis was a multivariate linear regression model including variables moderately associated with the primary outcome in bivariate analyses and controlled for age, sex, and asthma control and severity. The variables that minimized the Akaike information criterion, which adjusts for overfitting, were retained in the final model.40 An intent-to-treat analysis was used for patients without the closeout by carrying forward their last energy expenditure value. Few patients had missing closeout values, and other imputation methods gave the same results. Secondary analyses assessed repeated measurement of energy expenditure every 2 months with a mixed-effects model with the energy expenditure value from each follow-up call as the dependent variable. Each patient constituted a random factor, and time from enrollment was a fixed-time variable. We also assessed values longitudinally at each follow-up with a linear mixed model using a flexible spline-based time component to discern the physical activity pattern over time. Analyses were performed using SAS software, version 5.41
Results
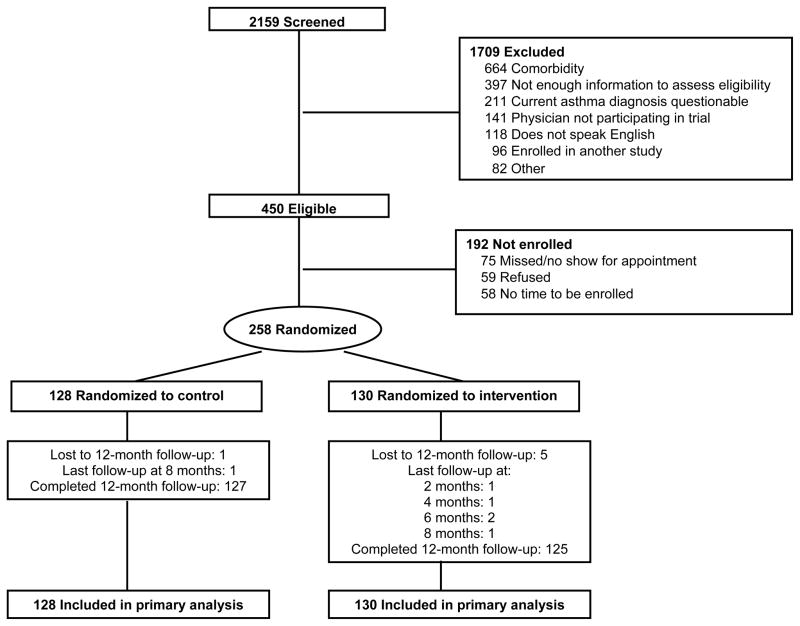
Two hundred fifty-eight patients were enrolled from September 28, 2004, through July 5, 2007 (16 men were enrolled in addition to the projected sample size to match the percentage of men with asthma in this practice). Of these, 252 patients (97.7%) completed the 12-month closeout, and each of the remaining 6 patients had a least 1 follow-up (Figure 1). At enrollment, controls (n=128) and intervention patients (n=130) were comparable regarding demographic and clinical characteristics (Table 1). Similar percentages reported a history of exercise-related respiratory symptoms (53.9% and 55.4%, p=.81). For most controls (68.8%) and intervention patients (59.2%, p=.11) the activity contract was to walk several more blocks several times per week. Mean (SD) self-efficacy for fulfilling the contract was 8.2 (1.8) and 8.4 (1.7) (p=.47), respectively, on a scale of 0 (no confidence) to 10 (most confidence).
Figure 1.
Flow diagram of participants.
Table 1.
