Abstract
We previously showed that while EGFR mutations are not a feature of pure squamous cell carcinomas of lung (SQC), these mutations do occur in adenosquamous carcinomas (AD-SQC) and in rare solid adenocarcinomas (ADC), both of which can mimic SQC in small samples. Here we present an expanded series of these cases with a focus on sensitivity to erlotinib. The study included 13 patients with EGFR mutant lung carcinomas, which after detailed pathologic review were classified as AD-SQC (n=11) or solid ADC (n=2). The majority received a diagnosis of “SQC” in at least one sample. All patients were treated with erlotinib. 8 of 11 patients with AD-SQC were evaluable for response. Their overall response rate was 88% (7/8; 95% CI: 47%–99%). One of 2 solid ADC patients responded to erlotinib. As a group, median PFS was 12 months (95% CI: 8-NR); median OS was 29 months (95% CI: 27-NR). In conclusion, EGFR mutant AD-SQC and solid ADC show a response to erlotinib that is comparable to that seen in patients with conventional ADC. These tumors can mimic SQC in small samples. We propose an approach to increase the capture of these rare histology patients for EGFR mutation testing.
Keywords: squamous cell lung carcinoma, EGFR, never smoker
Introduction
The sensitivity of a subset of non-small cell lung cancers (NSCLC) to EGFR tyrosine kinase inhibitors (TKIs) is firmly linked to the presence of activating EGFR mutations (1). EGFR mutations occur almost exclusively in conventional adenocarcinomas of lung (ADC). The majority of the data on TKI sensitivity is thus derived from mutations that arise in this histology, with radiographic response rates ranging from 55% to 91% and progression-free survival ranging from 7 to 13 months (1, 2).
In contrast to TKI sensitivity in conventional ADCs, TKI sensitivity in EGFR-mutant carcinomas of unusual histology is not well established. Recent data suggest that histology can modify the sensitivity of EGFR-mutant tumors to TKIs. For example, carcinomas with epithelial-mesenchymal transition and small cell carcinomas may be inherently TKI-resistant despite the presence of activating EGFR mutations (3–5). The impact of other non-adenocarcinoma histologies, particularly squamous, on determining response to EGFR TKIs is not well established.
Whether EGFR mutations do arise in squamous cell carcinomas of the lung (SQC) is itself a controversial topic. While several large series of surgically-resected SQC tumors found no EGFR mutations (6, 7), a number of reports, primarily from small biopsy/cytology samples, have found EGFR mutations in a small proportion of SQCs. We have recently shown that the two main settings in which clinical small biopsy/cytology samples with a diagnosis of SQC are found to harbor EGFR mutations include 1) undersampling of adenosquamous carcinoma (AD-SQC), and 2) morphologic mimicry by solid ADC (8). We ourselves have found no EGFR mutations among 95 surgically-resected and pathologically-verified SQCs at our institution (8). This suggests that when abundant primary tumor is available for rigorous pathologic evaluation, the low rate of EGFR mutations collapses.
Adenosquamous carcinoma is a rare type of lung cancer, representing 0.4–4% of NSCLCs, and consists of a mixture of both adeno and squamous components. EGFR mutations occur in AD-SQCs with a similar frequency as in ADC, and with a similar predilection for never-smokers. Notably, EGFR mutations are present in both the adeno and squamous components of these tumors (9–11). The well-known diagnostic limitation inherent to small biopsy/cytology specimens is that such samples may contain only a single component. This may result in a detection of EGFR mutations in a sample diagnosed as “SQC”.
The second, less common, explanation for the detection of EGFR mutations in SQC is an unusual morphologic variant of ADC marked by a solid growth pattern. This can closely mimic SQC (we termed this squamous-like variant of ADC “pseudosquamous” or “squamoid”) (8). Despite a morphologic similarity to SQC, immunohistochemistry (IHC) can readily distinguish between these two histologies. Given the increasing utilization of IHC to characterize poorly-differentiated NSCLCs, this morphologic mimic is unlikely to appear under the guise of “SQC” in the future.
