Abstract
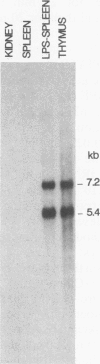
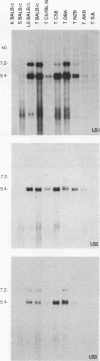
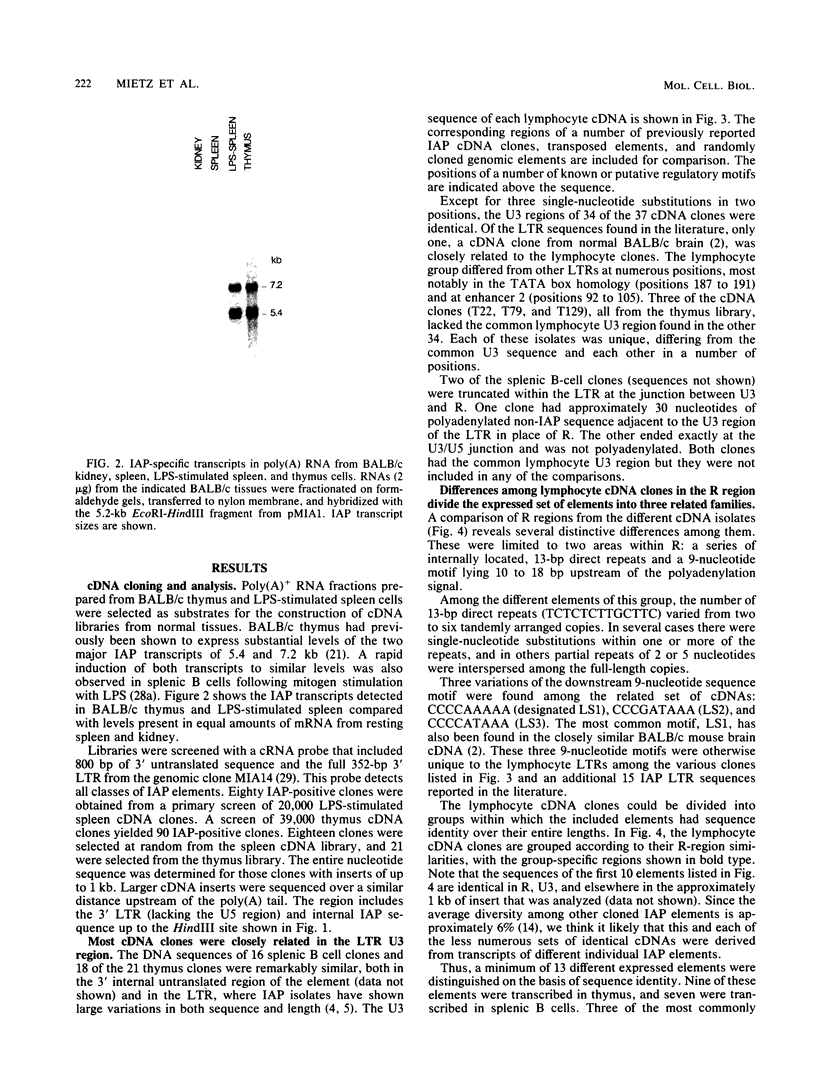
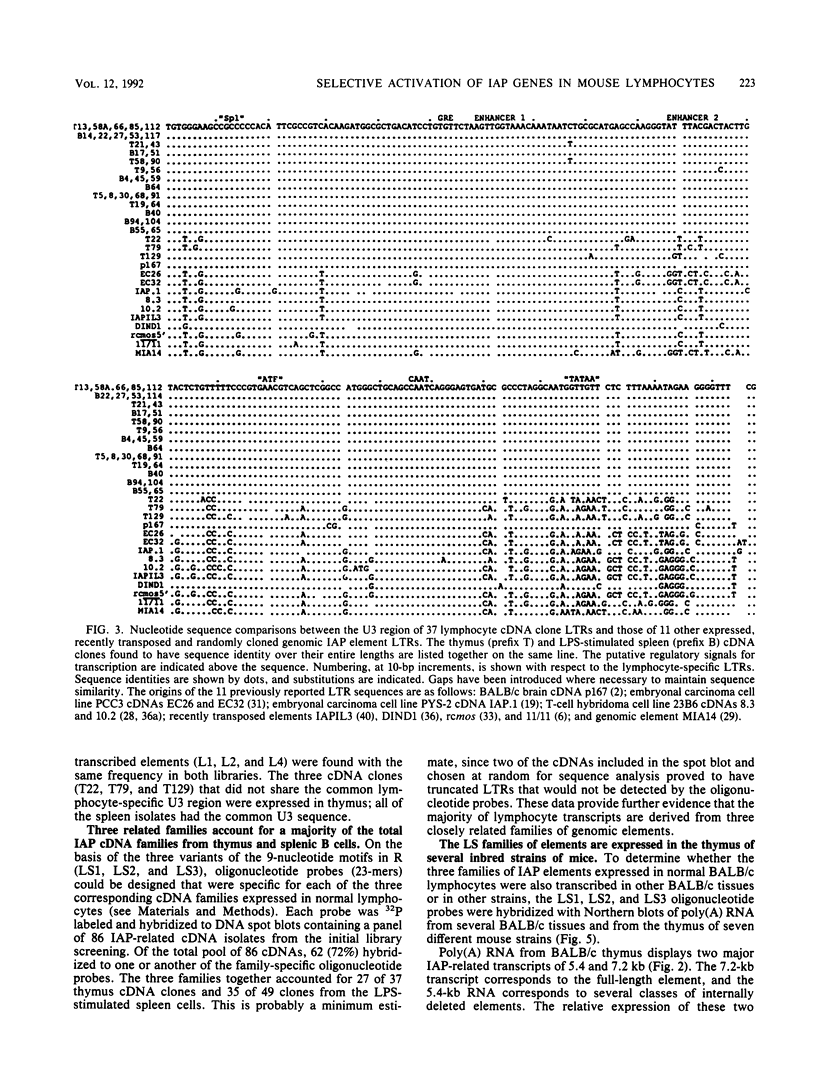
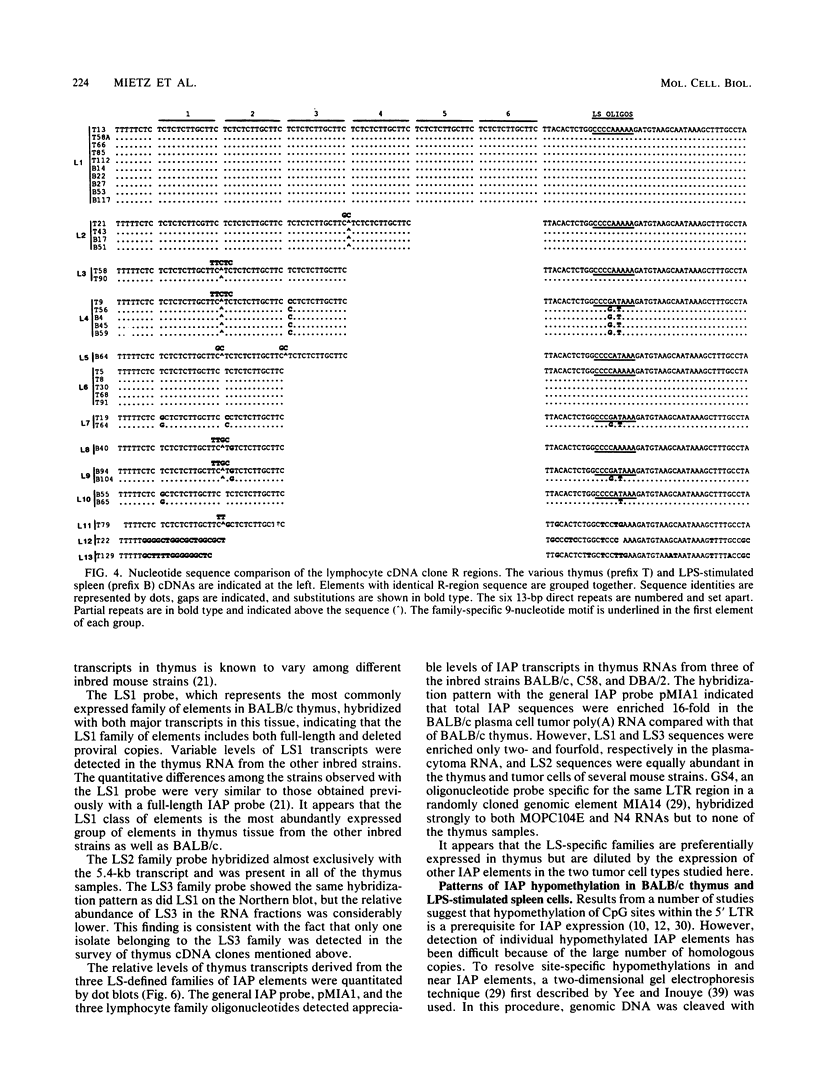
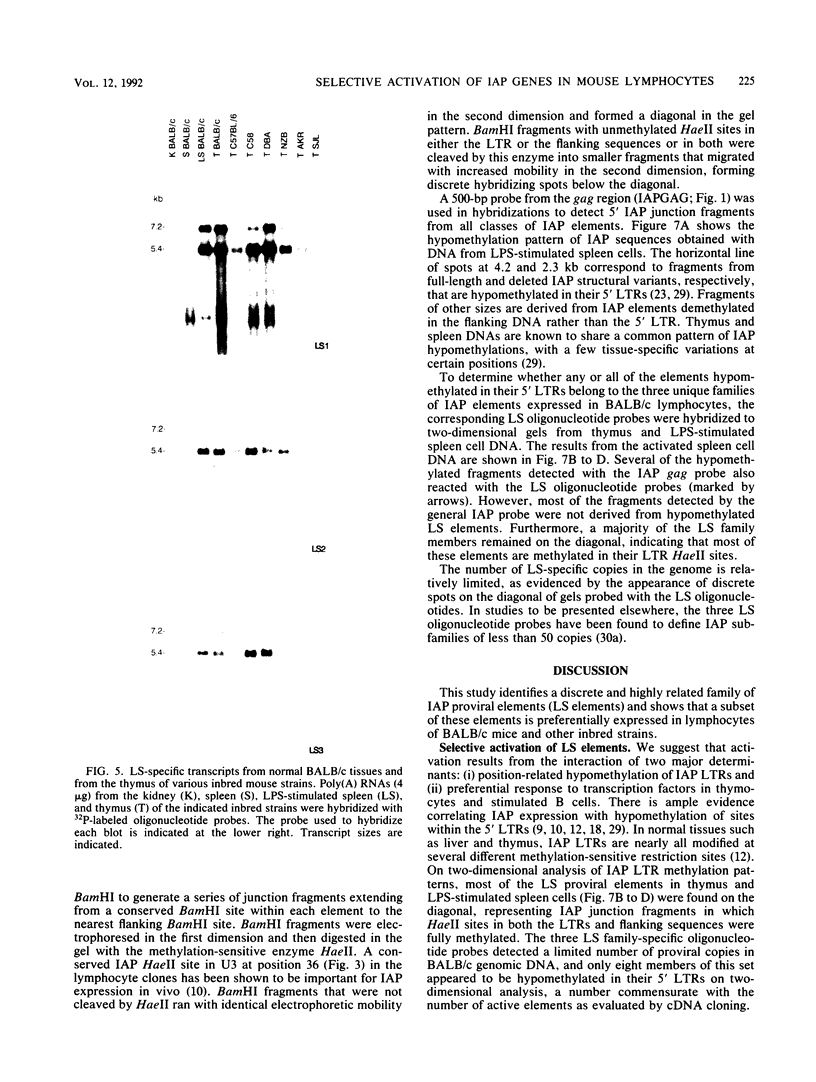
Intracisternal A-particle (IAP) proviral elements are abundant and widely dispersed in the mouse genome. IAP-related transcripts have been detected in normal mouse tissues where expression is under genetic control. In this study, we sought to determine whether IAP expression in BALB/c thymus and lipopolysaccharide-stimulated B cells was due to selective or indiscriminate activation of IAP elements. cDNA libraries were prepared from each source. A total of 86 IAP cDNA clones were isolated from both libraries, and 37 of these were sequenced over a common 0.7- to 1.0-kb region of the IAP genome that included the 3' long terminal repeat (LTR). Three highly related families of elements were found to be expressed in the two cell types examined. All of the related elements had a distinctive U3 regulatory region. Thirteen individual IAP proviral elements were distinguished on the basis of sequence differences within the R region of the LTR. Hybridization of genomic DNA with element-specific oligonucleotide probes confirmed the presence of a restricted number of proviral copies in the lymphocyte-specific family of elements. Most of these copies were found to be methylated in the lymphocyte DNA, but at least seven were hypomethylated in their 5' LTRs. This study shows that activation of IAP elements in normal normal mouse lymphocytes is highly selective. Activation is probably a function of both sequence specificity and methylation status of the proviral LTR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aota S., Gojobori T., Shigesada K., Ozeki H., Ikemura T. Nucleotide sequence and molecular evolution of mouse retrovirus-like IAP elements. Gene. 1987;56(1):1–12. doi: 10.1016/0378-1119(87)90153-3. