Abstract
Prior research found that Americans born in 6 southeastern states (the AF-risk zone) had elevated risk of AF-related mortality, but no mechanisms were identified. We hypothesized the association between AF-related mortality and AF-risk zone birth is explained by indicators of childhood social disadvantage or adult risk factors. In 24,323 participants in the US Health and Retirement Study, we found that birth in the AF-risk zone was significantly associated with hazard of AF-related mortality. Among whites, the relationship was specific to place of birth, rather than place of adult residence. Neither paternal education nor subjectively assessed childhood SES predicted AF-related mortality. Conventional childhood and adult cardiovascular risk factors did not explain the association between place of birth and AF-related mortality.
Keywords: Atrial Fibrillation, Mortality, Residence, Geographic, Lifecourse
INTRODUCTION
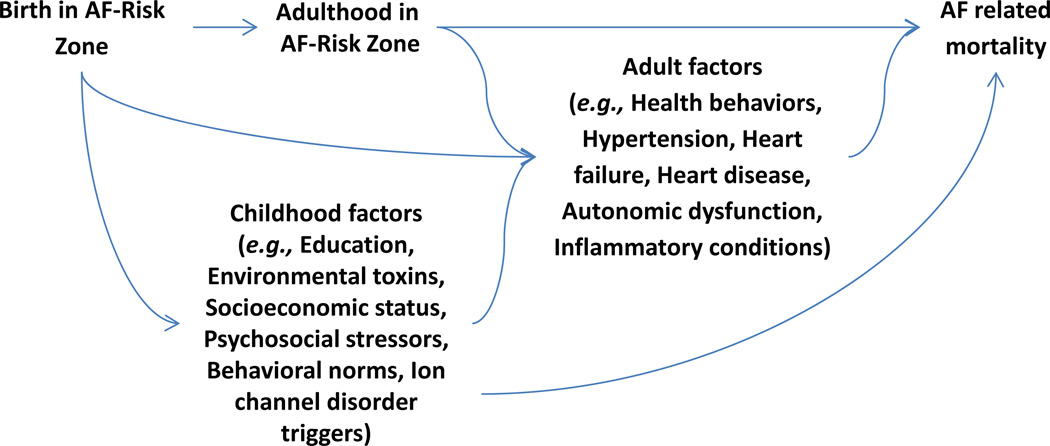
Lifecourse cardiovascular epidemiology has demonstrated that early life risk factors such as low birthweight and childhood socioeconomic adversity predict greater risk for angina and atherosclerosis as well as adult mortality from coronary heart disease and stroke. (Galobardes et al., 2006, Fabsitz and Feinleib, 1980, Batty et al., 2007, Glymour et al., 2007) Atrial fibrillation (AF) is the most common cardiac arrhythmia, (Benjamin et al., 2009, Magnani et al., 2011) and is responsible for significant morbidity from heart failure, dementia, and stroke, and increased mortality. Few articles have addressed whether early life conditions contribute to the development of AF. Preliminary evidence suggests that early life factors may influence AF, but via mechanisms distinct from those established for most other cardiovascular outcomes. For example, higher birthweight predicted increased risk of AF in the Women’s Health Study. (Conen et al., 2010) We recently showed that birth in a band of six Southeastern states (the AF-risk zone) predicted AF-related mortality. (Patton et al., 2011) Surprisingly, given the close association between AF and stroke, the geography of the AF-risk zone only partially overlapped with the U.S. Stroke Belt. (Glymour et al., 2009, Glymour et al., 2007, Howard, 1999, Casper et al., 1995, Howard et al., 1997) Place of birth sets the stage for a host of subsequent childhood and adult exposures that may influence risk of AF (figure 1). No prior study has examined whether the association between place of birth and AF-related mortality may be explained by childhood socioeconomic disadvantage or common cardiovascular risk factors.
Figure 1.
Hypothesized causal structure linking place of birth and Atrial Fibrillation related mortality.
In the current analysis, we used a national cohort to confirm and further examine our previous report that individuals born in the AF-risk zone had elevated risk of AF-related mortality, regardless of state of adult residence. (Patton et al., 2011) We tested the following hypotheses: 1) birth in any of 6 high risk states -- previously identified from the national mortality files to have the strongest relationship with AF-related mortality -- predicts AF-related mortality in this nationally representative cohort; 2) indicators of childhood adversity, previously associated with stroke and heart disease mortality, predict AF-related mortality; and 3) associations of place of birth with AF-related mortality are attenuated when adjusted for indicators of childhood adversity and for adult behavioral and physical risk factors.
METHODS
The national Health and Retirement Study (HRS) is an ongoing cohort study of US adults age ≥50 years and their spouses (regardless of spousal age). Details on study design and measures have been previously published. (Juster and Suzman, 1995, Heeringa and Connor, 1995) Briefly, the study was initiated in 1992, with additional enrollments in 1993, 1998, and 2004, depending on birth cohort. Participants are interviewed biennially; following each wave a linkage to National Death Index (NDI) records is conducted. (2009) NDI records are searched for participants lost to follow-up and individuals previously reported as deceased but not yet successfully linked to NDI data. (Health and Retirement Study, 2011) We followed HRS participants with non-missing data from their date of first interview to date of death, drop-out, or last HRS interview (in the 2008 wave).