In this study, we expanded on data from our initial series of EGFR-mutant carcinomas with squamous and pseudosquamous histologies. Because the sensitivity to EGFR TKIs in carcinomas with these unusual histologies is not established, we sought to retrospectively determine the response of these tumors to erlotinib.
Material and Methods
Study Design, Patients, and Radiographic Response
We identified 13 patients with EGFR-mutant NSCLCs that had a true squamous component (n=11) or solid/pseudosquamous ADC histology (n=2). Based on our recent study (8), we refer to all EGFR-mutant samples that had a true squamous component (as confirmed by morphology and IHC) as representative of AD-SQC, irrespective of whether a glandular component could (n=9) or could not (n=2) be found on pathologic re-review. All pathologic samples were re-reviewed by two thoracic pathologists (NR, ALM) using light microscopy and IHC, as described in our recent publication (8). All patients were diagnosed with recurrent or metastatic disease and treated with erlotinib. Where available, baseline and follow-up CT scans were reviewed to determine radiographic response to erlotinib as per RECIST 1.1. The study was approved by the MSKCC Institutional Review Board.
Genotype Analysis
Briefly, EGFR exon 19 deletions were identified through a PCR-based assay (12). EGFR exon 21 mutations, including secondary T790M mutations, as well as mutations in AKT1, BRAF, ERBB2, KRAS, MEK1, NRAS, and PIK3CA were assayed by Sequenom (Sequenom, Inc., San Diego, CA), as described previously (8).
Statistical Analysis
Progression-free survival (PFS) was measured from the date at which treatment with erlotinib began to the date at which there was evidence of radiographic progression. Overall survival (OS) was measured from the date of diagnosis of stage IV disease until the date of death. Survival probabilities were calculated using the Kaplan-Meier method. Group comparison was performed with log-rank tests and Cox proportional hazards methods. Statistical analyses were performed using SAS statistical software (SAS Institute, Inc, Cary, NC).
Results
Patient and tumor characteristics
Clinicopathologic characteristics for the 11 patients with EGFR-mutant AD-SQC are summarized in Table 1. Details of the pathologic review of samples from patients 1 through 7 are provided in our recent publication (corresponding patient IDs are indicated in Table 1) (8). An analogous pathologic review was performed for patients newly identified in this series (patients 8–11). Overall, 9 of 11 patients had at least one sample with a pathologic diagnosis of SQC, highlighting the difficulty in the diagnosis of AD-SQC in small samples. Clinicopathologic characteristics for the 2 patients with solid/pseudosquamous ADC are summarized in Table 2; their detailed morphologic and IHC characteristics are described in reference (8). Eleven of 13 (85%) patients in the cohort were never smokers.
Table 1.
Clinicopathologic findings for patients with EGFR-mutant adenosquamous carcinomas. The majority were diagnosed with squamous cell carcinoma in at least 1 sample.
| Patient# | Age | Gender | Race | Smoking | Stage‡ | Biopsy #1^ | Biopsy #1 Mutation | Biopsy #2^ | Biopsy #2 Mutation | Lines of therapy | EGFR TKI line | Best response to EGFR TKI | TTP on TKI(months) |
OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (1) | 61 | M | White | Never | IV | Squamous (L1)† | exon 19 del | Adenosquamous (LLL lung) | exon 19 del | 3 | 2nd line | PR | 12.1 | 27.5 |
| 2 (2) | 71 | F | White | Never | IV | Squamous (RLL lung)† | exon 19 del | Adenocarcinoma (RLL lung) | exon 19 del | 2 | 1st line | Unavailable | 19.6 | 32.9+ |
| 3 (3) | 58 | F | White | Never | IV | Squamous (RUL lung) | exon 19 del | Adenosquamous (LLL lung)† | exon 19 del | 3 | 2nd line | SD | 23.6 | 32.2+ |
