Abstract
Impulsive behaviors are closely linked to drug use and abuse, both as contributors to use and as consequences of use. Trait impulsivity is an important determinant of drug use during development, and in adults momentary ‘state’ increases in impulsive behavior may increase the likelihood of drug use, especially in individuals attempting to abstain. Conversely, acute and chronic effects of drug use may increase impulsive behaviors, which may in turn facilitate further drug use. However, these effects depend on the behavioral measure used to assess impulsivity. This article reviews data from controlled studies investigating different measures of impulsive behaviors, including delay discounting, behavioral inhibition and a newly proposed measure of inattention. Our findings support the hypothesis that drugs of abuse alter performance across independent behavioral measures of impulsivity. The findings lay the groundwork for studying the cognitive and neurobiological substrates of impulsivity, and for future studies on the role of impulsive behavior as both facilitator and a result of drug use.
Keywords: Attention, behavioral inhibition, decision making, delay discounting, drug use, impulsivity
INTRODUCTION
Impulsivity appears to function as both a determinant and a consequence of drug use. As a determinant, trait impulsivity is a risk factor for drug experimentation, problematic drug use and inability to abstain from drug use (e.g. McGue & Bouchard 1984; Tarter et al. 2007). In addition, brief, state-dependent increases in impulsive behaviors may also increase drug use. These momentary increases in impulsive behavior may impair the ability to abstain among individuals who are trying to control their use. Momentary fluctuations in decision-making or inhibition may have especially negative consequences for drug users who are trying to abstain from drug use, because momentary lapses in control or inhibition could increase the risk of drug use.
Conversely, drug use itself may increase maladaptive behaviors, either through their direct, acute effects or because of long-term sequelae of drug use. Acutely, drugs may impair inhibition or decision-making that could result in an increased likelihood of engaging in risky behaviors such as risky sex or driving while intoxicated. In addition to these risky behaviors, the drug-induced impairments could also affect the likelihood of further drug use. That is, the direct effects of a drug on decision-making may in turn lead to unplanned continuation or escalation of drug use. Extended exposure to a drug may also result in impaired inhibitory capacity, perhaps because of lasting neurological sequelae of chronic drug use (Jentsch &Taylor 1999). It is clear that impulsivity and drug use has a rich but complex association. Considering the public health consequences of both pathological impulsivity and drug use, it is clear that we need a better understanding of this complex relationship.
Unfortunately, impulsivity research has historically been limited by a lack of consistency in defining and assessing the construct. The term ‘impulsivity’ has been used to refer to a wide range of seemingly unrelated mal-adaptive behaviors including, for example, inability to wait, difficulty withholding responses and insensitivity to negative or delayed consequences. There is now a growing consensus that impulsivity is a multi-dimensional construct, and that the various impulsivity measures probably reflect separate underlying processes (Castellanos & Tannock 2002; Swann et al. 2002; Reynolds et al. 2004). The two most commonly identified processes are behavioral inhibition and impaired decision-making, but other dimensions are likely to be identified in the future. We propose one additional process that may result in apparently impulsive behaviors in this paper, namely, a dimension of lapses of attention. These separate and dissociable processes that contribute to ‘impulsive’ behaviors need to be characterized and understood to study their potential roles in the development and consequences of drug use. Figure 1 illustrates how different processes may lead to observed impulsive behaviors, and shows the specific measures that have been developed to quantify each of these processes. This article will review the literature on the relationship between drug use and impulsivity focusing on measures of behavioral inhibition, impulsive decision-making and attentional lapses.
Figure 1.
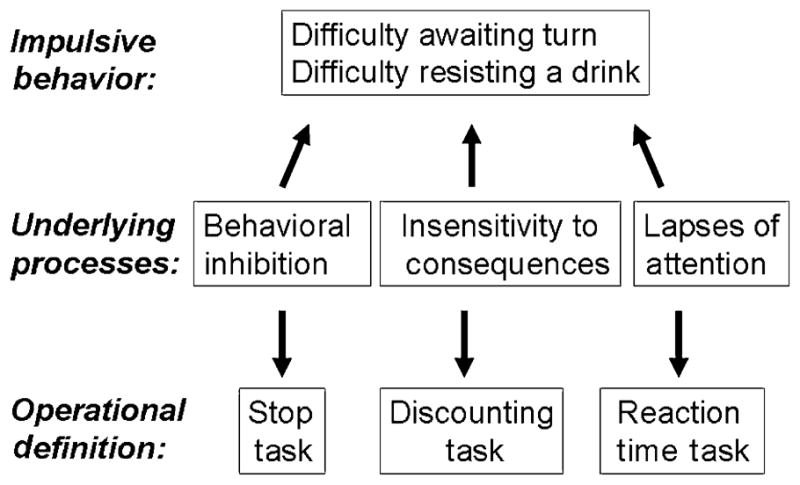
Schematic of relationships between observable ‘impulsive’ behaviors, processes that may lead to these behaviors and laboratory tasks that measure the processes. ‘Difficulty awaiting turn’ refers to a common symptom of ADHD, ‘difficulty resisting drink’ refers to a drug-related behavior that involves impulsivity. Any of the three processes, behavioral inhibition, delay discounting and inattention, may contribute to these maladaptive behaviors
DEFINITIONS OF IMPULSIVITY
Most broadly defined, impulsivity refers to the tendency to engage in inappropriate or maladaptive behaviors. Impulsivity functions as a dimension of normal behavior. Very high levels of impulsivity also are symptomatic of many psychiatric disorders, including attention deficit hyperactivity disorder (ADHD) or borderline personality disorder. Because ‘impulsivity’ has been used to refer to a variety of behaviors, a correspondingly wide assortment of measures has been used to assess impulsivity. These measures include electrophysiological assessment, questionnaires, interviews and a variety of objective behavioral tasks. Self-report personality measures are valuable but they are limited by many factors, including the fact that they rely on the individuals’ ability to accurately assess and report their own behavioral tendencies. Operationally defined behavioral tasks have the advantage that they are not influenced by subjective bias.