From 31022 respondents, we excluded 6696 (21.6%) participants who were not age eligible (younger than 49.9 years at baseline) and 3 (0.01%) missing date of last interview. The analytic sample comprised 24,323 respondents followed an average of 9.7 years. The parent study is approved by the University of Michigan Institutional Review Board and the current analyses were approved by the Human Subjects Committee of the Harvard School of Public Health.
Data collection and measurements
The outcome was defined as death with AF (ICD-9 code 427.3 or ICD-10 code I48) listed as a contributing cause. At enrolment (average age 63.7), respondents identified their state of current residence and state of birth. The AF-risk zone was defined to include 5 states (Maryland, North Carolina, South Carolina, Virginia, West Virginia) and the District of Columbia (henceforth referred to collectively as the AF-risk zone). Study participants were classified as having lived in the AF-risk zone at birth or at study enrolment based on self-reported state of residence. These states were selected based on our previous analyses in a separate data set (National Center for Health Statistics mortality records), which identified these six states as the highest risk states of birth for AF-related mortality. (Patton et al., 2011) In supplemental analyses, we examined all-cause mortality, death with stroke (ICD-9 codes: 430 to 438 or ICD-10 codes: I60 to I69, or G458, or G459) as a contributing cause and death with heart failure (ICD-9 428 or ICD-10 I50) as a contributing cause.
In the current paper, we conduct supplemental analyses contrasting AF-related mortality in the AF-risk zone against AF-related mortality in Stroke Belt states not in the AF-risk zone. We defined the Stroke Belt exactly as in our previous publications to include the states of: North Carolina, South Carolina, Georgia, Tennessee, Arkansas, Mississippi, or Alabama. (Glymour et al., 2009, Glymour et al., 2007)
We examined years of completed schooling (0–17) and three indicators of childhood socioeconomic adversity (father’s education, perceived childhood socioeconomic status (SES), and height) previously shown to predict other aspects of health in HRS or other studies. Father’s education (<8, ≥8 years, unknown) predicted stroke onset in HRS (Glymour et al., 2008) and several cardiovascular outcomes in other studies. (Lemelin et al., 2009, Galobardes et al., 2006) To assess perception of childhood socioeconomic disadvantage, respondents were asked: “Now think about your family when you were growing up, from birth to age 16. Would you say your family during that time was pretty well off financially, about average, or poor?” Responses were scaled from 1 (pretty well off) to 5 (poor), and the small fraction of participants who volunteered “it varied” were coded as 3. Three additional questions related to childhood SES were asked including “did financial difficulties ever cause you or your family to move to a different place?”, “was there a time when you or your family received help from relatives because of financial difficulties?” and “was there a time of several months or more when your father had no job?” These measures were combined into a single scale from 1 to 5, with a missing indicator for respondents who did not complete the question. These items regarding perceived childhood SES predict several domains of adult health. (Luo and Waite, 2005) Height was self-reported at baseline interview. Adult height is sensitive to childhood nutrition, infections, and psychosocial conditions, (Steckel, 1995, Kuh and Wadsworth, 1989, Peck and Lundberg, 1995) and short stature has been linked to cardiovascular conditions and stroke. (Hebert et al., 1993, Krahn et al., 1994, Glymour et al., 2008) Based on prior evidence linking tall stature to elevated AF risk, we first considered height in sex specific categories and then as a single linear term expressed in inches. We found no evidence that the association between height and AF-related mortality differed by sex (p-value for test of interaction between height and sex=0.69; detailed sex-stratified results in web table 1), so we estimated a common coefficient.
We also examined adult risk factors including first available report of: household wealth (in 1992 dollars, divided by the square root of household size and natural log transformed); smoking status (never, past, current); self-reported body mass index (BMI); and self-reported physician’s diagnoses of hypertension, diabetes, stroke and heart disease. We used the missing indicator method to retain observations missing BMI information (n=203, 0.8%). All models were adjusted for age at enrolment and sex. We present models stratified or adjusted for race (black vs all other; nearly all “others” described themselves as white).