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Huang R. C. Functional analysis of the long terminal repeats of intracisternal A-particle genes: sequences within the U3 region determine both the efficiency and direction of promoter activity. Mol Cell Biol. 1988 Mar;8(3):1093–1102. doi: 10.1128/mcb.8.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dührsen U., Stahl J., Gough N. M. In vivo transformation of factor-dependent hemopoietic cells: role of intracisternal A-particle transposition for growth factor gene activation. EMBO J. 1990 Apr;9(4):1087–1096. doi: 10.1002/j.1460-2075.1990.tb08214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton L., Norton J. D. Independent regulation of mouse VL30 retrotransposon expression in response to serum and oncogenic cell transformation. Nucleic Acids Res. 1990 Apr 25;18(8):2069–2077. doi: 10.1093/nar/18.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. A variant binding sequence for transcription factor EBP-80 confers increased promoter activity on a retroviral long terminal repeat. J Biol Chem. 1990 Aug 5;265(22):13084–13090. [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Binding of the transcription factor EBP-80 mediates the methylation response of an intracisternal A-particle long terminal repeat promoter. Mol Cell Biol. 1991 Jan;11(1):117–125. doi: 10.1128/mcb.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzon M., Kuff E. L. Multiple protein-binding sites in an intracisternal A particle long terminal repeat. J Virol. 