Abstract
We report simultaneous measurements of fluorescence lifetimes at multiple excitation wavelengths with a Fourier transform frequency domain fluorescence lifetime spectrometer. The spectrometer uses a Michelson interferometer with its differential optical path length scanning at 22,000 Hz scan rate. The scan speed of the optical delay varies linearly during each scan and creates interference modulations that sweep from −150 to 150 MHz in 45.5 μs. The frequency-sweeping modulation allows nanosecond fluorescence lifetime measurements within 45.5 microseconds. Because the interference modulation frequency is wavelength-dependent, under the Fourier multiplexing principle, the spectrometer can perform lifetime measurements on multiple excitation wavelengths simultaneously.
Multiplexed fluorescence imaging simultaneously visualizes multiple molecular components of interests within the same sample [1, 2]. The most common multiplexing method is to differentiate fluorophores by their emission spectra. Fluorescence lifetime imaging provides additional means to distinguish multiple fluorescent components by their lifetimes [3]. However, multiplexing by excitation remains a bottleneck in fluorescence lifetime imaging. Multi-excitation fluorescence lifetime instruments often use a time sharing scheme, which uses switching mechanisms to limit the excitation wavelength to be one at a time to obtain spectrally resolved lifetimes [4–6]. In these instruments, the complexity of the optical systems and the data acquisition time increases linearly with growing numbers of excitation lines.
Fourier excitation spectroscopy allows true parallel excitation detection [7, 8] by modulating a multi-wavelength excitation source with time-varying interference in a path-length modulated interferometer. Because the interference modulation frequency is linear to the path-length scanning speed and wavenumber, multiple excitation lines are naturally separated by their different modulation frequencies. When fluorophores are excited by the Fourier modulated multiple excitation sources, fluorescence emission associated with different excitation wavelengths can be resolved by the inverse Fourier analysis. By changing the path-length scanning speed, which changes interferometric modulation frequencies, emission responses at different modulation frequencies can be measured. The fluorescence lifetimes at multiple excitation lines can then be extracted with frequency domain lifetime methods [7, 8]. However, the previous Fourier lifetime spectrometers can only measure μs lifetimes at 50 points per second, whereas lifetime imaging applications require ns lifetime measurements at a high pixel rate. In this letter we present a novel Fourier fluorescence lifetime system that can perform nanosecond fluorescence lifetime measurements with multiple excitation lines simultaneously within 45.5 μs.
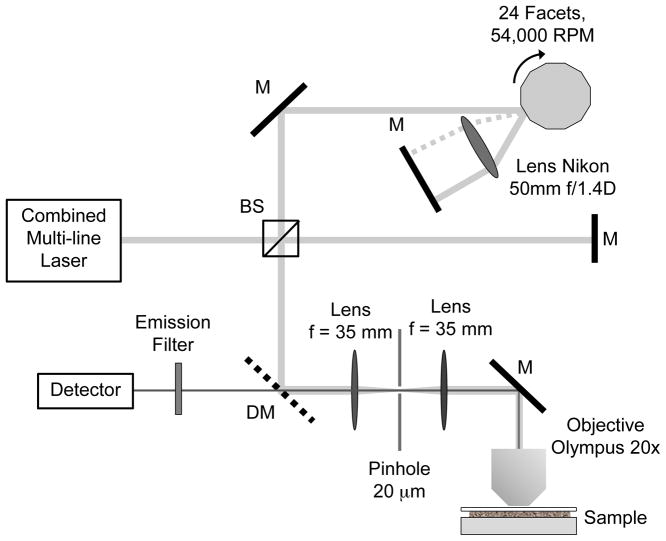
Figure 1 shows a schematic of the frequency-sweeping fluorescence lifetime spectrometer. Because the intensity modulations of the excitation source are generated by interference, the system accepts any laser with a coherence length longer than the maximum differential optical path length (~2 mm). We choose a combination of four continuous wave lasers at wavelength 405 nm, 488 nm, 561 nm and 638 nm, which is a common 4-color configuration in confocal microscopes. The four laser lines are combined by three dichroic mirrors (Semrock) and directed into a Michelson interferometer, whose differential path-length scans between 0 and 2 mm at 22,000 Hz by a double-passing high-speed optical delay line [9]. The delay line consists of a spinning 24-facet polygon mirror array (2.5 inch diameter, Lincoln Laser) scanning at 55,000 RPM (45.5 μs per facet), a lens (Nikon 50mm f/1.4D) and a mirror. The modulated multi-line output of the interferometer is focused onto the fluorescence sample by a 20x, 0.75 NA microscope objective (Olympus). The emitted fluorescence light is collected by the same microscope objective. After spatial filtering by a 20 μm pinhole and a pair of 35 mm focus lenses, the emission is detected by a PMT detector (Hamamatsu H7422, 0.78 ns rise time), amplified and recorded by a 1.5 GHz digitizer.