Characteristics at enrollment
| Variables
|
Patient Education Control (n=128)
|
Positive Affect Intervention (n=130)
|
p
|
|
|---|---|---|---|---|
| Age, mean (SD), years | 42 (12) | 42 (12) | .78 | |
| Women | 73.4% | 76.2% | .62 | |
| College graduates | 60.2% | 62.3% | .72 | |
| Never married | 49.2% | 43.9% | .39 | |
| Race a | ||||
| White | 52.3% | 56.2% |
|
.84 |
| African American | 24.2% | 19.2% | ||
| Other | 23.5% | 24.6% | ||
| Latino a | 30.5% | 32.3% | .75 | |
| Body mass index, mean (SD) b | 30 (8) | 29 (7) | .51 | |
| < 25 (normal weight) | 32.8% | 30.0% |
|
.87 |
| ≥ 25, < 30 (overweight) | 25.0% | 32.3% | ||
| ≥ 30 (obese) | 42.2% | 37.7% | ||
| Asthma duration, mean (SD), years | 23 (14) | 21 (15) | .14 | |
| Forced expiratory volume in one second (FEV1), mean (SD) | 88% (18%) | 92% (18%) | .06 | |
| Current asthma medications | ||||
| none | 4.7% | 6.2% | .60 | |
| inhaled beta agonist only | 46.1% | 38.5% | .22 | |
| inhaled corticosteroid | 45.3% | 48.5% | .61 | |
| leukotriene modifier | 14.8% | 16.2% | .77 | |
| other c | 2.3% | 4.6% | .32 | |
| Long-term asthma severity, mean (SD) d | 6 (5) | 6 (4) | .55 | |
| < 5 | 46.1% | 43.9% |
|
.42 |
| ≥ 5, < 10 | 32.8% | 42.3% | ||
| ≥ 10, < 14 | 1.5% | 9.2% | ||
| ≥ 14 | 8.6% | 4.6% | ||
| Short-term asthma control, mean (SD) e | 1.5 (1.3) | 1.3 (1.1) | .21 | |
| ≤ .75, well controlled | 38.3% | 36.2% |
|
.82 |
| > .75, < 1.5, partially controlled | 20.3% | 26.9% | ||
| ≥ 1.5 not well controlled | 41.4% | 36.9% | ||
| Current smoker | 10.9% | 10.8% | .97 | |
| Any major comorbidity | 11.7% | 10.0% | .66 | |
| PANAS Positive affect, mean (SD) f | 36 (9) | 35 (8) | .50 | |
| PANAS Negative affect, mean (SD) f | 19 (7) | 20 (8) | .25 | |
| Depressive symptoms, mean (SD) g | 6 (6) | 6 (7) | .74 | |
| Perceived social support, mean (SD) h | 80 (22) | 77 (22) | .26 | |
| Perceived stress, mean (SD) i | 15 (8) | 15 (9) | .71 | |
self-described
calculated in kilograms/meters2
theophylline, cromolyn sodium, ipratropium bromide
Severity of Asthma Scale, possible score range 0–28, higher score means more severe asthma, 5-point increments correspond to clinically important differences
Asthma Control Questionnaire, possible score range 0–6, higher score means less control, divided into 3 clinical categories
Positive and Negative Affect Schedule, possible score range for each subscale 10–50, higher score means more of that attribute
Geriatric Depression Scale, possible score range 0–30, higher score means more depressive symptoms
MOS Social Support Scale, possible score range 0–100, higher score means more support
Perceived Stress Scale, possible score range 0–40, higher score means more stress
The shared behavioral maneuvers were equally administered in both groups, that is, most patients in both groups received bimonthly follow-up calls and reported wearing the pedometer, fulfilling the contract, and reading the workbook (Table 2). Pedometer use was greatest at 2 months, which coincided with the peak of self-efficacy for the activity (9.2 [1.5]). Most intervention patients reported thinking the positive affect and self-affirmation thoughts and receiving the mailed gifts. The most common reason for not receiving a gift was that there was no one at home at the time of mail delivery to receive the package. At closeout, 82.4% of controls and 85.4% of intervention patients (p=.53) reported currently fulfilling their contract completely or to some extent.
Table 2.