The most commonly used behavioral measures of impulsivity are delay discounting, which assesses impulsive decision-making, and behavioral-inhibition tasks. Measures of delay discounting are based on the operational definition of a relative preference for smaller, more immediate rewards over larger, more delayed rewards (Rachlin & Green 1972; Ainslie 1975; Herrnstein 1981; Logue 1988; Rachlin, Raineri & Cross 1991). Although all organisms ‘discount’ the value of delayed consequences relative to immediate consequences, this tendency is more pronounced in impulsive individuals. This behavioral measure is illustrated in Fig. 2, showing that delayed rewards have a smaller perceived value among impulsive individuals, including drug users.
Figure 2.
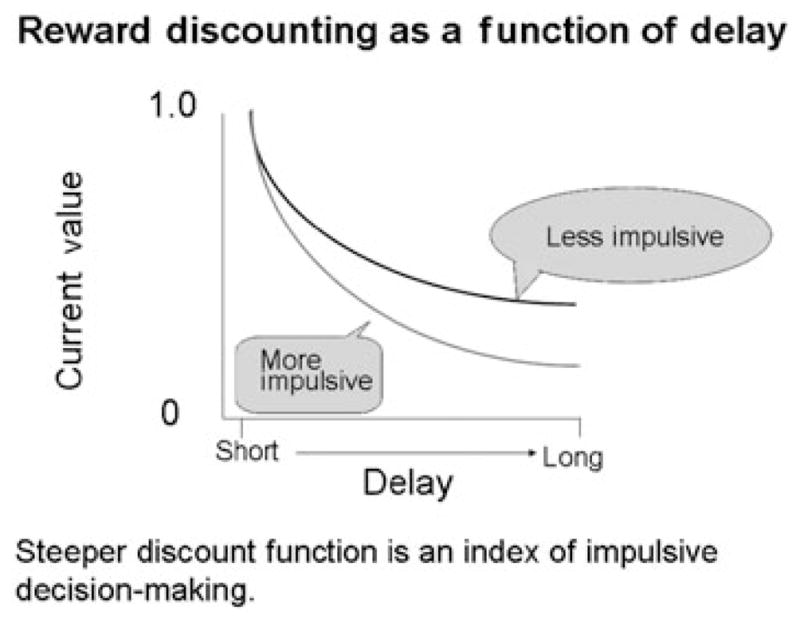
Schematic of delay discounting. The graph shows the indifference points, or points of equivalence, from choices between immediate (smaller) rewards and delayed (larger) rewards. More impulsive individuals ‘discount’ delayed rewards more steeply
Behavioral inhibition tasks measure the ability (or inability) to stop a prepotent response, i.e. a response that the individual is ready to emit. Figure 3 shows a schematic of the procedure used in the Stop Task, one of the most commonly used measures of behavioral inhibition (Logan 1994). Subjects are required to ‘stop’ their response after receiving a ‘go’ signal to make the response. The procedure allows the investigator to calculate the amount of time needed to inhibit a response once its execution has been initiated. Children with ADHD, who typically exhibit poor behavioral control, are impaired on this task (Tannock et al. 1989; Tannock, Ickowicz & Schachar 1995). Interestingly, their impairment is reversed by the psychomotor stimulant methylphenidate, which also reduces ADHD symptoms in these patients (Tannock et al. 1989, 1995). The task has also been proven useful in studies with adults, including studies relating to drug abuse (see following discussion of previous studies). Thus, whereas self-report measures assess an individual’s perception of their impulsive tendencies, the most commonly used measures of actual impulsive behavior are associated with two well-characterized impulsivity dimensions. However, as described in the next paragraph, there is new evidence that inattention may also be a facet of impulsivity.
Figure 3.