Methods of analysis
In models adjusted only for demographic variables, we used Cox proportional hazard models to assess whether birth or adult residence in the AF-risk zone predicted AF-related mortality. We considered birth and adult residence in the AF-risk zone first in separate models and then in the same model. Because place of birth is temporally prior to place of adult residence and also influences place of adult residence (figure 1), we conceptualize adult residence in the AF-risk zone as a potential mediator of the effect of birth in the AF-risk zone. The overall effect of birth in the AF-risk zone is therefore best estimated in a model without adjustment for place of adult residence. The AF-risk zone overlaps with the Stroke Belt. Therefore, AF might be observed more frequently in people who died in states in the AF-risk zone because they were more likely to die of stroke, if AF ascertainment was better among individuals with stroke. To rule this out, we repeated the primary analyses excluding respondents who died with stroke listed as a contributing cause of death. The exclusion of stroke death is an overly conservative analysis because AF is an established cause of stroke. (Patton et al., 2011) We also repeated analyses distinguishing between states in the AF-risk zone and states in the Stroke Belt but not the AF-risk zone (Georgia, Tennessee, Arkansas, Mississippi, or Alabama). We present these models stratified by race and pooled for all respondents because of prior evidence that the geographic patterns of AF may differ for blacks and whites. (Patton et al., 2011, Cushman et al., 2008) We interpret results for blacks very cautiously, because of the close correspondence between region of birth and region of adult residence and small number of events.
We next assessed whether childhood risk factors predicted AF-related mortality, and whether birth in the AF-risk zone was associated with AF-related mortality after adjustment for childhood risk factors. Finally, we repeated analyses including adjustment for adult cardiovascular risk factors. We present hazard ratios (HRs) and 95% CIs comparing AF-related mortality risk in each exposure category to AF-related mortality risk for the reference group. We estimate percent attenuation in adjusted versus unadjusted models as 1-(HRadjusted-1)/(HRunadjusted-1). Survival was defined as time from baseline interview to AF-related mortality, mortality due to other causes, or last interview date. Analyses were conducted using SAS 9.1 and 9.2 (SAS Institute Incorporated, Cary, NC, USA).
RESULTS
Characteristics of the HRS population, stratified by mortality status, are shown in Table 1. Of 230 AF-related deaths, 206 were among whites and 24 were among blacks. Descriptive characteristics stratified by birth or adult residence in the AF-risk zone are shown in the Appendix Table. Pooling blacks and whites, respondents born in the 6 states designated as the AF-risk zone had 78% elevated hazard of AF-related mortality (95% CI 1.20 to 2.64; Table 2). Restricting to blacks, birth in the AF-risk zone was associated with over a doubling of the risk of AF-related mortality, although this estimate was very imprecise due to the small number of events (HR=2.64; 1.18 to 5.91). Among whites, the point estimate suggested birth in the AF-risk zone was associated with a 56% increase in hazard of AF-related mortality, but the CI included the null (0.98 to 2.51). Whites who lived in the AF-risk zone in adulthood (specifically, at study enrollment, 65% of whom were born in the risk-zone) had a non-significant elevation in AF-related mortality risk (HR=1.28; 0.78 to 2.10). For blacks, living in the AF-risk zone at study enrollment (89% of whom were born in the risk-zone) was associated with even greater elevation in risk than birth in the AF-risk zone (HR=3.82; 1.64 to 8.92). When simultaneously adjusting for birth and adult residence in the AF-risk zone separately for blacks and whites, the point estimate of the HR for region of birth was larger for whites, while the point estimate for region of adult residence was larger for blacks. Overall, in the race-pooled model including both place of birth and place of adult residence, neither birth nor adult residence in the AF-risk zone was significantly associated with AF-related mortality, but the point estimate was higher for birth in the AF-risk zone (HR=1.59; 0.93 to 2.72) than for adult residence in the AF-risk zone (HR=1.20; 0.69 to 2.09).
Table 1.
Characteristics of HRS participants, stratified by atrial-fibrillation related vs all other cause mortality
| Sample Members Who Did Not Die During Follow-Up |
Sample Members With Non-AF-Related Death |
Sample Members With AF-Related Death |
||||
|---|---|---|---|---|---|---|
| n or mean | % | n or mean | % | n or mean | % | |
| n | 17,041 | 7,052 | 230 | |||
| Follow-up, years (SD) | 11.1 | (5.1) | 6.3 | (3.7) | 6.7 | (3.4) |
| Enrollment age, years (SD) | 59.8 | (8.9) | 72.8 | (11.2) | 77.7 | (9.5) |
| Male | 7,499 | 44.0 | 3,371 | 47.8 | 90 | 39.1 |
| Black | 2,548 | 15.0 | 1,213 | 17.2 | 24 | 10.4 |
| Childhood Risk Factors | ||||||
| Born in AF-risk zone | 1,771 | 10.4 | 795 | 11.3 | 30.0 | 13.0 |
| Father's Education | ||||||
| < 8 years | 5,649 | 33.1 | 4,693 | 66.5 | 179 | 77.8 |
| 8+ years | 8,797 | 51.6 | 1,203 | 17.1 | 22 | 9.6 |
| Unknown | 2,595 | 15.2 | 1,156 | 16.4 | 29 | 12.6 |
| Self-reported childhood socioeconomic disadvantage (range 0–4; 0=most advantaged) | 1.8 | 0.9 | 1.8 | 0.9 | 1.8 | 0.9 |
| Self-reported childhood socioeconomic disadvantage unknown | 939 | 5.5 | 2,585 | 36.7 | 69 | 30.0 |
| Male height, inches (SD) | 69.7 | 3.8 | 69.2 | 3.8 | 69.5 | 2.7 |
| Female height, inches (SD) | 63.8 | 4.1 | 63.0 | 5.5 | 63.6 | 2.9 |
| Education, years (SD) | 12.3 | 3.4 | 10.8 | 3.7 | 10.8 | 3.8 |
| Risk Factors at Study Enrollment | ||||||
| Median household wealth (interquartile range)^ | 68,236 | (146,140) | 45,396 | (107,760) | 52,476 | (124,051) |
| Current smoker | 3,351 | 19.7 | 1,523 | 21.6 | 21 | 9.1 |
| Past smoker | 6,335 | 37.2 | 2,915 | 41.4 | 102 | 44.4 |
| Body mass index, kg/m2 (SD) | 27.0 | 5.7 | 25.7 | 5.7 | 25.1 | 5.5 |
| Diabetes | 1,434 | 8.4 | 1,278 | 18.1 | 29 | 12.6 |
| Hypertension | 6,022 | 35.3 | 3,389 | 48.1 | 122 | 53.0 |
| Stroke | 466 | 2.7 | 817 | 11.6 | 26 | 11.3 |
| Heart disease | 1,915 | 11.2 | 2,156 | 30.6 | 104 | 45.2 |
Wealth is standardized by dividing by the square root of household size and Consumer Price Index adjusted to US 1992 dollars.
