Abstract
Loss of liver fatty acid binding protein (L-FABP) decreases long chain fatty acid uptake and oxidation in primary hepatocytes and in vivo. On this basis, L-FABP gene ablation would potentiate high-fat diet-induced weight gain and weight gain/energy intake. While this was indeed the case when L-FABP null (−/−) mice on the C57BL/6NCr background were pair-fed high fat diet, whether this would also be observed under high-fat diet fed ad libitum was not known. Therefore, this possibility was examined in female L-FABP (−/−) mice on the same background. L-FABP (−/−) mice consumed equal amounts of defined high-fat or isocaloric control diets fed ad libitum. However, on the ad libitum fed high-fat diet the L-FABP (−/−) mice exhibited: 1) Decreased hepatic long chain fatty acid (LCFA) β-oxidation as indicated by lower serum β–hydroxybutyrate level; 2) Decreased hepatic protein levels of key enzymes mitochondrial (rate limiting carnitine palmitoyl acyltransferase A1, CPT1A; HMG-CoA synthase) and peroxisomal (acyl CoA oxidase 1, ACOX1) LCFA β-oxidation; 3) Increased fat tissue mass (FTM) and FTM/energy intake to the greatest extent; and 4) Exacerbated body weight gain, weight gain/energy intake, liver weight, and liver weight/body weight to the greatest extent. Taken together, these findings showed that L-FABP gene-ablation exacerbated diet-induced weight gain and fat tissue mass gain in mice fed high-fat diet ad libitum—consistent with the known biochemistry and cell biology of L-FABP.
Keywords: Liver, fatty acid binding protein, fat, body weight, fat/lean tissue mass
INTRODUCTION
Liver fatty acid binding protein (L-FABP) is a small 14 kDa soluble protein highly expressed in liver, intestine, and kidney (0.1–0.4 mM) (rev. in (1). It has two binding sites for lipidic ligands such as long chain fatty acids (LCFAs) or long chain fatty acyl CoAs (LCFA-CoAs) (rev. in (1). Studies in vitro, with transfected cells, and in vivo suggest roles for L-FABP in LCFA/LCFA-CoA transport, β-oxidation, esterification, and nuclear targeting (rev. in (1, 2).
Initial studies of L-FABP function were largely in vitro. Purified L-FABP facilitated extraction of LCFA/LCFA-CoA from isolated membranes and prevented hydrolysis of LCFA-CoAs as expected from its ability to bind these lipids (3–7). Further, L-FABP directly interacted with purified carnitine palmitoyltransferase-1 (CPT-1), the outer mitochondrial membrane enzyme rate-limiting in LCFA-CoA β-oxidation, as well as prevented inhibition of CPT1 by excess substrate LCFA-CoA (8–10). L-FABP also potentiated peroxisomal LCFA oxidation (11). L-FABP bound LCFA-CoA for stimulating synthesis of phosphatidic acid and cholesteryl ester via GPAT and ACAT, respectively (3–7). Finally, L-FABP stimulated delivery of bound LCFA to purified nuclei (12). The latter proved especially important since L-FABP also directly interacted with peroxisome proliferator activated receptor-α (PPARα) (2, 13–15), a key nuclear receptor in hepatic transcription of genes in LCFA β–oxidation (CPT1A, CPT2, ACOX1), LCFA uptake (FATPs, L-FABP), and plasma VLDL triglyceride hydrolysis (LPL) (16–19).
Real-time imaging of fluorescent LCFA and radiolabeled LCFA studies in transfected cells were also consistent with roles for L-FABP in LCFA uptake, transport, β-oxidation, esterification, and nuclear targeting in living cells. Overexpression of murine or human wild-type L-FABP in transformed L-cell fibroblasts or Chang liver cells, respectively, as well as increased expression of human wild-type L-FABP in HepG2 cells correlated with increased LCFA uptake, intracellular transport, and nuclear targeting (14, 20–28). In contrast, overexpression of the human L-FABP T94A variant in Chang liver cells did not increase LCFA uptake (28). Murine L-FABP overexpression also increased intracellular LCFA transport diffusion (23, 25), LCFA β oxidation (29, 30), peroxisomal oxidation of branched-chain LCFA (30), LCFA esterification (24, 26, 31), and LCFA distribution to nuclei (14, 21, 22).
Since transformed tumor cell lines are dedifferentiated cells that typically exhibit abnormal expression levels of multiple proteins in LCFA and glucose metabolism, the physiological relevance of the above findings remained to be shown. This issue was addressed with cultured primary mouse hepatocytes and in vivo. Western blotting showed that primary mouse hepatocytes maintained constant similar levels (up to 3 days in culture) as liver for proteins in transmembrane transport of LCFAs (FATP-5, GOT, FATP-2, FATP-4) (32–34) and cholesterol (SRB1, ABCA1, ABCG1, ABCG5, ABCG8, and NPCL1) (34), cytosolic LCFA/LCFA-CoA transport (L-FABP, SCP-2) (35), and nuclear receptors regulating transcription of these proteins (PPARα, PPARβ, LXR, and SREBP) involved in expression of these proteins (35). L-FABP null hepatocytes exhibit decreased LCFA uptake, intracellular transport/diffusion, β-oxidation, esterification, nuclear targeting, and transcription/protein expression of PPARα-regulated genes in LCFA β-oxidation (34, 36, 37). Physiological relevance was further solidified by the demonstration that L-FABP gene ablation inhibited hepatic LCFA uptake and oxidation in vivo (1, 38–41).