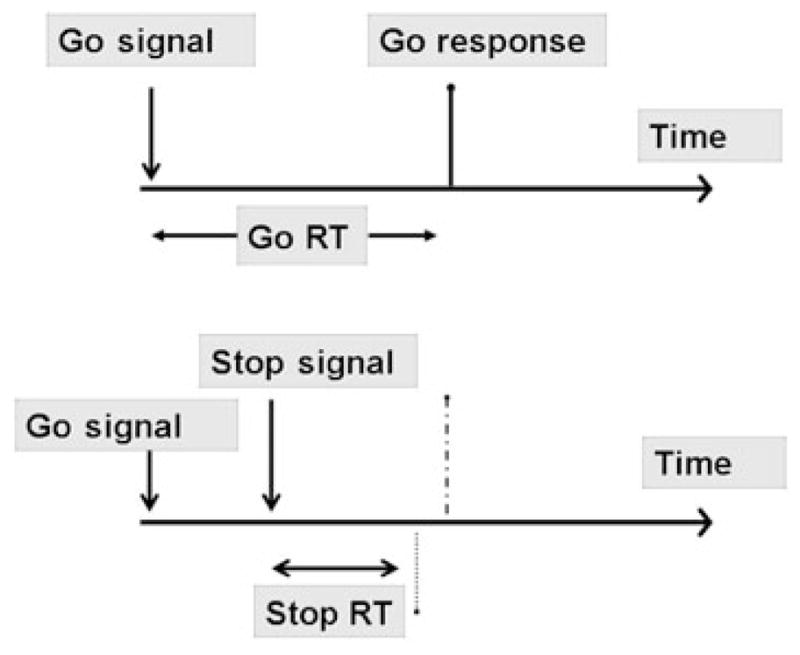
Schematic of the Stop Task procedure. Subjects are presented with either Go trials (75% of trials; top panel) or Stop trials (25% of trials; bottom panel). A simple Go reaction time is calculated from the Go trials. On Stop trials, subject is give a Stop signal shortly after the Go signal, and instructed to inhibit their ‘Go’ response on these trials. The time between the Go and Stop signal is varied systematically until the delay is reached at which the subject is able to inhibit the response on 50% of trials. This provides an estimate of the time needed to stop a response. Longer stop times are indices of poorer inhibitory control
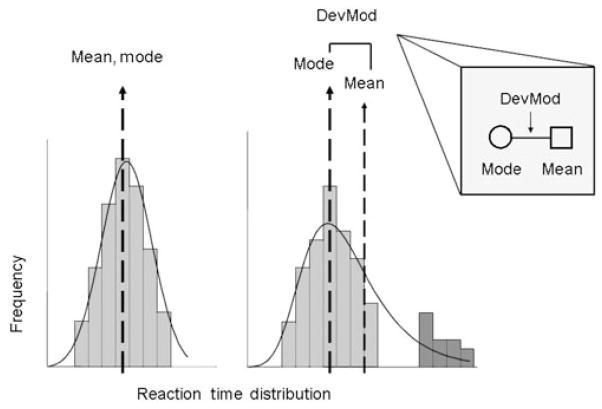
This laboratory has recently explored one other cognitive process that may result in maladaptive behaviors resembling impulsivity in healthy adults. Attention is a cognitive process closely related to impulsivity. Populations with high levels of impulsivity, such as children or adults with ADHD, are often found to also have deficits of attention. Attention, broadly defined, refers to the ability to selectively concentrate on one aspect of the environment while ignoring potential distracters. There are many forms of attention, and many ways to measure them (Knudsen 2007), but their relationship to drug use has rarely been studied. It is likely that various aspects of attentional function influence drug use. As a first step to explore this relationship we have begun to study a form of inattention that can be measured by examining the positive skew in a distribution of simple reaction times (Leth-Steensen, Elbaz & Douglas 2000). This form of inattention results in an inability to focus on completion of a specific task for extended periods of time, or perhaps a tendency to be distracted by other stimuli. The simplest way to measure this form of inattention is by examining the variability in reaction times on a simple reaction time task: ‘lapses in attention’ are characterized by a higher proportion of long reaction times. These lapses can be quantified and differentiated from the inability to respond quickly by examining the difference between the mean and the mode of the distribution. Lapses of attention may represent a measure of impulsive tendencies that is separate from the other measures described earlier. A similar measure has been validated in ADHD children who exhibit a higher proportion of lapses in attention (Leth-Steensen et al. 2000). Figure 3 illustrates a schematic of this definition of lapses of attention, and the methods used to quantify them. The measure is derived from the frequency distribution of response times on a simple visual reaction time task. Mean reaction time may vary as a result of two independent processes: (1) a shift in the overall distribution of reaction times; or (2) a change in long reaction times. The long reaction time, which may be calculated as the mean deviation from the mode (DevMod), is our operational definition of lapses. Later in this paper we present data supporting the use of this measure. It remains to be determined in future studies whether other forms of attention separately, or in concert with this measure, influence the likelihood of using drugs.
Any of these three processes—delay discounting, behavioral inhibition or lapses in attention—may contribute to an observed, maladaptive behavior. For example, Fig. 1 shows schematically how an observed impulsive behavior, such as ‘difficulty awaiting turn’ in ADHD children, or ‘inability to refrain from using a drug’ in drug users, might be mediated by any of these underlying processes, and how these processes may be assessed with specific behavioral tasks. Although each of these measures has empirical support as a behavioral index of impulsivity, they probably reflect separate underlying processes. We and others have found that the measures are not related to one another in samples of young adults (Swann et al. 2002; Reynolds et al. 2006). We found no correlation between Stop reaction time and delay discounting in a sample of 165 young adults (unpublished), and in a principal components analysis with four tasks in 99 volunteers we also found that the Stop Task performance was unrelated to delay discounting (Reynolds et al. 2006). The lapses of attention measure was not significantly correlated with either Stop reaction time or delay discounting in 60 healthy volunteers (unpublished; Table 1). The lapses of attention measure was correlated with the motor subscale of the Barratt Impulsiveness Scale (Barratt & Patton 1983), suggesting that it is related to certain forms of self-reported impulsive tendencies. Dissociations between different measures have been reported in other populations. Sonuga-Barke (2002) found that ADHD children were impaired on both sensitivity to delay and behavioral inhibition relative to control children, but that impairments on the two measures were not correlated. Instead, ADHD patients exhibited sensitivity to delay or exhibited poor inhibitory control. Their findings that disinhibition and delay discounting were dissociable are similar to our findings with healthy young adults. Thus, there is growing evidence that the processes of delay discounting and behavioral inhibition are independent of each other (Sonuga-Barke 2002; Swann et al. 2002; de Wit & Richards 2004; Castellanos et al. 2006; Reynolds et al. 2006). However, it is not yet known how each of the processes contribute to problem behaviors, such as initiation of drug use or inability to stop using, and further research is likely to reveal additional as-yet-unidentified underlying processes.