Figure 1.
Optical setup of the frequency-sweeping fluorescence lifetime fluorometer. The combined multi-line laser is directed into a Michelson interferometer. The path length of the interferometer is modulated by an optical delay line consisting of a rotating polygon mirror, a lens and a mirror.
The optical delay d of the polygon delay line is a parabolic function of rotational angle, given by d=2Rθ2, where R is the radius of the mirror array and θ is the incident angle (±7.5°). The polygon mirror produces 22,000 facet scans per second. Within a single facet scan, the instantaneous delay-scan speed changes linearly from approximately −94 m/s to +94 m/s. At the interferometer’s output, the instantaneous frequency of the wavelength-dependent modulation is given by f= λ/v, where λ is the wavelength and v is the instantaneous velocity of the optical delay. For laser lines at 400 ~ 600 nm wavelengths, the modulation frequency linearly changes from approximately 230~150 MHz to 0 then back to 230~150 MHz. The frequency sweeping modulation allows frequency domain lifetime measurements over a continuous frequency span at a rate of 22,000 points per second. To our knowledge, it is the fastest frequency domain lifetime fluorometer currently available.
The detected fluorescence signal contains emission driven by all excitation lines, each modulated at a different frequency. Fluorescence emissions from different excitation lines are therefore orthogonal to each other in the frequency domain. By correlating fluorescence emission with the interference signal from a single excitation line at λ, the lifetime property of the sample at the excitation wavelength λ is revealed.
| (1) |
where Iem is the total emission signal, Iλex is the interference signal from the excitation line at λ, H refers to the Hilbert transform, m is the modulation intensity of fluorescence emission, and φ is the phase delay between the fluorescence emission and the excitation modulation. Lifetime of the sample at the excitation wavelength equals to λ can be obtained by fitting m and φ with the frequency domain lifetime model [10].
Since ω changes linearly with time during each scan, in the data analysis, signal from a single scan is divided into short segments, in which modulation frequencies are approximately constants. In these segments, the fluorescence emission is cross-correlated with the excitation modulation at wavelength λ to obtain m and φ responses at different modulation frequencies. The fluorescence lifetime is then calculated by fitting m and φ with a decay model. The process is repeated for all excitation lines, and the lifetime of the sample is obtained as a function of excitation wavelengths. The Fourier spectral resolution in excitation wavelengths is set by the length of the delay-scan during a segment, which is 10 μm or higher for 1-μs time segments. The spectral resolution under 1-μs segmenting is 500 cm−1 (12 nm at 488 nm). Segment durations and frequency points can be freely adjusted depending on the signal to noise ratio, the excitation line separation and the lifetime model. Such flexibility could benefit the study of complex lifetime phenomena such as multi-exponential decays.