Table 2.
Birth or adult residence in the AF-risk zone and hazard ratios for AF-related mortality, HRS participants.
| Whites | Blacks | Pooled | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Model 1: Birth in AF-risk zone* | ||||||
| AF-risk zone birth | 1.56 | (0.98 to 2.51) | 2.64 | (1.18 to 5.91) | 1.78 | (1.20 to 2.64) |
| Model 2: Adult residence in AF-risk zone* | ||||||
| AF-risk zone adult residence | 1.28 | (0.78 to 2.10) | 3.82 | (1.64 to 8.92) | 1.63 | (1.08 to 2.46) |
| Model 3: Modeled together* | ||||||
| AF-risk zone birth | 1.64 | (0.87 to 3.09) | 1.32 | (0.39 to 4.46) | 1.59 | (0.93 to 2.72) |
| AF-risk zone adult residence | 0.92 | (0.48 to 1.80) | 3.06 | (0.86 to 10.92) | 1.20 | (0.69 to 2.09) |
| Model 4: Birth in AF-risk zone, excluding stroke related deaths† | ||||||
| AF-risk zone birth | 1.80 | (1.05 to 3.08) | 1.06 | (0.29 to 3.91) | 1.65 | (1.00 to 2.73) |
n= 20,538 white and 3,785 black HRS sample members.
n= 19,850 white and 3,621 black HRS sample members without stroke related deaths.
All models are adjusted for demographic covariates: baseline age, sex, and (in pooled models) black race.
We repeated analyses excluding individuals with stroke as a contributing cause of death. Although this should be an overly conservative effect estimate, we found that birth in the AF-risk zone was associated with a HR of 1.65 (95% CI 1.00 to 2.73) even when excluding all events with stroke as a contributing cause of death (Table 2, Model 4). The AF-risk zone partially overlaps with the Stroke Belt. To assess whether AF-related mortality was elevated throughout the Stroke Belt, we estimated the HR for AF-related mortality associated with birth in a Stroke Belt state which was not in the AF-risk zone. Birth in this region was not associated with elevated AF-related mortality among whites (HR=0.85; 0.52 to 1.38) or blacks (HR=1.21; 0.40 to 3.70) compared to birth elsewhere in the US (i.e., not the Stroke Belt and not the AF-risk zone). Birth in the AF risk zone was associated with small elevations in all-cause mortality in race-pooled models (HR= 1.19; 95% CI: 1.10, 1.28), reflecting a significant association among whites (HR=1.29; 95% CI: 1.18, 1.42) but not among blacks (HR=1.02; 0.90 to 1.16) (results not shown in tables). In race pooled models, birth in the AF-risk zone was associated with both death related to heart failure (HR=1.23; 1.00, 1.50) and death related to stroke (HR=1.43; 1.17, 1.76) (not shown in tables).
AF-related mortality was higher among whites and was not associated with paternal education or subjective assessment of childhood socioeconomic disadvantage (Table 3). In models examining height in sex-specific categories, we found a progressive gradient of increasing height associated with higher risk of AF-related mortality regardless of sex (Web Only Table 1). AF-related mortality risk was associated approximately linearly with stature; although contrary to associations with other cardiovascular outcomes, taller stature was associated with higher AF risk (HR per inch=1.08; 95% CI 1.03 to 1.13). Finally, years of education did not predict AF-related mortality (HR=0.99; 0.95 to 1.02). Adjusting for all of these risk factors, birth in the AF-risk zone retained an association with AF risk (HR=1.66; 1.12 to 2.46) an attenuation of only 15% from the unadjusted model HR of 1.78 (Table 2).