Taken together the above biochemical, cell biological, and mouse studies suggested that loss of L-FABP would increase weight-gain and obesity, especially in the context of high-fat diet. However, previous studies on the effect of high-fat diet on L-FABP null mice have been conflicting thought potentially due to differences in dietary feeding regimen (pair-fed vs ad libitum with the control being mouse chow) and/or background stain (C57BL/6NCr vs C57BL/6J) (rev. in (1). To begin to resolve this issue, our earlier study reported that L-FABP (−/−) mice on the C57BL/6NCr background exhibit exacerbation of weight-gain and obesity when pair-fed high-fat diet (42). The present investigation extended these studies by examining how L-FABP gene ablation impacted the effect of ad libitum fed high-fat diet on: (i) LCFA β-oxidation; ii) expression of proteins involved in LCFA uptake, intracellular transport, oxidation, esterification, and regulation; iii) whole body and liver weight gain, and (iv) distribution between fat tissue mass (FTM) and lean tissue mass (LTM).
MATERIALS AND METHODS
Materials
Antibodies against ACBP (sc-23474), Acox1 (sc-98499), Cpt1a (sc-31128), Cpt2 (sc-20671), FATP2 (sc-161310), FATP4 (sc-25670), HMGCoA cytosolic (sc-33829), were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Antibodies against FATP-5 (ab89008) were purchased from Abcam (Cambridge, MA). COX IV and GAPDH antibodies were from Life Technologies (Grand Island, NY) and Millipore (Billirica, MA), respectively. Antibodies against PPARα (PA1-822A) were from Pierce Antibodies (Rockford, IL).
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University. L-FABP (−/−) mice were generated by homologous recombination deleting all four exons of the L-FABP gene as we described (38). L-FABP (−/−) mice were maintained on a C57Bl/6NCr background for at least N11 generations (99.95% homogeneity). Studies were performed with 8 wk old female mice because, as compared to the WT L-FABP (+/+) controls, the L-FABP (−/−) females exhibit greater weight gain and fat tissue mass than male counterparts when pair-fed high fat diet (42). Age matched female inbred C57BL/6NCr mice obtained from the National Cancer Institute (Frederick Cancer Research and Developmental Center, Maryland) were used as controls. Up until 1 week prior to the start of the dietary studies, the mice were housed in individual ventilated cage (3–5 animals per cage) with a 12 hr light/dark cycle in a temperature-controlled facility (25°C). The mice had access to a fixed formula rodent diet (Harlan Teklad Rodent Diet 8604, Madison, WI, USA) and water ad libitum. The mice were monitored by the presence of sentinels quarterly and found negative for all known mouse pathogens. Interpretations of results relating to L-FABP function apply specifically to C57BL/6NCr mice and may not reflect the general function of L-FABP among mammals. Likewise, results relating to L-FABP function in female C57BL/6NCr mice may not reflect those in male mice not studied herein.
Diet Composition
Defined high-fat and control pelleted diets were custom prepared by Research Diets, Inc. (New Brunswick, NJ) for this study. Two approximately isocaloric diets, a control and a high fat diet, were used for ad libitum-fed studies. The custom control diet (# D09010501, Research Diets, Inc., New Brunswick, NJ) was comprised of 20, 70, and 10 kcal% protein, carbohydrate and fat, respectively, with caloric value of 4057 kcal/1055.05 g or 3.85 kcal/g diet. This custom control diet consisted of the following modification of the carbohydrate mixture of diet D12450B (Research Diets, Inc., New Brunswick, NJ): corn starch (17 kcal%), maltodextrin 10 (4 kcal%), sucrose (28 kcal%), and fructose (21 kcal%). Fat in the control diet amounted to 5.5 kcal% and 4.5 kcal% derived from soybean oil and lard, respectively. The custom high-fat diet (#D09010502, Research Diets, New Brunswick, NJ) was comprised of 20, 55, and 25 kcal% of protein, carbohydrate and fat, respectively, with a caloric value of 4058 kcal/969.05 g or 4.19 kcal/g. This custom high fat diet consisted of the following changes to the control diet: (i) an increase in fat from 10 to 25 kcal% which was derived from an increase in the lard content, basically in the form of triglycerides; (ii) a decrease in fructose from 21 kcal% to 6 kcal%. The change in carbohydrates was used to balance the caloric increase due to higher fat content in order to keep the diet near isocaloric. Since the amount of carbohydrate (glucose and the more lipogenic fructose) is not equivalent in the control and high-fat diets, the difference in carbohydrate composition in addition to a difference in fat composition may contribute to the severity of fat mass increase. While this could be a potential confounder in this study, to maintain the high-fat diet isocaloric with respect to the control diet it was necessary to decrease other dietary components such as carbohydrate (as shown herein) or protein (maintained constant herein). The rest of the diet contents remained unchanged. Both diets had very low cholesterol, 0.002% and 0.009%, respectively, by weight.