| 4 (4) | 45 | F | Hispanic | Never | IV | Squamous (sacrum) | exon 19 del | Adenocarcinoma (pleural fluid) | exon 19 del | 2 | 2nd line | Unavailable | Unavailable | 15.3 |
| 5 (5) | 46 | M | Asian | Never | IV | Squamous (R lung) | exon 19 del | Adenocarcinoma (SC LN) | exon 19 del | 1 | 1st line | PR | 5.0+ | 6.6+ |
| 6 (6) | 73 | M | White | Former (25 PY) | IV | Squamous (adrenal) | exon 19 del | Adenocarcinoma (SC LN) | insufficient | 2 | 3rd line | Unavailable | Unavailable | 29.8 |
| 7 (10) | 58 | M | Asian | Never | IV | Squamous (bronchus) | L858R | Squamous (T8) | insufficient | 1 | 1st line | PR | 1.9 | 2.5 |
| 8 (new) | 76 | M | White | Never | IV* | Squamous (R lung)† | insufficient | Adenocarcinoma (L lung) | exon 19 del | 1 | 1st line | PR | 5.3 | 5.3+ |
| 9 (new) | 68 | M | White | Never | IV | Squamous (L lung) | L858R | None | N/A | 4 | 4th line | PR | 2.8+ | 24.0+ |
| 10 (new) | 30 | F | Asian | Never | IV | Adenosquamous (R lung) | L858R | None | N/A | 2 | 1st line | PR | 8.4 | 10.9+ |
| 11 (new) | 50 | M | White | Never | IV | Adenosquamous (L lung) | exon 19 del | None | N/A | 1 | 1st line | PR | 9.2+ | 9.6+ |
In parentheses are corresponding patient IDs in reference (8).
Biopsy numbers are not chronologic: biopsy #1 represents the index case (EGFR mutant “SQCLC”)
Stage at the time of TKI treatment.
Prior Stage IIA treated with induction cisplatin + pemetrexed followed by LLL lobectomy
Acquired resistance biopsy
Abbreviations: SC LN supraclavicular lymph node, PR partial response, SD stable disease, PY pack years
Table 2.
Clinicopathologic findings for patients with EGFR-mutant solid “pseudosquamous” adenocarcinomas. Both were initially diagnosed as squamous cell carcinomas.
| Patient # | Age | Gender | Race | Smoking status | Stage‡ | Initial diagnosis (site) | Mutation | Re-review | Lines of therapy | EGFR TKI line | Best response to EGFR TKI | TTP on TKI (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 (11) | 89 | F | Asian | Never | IV | Squamous (lung) | L858R | Adenocarcinoma | 1 | 1st line | SD | 7.6 | 16.5 |
| 13 (12) | 53 | F | White | Former (31) | IV** | Squamous (lung) | exon 19 del | Adenocarcinoma | 1 | 1st line | PR | 12.4 | 20.6+ |
In parentheses are corresponding patient IDs in reference (8)
Prior Stage IB s/p adjuvant cis/doce followed by RLL lobectomy
EGFR mutation status
As shown in Tables 1 and 2, EGFR mutations included exon 19 deletions (n=9) and exon 21 L858R substitutions (n= 4). No other mutations were detected. Eight patients with AD-SQC (patients 1–8) had paired biopsies from other sites or time-points which were used to demonstrate the presence of both squamous and glandular components in different samples from the same patient. Of these 8 patients, 5 had sufficient material for genotyping in both biopsies, which revealed identical EGFR mutations in all paired samples, supporting their clonal relationship despite the heterogeneous histology.
Of note, 3 samples in this series (from patients 1, 2 and 3) were biopsies taken at the time of acquired resistance (AR) to erlotinib. Two of the AR samples were entirely squamous (patients 1 and 2) and one was adenosquamous (patient 3). Notably, a squamous histology was also present in 2 of 3 pre-treatment biopsies (patients 1, 3). None of the 3 AR samples harbored a secondary T790M mutation, while the original sensitizing EGFR mutation was detected in all 3 samples.
Response to erlotinib
Of the 11 patients with AD-SQC, 8 were evaluable for response. Their overall response rate (ORR) was 88% (7/8 partial responses; 95% CI: 47%–99%). One of 8 patients had stable disease. Of the 2 patients with solid ADC, one patient had a partial response to erlotinib and the other, stable disease. A waterfall plot of response is shown in Figure 1.