Table 3.
Birth in the AF-risk zone, childhood risk factors, and hazard ratios for AF-related mortality, HRS participants.
| Model 1: Paternal Education |
Model 2: Childhood Poverty |
Model 3: Short Stature | Model 4: Own Education | Model 5: All Childhood | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Birth in AF-risk zone | 1.77 | (1.20 to 2.64) | 1.74 | (1.17 to 2.58) | 1.72 | (1.16 to 2.56) | 1.77 | (1.19 to 2.63) | 1.66 | (1.12 to 2.46) |
| Age at enrollment | 1.14 | (1.12 to 1.16) | 1.13 | (1.11 to 1.14) | 1.15 | (1.13 to 1.17) | 1.14 | (1.13 to 1.16) | 1.13 | (1.11 to 1.15) |
| Male (vs female) | 1.21 | (0.93 to 1.58) | 1.14 | (0.87 to 1.50) | 0.77 | (0.52 to 1.12) | 1.21 | (0.93 to 1.59) | 0.69 | (0.47 to 1.02) |
| Black (vs. all other) | 0.66 | (0.43 to 1.13) | 0.58 | (0.38 to 0.90) | 0.65 | (0.42 to 1.00) | 0.64 | (0.41 to 1.00) | 0.57 | (0.37 to 0.90) |
| Paternal education <8 years | 0.79 | (0.47 to 1.32) | - | - | - | 0.77 | (0.46 to 1.29) | |||
| Self-reported childhood socioeconomic disadvantage | 1.12 | (0.95 to 1.33) | - | - | 1.13 | (0.95 to 1.33) | ||||
| Height, inches | 1.08 | (1.03 to 1.13) | - | 1.09 | (1.04 to 1.14) | |||||
| Education completed, years | 0.99 | (0.95 to 1.02) | 0.99 | (0.96 to 1.03) | ||||||
Each column presents coefficients from a single regression model. No covariates other than those listed were included in the models.
Among adult risk factors examined (Table 4), lower household wealth, diagnoses of hypertension, and heart disease all significantly predicted hazard of AF-related mortality. Adjusting for all adult measures, birth in the AF-risk zone remained associated with AF-related mortality (HR=1.66; 95% CI 1.12 to 2.47), an attenuation of only 15% from the unadjusted model HR.
Table 4.
Birth in the AF-risk zone, adult risk factors and hazard ratios for AF-related mortality, Health and Retirement Study
| Model 1: Adjusted for social factors |
Model 2: Adjusted for BMI and smoking status |
Model 3: Adjusted for adult cardiovascular risk factor |
||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Birth in AF-risk zone | 1.71 | (1.15 to 2.53) | 1.71 | (1.15 to 2.54) | 1.66 | (1.12 to 2.47) |
| Age | 1.15 | (1.13 to 1.16) | 1.14 | (1.13 to 1.16) | 1.14 | (1.12 to 1.16) |
| Men vs. women | 0.78 | (0.53 to 1.14) | 0.80 | (0.54 to 1.17) | 0.83 | (0.56 to 1.21) |
| Black vs. white race | 0.55 | (0.35 to 0.86) | 0.55 | (0.35 to 0.87) | 0.59 | (0.38 to 0.94) |
| Height, inches | 1.09 | (1.04 to 1.14) | 1.09 | (1.04 to 1.14) | 1.08 | (1.03 to 1.13) |
| Education, years | 1.00 | (0.96 to 1.04) | 1.00 | (0.96 to 1.04) | 1.01 | (0.97 to 1.05) |
| Log household wealth* | 0.91 | (0.84 to 0.98) | 0.91 | (0.84 to 0.98) | 0.93 | (0.86 to 1.00) |
| Current smoker | 0.94 | (0.59 to 1.50) | 1.04 | (0.65 to 1.67) | ||
| Body mass index | 0.99 | (0.96 to 1.02) | 0.98 | (0.95 to 1.01) | ||
| Diabetes | 1.28 | (0.86 to 1.91) | ||||
| Hypertension | 1.48 | (1.13 to 1.94) | ||||
| Stroke | 1.32 | (0.87 to 2.01) | ||||
| Heart disease | 2.61 | (1.99 to 3.41) | ||||
Each column presents coefficients from a single regression model. No covariates other than those listed were included in the models.
In thousands divided by the square root of the household size, CPI adjusted to 1992 US dollars, and natural log transformed.
All covariates were assessed at baseline (enrollment).
DISCUSSION
We observed significant elevations in odds of AF-related mortality associated with birth in the AF-risk zone. Other early life cardiovascular risk factors, including risk factors previously associated with stroke, did not predict AF-related mortality. To the contrary, shorter stature, an indication of early life disadvantage associated with stroke and heart disease, was associated with lower risk of AF relative to taller stature. The elevated risk associated with birth in the AF-risk zone was not attenuated by adjustment for any of the measured early life or adult risk factors.