Dietary Study
One week prior to the start of the experimental feeding, mice were housed individually in static microisolators with wire grid tops for holding food pellets and switched from the standard rodent chow mix to the defined control diet (D09010501, Research Diets, Inc., New Brunswick, NJ, USA), free of significant amounts of phytoestrogens or cholesterol. Two days before the start of the experimental feeding, mice were examined by dual-energy X-ray absorptiometry (DEXA). For the ad libitum feeding, all mice were then divided into two groups of 16 mice: LFABP (−/−) and L-FABP (+/+) with 8 mice of each group receiving a high-fat diet (D09010502, Research Diets, Inc., New Brunswick, NJ) and 8 mice receiving the low-fat control diet (D09010501, Research Diets). Mice had free access to food and water, food intake and body weight were measured every other day at the same time of day (morning). At the end of the 12-week dietary study, all mice were fasted overnight (12h) to reduce fluctuations and minimize deviations within each group. Mice were weighed and then anesthetized by intraperitoneal (IP) injection of ketamine (0.1 mg/g body weight) and xylazine (0.01mg/g body weight). Blood was collected using cardiac puncture and the mice killed by cervical dislocation. DEXA analysis was immediately performed and the liver removed and flash frozen using dry ice and stored at −80°C. Also, serum was isolated from serum and stored at −80°C.
DEXA Analysis
Dual-energy X-ray absorptiometry (DEXA) was performed on the mice using a Lunar PIXImus densitometer (Lunar Corp., Madison, WI) to determine the relative proportion of fat tissue mass (FTM) and bone-free lean tissue mass (LTM) as described previously(42, 43). A phantom mouse with known bone mineral density and FTM was used for calibration.
Prior to the initial DEXA analysis 2 days before the feeding study, each mouse was anesthetized as described in the previous section. After the procedure, mice were injected with yohimbine (0.11 μg/g body weight), as well as with warm saline solution for rehydration. During the recovery, the mice were kept warm with heat pads to minimize heat loss with subsequent check-ups every 10 min until the mice had fully recovered. After 12 weeks, the mice were terminated as described above and DEXA was performed again. The data collected at the initiation of the dietary studies was subtracted from the data collected at the termination of the study to calculate the changes in FTM and LTM.
Western blotting analysis
Western blotting was performed on liver homogenates to determine the relative liver content of the fatty acid transport proteins (FATP-2, FATP-4, FATP-5, and GOT), ACOX1, SCP-X, p-thiolase, PPARα, CPT1, CPT2, and HMG-CoA synthase with the relative proteins levels expressed as integrated density values. The proteins levels were normalized using either GAPDH or COX IV. Western blot analysis of the intracellular lipid-binding proteins, L-FABP, ACBP, and SCP-2, was performed similarly as described previously except that standard curves were performed using recombinant ACBP, SCP-2, or L-FABP purified as described (44–46). Briefly, each liver homogenate isolated from minced liver (aliquots of 10 μg protein) was loaded onto tricine gels (12%), ran on a Mini-Protean II cell (Bio-Rad lab, Hercules, CA) at 100 V constant voltage for 1.5 to 2 hrs (30 mA per gel initially). For 2 hrs, the proteins were transferred to nitrocellulose membranes (Bio-Rad) at a constant voltage of 100 V. The transferred blots were blocked for 1hr with 3 % gelatin in TBST (10 mM Tris-HCL, pH 8, 100 mM NaCl, 0.05% Tween-20) at room temperature. After several washings with TBST and overnight incubation with the necessary dilutions of primary antibodies (see Materials) in 1% gelatin in TBST and again washing with TBST, blots were incubated for 2h at room temperature with a secondary antibody (alkaline-phosphatase conjugate of goat anti-rabbit IgG) diluted 1:4500 in 1% gelatin TBST. The TBST washed blot was stained with Sigma Fast 5-bromo-4chloro-3-indolyl phosphate/nitro blue tetrazolim tablets (Sigma, St. Louis, MO), and imaged using an IS-500 system (Alpha Innotech, San Leandro, CA). NIH Image (http://rsbweb.nih.gov/nih-image) was used for densitometric analysis of each blot.
Measurement of serum β-hydroxybutyrate
β-hydroxybutyrate (3-HB), a ketone body created in the liver from acetyl CoA produced by LCFA β-fatty oxidation, was used as a gauge of fatty acid oxidation in the liver (38, 40, 41, 43). The levels of β-hydroxybutyrate in the serum were measured using β-hydroxybutyrate LiquiColor Test (StanBio Laboratory, Boerne, TX) according to the manufacturer’s protocol where the resultant enzymatic reaction was measured spectrophotometrically at 505 nm.
Statistical Analysis
Data analysis was performed on the dietary groups as follows: control diet pair-fed L-FABP (+/+), control diet L-FABP (−/−), high fat pair-fed L-FABP (+/+), high fat pair-fed L-FABP (−/−) mice, control diet ad libitum fed L-FABP (+/+), control diet ad libitum fed L-FABP (−/−), high fat ad libitum fed L-FABP (+/+), and high fat ad libitum fed L-FABP (−/−) mice. All data were analyzed by either t-test in between groups (e.g. L-FABP (−/−) compared to L-FABP (+/+)) or one-way ANOVA using statistical software (GraphPad Prism, San Diego, CA) as stated. Values represent the mean ± SEM, with p<0.05 considered statistically significant.
RESULTS
Effect of L-FABP gene-ablation on food consumption in ad libitum-fed diets
Food consumption for each mouse was measured over the 12 wk period (see Methods) and the cumulative mass (g) and caloric (kcal) intake of control or high-fat diets were plotted (Supplementary Fig. 1). WT L-FABP (+/+) mouse total food consumption and thus the caloric intake was similar between the ad libitum fed high-fat (Supplementary Fig. 1B, D) and control (Supplementary Fig. 1A, C) diets. L-FABP gene-ablation did not significantly affect total mass or caloric intake of control or high-fat diet consumed under the ad libitum fed conditions. Thus, L-FABP (+/+) and L-FABP (−/−) mice consumed the same amount of food (mass or energy) whether it was control or high-fat diet.