Table 1.
Correlations between behavioral inhibition as measured by Stop reaction time (RT) on the Stop Task, delay discounting as measured by the k parameter, and lapses in attention on a simple reaction time task, as measured by mean deviation from the mode (DevMod) and scores on the motor subscale of the Barratt Impulsivity Scale (BIS).
| Stop RT | k | DevMod | |
|---|---|---|---|
| k | r = 0.03 (N = 165) | ||
| DevMod | r = 0.20b (N = 80) | r = −0.06 (N = 52) | |
| BIS motor | r = 0.19b (N = 86) | r = −0.11 (N = 57) | r = 0.24a (N = 104) |
Data were obtained from healthy young adults (n = 52 to 165);
P <0.05;
P < 0.10.
The next sections will review empirical findings on how the different measures of impulsive behaviors relate to drug use and abuse, and examine the extent to which the various measures assess the same or different underlying processes. First is a section reviewing the role of the trait of impulsivity in the development of drug use. Following is a section comparing measures of impulsivity in drug users and non-users. This is followed by a review of laboratory-based studies investigating the acute effects of abused drugs on specific tasks measuring impulsive behaviors, and a section on non-drug stimuli that could transiently increase impulsive behavior.
DEVELOPMENTAL STUDIES LINKING TRAIT IMPULSIVITY TO DRUG USE
Both longitudinal and cross-sectional studies support the idea that more impulsive youth are more likely to experiment with drugs, use drugs regularly and develop substance-use disorders. Although these studies use a variety of self-report behavioral measures of impulsivity, they provide consistent evidence that a cluster of impulsive traits increase risk for onset of drug use. For example, Sher, Bartholow & Wood (2000) reported that a trait referred to as ‘behavioral disinhibition’ as measured by standardized personality questionnaires (Eysenck & Eysenck 1968; Cloninger 1986; Eysenck 1993) predicted substance use disorder 6 years later in a sample of 457 young adults. Kirisci et al. (2007) and Tarter et al. (2007) conducted a longitudinal study showing that childhood ‘neurobehavioral disinhibition’ directly predicted substance use disorder at age 22. ‘Neurobehavioral disinhibition’ was measured using a composite of symptoms of childhood behavioral disorders, questionnaires indexing and standardized neuropsychological tests. Using adolescent twins, Iacono & McGue (2006) reported that P3 event-related brain potential amplitude, an electrophysiological measure associated with disinhibited behavior, was related to future problem behaviors including drug use. Using a different physiological measure, Habeych et al. (2006) reported that children at high risk for alcohol-use disorder were impaired on an oculomotor response inhibition task that is sensitive to prefrontal cortex dysfunction. These and many other developmental studies suggest that both trait measures and physiological measures of impulsive behaviors predict early onset and higher likelihood of drug use. Clearly, the studies have utilized widely varying operational definitions of impulsive tendencies, and it is not clear to what extent these represent similar underlying processes. Until now, few developmental studies have examined the relationship between the specific behavioral measures of delay discounting and behavioral inhibition in relation to onset of drug use in youth.
STUDIES COMPARING DRUG USERS AND NON-USERS ON MEASURES OF IMPULSIVITY
As expected, levels of impulsivity as measured by personality trait measures and most of the behavioral tasks are high in individuals with a history of drug use or abuse. Many studies have shown that drug users score higher on self-report personality measures of impulsivity (e.g. von Knorring, Oreland & von Knorring 1987; Sher & Trull 1994; Moeller et al. 2001). More recently, quite a number of studies have shown using objective behavioral tasks that drug users value immediate rewards more than delayed rewards. Greater discounting has been reported with opioid-dependent individuals (Madden et al. 1997; Kirby, Petry & Bickel 1999), cocaine users (Coffey et al. 2003), alcohol abusers (Vuchinich & Simpson 1998), cigarette smokers (Bickel, Odum & Madden 1999; Mitchell 1999) and individuals with unspecified histories of drug dependence (Allen et al. 1998). Although fewer studies have compared users and non-users on measures of behavioral inhibition, there is evidence that both cocaine and methamphetamine abusers perform more poorly than control subjects on the Stop Task (Fillmore & Rush 2002; Monterosso et al. 2005). Unfortunately, comparisons between drug users and non-users do not permit investigators to determine the causal relation between impulsivity and drug use. The group differences may reflect stable behavioral patterns that pre-dated, and perhaps contributed to the drug use, they may reflect consequences of the drug using lifestyle, or they may reflect the direct or indirect effects of the drugs themselves. The developmental studies referred to earlier suggest that impulsive tendencies lead to drug use. On the other hand, Jentsch & Taylor (1999) reviewed data from laboratory animals indicating that chronic drug use may lead to frontal cortical cognitive dysfunction that results in an inability to inhibit inappropriate responses. It is likely that both pre-existing differences and consequences of drug use contribute to the observed differences between users and non-users. These studies are valuable to document differences in user and non-user samples, but they provide little information about the causality of these differences.
STUDIES INVESTIGATING THE ACUTE EFFECTS OF DRUGS OF ABUSE ON MEASURES OF IMPULSIVITY
Drugs of abuse have varying effects on behavioral measures of impulsivity in humans. The drugs’ effects depend on dose, subject samples, and specific testing parameters. This section reviews some of the findings on the effects of acute drug administration on measures of delay discounting, behavioral inhibition and lapses in attention. As noted earlier, drug effects on any of these dimensions of impulsivity may increase the likelihood of engaging in risky behaviors in the non-laboratory setting, including an increased tendency for the individual to take more of the drug than originally planned.