There has been little prior research on lifecourse determinants of AF. Using the National Center for Health Statistics mortality files and the US Census microsample, we previously identified a cluster of US states with elevated AF-related mortality risk. (Patton et al., 2011) The current research confirms that finding and addresses several limitations of the prior study by using a longitudinal cohort. These advantages include ascertainment of place of birth directly from respondents, and the opportunity to examine several plausible mechanisms linking place of birth to AF-related mortality. Because of the close association between AF and stroke, we anticipated that childhood risk factors associated with stroke incidence and mortality would also predict AF. We found no support for this hypothesis. Remarkably, indicators of early life disadvantage linked to stroke did not predict AF-related mortality.
One prior study examining early life antecedents of AF found that higher birthweight predicted elevated AF risk (Conen et al., 2010), consistent with evidence that tall stature is associated with increased risk of AF. (Chamberlain et al., 2011, Mont et al., 2008, Rosengren et al., 2009) However, this finding contrasts with much lifecourse epidemiology, which implicates low birthweight and short adult stature in increased risk of adult cardiovascular disease. In recent years, low birthweight has been shown to be more common among southerners (Thompson et al., 2005, Nepomnyaschy, 2010) although it is not known if this association applied in earlier birth cohorts. This suggests that early life determinants of AF may be distinct from pathways related to coronary heart disease. (Fabsitz and Feinleib, 1980, Roux et al., 2001, Batty et al., 2007, Glymour et al., 2007, Galobardes et al., 2006, Conen et al., 2010)
Previous research indicates that AF prevalence differences do not explain the Stroke Belt, but AF may contribute to elevated stroke risk in the region considered the “buckle” of the Stroke Belt. Cushman et al. reported elevated AF prevalence in white residents of the Stroke Buckle, but not when considering the Stroke Belt as a whole. (Cushman et al., 2008) We also found evidence that states at especially high risk for AF-related mortality overlap to some extent with the Stroke Buckle.
If measured indicators of early life adversity do not predict AF-related mortality, the AF-risk zone effects must be mediated by some other factor. Because AF is a strong risk factor for both stroke and heart failure, we expected that birth in the AF-risk zone would also predict those outcomes and indeed we find evidence to support this. However, for all-cause mortality (HR=1.19), stroke-related mortality (HR=1.43), and heart-failure-related mortality (HR=1.23), the association was attenuated compared to AF-related mortality (HR=1.78), suggesting the association might be via independent mechanisms related specifically to AF. Plausible factors might be conditions that are not well measured in epidemiologic studies of adult cardiovascular disease, such as early life dietary patterns, environmental exposures, gestational exposures, latent infectious diseases, psychological traumas, and institutional resources, such as schools or occupational conditions. (Howard, 1999, Brunner, 2000, Seeman et al., 2010, Marks, 2003, Alt, 1994) Normal electrophysiologic function is dependent on cardiac cellular structure and the function of multiple ion channels. (Iwasaki et al., 2011) AF is also related to enlarged atrial size but the etiology of AF is not fully understood; it is not clear what early life risk factors would differentially affect AF over stroke. The most rapid development of cardiac tissue occurs prenatally, suggesting the possibility that prenatal environmental exposures may subtly disrupt proper development during gestation. This hypothetical developmental change would have to be quite subtle, with manifestation only in much later life when AF emerges as a common event. Examination of regional differences in other indicators of cardiac electrophysiology may provide better insight into specific aspects of cardiac development that are geographically patterned.
Isolating the relevant timing of exposure could be a valuable clue into plausible mechanisms, but unfortunately we do not have detailed information on residential history. Nearly all children born in the AF-risk zone would have lived there at least through adolescence; for most, their mothers would have resided in the AF-risk zone during pregnancy. (Ruggles et al., 2004, Glymour and Manly, 2008) This period spans many important developmental periods associated with rapid physical (e.g., gestation, childhood growth spurts, puberty) and social (e.g., formal education, labor market entry) changes. With these data, we cannot pinpoint the etiologically relevant lifecourse period.
To our knowledge, our study is the first to examine childhood socioeconomic conditions and adult AF outcomes, but we faced several limitations. First, we did not have AF diagnoses or incidence as an outcome, but could only use AF-related mortality as a surrogate endpoint. AF-related mortality may not correspond well with incidence or prevalence of AF because it reflects the combined influence of incidence and case-fatality. We therefore cannot distinguish predictors of incidence from predictors of fatality rate among individuals with AF. AF may be underreported on death certificates. (Goldacre, 1993, Lloyd-Jones et al., 1998) However, our results are largely consistent with prior research on several previously investigated AF risk factors, including age, race, height, and hypertension. (Benjamin et al., 2009, Kannel and Benjamin, 2008) Differences in cause of death coding seem unlikely to fully explain our results, because such differences would primarily pertain to place of death, rather than place of birth. We caution that these results require replication in a geographically diverse dataset with improved assessment of AF. Competing risks and survivor bias may also have attenuated the associations we observe. There is no perfect solution to these problems, but we believe the bias would almost certainly be towards the null. We used a definition of the AF-risk zone based on prior research on place of birth effects. (Patton et al., 2011) Although the results for this definition of the AF-risk zone seem most consistent for exposure at birth, our ability to distinguish birth from adult risk is based on the relatively small number of individuals (11% of blacks and 5% of whites) who migrated between the AF-risk zone and other states. Our findings do not preclude a distinct geography of adult exposures that influences AF incidence or mortality.