Effect of L-FABP gene-ablation on terminal body weight, percent weight gain, and liver weights in mice on ad libitum-fed control or high-fat diet
To determine if ad libitum-fed dietary type (control versus high fat) altered the impact of L-FABP gene ablation on final body weight and % weight gain, these parameters were measured in L-FABP (+/+) and L-FABP (−/−) mice after an overnight fast. Qualitatively the final body weights were higher for L-FABP (−/−) mice fed either diet ad libitum fed (Figs. 1A, B). However, quantitatively the final body weights were highest in L-FABP (−/−) mice fed the high-fat diet ad libitum. This effect was especially prominent when data were expressed as % gain in body to account for any slight differences in initial body weights between mouse groups (Fig. 1A, B).
Figure 1. Effect of L-FABP gene-ablation and high-fat diet on body weight, percent weight gain, and liver weight in ad libitum-fed mice.
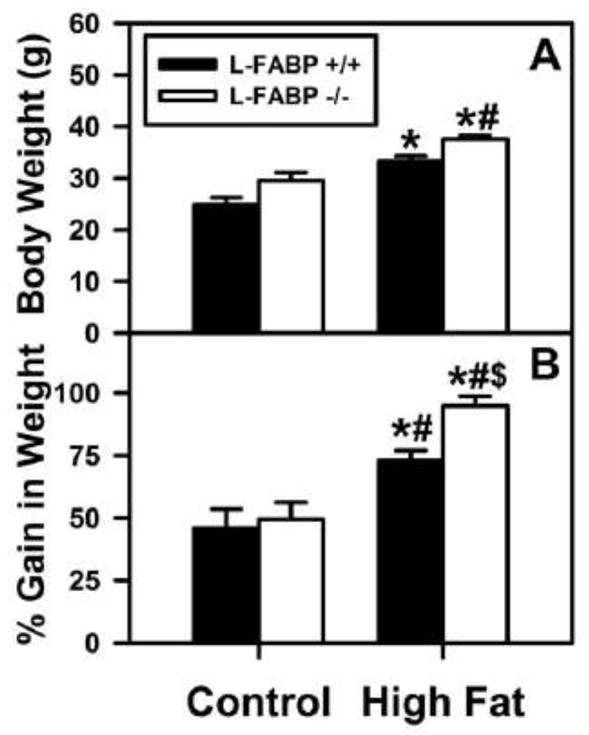
Graphs of final body weight (A) and % weight gain (B) for L-FABP (+/+) (solid bars) and L-FABP (−/−) (open bars) mice on either control or high-fat diets were results from ad libitum-feeding control (black bars) or high-fat (open bars) diets. At the end of the 12 wk dietary study, livers were removed as described in the Methods. Values represent the mean ± SEM, n=5–8. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p≤0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
To determine if the above effects of L-FABP gene ablation together with high-fat diet on increased body weight and weight gain resulted in enlarged livers, liver weights and liver weight/body weight were calculated. In L-FABP (−/−) mice ad libitum fed high-fat diet the liver weight (Supplementary Fig. 2A) and liver weight/body weight (Supplementary Fig. 2B) were both significantly increased. This indicated that in the context of ad libitum feeding high-fat diet the loss of L-FABP increased not only body weight, but even more so liver weight.
Thus, the L-FABP (−/−) mice fed high-fat diet ad libitum were about 20–25% heavier than the WT mice. L-FABP gene ablation did not protect the mice from increased body weight gain. In fact ad libitum fed high fat diet exacerbated gain in body weight, weight gain/energy intake, liver weight, and liver weight/body weight in L-FABP (−/−) mice.
Effect of L-FABP gene-ablation on the rate of body weight gain in mice ad libitum-fed control or high-fat diet
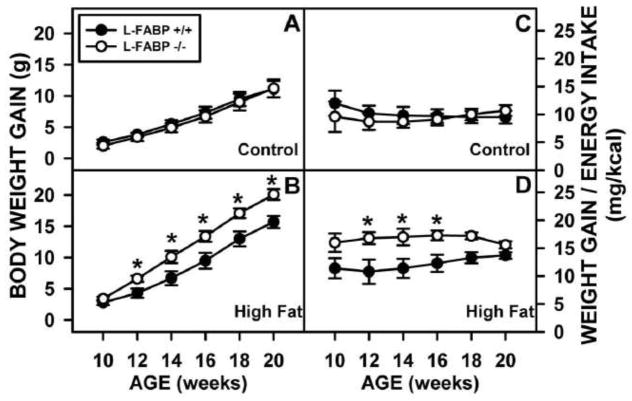
When examined over time on the respective diets, in WT L-FABP (+/+) mice ad libitum fed high-fat diet the weight gain (Fig. 2B vs 2A, solid circles) was faster and cumulative weight gain was also higher (Fig. 2D vs 2C, solid circles) as compared to control-diet. While L-FABP gene ablation did not significantly alter weight gain or cumulative weight gain in control-diet fed mice, this was not the case in L-FABP (−/−) mice ad libitum fed high fat diet. L-FABP gene ablation exacerbated the rate of weight gain (Fig. 2B vs 2A, open circles) and cumulative weight gain (Fig. 2D vs 2C, open circles).