Figure 1. Radiographic response to erlotinib in patients with adenosquamous and solid “pseudosquamous” adenocarcinomas harboring EGFR mutations.
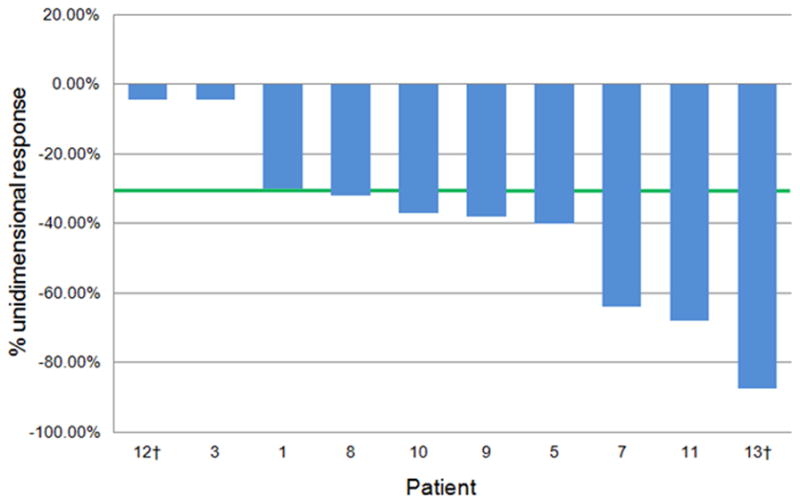
† Denotes solid (pseudosquamous) adenocarcinomas; other cases are carcinomas with a squamous component (confirmed or presumed adenosquamous carcinomas).
Only one patient (patient 4) had evidence, by outside report, of a divergent response to erlotinib at 2 histologically distinct biopsy sites, where a parenchymal lung tumor shrank (ADC) while a sacral metastasis (SQC) increased in both size and FDG-avidity. Other patients in this group had no evidence of heterogeneous radiologic responses, although no other patient in this series had distinct histologies at different sites of disease at the time of erlotinib treatment.
The median PFS of all evaluable patients (AD-SQC and solid ADC) treated with erlotinib was 12 months (95% CI: 8-NR) (Figure 2). Median OS was 29 months (95% CI: 16-NR) (Figure 3). For patients with AD-SQC, median PFS was 12 months (95% CI: 8-NR) and median OS was 29 months (95% CI: 27-NR).
Figure 2.
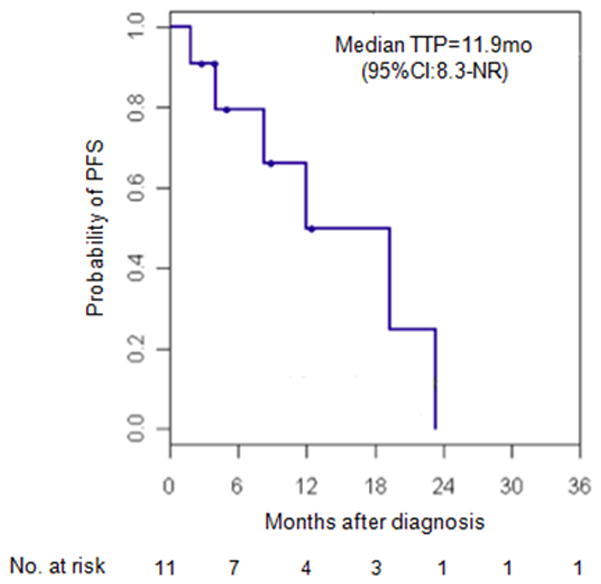
Kaplan Meier survival curve for PFS in patients with EGFR-mutant adenosquamous and solid “pseudosquamous” adenocarcinomas treated with erlotinib.
Figure 3.