Administration of behavioral components
| Components
|
Patient Education Control
|
Positive Affect Intervention
|
p
|
|---|---|---|---|
| Received 5–6 telephone follow-ups (of possible 6, excluding close-out) | 90% | 90% | .97 |
| Wore pedometer at any time during trial a | 92% | 90% | .73 |
| Pedometer helped carry out activity b | 73% | 79% | .27 |
| Fulfilled contract, mean (SD) a,c | 3.4 (1.0) | 3.6 (0.9) | .10 |
| Workbook helped carry out activity b | 88% | 92% | .40 |
| How well able to: mean (SD) a,c | |||
| think positive affect thoughts | -- | 3.9 (0.8) | -- |
| think self-affirmation thoughts | -- | 3.6 (0.9) | -- |
| Number of gifts received (of possible 6) a | |||
| 5–6 | -- | 66% | -- |
| 1–4 | -- | 32% | -- |
| 0 | -- | 2% | -- |
reported during bimonthly follow-ups
reported at close-out
possible responses 1=not at all, 5=completely
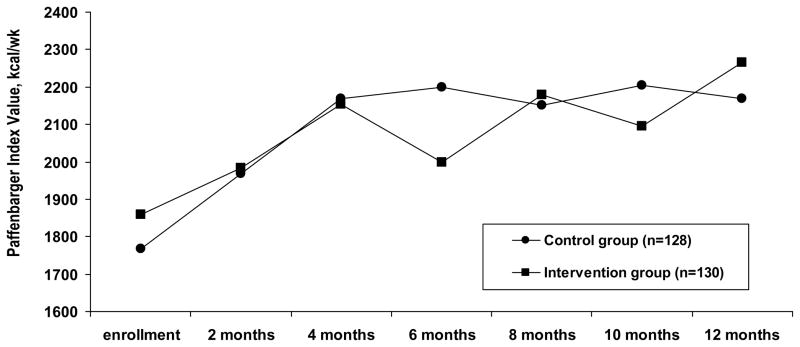
Energy expenditure at enrollment was comparable between groups (Table 3). Most expenditure was derived from walking, with only 39.8% of controls and 48.5% of intervention patients participating in any sports/exercise (p=.16).1 Variables associated with more energy expenditure at enrollment were younger age, male sex, being a college graduate, and lower body mass index.26 The longitudinal pattern of energy expenditure was similar for both groups (Figure 2).
Table 3.
Energy expenditure from the Paffenbarger Index at enrollment and close-out
| Patient Education Control
|
Positive Affect Intervention
|
Between group difference
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Enrollment
|
Close-out
|
Within-patient change
|
Enrollment
|
Close-out
|
Within-patient change
|
Enrollment
|
Close-out
|
Within-patient change
|
|
| Meana | 1767 | 2182 | 415 | 1860 | 2258 | 398 | 93 | 77 | 16 |
| Standard deviationa | 1686 | 1725 | 1938 | 1633 | 1760 | 1462 | 1660 | 1742 | 1715 |
| p value | -- | -- | .02b | -- | -- | .002b | .65c | .72c | .94c |
kilocalories/week
paired t-test
t-test
Figure 2.
Paffenbarger Physical Activity and Exercise Index values at each follow-up for the entire sample.
The primary outcome - the mean within-patient change in energy expenditure from enrollment to 12 months - increased by 415 (95% CI, 76–754; p=.02) kcal/wk in controls and 398 (95% CI, 145–652; p=.002) kcal/wk in intervention patients, with no difference between groups (p=.94). This was above the threshold for a clinically important difference (336 kcal/wk) for both groups, but was not different between groups (Table 3). An increase of 336 kcal/wk or more was reported by 44.5% of controls and 49.2% of intervention patients (p=.45).
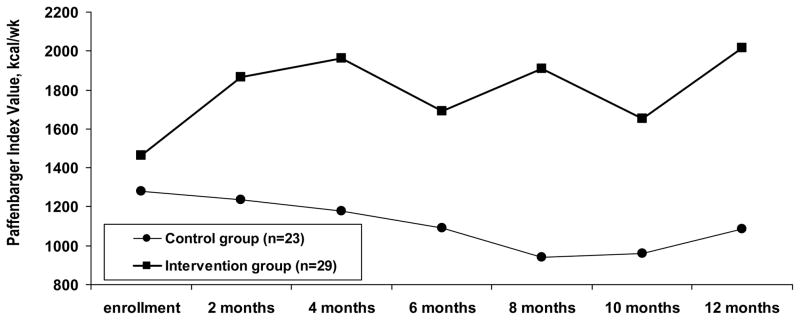
Fifty two patients (23 controls, 29 intervention patients; p=.39) reported interval medical care during the trial. Among these, energy expenditure decreased by 198 kcal/wk for controls but increased by 552 kcal/wk for intervention patients (p=.05) (Figure 3).