1988 Nov;62(11):4070–4077. doi: 10.1128/jvi.62.11.4070-4077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Hu W. S., Cardiff R. D. Analysis of tissue-specific methylation patterns of mouse mammary tumor virus DNA by two-dimensional Southern blotting. J Virol. 1985 Jun;54(3):726–730. doi: 10.1128/jvi.54.3.726-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenstra A., Fewell J., Lueders K., Kuff E. In vitro methylation inhibits the promotor activity of a cloned intracisternal A-particle LTR. Nucleic Acids Res. 1986 May 27;14(10):4343–4352. doi: 10.1093/nar/14.10.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U., Mohit B., Tomkins G. M. Glucocorticoid action on hybrid clones derived from cultured myeloma and lymphoma cell lines. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3124–3127. doi: 10.1073/pnas.69.11.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z., Mietz J. A., Kuff E. L. Nearly identical members of the heterogeneous IAP gene family are expressed in thymus of different mouse strains. Nucleic Acids Res. 1987 May 11;15(9):3823–3834. doi: 10.1093/nar/15.9.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Heidmann O., Heidmann T. Retrotransposition of a mouse IAP sequence tagged with an indicator gene. Cell. 1991 Jan 11;64(1):159–170. doi: 10.1016/0092-8674(91)90217-m. [DOI] [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Lasneret J., Dianoux L., Canivet M., Ravicovitch-Ravier R., Périès J. Regulation of intracisternal A particles in mouse teratocarcinoma cells: involvement of DNA methylation in transcriptional control. Biol Cell. 1984;52(3):199–204. doi: 10.1111/j.1768-322x.1985.