Delay discounting
Many studies have investigated the effect of acute drug administration on delay discounting in animal models (de Wit & Mitchell in press), but relatively few studies have examined drug-induced changes in discounting with humans. Two studies (Richards et al. 1999; Ortner, MacDonald & Olmstead 2003) have examined the effects of moderate doses of alcohol (equivalent to 3–4 standard drinks) in healthy young adults, using a discounting procedure where subjects make choices between smaller amounts of money to be provided immediately and larger amounts of money to be provided after delays of 1 to 365 days. Alcohol failed to affect delay discounting. Several other drugs have been tested using a similar procedure, including d-amphetamine, tetrahydrocannabinol, diazepam, bupropion and naltrexone (de Wit, Enggasser & Richards 2002; McDonald et al. 2003; Reynolds et al. 2004; Acheson et al. 2006; Mitchell et al. 2007). Of these only d-amphetamine had an effect on delay discounting (de Wit et al. 2002), producing a small decrease in discounting in 36 healthy volunteers. However, this finding was not replicated in a second study (Acheson & de Wit 2008). The absence of acute drug effects on discounting in humans is in marked contrast to studies with laboratory animals, in which the drugs have pronounced, if variable, effects. This raises some question about the sensitivity of the procedure used with humans, to detect state changes in impulsive behavior. One possible explanation is that the drugs have little effect because, unlike in the animal procedures, the human subjects do not experience the delays or receive the delayed rewards under the influence of the drug. To address this issue, Reynolds & Schiffbauer (2004) designed an alternative procedure involving delays in the range 0–60 seconds, so that the subject experiences the delays and delayed rewards in the presence of the drug. Ethanol increased delay discounting in a sample of social drinkers using this modified procedure (Reynolds et al. 2006), although, as noted by the authors, the procedure also has other features that complicate the interpretation.
Several investigators have examined the effect of acute drug withdrawal on discounting, with mixed results. Mitchell (2004) reported that abstinence from cigarette smoking increased discounting for cigarettes but not money, whereas Field et al. (2006) reported that nicotine deprivation increased impulsive choices for both cigarette and monetary rewards in a delay-discounting task. Giordano et al. (2002) reported that heroin users undergoing withdrawal exhibited an increase in discounting of both heroin and money. Thus, despite a lack of evidence for the effects of acute drug administration on discounting, acute drug withdrawal does appear to produce steeper discounting for the drug and, to a lesser extent, other rewards.
Behavioral inhibition
Several studies have found that acute doses of abused drugs affect performance on the Stop Task. We (de Wit, Crean & Richards 2000) have found that alcohol increases Stop time (i.e. the ability to inhibit responses) without affecting Go time in healthy young adults, consistent with the idea that alcohol impairs inhibitory control. In contrast, d-amphetamine decreased Stop times, i.e. improved the ability to inhibit prepotent responses, although this was observed only in individuals whose initial Stop times were slow. The decrease in Stop time after amphetamine is consistent with the decrease in impulsivity that is also observed with this class of drugs in children treated for ADHD (Tannock et al. 1989). This is also consistent with a recent report that the non-abused noradrenergic drug atomoxetine improved the ability to inhibit responses in adults with ADHD (Christman, Fermo & Markowitz 2004).
One study has examined the effect of drug withdrawal on behavioral inhibition. Dawkins et al. (2007) reported that acute nicotine abstinence in smokers impaired the ability to inhibit prepotent responses. These results suggest behavioral inhibition may be more sensitive to acute drug effects than discounting.
Lapses of attention
Inattention, or more specifically lapses of attention, represents a potentially separate process that may result in behaviors that appear to be ‘impulsive’. For an experienced drug user, abstaining from drugs for an extended period of time requires active and sustained attention. Sustained attention is needed to continuously suppress or inhibit drug-seeking responses. Drug-seeking responses are not only well-learned habits, but they can also be readily reinforced with use of the drug, making them especially compelling to the user. Either a chronic (trait) inability to sustain attention for extended periods or a momentary lapse in attention precipitated by an environmental event may increase the difficulty of abstaining. Thus, lapses of attention could result in lapses to drug use. Lapses in attention can be determined from the distribution of reaction times of a simple visual reaction time task. As noted previously, Fig. 4 shows that the measure of lapses of attention corresponds to the mean DevMod, or the mean of each individual reaction time from the modal value. The mean DevMod is equivalent to the difference between the mean and the mode of a reaction time distribution. The larger the DevMod, the greater the proportion of long reaction times.
Figure 4.