Figure 3.
Paffenbarger Physical Activity and Exercise Index values at each follow-up for patients who required urgent care for asthma or any hospitalization during the trial.
In multivariate analysis, increased mean energy expenditure was associated with less social support at enrollment, decreased depressive symptoms from enrollment to closeout, and being in the intervention group for those requiring interval medical care during the trial (Table 4). Receiving more follow-up telephone calls, fulfilling the contract more often, and using the pedometer also were associated with increased energy expenditure.
In a mixed effects model with each bimonthly Paffenbarger value as the dependent variable, increased energy expenditure was associated with time of follow-up (p<.001) for both groups. Using a spline model with an adaptive slope, energy expenditure increased from enrollment to 4 months and then continued at a stable level to 12 months (p=.02). This pattern was the same for both groups.
At closeout, asthma remained well controlled or improved in 68.4% of patients and asthma-related quality of life stayed the same or improved in 81.2%. In addition, 50.4% thought the trial made their asthma better, 49.6% thought it had no effect, and none reported it made their asthma worse. There were no differences between groups for these assessments.
Regarding adverse events, 29 controls (22.7%) and 36 intervention patients (27.7%; p=.35) had a serious adverse event during the trial, defined as any event that required emergency department care or hospitalization or any musculoskeletal complaint that required care in any setting. Forty-four events occurred in controls and 69 in intervention patients (p=.12); no events were attributed to the trial.
Comments
A multiple-component protocol aimed at increasing lifestyle physical activity in asthma patients resulted in clinically important increases in energy expenditure after 12 months. These gains were achieved without worsening asthma, and in most cases asthma improved. These findings are clinically relevant because they address a dilemma faced by asthma patients: although patients acknowledge that physical activity is beneficial for cardiovascular health, many avoid it for fear it will exacerbate respiratory symptoms.42,43 This trial is the first to apply several tenets of health behavior theory to address this critical yet largely overlooked dilemma for asthma patients.
We did not find that the intervention was more effective than the control protocol. Possible reasons for this may be characteristics of both protocols. For example, it is possible that the intervention simply was not an effective mediator of behavior change in our relatively healthy young patients with stable asthma. It also is possible that methods we used to foster positive affect and self-affirmation were not ideal for this sample and that other techniques would have been more effective. With regard to the control protocol, it is likely that not having a traditional control group but rather having a multiple-component background protocol was particularly effective in our sample. We chose this comprehensive control protocol because multiple studies have shown that meaningful increases in physical activity do not occur in the absence of specific interventions.27–32 In a previous 12-month observational study performed in the same medical practice as this trial, energy expenditure increased by only 162 kcal/wk in healthy patients in the absence of any intervention.30
Thus, both groups in the current trial received behavioral maneuvers that, although not previously tested in asthma patients, are well-established facilitators of behavior change that exceed usual practice.44 We believe these maneuvers were effective in both groups and most likely dwarfed effects of the intervention. There are several possible reasons for the particular effectiveness of these maneuvers in our sample. First, because our patients had few comorbidities and were not coping with multiple health concerns, they probably were better able to focus on the maneuvers we presented to them. Second, our sample consisted predominately of young adults who probably were better able to master all proactive maneuvers such as managing the contract and using the pedometer and workbook. In addition, younger adults typically engage in more diverse lifestyle activities and thus have more opportunity to incorporate physical activity into daily routines. For example, we encouraged integrating physical activity into workplace functions, commuting, and recreational activities with children. Finally, some physical activities, such as exercising with others and in health clubs, are more socially acceptable for younger individuals. We also encouraged these activities to simultaneously derive reinforcement from others. Thus, our multiple-component control protocol administered to both groups was particularly well suited to younger patients with fewer comorbidities and potentially overshadowed the positive affect and self-affirmation intervention.