Figure 2. Effect of L-FABP gene-ablation and high-fat diet on accumulated weight gain in ad libitum-fed mice.
Mice were fed control and high-fat diets ad libitum mice as described in the Methods. The cumulative body weight wise plotted in bi-weekly intervals for L-FABP (+/+) (solid circles) and L-FABP (−/−) (open circles) mice on control (A) and high-fat (B) diets. Similarly, the weight gain/energy intake was plotted in bi-weekly intervals for L-FABP (+/+) (solid circles) and L-FABP (−/−) (open circles) mice on control (C) and high-fat (D) diets. Statistical analysis was performed by Student’s t-test with significance p ≤0.05 represented by *.
Thus, on control diet, energy utilization was not different between the WT L-FABP (−/−) and L-FABP (−/−) mice. However, energy utilization of high-fat diet was more efficient in L-FABP (−/−) mice as evidenced by greater weight gain (total and per Kcal).
Effect of diet and L-FABP gene-ablation on fat and lean tissue mass in ad libitum-fed mice
To determine if the increased body weight of L-FABP (−/−) mice, especially on high fat diet, was due to increased lean tissue mass (LTM) or fat tissue mass (FTM), the mice were examined by non-invasive dual emission x-ray absorptiometry (DEXA) and analysis as in Methods.
Representative DEXA images of L-FABP (+/+) (Supplementary Fig. 3A and B) and L-FABP (−/−) (Supplementary Fig. 3C and D) mice on control and high-fat diets, respectively, suggested that the L-FABP (−/−) mice fed high-fat diet were more obese. Quantitative analysis of multiple DEXA images showed that in the ad libitum-fed mice on the control diet there was no significant difference in either the lean tissue mass (LTM) or the fat tissue mass (FTM) resulting from L-FABP gene-ablation (Fig. 3A). On the high-fat diet, the L-FABP (−/−) mice had a higher proportion of the increased body weight distributed as FTM with little change in the LTM (Fig. 3B). Due to the similarity in food consumption, similar results were obtained when the data corrected for total caloric intake (Fig. 3C and D).
Figure 3. Effect of L-FABP gene-ablation and high-fat diet on distribution of lean tissue mass (LTM) and fat tissue mass (LFM) in ad libitum-fed mice.
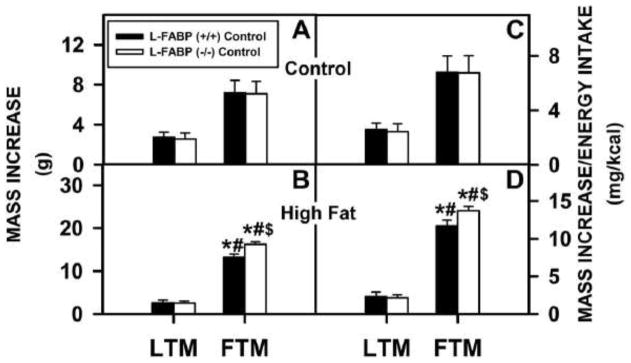
Dual emission X-ray absorptiometry (DEXA) was performed at the beginning and end of the 12 wk ad libitum-fed study. DEXA analysis results were reported as mass increases in LTM and FTM for L-FABP (+/+) and L-FABP (−/−) mice as for the (A) control and (B) high-fat diets. The mass increase per energy intake (mg/kcal) was reported as well for (C) control and (D) high-fat diets. Values represent the Mean ± SEM, n=5–7. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤ 0.05 as compared to L-FABP (+/+) LTM; # as compared to L-FABP(−/−) LTM; and $ as compared to L-FABP (+/+) FTM.
Thus, when ad libitum fed high-fat diet the L-FABP (−/−) deposited more fat and more efficiently utilized dietary fat as suggested by the data presented in Figs. 1 and 2.
Effect of ad libitum feeding of high-fat diet on hepatic levels of membrane fatty acid transporters
Since the liver is a key organ in fatty acid uptake, oxidation, and secretion as VLDL, the possibility that the impacts of L-FABP gene ablation on increased body weight and FTM may be associated with altered expression of integral membrane LCFA transport proteins was examined by western blotting. Two integral membrane LCFA transporters enhance LCFA uptake across the hepatic plasma membrane, fatty acid transport protein-5 (FATP-5) and glutamic-oxaloacetic transaminase (GOT) (47–50). Although localized in hepatic peroxisomal membranes, fatty acid transport protein-2 (FATP-2) and fatty acid transport protein-4 (FATP-4) also enhance hepatic fatty acid uptake (47).
High-fat diet fed ad libitum increased the expression of FATP-2 (Fig. 4A) and GOT (Fig. 4D), but not the other membrane fatty acid transporters FATP-4 (Fig. 4B) or FATP-5 (Fig. 4C) in WT L-FABP (+/+) mice. L-FABP gene ablation increased expression of GOT (Fig. 4D) while decreasing that of FATP-5 (Fig. 4C) in control-fed mice. In contrast, L-FABP gene ablation elicited relatively minor effects if any on expression of FATP-2, FATP-4, FATP-5, or GOT (Fig. 4A–D) in high-fat diet fed mice.
Figure 4. Effects ad libitum feeding as well as L-FABP gene-ablation on key fatty acid transporters/enzymes.