Schematic of lapses of attention. This figure shows two hypothetical distributions of simple reaction times, including the mean and the mode. The left panel shows the distribution without long reaction times, the right panel shows the separation of the mode and the mean when there are long reaction times or ‘lapses in attention’. It shows that long reaction times change the mean while leaving the mode relatively unaffected. The difference between the mean and the mode provides a measure of the skew, and the deviation from the mode (DevMod) is considered a measure of inattention. The inset shows the symbols to be used for mode, mean and DevMod in Figs 5, 6 and 7
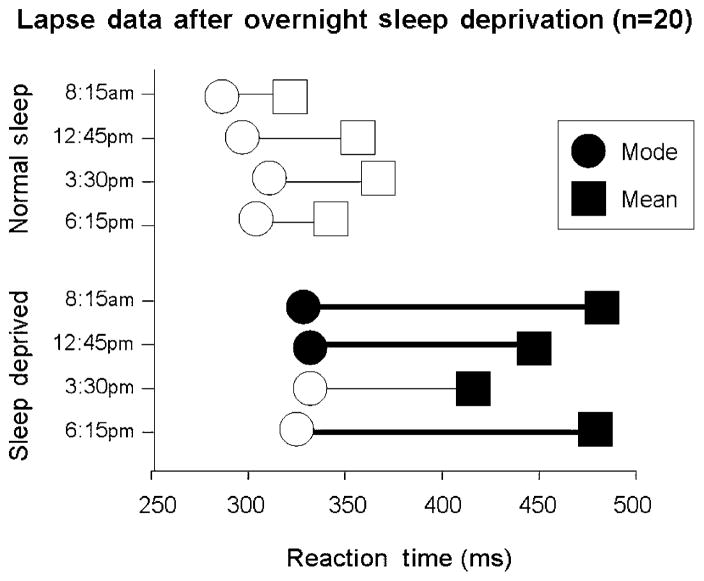
In three studies using healthy young adults, we have examined the effects of (1) overnight sleep deprivation; (2) acute administration of d-amphetamine (20 mg oral); (3) acute administration of bupropion (150 mg oral); and (4) caffeine (200 mg) in fatigued individuals (Child & de Wit 2008, Acheson & de Wit 2008, Acheson, Richards & de Wit 2007). In the first study, subjects completed the reaction time task at four timepoints on the day after remaining awake all night. Figure 5 shows that sleep deprivation not only increased both mean and modal reaction times at most timepoints, but it also significantly increased the number of lapses of attention, as measured by the increase in the DevMod. In the next study, subjects received acute doses of d-amphetamine (20 mg), bupropion (150 mg) or placebo on three sessions. Figure 6 shows that, relative to placebo, d-amphetamine decreased the mean, the mode and the DevMod, indicating that it decreased mean reaction times and reduced the number of lapses. In contrast, bupropion decreased the mean and the DevMod without changing the mode, indicating that this drug specifically reduced the number of lapses. These results show that this measure of lapses of attention may be used to distinguish overall shifts in reaction time distributions which result in changes in the mode, from the effects of the occasional, long reaction times that might indicate lapses in attention which results in changes in DevMod. In the last study, subjects were tested on a simple reaction time task at 3 and 4 a.m. after remaining awake for about 19 hours, on two sessions. At 3 a.m. they received either caffeine (200 mg) or placebo. Figure 7 shows that as subjects became more fatigued, both their mean and the modal reaction times increased. Caffeine (200 mg oral) administered at 3 a.m. reduced both the mean and modal reaction times relative to the placebo session. The fact that there was no significant effect on DevMod indicates that neither sleep deprivation (in the first study) nor acute caffeine specifically affected lapses in attention. However, the contrasting results with d-amphetamine and bupropion show that the two processes can be differentiated with different pharmacological challenges.
Figure 5.
Effects of sleep deprivation on lapses of attention. This figure shows the mean of the mode (circles), deviation from the mode (DevMod; horizontal line) and mean (squares) of reaction time (RT) distributions after 24 hours of sleep deprivation and after a normal night’s sleep. Healthy volunteers performed simple RT tasks at regular intervals on the day after sleep and no sleep. The mean RT = mode +DevMod. Therefore, the horizontal line connecting the mode to the mean RT corresponds to the DevMod. Filled symbols indicate significant differences between the normal sleep and deprivation conditions, at the corresponding timepoints (P < 0.05). A heavy horizontal line indicates that the DevMod is significantly different in the two conditions. The mean RT was increased at all four timepoints. Although there were small increases in modal RT, the plots indicate that most of the increase in mean RT was because of the increases in the DevMod measure, indicating more frequent long RTs
Figure 6.
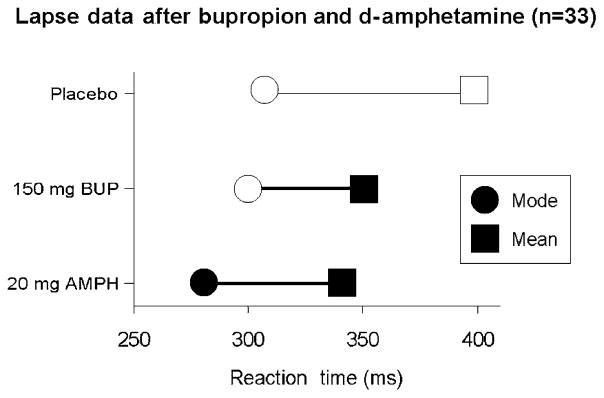
Effects of bupropion (BUP) and amphetamine (AMPH) on lapses of attention. This figure shows the means of the mode (circle), deviation from the mode (DevMod; horizontal line) and mean (square) of reaction time (RT) distributions after placebo, bupropion (150 mg) and d-amphetamine (20 mg). The mean RT = mode +DevMod. Filled symbols indicate significant differences between the drug and placebo conditions, at the corresponding timepoints (P <0.05). A heavy horizontal line indicates that the DevMod is significantly different in the two conditions. Both bupropion and d-amphetamine decreased the mean RT. However, bupropion selectively decreased the DevMod measure, whereas d-amphetamine decreased both the modal RT and the DevMod measure
Figure 7.