For some patients, specifically those who required medical care during the trial, the intervention potentially had an incremental benefit. Possible reasons for this may be that positive affect and self-affirmation buffer patients from psychological consequences of acute illness and help prevent abandoning elective healthy behaviors during periods of medical adversity.
In the multivariate analysis, we found that using the pedometer, fulfilling the contract, and receiving more telephone contacts were associated with more activity. Patients reported the pedometer provided information about actual activity level and motivated them to match, and preferably exceed, this level each day. Thus, by providing real-time feedback, the pedometer increased self-efficacy for the activity. These findings confirm other trials that showed pedometers were associated with increased walking, even among those who were previously sedentary.45,46 Our trial also confirms previous studies that demonstrated the effectiveness of setting goals, making a contract, and using telephone follow-up calls to promote physical activity.44,47,48
Of the patient characteristics we considered, less social support at enrollment was associated with greater increases in physical activity. A reason for this counterintuitive result may be that having more social support often is accompanied by having more obligations to provide support to others. This hypothesis is endorsed by our previous finding that having extensive family obligations, which was common in our predominately young female population, was a barrier to exercise, whereas having more personal time was a facilitator.42 Of the other characteristics we studied, a decrease in depressive symptoms from enrollment to closeout was associated with increased physical activity, most likely because depressive symptoms affect the motivation to exercise and the ability to self-manage asthma.49
We found that energy expenditure increased during the first several months and then was sustained at a relatively constant level to closeout. In another trial to increase physical activity, researchers found an initial increase in energy expenditure, but then a gradual decline to closeout.47 In that trial, the frequency of follow-ups decreased over time compared with our trial in which the frequency was maintained until closeout. This finding supports the psychosocial principle that sustained behavior change requires ongoing counseling and support.50 Another possible reason for sustained activity in our trial was that patients chose their own activity goal based on what they thought was prudent and practical. In contrast, in the other trial all patients were assigned the same goal of meeting national exercise guidelines. Perhaps a better way to meet guidelines is to incrementally increase activity based on evidence of success, such as from daily pedometer readings.51
This trial has several limitations. First, our study design precludes our ability to conclude whether positive affect and self-affirmation interventions are effective without other behavioral maneuvers. Second, this study was conducted in an urban primary care practice with patients with minimal comorbidities and mild to moderate asthma and may not be generalizable to patients in different settings with more severe disease. Third, the same study personnel administered the follow-up calls to maintain continuity and therefore were not blinded to randomization group and any protocol-specific issues. Fourth, although we used a well-established and widely-used scale to measure physical activity, this scale relied on self-report and may have been subject to over-reporting or under-reporting.
In summary, a composite of several behavioral maneuvers was effective in increasing physical activity in patients with stable asthma. Physical activity increased to 4 months and was sustained through closeout at 12 months. These gains were achieved without exacerbating asthma. An intervention to increase positive affect and self-affirmation was not effective in increasing physical activity above the control protocol but may have had an incremental benefit in patients who required interval medical care during the trial.
Acknowledgments
The authors thank B. Robert Meyer MD and the physicians and patients at the Cornell Internal Medicine Associates at the Weill Cornell Medical College for their participation, Mary Murray-Weir PT, MBA at the Hospital for Special Surgery for her assistance in categorizing physical activities, and William M. Briggs PhD at New York Methodist Hospital and Bohumir Prikryl at the Weill Cornell Medical College for their assistance in managing databases. Dr. Briggs received salary support for his contributions during the initial phase of the trial.
Supported by contract N01 HC 25196 from the National Heart Lung and Blood Institute.
The funding agency participated in the design of the study, in the analysis and interpretation of the data, and in the preparation of the manuscript. The funding agency had no rolein the conduct of the study or the collection or management of the data. The funding agency reviewed and approved the final manuscript.
Footnotes
Presented at the American Thoracic Society 2009 Annual Meeting, International Conference, San Diego, California, May 19, 2009.
None of the authors has financial disclosures associated with this work.
This trial is registered in ClinicalTrials.gov (NCT00195117).
The authors have of no conflicts of interest to disclose.