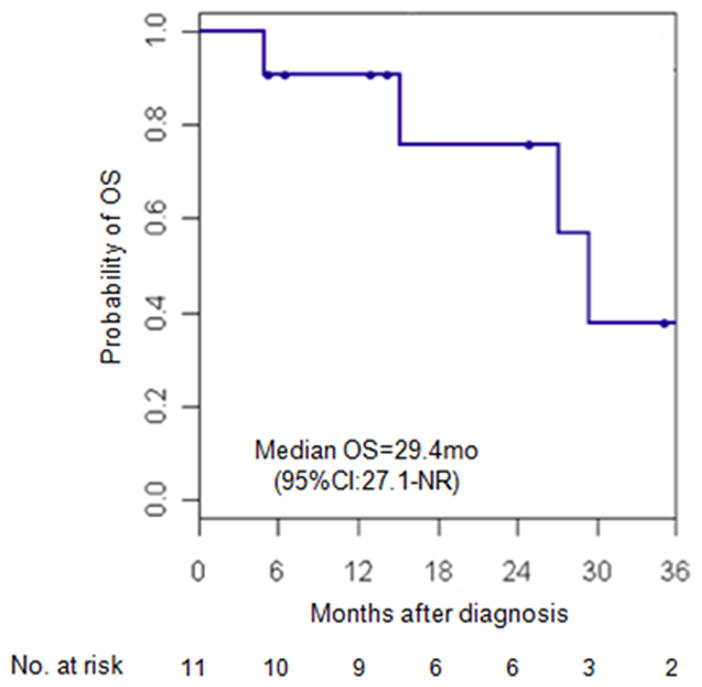
Kaplan Meier survival curve for OS in patients with EGFR-mutant adenosquamous and solid “pseudosquamous” adenocarcinomas treated with erlotinib.
Discussion
We recently demonstrated that EGFR-mutant SQCs of lung usually represent undersampled AD-SQC or, less commonly, a solid variant of ADC (8). Here we expand on this observation, and show that these unusual tumors have an overall sensitivity to erlotinib that is similar to that seen in patients with conventional ADCs.
Prior reports on the sensitivity of EGFR-mutant carcinomas with squamous histology (which our study suggests represent, in the majority of cases, undersampled AD-SQC) to EGFR TKIs include only several small case series. Based on a pooled analysis of 15 publications, Shukuya et al. (13) suggested that SQCs with sensitizing EGFR mutations have a diminished sensitivity to EGFR TKIs, with an ORR of 38% (n=16 patients) and median PFS of 3.1 months (n=10 patients). In addition, several studies have described TKI responses in SQCs that harbor atypical or complex EGFR mutations – mutations which are thought to have no or uncertain TKI sensitizing potential (13), and SQCs lacking EGFR mutations (14, 15), suggesting that TKI responses in some SQCs may be related to factors other than activating EGFR mutations.
Our study is the largest single series to report on the response to erlotinib in patients with sensitizing EGFR mutations in NSCLCs with a squamous component. In contrast to the lower response seen in aggregate from prior studies, we found that these patients have an ORR of 88% and a median PFS of 12 months. Responses appeared to be uniform in almost all cases. We do note that one patient (Patient 4) in our series had a divergent radiographic response to erlotinib, with what appeared to be primary resistance at a sacral lesion that was histologically-confirmed as squamous carcinoma.
This series also included 3 patients who had a squamous component in samples obtained at the time of AR to erlotinib. Unlike cases of small cell and epithelial-mesenchymal transformation, there have been no reports correlating squamous histology with the development of AR to EGFR TKIs (3, 4). Notably, in 2 of 3 of our patients, a squamous component was also present in a pre-treatment sample, suggesting that the squamous histology seen at the time of AR is more likely a manifestation of the patient’s underlying AD-SQC than a result of histologic transformation. Selection for the squamous component of the underlying AD-SQC remains a possibility which we cannot exclude, however, particularly given the absence of the most common mechanism of resistance (T790M) in all 3 AR samples with squamous histology.
Given the clinical benefit demonstrated herein, an important practical question is how best to capture these rare unusual-histology patients for EGFR mutation testing. As a first step, we recommend using strict morphologic criteria and, if needed, widely-advocated IHC markers to establish a diagnosis of SQC and to exclude solid/pseudosquamous ADC (8, 16, 17). Cases found to represent solid ADC should be tested for EGFR mutations and treated with TKI based on the responses demonstrated herein. For pathologically-verified SQC in primary resections (where the likelihood of undersampled AD-SQC is low), we do not advocate routine EGFR testing, which is supported by the lack of EGFR mutations in such samples in prior studies (6, 7).