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Overton G. C. Expression of the intracisternal A-particle is elevated during differentiation of embryonal carcinoma cells. Mol Cell Biol. 1986 Jan;6(1):150–157. doi: 10.1128/mcb.6.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. E., Lueders K. K., DiPaolo J. A., Amsbaugh S. C., Popescu N. C. Chromosome distribution of intracisternal A-particle sequences in the Syrian hamster and mouse. Chromosoma. 1986;93(3):213–219. doi: 10.1007/BF00292740. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Kuff E. L. Intracisternal Type A particles in murine pancreatic B cells. Immunocytochemical demonstration of increased antigen (p73) in genetically diabetic mice. Am J Pathol. 1984 Jan;114(1):46–55. [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Fewell J. W., Kuff E. L., Koch T. The long terminal repeat of an endogenous intracisternal A-particle gene functions as a promoter when introduced into eucaryotic cells by transfection. Mol Cell Biol. 1984 Oct;4(10):2128–2135. doi: 10.1128/mcb.4.10.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens C. L., Huff T. F., Jardieu P., Trounstine M. L., Coffman R. L., Ishizaka K., Moore K. W. cDNA clones encoding IgE-binding factors from a rat-mouse T-cell hybridoma. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2460–2464. doi: 10.1073/pnas.82.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Kuff E. L. Tissue and strain-specific patterns of endogenous proviral hypomethylation analyzed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2269–2273. doi: 10.1073/pnas.87.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Christy R. J., Huang R. C. Murine A type retroviruses promote high levels of gene expression in embryonal carcinoma cells. Development. 1988 Jan;102(1):23–30. doi: 10.1242/dev.102.1.23. [DOI] [PubMed] [Google Scholar]