Author Contributions: Carol A. Mancuso MD had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mancuso, Hollenberg, Isen, Jobe, Allegrante, Charlson
Acquisition of data: Mancuso, Choi, Westermann, Wenderoth, Hollenberg, Charlson
Analysis and interpretation of data: Mancuso, Choi, Westermann, Hollenberg, Wells, Isen, Charlson
Drafting of the manuscript: Mancuso, Choi, Westermann, Wells, Hollenberg, Charlson
Critical revision of the manuscript for important intellectual content: Mancuso, Choi, Westermann, Wenderoth, Hollenberg, Wells, Isen, Jobe, Allegrante, Charlson
Statistical analysis: Mancuso, Wells, Charlson
Study supervision: Mancuso, Jobe, Charlson
Obtained funding: Mancuso, Isen, Jobe, Allegrante, Charlson
Administrative, technical, or material support: Mancuso, Choi, Westermann, Wenderoth, Hollenberg, Charlson
Contributor Information
Carol A. Mancuso, Email: mancusoc@hss.edu, Hospital for Special Surgery, Weill Cornell Medical College.
Tiffany N. Choi, Email: tiffany.choi@gmail.com, Weill Cornell Medical College.
Heidi Westermann, Email: heidi.gortakowski@gmail.com, Weill Cornell Medical College.
Suzanne Wenderoth, Email: swendero@med.cornell.edu, Weill Cornell Medical College.
James P. Hollenberg, Email: DrJimHollenberg@aol.com, Weill Cornell Medical College.
Martin T. Wells, Email: mtw1@cornell.edu, Department of Statistical Sciences, Cornell University.
Alice M. Isen, Email: ami4@cornell.edu, Department of Psychology and Johnson Graduate School of Management, Cornell University.
Jared B. Jobe, Email: jobej@mail.nih.gov, National Institutes of Health.
John P. Allegrante, Email: jpal@columbia.edu, Teachers College and Mailman School of Public Health, Columbia University.
Mary E. Charlson, Email: mecharl@med.cornell.edu, Weill Cornell Medical College.
References
- 1.Westermann H, Choi TN, Briggs WM, Charlson ME, Mancuso CA. Obesity and exercise habits of asthmatic patients. Ann Allergy Asthma Immunol. 2008;101:488–494. doi: 10.1016/S1081-1206(10)60287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiler JM, Bonini S, Coifman R, et al. American Academy of Allergy, Asthma & Immunology Work Group Report: Exercise-induced asthma. J Allergy Clin Immunol. 2007;119(6):1349–1358. doi: 10.1016/j.jaci.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute National Asthma Education and Prevention Program. 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. [Google Scholar]
- 4.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women. JAMA. 1999;281(4):335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 5.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347(10):716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 6.King AC, Blair SN, Bild DE, Dishman RK, Dubbert PM, Marcus BH, Oldridge NB, Paffenbarger RS, Jr, Powell KE, Yeager KK. Determinants of physical activity and interventions in adults. Med Sci Sports Exer. 1992;24(6):S221–S236. [PubMed] [Google Scholar]
- 7.Sallis JF, Haskell WL, Fortmann SP, Vranizan KM, Taylor CB, Solomon DS. Predictors of adoption and maintenance of physical activity in a community sample. Prev Med. 1986;15(4):331–341. doi: 10.1016/0091-7435(86)90001-0. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LH, Wing RR, Thompson JK, Griffin W. Attendance and fitness in aerobics exercise: The effects of contract and lottery procedures. Behav Mod. 1980;4(4):465–479. [Google Scholar]
- 9.Anderson ES, Wojcik JR, Winett RA, Williams DM. Social-cognitive determinants of physical activity: the influence of social support, self-efficacy, outcome expectations, and self-regulation among participants in a church-based health promotion study. Health Psychol. 2006;25:510–520. doi: 10.1037/0278-6133.25.4.510. [DOI] [PubMed] [Google Scholar]
- 10.Erez A, Isen AM. The influence of positive affect on the components of expectancy motivation. J Applied Psychol. 2002;87:1055–1067. doi: 10.1037/0021-9010.87.6.1055. [DOI] [PubMed] [Google Scholar]