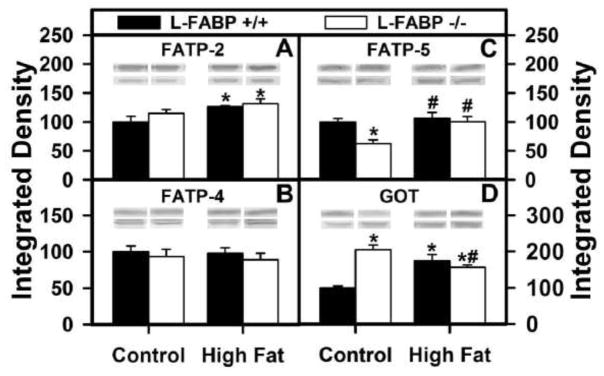
After termination of the 12 wk dietary study, livers from L-FABP (+/+) and L-FABP (−/−) mice on each of the different diets were harvested and expression of the several proteins involved fatty acid transport. Western blotting as described in Methods determined the levels of: (A) FATP-2, (B) FATP-4, (C) FATP-5, and (D) GOT. Representative western blots were included of each respective protein (lower lane) and normalization protein (upper lane). Values represent the mean ± SEM, n=5–8. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤ 0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
In summary, L-FABP gene ablation induced no or offsetting changes in expression among the LCFA membrane transporters—suggesting no net effect. Thus, there was no concomitant net upregulation of integral membrane LCFA transport proteins to account for L-FABP gene ablation induced increases in body weight and FTM in the context of high fat diet fed ad libitum.
Effect of ad libitum feeding high-fat diet on hepatic levels of cytosolic LCFA binding/transport proteins
The possibility that the impact of L-FABP gene ablation and/or high fat diet on body weight and FTM may be associated with altered expression of cytosolic LCFA/LCFA-CoA transport proteins such as L-FABP, sterol carrier protein-2 (SCP-2), or acyl CoA binding protein (ACBP) was examined by western blotting. Not only L-FABP, but also SCP-2, exhibits high affinity for LCFA and enhances LCFA uptake (1, 2, 13, 20, 23, 29, 30, 36, 38, 51, 52). Furthermore, all three proteins (L-FABP, SCP-2, and ACBP) exhibit high affinity for LCFA-CoAs (46, 53–57).
As shown by quantitative western blotting, the wild-type mouse hepatic mass levels of these cytosolic LCFA/LCFA-CoA binding proteins was in the order L-FABP > ACBP ≫ SCP-2 (Figs. 5). L-FABP gene ablation reduces liver cytosolic LCFA and LCFA-CoA binding capacity by 80–90% (38, 39). Further, L-FABP overexpression increases while ablation decreases cytosolic L-FABP transport/diffusion (23, 25, 36). Taken together these data suggested that L-FABP is the major LCFA/LCFA-CoA binding protein in liver. Under ad libitum feeding regimen, high fat diet slightly decreased the level of L-FABP in wild-type L-FABP (+/+) mice (Fig. 5A) while that of ACBP and SCP-2 was unaltered (Figs. 5B, C). L-FABP gene ablation resulted in complete loss of L-FABP (Fig. 5A) concomitant with slight increases in ACBP and SCP-2 on control diet, but 2-fold increased level of SCP-2 on high fat diet (Figs. 5B, C).
Figure 5. Effects of ad libitum feeding and L-FABP gene ablation on the expression levels of important fatty acid/fatty acyl CoA binding proteins.
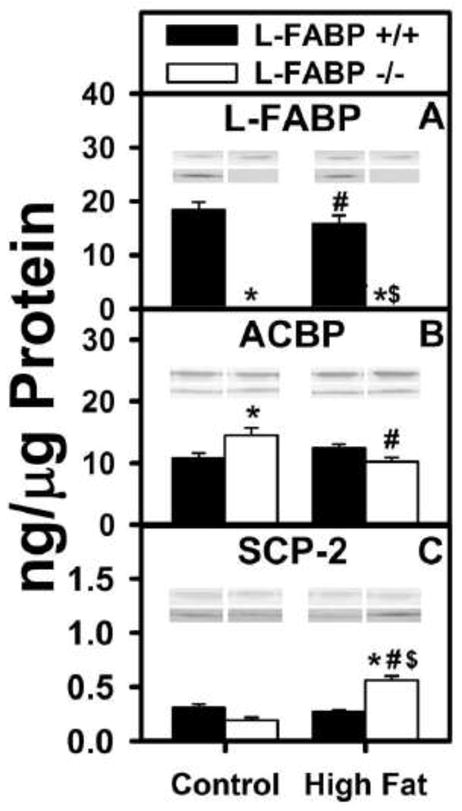
The expression levels of three LCFA/LCFA-CoA binding proteins (A) L-FABP, (B) ACBP, and (C) SCP-2 were examined by western blotting in the liver homogenates from L-FABP (+/+) and L-FABP (−/−) mice on ad libitum-fed control and high-fat diets as described in Methods. Representative western blots were included of each respective protein (lower lane) and normalization protein (upper lane). Values represent the mean ± SEM, n=5–8. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤ 0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
Taken together with the relative mass levels of these cytosolic LCFA/LCFA-CoA binding proteins (i.e. L-FABP > ACBP ≫ SCP-2), these data indicated that the increased body weight and body weight/energy intake noted in L-FABP (−/−) mice ad libitum fed high fat diet was not due to massive upregulation of ACBP or SCP-2 concomitant with loss of L-FABP.