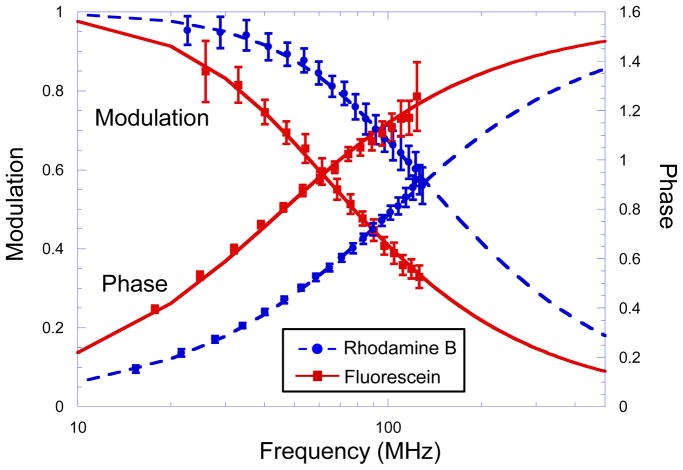
The above algorithm requires high-speed digitizing of both the fluorescence emission and interference modulations of all excitation lines. If the interference signals are highly repeatable, interference signals from the excitation lines can be pre-calibrated instead of measured in real-time, which greatly reduces the data volume. To determine whether pre-calibration is sufficient, we tested the system with a mixture of two fluorescence lifetime standards (2 μM fluorescein and 5 μM rhodamine B (Sigma) in DI water). The sample was excited by 488 nm and 561 nm laser lines simultaneously. Figure 2 plots normalized fluorescence modulation intensities of the mixture at 488 nm and 561 nm excitation, calculated with pre-recorded laser interference signals. Modulation intensity was analyzed in 1-μs segments, in which the modulation frequency swept across an approximately 7-MHz span. The error bars represents the standard deviation of the scan-to-scan variation. Modulation responses were fitted with the single exponential fluorescence decay model at each excitation lines [10]. At 488 nm excitation, which excites fluorescein, the fluorescence lifetime measured with a single facet scan is 3.6±0.4 ns. At 561 nm, which excites rhodamine B, the fluorescence lifetime is 1.7±0.2 ns. Both lifetimes match results in the literature [11]. The standard deviation in lifetime measurements is 10% with single 45.5-μs facet scans.
Figure 2.
Fluorescence intensity modulation and phase delay as a function of frequency measured within 45.5 μs at 488 nm and 561 nm excitation lines simultaneously. The sample was a mixture of 2 μM of rhodamine B and 5 μM of fluorescein in DI water. Error bars represent variations between 24 facet scans.
Figure 2 also plots fluorescence phase delays of the mixture at the two excitation lines, calculated with real-time laser interference signals. The resulting lifetimes are the same as measured by modulation, with lifetime of fluorescein at 3.6±0.4 ns, and rhodamine B at 1.6±0.2 ns. We found that real-time interference signals are required for accurately calculating the phase delay, whereas pre-recorder interference signals are sufficient for calculating the intensity modulation m. Laser modulation phases are very sensitive to random mechanical vibrations and motions of the delay line as the mirror spins. Therefore, real-time measurements of laser interference phases are required for precise phase analysis. On the other hand, modulation intensity analysis is much less sensitive to the instantaneous variations in the optical path. The algorithm to calculate m is equivalent to a frequency sweeping spectrum analyzer, whose bandwidth is the inverse of the segment duration and the center of the band tracks the laser modulation frequency. With 1-μs segments, the bandwidth is approximately 0.5 MHz. As long as the laser modulation frequency is repeatable within the segment bandwidth, frequency tracking is maintained in modulation analysis. We observed 0.05% variations in interference modulation frequencies between scans, which is mainly caused by the scanner motor speed instability (0.03%). When the modulation frequency changes between 0 and 230~150 MHz, the 0.05% frequency variation remains much smaller than the 0.5-MHz bandwidth during the entire scan. Because there is no observable frequency mismatch, pre-calibrating the optical delay line is sufficient for measuring lifetimes with modulations. We also observed 2% interference modulation amplitudes variation, which is caused by mirror tilting and vibration. The modulation amplitudes variation is the major noise source when the fluorescence is strong. It is the source of the 10% lifetime uncertainty and also limits the shortest detectable lifetime to be 0.4 ns. When the fluorescence is weak and the photon shot noise exceed the modulation amplitudes variations, a Monte-Carlo simulation shows that the photon efficiency of the frequency sweeping lifetime measurement is similar to the photon efficiency of a single frequency measurement at the center of the frequency sweeping span. Within the frequency range of our system, modulation measurements are more photon efficient than phase measurements when lifetimes are longer than 1.5 ns [12]. We conclude that although phase analysis provides additional information about fluorescence lifetime heterogeneity, modulation-only analysis will be better suited for imaging applications of the current system, because it does not need real time data of the laser interference. In the future, on-board hardware data processing may allow both phase and modulation analysis.