- 11.Isen AM, Rosenzweig AS, Young MJ. The influence of positive affect on clinical problem solving. Med Decision Making. 1991;11:221–227. doi: 10.1177/0272989X9101100313. [DOI] [PubMed] [Google Scholar]
- 12.Cohen GL, Aronson J, Steele CM. When beliefs yield to evidence: Reducing biased evaluation by affirming the self. Personality Soc Psychol Bull. 2000;26:1151–1164. [Google Scholar]
- 13.Isen AM. An influence of positive affect on decision making in complex situations: theoretical issues with practical implications. J Consumer Psychol. 2001;11(2):75–85. [Google Scholar]
- 14.Sherman DAK, Nelson LD, Steele CM. Do messages about health risks threaten the self? Increasing the acceptance of threatening health messages via self-affirmation. Personality Social Psychol Bulletin. 2000;26(9):1046–1058. [Google Scholar]
- 15.Charlson ME, Boutin-Foster C, Mancuso CA, Peterson JC, Ogedegbe G, Briggs WM, Robbins L, Isen AM, Allegrante JP. Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: Rationale, trial design and methods. Contemp Clin Trials. 2007;28(6):748–762. doi: 10.1016/j.cct.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Eisner MD, Katz PP, Yelin EH, Henke J, Smith S, Blanc PD. Assessment of asthma severity in adults with asthma treated by family practitioners, allergists, and pulmonologists. Medical Care. 1998;36(11):1567–1577. doi: 10.1097/00005650-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Mancuso CA, Sayles W, Allegrante JP. Development and testing of an asthma self-management questionnaire. Ann Allergy Asthma Immunol. 2009;102:294–302. doi: 10.1016/S1081-1206(10)60334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Personality Social Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 21.Rule BG, Harvey HZ, Dobbs AR. Reliability of the Geriatric Depression Scale for younger adults. Clin Gerontol. 1989;9(2):37–43. [Google Scholar]
- 22.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 23.Sherbourne CD, Stewart AL. The MOS Social Support Survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 24.Paffenbarger RS, Jr, Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exer. 1993;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exer. 2000;32(9 Suppl):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Mancuso CA, Choi TN, Westermann H, Briggs WM, Wenderoth S, Charlson ME. Measuring physical activity in asthma patients: Two-minute walk test, repeated chair rise test, and self-reported energy expenditure. J Asthma. 2007;44(4):333–340. doi: 10.1080/02770900701344413. [DOI] [PubMed] [Google Scholar]
- 27.Norris SL, Grothaus LC, Buchner DM, Pratt M. Effectiveness of physician-based assessment and counseling for exercise in a staff model HMO. Prev Med. 2000;30(6):513–523. doi: 10.1006/pmed.2000.0673. [DOI] [PubMed] [Google Scholar]
- 28.Smith BJ, Bauman AE, Bull FC, Booth ML, Harris MF. Promoting physical activity in general practice: A controlled trial of written advice and information materials. Br J Sports Med. 2000;34(4):262–267. doi: 10.1136/bjsm.34.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, McDermott S, DePue JD, Milan FB, Dube C, Tennstedt S. Physician-based physical activity counseling for middle-aged and older adults: A randomized trial. Ann Behav Med. 1999;21(1):40–47. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 30.Mancuso CA, Rincon M, Sayles W, Paget SA. Comparison of energy expenditure from lifestyle physical activities between patients with rheumatoid arthritis and healthy controls. Arthritis Care Res. 2007;57(4):672–678. doi: 10.1002/art.22689. [DOI] [PubMed] [Google Scholar]