HRS participants born in the AF-risk zone are not a representative sample of all such Americans because, although HRS is nationally representative, it is not representative at the state level. Our findings are far more convincing in the context of previous results – based on national death records – showing very similar geographic patterns. We had relatively few AF-related deaths, so our effect estimates are quite imprecise. The number of events in blacks was especially small, so our analyses in blacks and racial comparisons must be viewed as preliminary. Finally, state is the lowest level geographic resolution available for place of birth in this study. However, risk probably varies at much more local scales, such as counties or even neighborhoods. Many of the limitations we discuss may be addressed in other data sets, such as the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study or with future data linkages in HRS.
In summary, we confirm prior findings of significant elevations in odds of AF-related mortality associated with geographic place of birth. These intriguing findings suggest there are as yet unidentified early life factors relevant to the development of AF. Identifying such factors may help inform prevention efforts. Given the substantial medical burden of the AF epidemic, advances in prevention could result in major public health benefits.
Supplementary Material
Highlights.
U.S. HRS participants (n=24323) were followed an average of 9.7 years for AF-related mortality.
Birth in the AF-risk zone was significantly associated with hazard of AF-related mortality.
Among whites, the relationship was specific to place of birth, rather than place of adult residence.
Major childhood and adult socioeconomic risk factors previously shown to predict stroke did not predict AF-related mortality.
Acknowledgments
Funding/Disclosures
Contributions of Dr. Glymour and Ms. Kosheleva to this study were supported by funding from the NIH/NIA (R21AG03438501).
Dr. Benjamin receives research support from 1RC1-HL101056; 1R01HL092577; 1R01HL102214; 1R01AG028321;
Ms. Gilsanz was supported by the Harvard School of Public Health Initiative to Maximize Student Diversity (IMSD) training grant (NIH/NIGMS) and by the National Heart Lung and Blood Institute (F31HL112613).
The Health and Retirement Study is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and conducted by the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Dr. Glymour initiated the idea for the research, supervised the analysis, interpreted results, and wrote most of the first draft of the manuscript.
Dr. Benjamin contributed to the conceptualization for the research, helped interpret results and provided critical feedback and revisions to the manuscript.
Ms. Kosheleva conducted the statistical analyses, helped with interpretation of results, and contributed critical revisions to the manuscript.
Ms. Gilsanz helped interpret results, wrote sections of the first draft of the manuscript, and provided critical revisions to the manuscript.
Dr. Curtis suggested additional analyses to clarify results and contributed to critical revisions of the manuscript.
Dr. Patton collaborated on planning the study, helped interpret findings, contributed key sections of the manuscript and provided critical revisions of the manuscript.
References
- National Death Index US Department of Health and Human Services. 2009 [Google Scholar]
- Alt JE. The impact of the voting rights act on black and white voter registration in the South. In: Davidson C, Grofman B, editors. Quiet Revolution in the South: The Impact of the Voting Rights Act, 1965–1990. Princeton University Press; 1994. [Google Scholar]
- Batty GD, Alves JG, Correia J, Lawlor DA. Examining life-course influences on chronic disease: the importance of birth cohort studies from low- and middle- income countries. An overview. Braz J Med Biol Res. 2007;40:1277–1286. doi: 10.1590/s0100-879x2007000900015. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EJ. Toward a New Social Biology. In: Berkman LF, Kawachi I, editors. Social Epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- Casper ML, Wing S, Anda RF, KnowleS M, Pollard RA. Changes in the Geographic Pattern of Stroke Mortality in the United- States, 1995–1962. Stroke. 1988;26:755–760. doi: 10.1161/01.str.26.5.755. [DOI] [PubMed] [Google Scholar]
- Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A Clinical Risk Score for Atrial Fibrillation in a Biracial Prospective Cohort (from the Atherosclerosis Risk In Communities [ARIC] Study) The American journal of cardiology. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122:764. doi: 10.1161/CIRCULATIONAHA.110.947978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, Cantrell RA, Mcclure LA, Howard G, Prineas RJ, Moy CS, Temple EM, Howard VJ. Estimated 10 year stroke risk by region and race in the United States. Annals of Neurology. 2008;64:507–513. doi: 10.1002/ana.21493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabsitz R, Feinleib M. Geographic Patterns in County Mortality Rates from Cardiovascular Diseases. Am. J. Epidemiol. 1980;111:315–328. doi: 10.1093/oxfordjournals.aje.a112903. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Glymour M, Kosheleva A, Boden-Albala B. Birth and adult residence in the stroke belt independently predict stroke mortality. Neurology. 