In conclusion, we have demonstrated an instrument that can perform simultaneous lifetime measurements on multiple excitation wavelengths. The Fourier frequency-sweeping fluorescence lifetime spectrometer is fully compatible with laser-scanning confocal microscopy. At 22,000 measurements per second, it could produce multiplexed lifetime images at 3 second per frame over a 256×256 pixel field of view if connected with a confocal scanning microscope. Because the instrument generates laser modulations by interference, it works with any continuous wave laser excitation line combinations. In the future, we will replace the color-blind detector with a multi-spectral photon-counting detector. The upgrade will make the instrument capable of simultaneously measuring fluorescence intensities and lifetimes in excitation emission matrices (EEMs) [6] at single molecule level. The instrument opens the door to multidimensional spectral and lifetime imaging, which will allow more fluorescent labels to be imaged simultaneously, and enable the investigation of complex fluorescence phenomena, such as multi-color Förster resonance energy transfer.
Acknowledgments
The authors thank Dr. Guillermo Tearney for inspiring discussions. This work was supported by the NIH Pathway to Independence Award (R00EB008737).
References
- 1.Verveer PJ, Bastiaens PIH. Quantitative microscopy and systems biology: seeing the whole picture. Histochemistry and Cell Biology. 2008;130(5):833–843. doi: 10.1007/s00418-008-0517-5. [DOI] [PubMed] [Google Scholar]
- 2.Yan YL, Marriott G. Analysis of protein interactions using fluorescence technologies. Current Opinion in Chemical Biology. 2003;7(5):635–640. doi: 10.1016/j.cbpa.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Suhling K, French PMW, Phillips D. Time-resolved fluorescence microscopy. Photochemical & Photobiological Sciences. 2005;4(1):13–22. doi: 10.1039/b412924p. [DOI] [PubMed] [Google Scholar]
- 4.Lee NK, Kapanidis AN, Wang Y, Michalet X, Mukhopadhyay J, Ebright RH, Weiss S. Accurate FRET measurements within single diffusing biomolecules using alternating-laser excitation. Biophysical Journal. 2005;88(4):2939–2953. doi: 10.1529/biophysj.104.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarrabi SEN, Düser MG, Golovina-Leiker A, Becker W, Erdmann R, Dunn SD, Borsch M. Simultaneous monitoring of the two coupled motors of a single FoF1-ATP synthase by three-color FRET using duty cycle-optimized triple-ALEX. Proc SPIE. 2009;7185:718505. [Google Scholar]
- 6.Owen DM, Auksorius E, Manning HB, Talbot CB, de Beule PAA, Dunsby C, Neil MAA, French PMW. Excitation-resolved hyperspectral fluorescence lifetime imaging using a UV-extended supercontinuum source. Optics Letters. 2007;32(23):3408–3410. doi: 10.1364/ol.32.003408. [DOI] [PubMed] [Google Scholar]
- 7.Peng L, Gardecki JA, Bouma BE, Tearney GJ. Fourier fluorescence spectrometer for excitation emission matrix measurement. Optics Express. 2008;16(14):10493–10500. doi: 10.1364/oe.16.010493. [DOI] [PubMed] [Google Scholar]
- 8.Peng LL, Motz JT, Redmond RW, Bouma BE, Tearney GJ. Fourier transform emission lifetime spectrometer. Optics Letters. 2007;32(4):421–423. doi: 10.1364/ol.32.000421. [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg AL, Reynolds JJ, Marks DL, Boppart SA. Fast-Fourier-domain delay line for in vivo optical coherence tomography with a polygonal scanner. Applied Optics. 2003;42(22):4606–4611. doi: 10.1364/ao.42.004606. [DOI] [PubMed] [Google Scholar]
- 10.Lakowicz JR. Principle of Fluorescence Spectroscopy. 2006. [Google Scholar]
- 11.Boens N, Qin WW, Basaric N, Hofkens J, Ameloot M, Pouget J, Lefevre JP, Valeur B, Gratton E, Vandeven M, Silva ND, Engelborghs Y, Willaert K, Sillen A, Rumbles G, Phillips D, Visser A, van Hoek A, Lakowicz JR, Malak H, Gryczynski I, Szabo AG, Krajcarski DT, Tamai N, Miura A. Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Analytical Chemistry. 2007;79(5):2137–2149. doi: 10.1021/ac062160k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder A, Schlachter S, Kaminski CF. Theoretical investigation of the photon efficiency in frequency-domain fluorescence lifetime imaging microscopy. Journal of the Optical Society of America a-Optics Image Science and Vision. 2008;25(2):452–462. doi: 10.1364/josaa.25.000452. [DOI] [PubMed] [Google Scholar]