Effect of ad libitum feeding high-fat diet on hepatic LCFA β–oxidation
L-FABP directly binds to outer mitochondrial membrane CPT1A to enhance LCFA-CoA transfer and LCFA β oxidation (8). Thus, it was important to measure the impact of diet type (control versus high-fat) on hepatic LCFA β-oxidation in L-FABP (+/+) and L-FABP (−/−) mice. Serum levels of β-hydroxybutyrate levels, an in vivo measure of LCFA β-oxidation, were determined as described in Methods. L-FABP gene ablation significantly reduced serum β-hydroxybutyrate levels, regardless of diet (Fig. 6D). Thus, reduced LCFA β-oxidation in L-FABP (−/−) mice significantly contributed to greater weight gain and weight gain/energy intake.
Figure 6. Effects of ad libitum feeding and L-FABP gene ablation on the expression levels of important enzymes in mitochondrial oxidation as well as on serum levels of β-hydroxybutyrate.
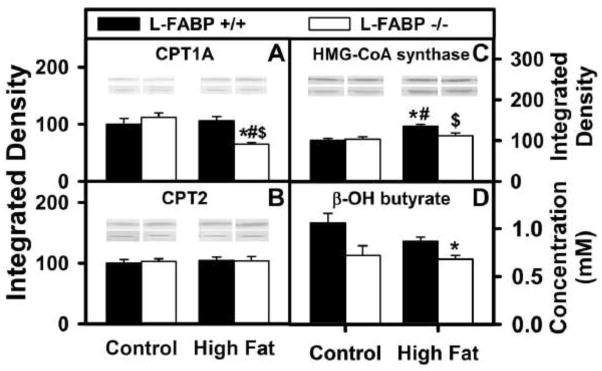
After the end of the 12 wk dietary study, livers from L-FABP (+/+) and L-FABP (−/−) mice fed control or high-fat diets ad libitum were harvested and expression of the key mitochondrial LCFA β-oxidation enzymes was measured by western blotting as described in Methods. The effect of L-FABP gene-ablation on (A) carnitine palmitoyl transferase-1 (CPT1), (B) carnitine palmitoyl transferase-2 (CPT2), and (C) HMG-CoA synthase was examined. At the end of the dietary study, serum was also collected and levels of β-hydroxybutyrate were measured for L-FABP(+/+) and L-FABP(−/−) mice ad libitum-fed control and high-fat diets (D) as described in Methods. Representative western blots were included of each respective protein (lower lane) and normalization protein (upper lane). Values represent the mean ± SEM, n=5–8. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤ 0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
Effect of ad libitum feeding high-fat diet on longer-term regulation of mitochondrial enzymes in fatty acid β-oxidation
L-FABP also enhances transport/cotransport of bound LCFA to the nucleus wherein L-FABP binds and facilitates transfer of bound LCFA to PPARα for inducing transcription of mitochondrial LCFA β-oxidative enzymes (2, 12–15, 21, 22, 37, 58, 59). Western blotting was therefore used to determine hepatic expression of key enzymes in mitochondrial LCFA β-oxidation: carnitine palmitoyl transferase-1A (CPT1A, outer mitochondrial membrane, rate limiting in mitochondrial LCFA β-oxidation); carnitine palmitoyl transferase-2 (CPT2, inner mitochondrial membrane); 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase. High-fat diet fed ad libitum increased the level of HMG-CoA CoA synthase (Fig. 6C), but not CPT1A or CPT2 (Figs. 6A and B). L-FABP gene ablation decreased the level of CPT1A, the rate limiting enzyme in mitochondrial LCFA β-oxidation (Fig. 6A) as well as HMG-CoA synthase (Fig. 6C) but not CPT2 (Fig. 6B) in livers of high-fat fed mice.
Thus, reduced level of the rate limiting enzyme in mitochondrial LCFA β-oxidation (i.e. CPT1A) and of HMG-CoA synthase contributed significantly to reduced LCFA β-oxidation in in L-FABP (−/−) mice fed high-fat diet ad libitum.
Effect of ad libitum feeding high-fat diet on hepatic levels of peroxisomal enzymes involved in peroxisomal LCFA β-oxidation
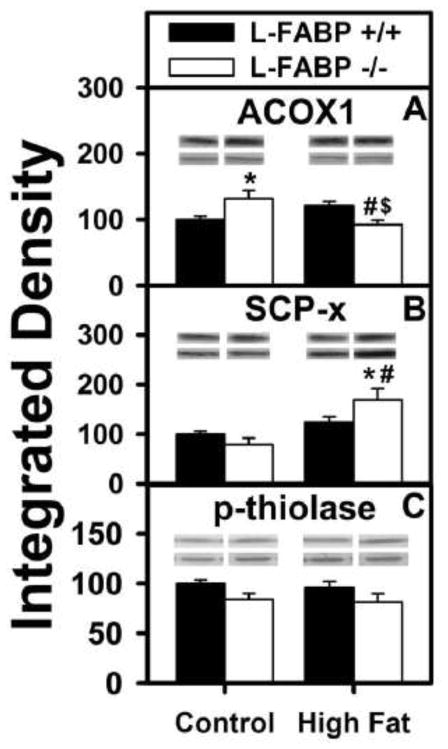
L-FABP also enhances ligand-mediated PPARα transcription of peroxisomal LCFA β-oxidative enzymes and peroxisomal LCFA β-oxidation (1, 30, 36, 58, 60, 61). Therefore, it was also important to determine the impact of diet-type and feeding regiment on L-FABP-mediated expression of peroxisomal LCFA oxidative enzymes. Western blotting as described in Methods was used to determine the levels of three enzymes involved in peroxisomal fatty acid oxidation, acyl CoA oxidase-1 (ACOX1, the rate limiting enzyme in peroxisomal β-oxidation of straight-chain fatty acids) and two thiolases, sterol carrier protein-x (SCP-x) and p-thiolase. Ad libitum fed high-fat diet did not increase transcription of ACOX1 (Fig. 7A, black), SCP-x (Fig. 7B, black), or p-thiolase (Fig. 7C, black). Hepatic level of ACOX1, but not SCP-x or thiolase, was increased in L-FABP (−/−) mice fed control diet ad libitum (Fig. 7A–C). In contrast, the hepatic level of ACOX1 was significantly decreased (Fig. 7A), that of SCP-x increased (Fig. 7B), and that of p-thiolase unaltered (Fig. 7C) when the L-FABP (−/−) mice were ad libitum fed high-fat diet.