2009;73:1858–1865. doi: 10.1212/WNL.0b013e3181c47cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Avendano M, Berkman LF. Is the 'stroke belt' worn from childhood?: risk of first stroke and state of residence in childhood and adulthood. Stroke. 2007;38:2415–2421. doi: 10.1161/STROKEAHA.107.482059. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Avendaño M, Haas SA, Berkman LF. Lifecourse Social Conditions and Racial Disparities in Incidence of First Stroke. Annals of Epidemiology. 2008;18:904–912. doi: 10.1016/j.annepidem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18:223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ. Cause-Specific Mortality - Understanding Uncertain Tips of the Disease Iceberg. Journal of Epidemiology and Community Health. 1993;47:491–496. doi: 10.1136/jech.47.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Retirement Study. Sample Size and Response Rates. [[Accessed 2/26/2012]];2011 Available: http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf. [Google Scholar]
- Hebert P, Rich-Edwards J, Manson J, Ridker P, Cook N, O'connor G, Buring J, Hennekens C. Height and incidence of cardiovascular disease in male physicians. Circulation. 1993;88:1437–1443. doi: 10.1161/01.cir.88.4.1437. [DOI] [PubMed] [Google Scholar]
- Heeringa SG, Connor J. Technical description of the Health and Retirement Study sample design. Ann Arbor, Michigan: Survey Research Center, University of Michigan; 1995. [Google Scholar]
- Howard G. Why do we have a stroke belt in the southeastern United States? A review of unlikely and uninvestigated potential causes. American Journal of the Medical Sciences. 1999;317:160–167. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Nishida K, Kato T, Nattel S. Atrial Fibrillation Pathophysiology. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- Juster F, Suzman R. An overview of the health and retirement study. Journal of Human Resources. 1995;30(suppl):S7–S56. [Google Scholar]
- Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Medical Clinics of North America. 2008;92:17–40. doi: 10.1016/j.mcna.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn AD, Manfreda J, Tate RB, Mathewson FAL, Cuddy TE. Evidence that height is an independent risk factor for coronary artery disease (the Manitoba follow-up study) The American Journal of Cardiology. 1994;74:398–399. doi: 10.1016/0002-9149(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Kuh D, Wadsworth M. Parental Height: Childhood Environment and Subsequent Adult Height in a National Birth Cohort. International Journal of Epidemiology. 1989;18:663–668. doi: 10.1093/ije/18.3.663. [DOI] [PubMed] [Google Scholar]
- Lemelin ET, Diez Roux AV, Franklin TG, Carnethon M, Lutsey PL, Ni H, O'meara E, Shrager S. Life-course socioeconomic positions and subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Social Science & Medicine. 2009;68:444–451. doi: 10.1016/j.socscimed.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2005;60:S93–S101. doi: 10.1093/geronb/60.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, Mcmanus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial Fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks HM. Epidemiologists Explain Pellagra: Gender, Race, and Political Economy in the Work of Edgar Sydenstricker. J Hist Med Allied Sci. 2003;58:34–55. doi: 10.1093/jhmas/58.1.34. [DOI] [PubMed] [Google Scholar]
- Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, Vidal B, Scalise A, Tejeira A, Berruezo A. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- Nepomnyaschy L. Race disparities in low birth weight in the US south and the rest of the nation. Social Science & Medicine. 2010;70:684–691. doi: 10.1016/j.socscimed.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Patton KK, Benjamin EJ, Kosheleva A, Curtis L, Glymour MM. Early Life Antecedents of Atrial Fibrillation: Place of Birth and Atrial Fibrillation Related Mortality. Annals of Epidemiology. 2011;21:732–738. doi: 10.1016/j.annepidem.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MN, Lundberg O. Short stature as an effect of economic and social conditions in childhood. Social Science & Medicine. 1995;41:733–738. doi: 10.1016/0277-9536(94)00379-8. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Hauptman PJ, Lappas G, Olsson L, Wilhelmsen L, Swedberg K. Big men and atrial fibrillation: effects of body size and weight gain on risk of atrial fibrillation in men. European heart journal. 2009;30:1113. doi: 10.1093/eurheartj/ehp076. [DOI] [PubMed] [Google Scholar]
- Roux AVD, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of Residence and Incidence of Coronary Heart Disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- Ruggles S, Sobek M, Alexander T, Fitch CA, Goeken R, Hall PK, King M, Ronnander C. Integrated Public Use Microdata Series: Version 3.0 [Machine-readable database] Minneapolis, MN: Minnesota Population Center; 2004. [Online]. Available: http://www.ipums.org [Accessed]. [Google Scholar]
- Seeman T, Epel E, Grueewald T, Karlamangla AS, Mcewen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Steckel RH. Stature and the Standard of Living. Journal of Economic Literature. 1995;33:1903–1940. [Google Scholar]
- Thompson LA, Goodman DC, Chang CH, Stukel TA. Regional variation in rates of low birth weight. Pediatrics. 2005;116:1114. doi: 10.1542/peds.2004-1627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.