- 31.Pinto BM, Goldstein MG, Papandonatos GD, Farrell N, Tilkemeier P, Marcus BH, Todaro JF. Maintenance of exercise after phase II cardiac rehabilitation. A randomized controlled trial. Am J Prev Med. 2011;41:274–283. doi: 10.1016/j.amepre.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemmler W, von Stengel S, Engelke K, Haberle L, Mayhew JL, Kalendaer WA. Exercise, body composition, and functional ability. A randomized controlled trial. Am J Prev Med. 2010;38:279–287. doi: 10.1016/j.amepre.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: a one-year randomized trial for patients with arthritis and fibromyalgia. Arth Care Res. 2008;59:1009–1017. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso CA, Sayles W, Robbins L, Allegrante JP. Novel use of patient-derived vignettes to foster self-efficacy in an asthma self-management workbook. Health Promotion Practice. 2010;11:44–53. doi: 10.1177/1524839907309865. [DOI] [PubMed] [Google Scholar]
- 35.Mancuso CA, Sayles W, Allegrante JP. Randomized trial of self-management education in asthma patients and effects of depressive symptoms. Ann Allergy Asthma Immunol. 2010;105:12–19. doi: 10.1016/j.anai.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancuso CA, Peterson MGE, Gaeta TJ, Fernandez JL, Birkhahn RH, Melniker LA, Allegrante JP. A randomized controlled trial of self-management education for asthma patients in the emergency department. Ann Emerg Med. 2011;57:603–612. doi: 10.1016/j.annemergmed.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Estrada CA, Isen AM, Young MJ. Positive affect improves creative problem solving and influences reported source of practice satisfaction in physicians. Motivation Emotion. 1994;18(4):285–298. [Google Scholar]
- 38.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death. The Multiple Risk Factor Intervention Trial. JAMA. 1987;258(17):2388–2395. [PubMed] [Google Scholar]
- 39.Sesso HD, Paffenbarger RS, Ha T, Lee IM. Physical activity and cardiovascular disease risk in middle-aged and older women. Am J Epidemiol. 1999;150(4):408–416. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 40.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 41.SAS User’s Guide: Statistics. Cary, NC: SAS Institute; 1985. Version 5 ed. [Google Scholar]
- 42.Mancuso CA, Sayles W, Robbins L, Phillips EG, Ravenell K, Duffy C, Wenderoth S, Charlson ME. Barriers and facilitators to healthy physical activity in asthma patients. J Asthma. 2006;43(2):137–143. doi: 10.1080/02770900500498584. [DOI] [PubMed] [Google Scholar]
- 43.Satta A. Exercise training in asthma. J Sports Med Phys Fitness. 2000;40(4):277–283. [PubMed] [Google Scholar]
- 44.Lombard DN, Lomard TN, Winett RA. Walking to meet health guidelines: the effect of prompting frequency and prompt structure. Health Psychol. 1995;14(2):164–170. doi: 10.1037//0278-6133.14.2.164. [DOI] [PubMed] [Google Scholar]
- 45.Hultquist CN, Albright C, Thompson DL. Comparison of walking recommendations in previously inactive women. Med Sci Sports Med. 2005;37(4):676–683. doi: 10.1249/01.mss.0000158993.39760.1b. [DOI] [PubMed] [Google Scholar]
- 46.Merom D, Rissel C, Phongsavan P, Smith BJ, Van Kemenade C, Brown WJ, Bauman AE. Promoting walking with pedometers in the community: The Step-by-Step Trial. Am J Prev Med. 2007;32(4):290–297. doi: 10.1016/j.amepre.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Simons-Morton DG, Blair SN, King AC, Morgan TM, Applegate WB, O’Toole M, Haskell WL, Albright CL, Cohen SJ, Ribisl PM, Shih JH. Effects of physical activity counseling in primary care. The Activity Counseling Trial: Randomized controlled trial. JAMA. 2001;286(6):677–687. doi: 10.1001/jama.286.6.677. [DOI] [PubMed] [Google Scholar]
- 48.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA. 2007;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 49.Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for future research. Clin Practice Epidemiol Mental Health. 2005 doi: 10.1186/1745-0179-1-18. http://www.cpementalhelth.com/content/1/1/18. [DOI] [PMC free article] [PubMed]
- 50.Wee CC. Physical activity counseling in primary care: The challenge of effecting behavioral change. JAMA. 2001;286(6):717–719. doi: 10.1001/jama.286.6.717. [DOI] [PubMed] [Google Scholar]
- 51.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]