- Papanicolaou C., Ripley L. S. Polymerase-specific differences in the DNA intermediates of frameshift mutagenesis. In vitro synthesis errors of Escherichia coli DNA polymerase I and its large fragment derivative. J Mol Biol. 1989 May 20;207(2):335–353. doi: 10.1016/0022-2836(89)90258-1. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Givol D., Canaani E. Activation of a cellular oncogene by DNA rearrangement: possible involvement of an IS-like element. Nature. 1982 Dec 16;300(5893):607–611. doi: 10.1038/300607a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Preston B. D., Johnston L. A., Soni A., Loeb L. A., Kunkel T. A. Fidelity of two retroviral reverse transcriptases during DNA-dependent DNA synthesis in vitro. Mol Cell Biol. 1989 Feb;9(2):469–476. doi: 10.1128/mcb.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C., Löliger C., Kawai M., Suciu S., Gough N., Ostertag W. Identification of genes involved in growth autonomy of hematopoietic cells by analysis of factor-independent mutants. Cell. 1988 Jun 17;53(6):869–879. doi: 10.1016/s0092-8674(88)90329-7. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Two-dimensional DNA electrophoresis applied to the study of DNA methylation and the analysis of genome size in Myxococcus xanthus. J Mol Biol. 1982 Jan 15;154(2):181–196. doi: 10.1016/0022-2836(82)90059-6. [DOI] [PubMed] [Google Scholar]
- Ymer S., Tucker W. Q., Campbell H. D., Young I. G. Nucleotide sequence of the intracisternal A-particle genome inserted 5' to the interleukin-3 gene of the leukemia cell line WEHI-3B. Nucleic Acids Res. 1986 Jul 25;14(14):5901–5918. doi: 10.1093/nar/14.14.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]