In small biopsy samples, however, neither morphology nor IHC can surmount the problem of incomplete sampling of an underlying AD-SQC, where the glandular component may simply not be represented. While analysis of multiple small samples (as in this retrospective series) increases the likelihood of detecting both components, it does not guarantee it. Thus, in a prospective setting, it may be impossible to distinguish pure SQC from a component of AD-SQC in a single (or several) small samples. Given this inherent limitation, the only way to ensure capture of all EGFR mutations would be to test all small samples with a diagnosis of SQC. This is unlikely to be cost-effective, given the low prevalence of AD-SQC relative to pure SQC. As almost all cases in this series were referred for EGFR mutation testing based on the atypical presentation of SQC in a never smoker, we believe that this single clinical factor, which heralds a higher likelihood of finding an underlying AD-SQC than true SQC (based on the low incidence of never smokers with pure SQC seen in our prior series) (8), can be used to guide whether or not these patients should undergo testing. This recommendation stems in part from a prioritization of resources, which may be obviated in the future with the introduction of routine multiplex genotyping of lung SQCs (18).
Footnotes
Conflicts of interest: Pfizer, Boehringer-Ingelheim, Roche China (Mark G. Kris)
References
- 1.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–74. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uramoto H, Iwata T, Onitsuka T, Shimokawa H, Hanagiri T, Oyama T. Epithelial-mesenchymal transition in EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res. 2010;30:2513–7. [PubMed] [Google Scholar]
- 6.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23:857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Sugio K, Uramoto H, Ono K, Oyama T, Hanagiri T, Sugaya M, et al. Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking. Br J Cancer. 2006;94:896–903. doi: 10.1038/sj.bjc.6603040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rekhtman N, Paik PK, Arcila ME, Tafe LJ, Oxnard GR, Moreira AL, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167–76. doi: 10.1158/1078-0432.CCR-11-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang SM, Kang HJ, Shin JH, Kim H, Shin DH, Kim SK, et al. Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer. 2007;109:581–7. doi: 10.1002/cncr.22413. [DOI] [PubMed] [Google Scholar]
- 10.Jia XL, Chen G. EGFR and KRAS mutations in Chinese patients with adenosquamous carcinoma of the lung. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.04.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Toyooka S, Yatabe Y, Tokumo M, Ichimura K, Asano H, Tomii K, et al. Mutations of epidermal growth factor receptor and K-ras genes in adenosquamous carcinoma of the lung. Int J Cancer. 2006;118:1588–90. doi: 10.1002/ijc.21500. [DOI] [PubMed] [Google Scholar]
- 12.Pan Q, Pao W, Ladanyi M. Rapid Polymerase Chain Reaction-Based Detection of Epidermal Growth Factor Receptor Gene Mutations in Lung Adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukuya T, Takahashi T, Kaira R, Ono A, Nakamura Y, Tsuya A, et al. Efficacy of gefitinib for non-adenocarcinoma non-small-cell lung cancer patients harboring epidermal growth factor receptor mutations: a pooled analysis of published reports. Cancer Sci. 2011;102:1032–7. doi: 10.1111/j.1349-7006.2011.01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77:128–33. doi: 10.1016/j.lungcan.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Shim HS, Park MS, Kim JH, Ha SJ, Kim SH, et al. High EGFR gene copy number and skin rash as predictive markers for EGFR tyrosine kinase inhibitors in patients with advanced squamous cell lung carcinoma. Clin Cancer Res. 2012;18:1760–8. doi: 10.1158/1078-0432.CCR-11-2582. [DOI] [PubMed] [Google Scholar]
- 16.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24:1348–59. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22–31. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 18.Paik P, Berger M, Hasanovic A, Rekhtman N, Ladanyi M, Kris M. Multiplex testing for driver mutations in squamous cell lung cancers. J Clin Oncol. 2012:abstr 7505. [Google Scholar]