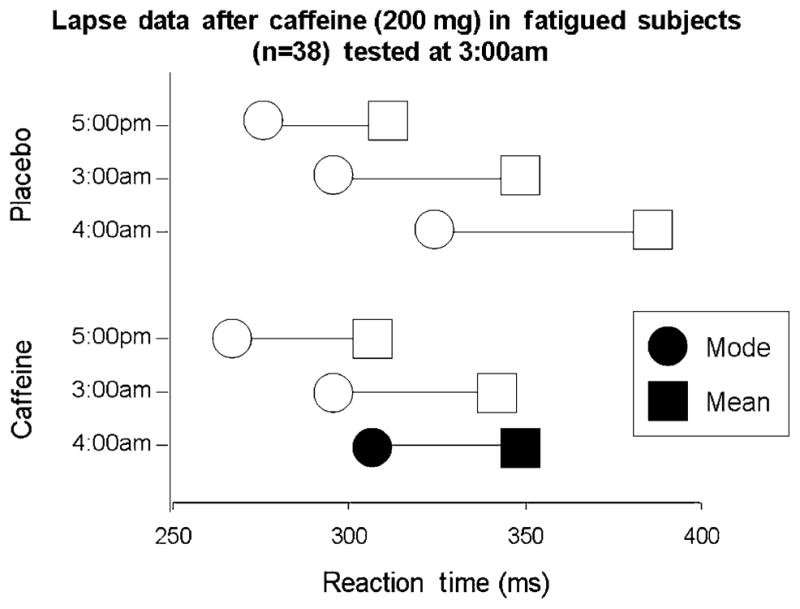
Effects of caffeine on lapses of attention in fatigued subjects. Healthy volunteers were tested on two sessions from 5 p.m. to 5 a.m. At 3 a.m. they ingested capsules with either caffeine (200 mg) or placebo. The mean reaction time (RT) = mode +deviation from the mode (DevMod). Therefore, the horizontal line connecting the mode to the mean RT corresponds to the DevMod. Filled symbols indicate significant differences between the placebo and caffeine conditions, at the corresponding timepoints (P <0.05). A heavy horizontal line indicates that the DevMod is significantly different in the two conditions. This figure shows that reaction times increased over the course of both sessions. Caffeine significantly decreased both the mode and the mean reaction time, relative to the same timepoint in the placebo condition. Caffeine did not affect the DevMod, indicating that it did not specifically decreases lapses in attention
STUDIES EXAMINING NON-DRUG-INDUCED TRANSIENT ‘STATE’ INCREASES IN IMPULSIVE BEHAVIOR
The effects of non-drug stimuli on impulsive behaviors have not been studied thoroughly. Stimuli other than the direct effects of drugs, including abrupt environmental, physiological or emotional events, may cause transient, ‘state’ changes in either self-control or inhibition that may result in re-initiation of drug use. For example, acute stress is known to increase the propensity to use drugs (Sinha 2001), and it may do this by increasing impulsive tendencies. Tice, Bratslavsky & Baumeister (2001) provide some evidence that emotional distress can increase the tendency to seek immediate gratification. They note that self-regulation of behavior may require a focus on long-term, relative to shorter-term goals, and that negative mood states may impair the ability to focus on long term goals by increasing a bias toward immediate pleasure. There is also some evidence that stressful environmental manipulations, such as sleep deprivation, may increase impulsive behaviors. Brown, Tickner & Simmonds (1970) showed that fatigued drivers made more ‘risky decisions’ even when other driving skills were unaffected. ‘Risky decisions’ were defined by participants’ willingness to engage in specific hazardous overtaking maneuvers (e.g. passing in low visibility, forcing other drivers to adjust speed to permit them to pass). Sicard, Jouve & Blin (2001) reported that pilots became more risk-prone as they became more drowsy after 24 hours of strenuous flights, and Killgore, Balkin & Wesensten (2006) reported that sleep deprivation impaired performance on the Iowa Gambling Task, a complex strategic task that requires integration of payoff-to-penalty ratios and may be related to risk taking. As described earlier, we found that sleep deprivation increased lapses of attention in healthy volunteers, whereas it did not affect either delay discounting or behavioral inhibition (Acheson & de Wit 2008). Thus, disruptive environmental events such as emotional states and sleep deprivation may increase impulsive behaviors, although the mechanisms by which they do this have yet to be determined.
CONCLUSIONS
This paper examines the associations between impulsive behavior and drug use. Impulsive behavior is closely linked to drug use, both as a determinant and as a consequence. As a determinant, both the trait of impulsivity and momentary ‘state’ increases in impulsivity may increase the tendency to use drugs. Conversely, impulsive behavior may also be a consequence of drug use, either acute or chronic. The sections in this paper provide evidence that several independent processes may contribute to observable impulsive behaviors, but that these measures are relatively independent of each other. Future research, including research on the neurobiological mechanisms for these behaviors, may provide more information about their contributions to maladaptive drug use. For example, certain forms of impulsive behavior may be important during initiation and early drug use, whereas other forms may be important during relapse and attempts to abstain. In particular, further investigations of the role of attention and inattention in drug use are warranted. Especially needed is behavioral research to refine the measures of attention, and how different forms of inattention relate to initiation, maintenance and relapse to drug use, and how they relate to behaviors described broadly, in the real world, as ‘impulsive’ behavior.
Acknowledgments
This research was supported by DA09133. Jerry Richards contributed to the conception, design and analysis of the studies conducted in the Chicago laboratory. Emma Childs and Ashley Acheson contributed to several of the studies, Jim Zacny and Mike McCloskey provided valuable comments on an earlier version of the manuscript, and Patricia Kriegel, Christy Casnar and Gina Beguhn assisted with manuscript preparation.
References
- Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy volunteers. Exp Clin Psychopharmacol. 2008;16:113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Reynolds B, Richards JB, de Wit H. Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Exp Clin Psychopharmacol. 2006;14:190–198. doi: 10.1037/1064-1297.14.2.190. [DOI] [PubMed] [Google Scholar]
- Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on performance. Physiol Behav. 2007;91:579–587. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Moeller FG, Rhoades HM, Cherek DR. Impulsivity and history of drug dependence. Drug Alcohol Depend. 1998;50:137–145. doi: 10.1016/s0376-8716(98)00023-4. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Patton JH. Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Bases of Sensation Seeking, Impulsivity, and Anxiety. Hillsdale, NJ: Earlbaum; 1983. pp. 77–121. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Brown ID, Tickner AH, Simmonds DC. Effect of prolonged driving on overtaking criteria. Ergonomics. 1970;13:239–242. doi: 10.1080/00140137008931137. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Enhanced mood and psychomotor performance by a caffeine containing energy capsule in fatigued individuals. Exp Clin Psychopharmacol. 2008;16:13–21. doi: 10.1037/1064-1297.16.1.13. [DOI] [PubMed] [Google Scholar]
- Christman AK, Fermo JD, Markowitz JS. Atomoxetine, a novel treatment for attention-deficit-hyperactivity disorder. Pharmacotherapy. 2004;24:1020–1036. doi: 10.1592/phco.24.11.1020.36146. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatry Dev. 1986;4:167–226. [PubMed] [Google Scholar]
- Coffey SF, Gudelski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Powell JH, West R, Powell J, Pickering A. A double-blind placebo-controlled experimental study of nicotine: II—Effects on response inhibition and executive functioning. Psychopharmacology (Berl) 2007;190:457–467. doi: 10.1007/s00213-006-0634-6. [DOI] [PubMed] [Google Scholar]
- de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Mitchell S. Drug effects on delay discounting. In: Madden GJ, Bickel WK, Critchfield TS, editors. Impulsivity: Theory, Science, and Neuroscience of Discounting. Washington, DC: American Psychological Association; (in press) [Google Scholar]
- de Wit H, Richards JR. Dual determinants of drug abuse: reward and impulsivity. In: Bevins RA, Bardo MT, editors. Nebraska Symposium on Motivation. Lincoln, NE: University of Nebraska Press; 2004. pp. 19–55. [PubMed] [Google Scholar]
- Eysenck HJ. The nature of impulsivity. In: McCowen WG, Johnson JL, Sure MB, editors. The Impulsive Client: Theory, Research, and Treatment. Washington, DC: American Psychological Association; 1993. pp. 57–70. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Eysenck Personality Inventory. San Diego, CA: Educational and Industrial Testing Service; 1968. [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Psychopharmacology (Berl) Vol. 186. School of Psychology, University of Liverpool; Liverpool, L69 7ZA, UK: 2006. Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation; pp. 255–263. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Berl) 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Habeych ME, Folan MM, Luna B, Tarter RE. Impaired oculomotor response inhibition in children of alcoholics: the role of attention deficit hyperactivity disorder. Drug Alcohol Depend. 2006;82:11–17. doi: 10.1016/j.drugalcdep.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Self-control as response strength. In: Bradshaw CM, Szabadi E, Lowe CF, editors. Quantification of Steady-State Operant Behavior. Amsterdam, the Netherlands: Elsevier; 1981. pp. 3–20. [Google Scholar]
- Iacono WG, McGue M. Association between P3 event-related brain potential amplitude and adolescent problem behavior. Psychophysiology Links. 2006;43:465–469. doi: 10.1111/j.1469-8986.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 1999;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Tarter R, Mezzich A, Vanyukov M. Developmental trajectory classes in substance use disorder etiology. Psychol Addic Behav. 2007;21:287–296. doi: 10.1037/0893-164X.21.3.287. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a users’ guide to the Stop Signal Paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory Processes in Attention, Memory, and Language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Logue AW. Research on self-control: an integrated framework. Behav Brain Sci. 1988;11:665–709. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self control choices in opioid dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of delta 9-tetrahydrocannabinol on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ., Jr Adjustment of twin data for the effects of age and sex. Behav Genet. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32:439–449. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ortner CN, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol Alcohol. 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Ortengren A, Richards JB, de Wit H. Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Dif. 2006;40:305–315. [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Process. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Process. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Wood MD. Personality and substance abuse disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sicard B, Jouve E, Blin O. Risk propensity assessment in military special operations. Mil Med. 2001;166:871–874. [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Psychological heterogeneity in AD/HD—a dual pathway model of behavior and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. J Am Acad Child Adolesc Psychiatry. 1995;34:886–896. doi: 10.1097/00004583-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD. Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol. 1989;17:473–491. doi: 10.1007/BF00916508. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Feske U, Vanyukov M. Modeling the pathways linking childhood hyperactivity and substance use disorder in young adulthood. Psychol Addict Behav. 2007;21:266–271. doi: 10.1037/0893-164X.21.2.266. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: if you feel bad, do it! J Pers Soc Psychol. 2001;80:153–167. [PubMed] [Google Scholar]
- von Knorring L, Oreland L, von Knorring AL. Personality traits and platelet MAO activity in alcohol and drug abusing teenage boys. Acta Psychiatr Scand. 1987;75:307–314. doi: 10.1111/j.1600-0447.1987.tb02793.x. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Delayed reward discounting in alcohol abuse. Presented at National Bureau of Economic Research Conference “The Economic Analysis of Substance Use and Abuse: An Integration of Econometric and Behavioral Research,”; Cambridge, MA. 1998. [Google Scholar]