Figure 7. Effects of ad libitum feeding and L-FABP gene-ablation on key enzymes involved in hepatic peroxisomal oxidation of fatty acids.
After termination of the 12 wk dietary study, livers from L-FABP (+/+) and L-FABP (−/−) mice ad libitum-fed control or high-fat diets were harvested and expression of the key peroxisomal fatty acid oxidation enzymes was measured by western blotting as described in Methods. The effect of L-FABP gene-ablation on (AD) ACOX1, (B) SCP-x, and (C) p-thiolase was examined. Representative western blots were included of each respective protein (lower lane) and normalization protein (upper lane). Values represent the mean ± SEM, n=5–8 in relative units of integrated density. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
Thus, these findings were consistent with attributing the higher gain in body weight and weight/energy intake in L-FABP (−/−) mice fed high-fat diet ad libitum as being due in part to reduced expression of ACOX1, the rate limiting enzyme in peroxisomal LCFA β-oxidation.
Effect of ad libitum feeding of high-fat diet on hepatic expression of peroxisome proliferator activated receptor-α (PPARα)—a key transcriptional regulator of proteins in LCFA uptake, transport, and β-oxidation
Since L-FABP is a PPARα regulated gene and L-FABP binds to and induces PPARα transcriptional activity (2, 13–15, 59, 61, 62), it was important to determine the impact of diet type and regimen on hepatic level of PPARα. Wild-type L-FABP (+/+) mice ad libitum fed control diet had unaltered expression of PPARα (Fig. 8, black). In contrast, L-FABP gene ablation decreased hepatic PPARα level in mice fed control diet while increasing that in mice fed high-fat diet ad libitum (Fig. 8).
Figure 8. Effects of ad libitum feeding and L-FABP gene-ablation on the nuclear receptor, PPARα.
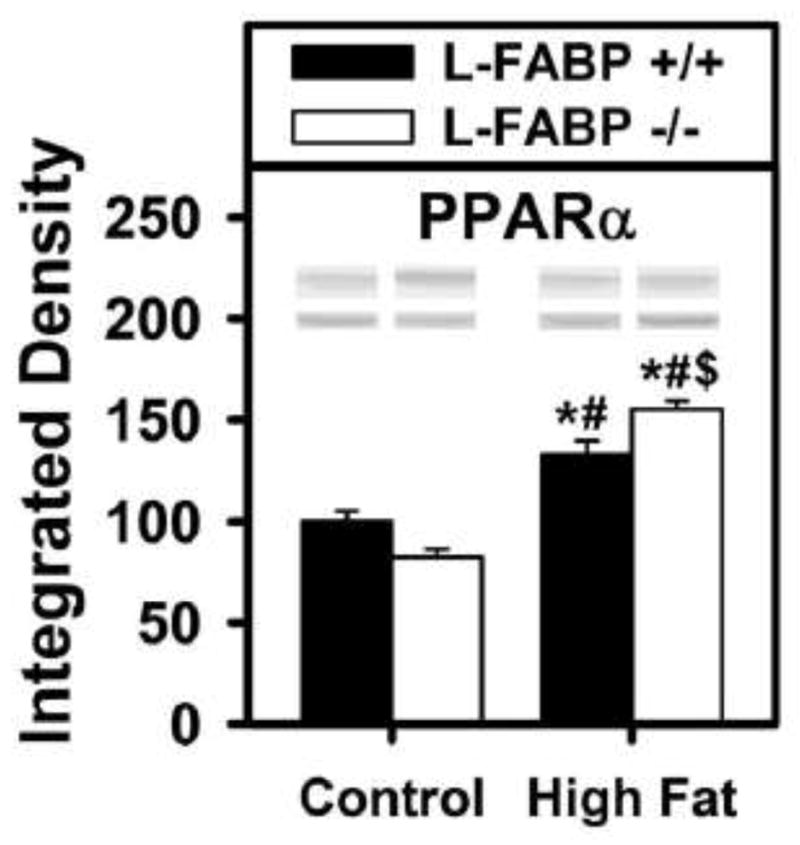
The expression level of PPARα was determined by western blotting using livers removed from L-FABP (+/+) and L-FABP (−/−) mice at termination of the ad libitum-fed control or high-fat dietary study. Representative western blots were included of each respective protein (lower lane) and normalization protein (upper lane). Values represent the mean ± SEM, n=5–8. Statistical analysis performed by one-way ANOVA with Bonferroni post-tests. The significance was as follows: * p ≤ 0.05 as compared to L-FABP (+/+) control diet; # as compared to L-FABP (−/−) control diet; and $ as compared to L-FABP (+/+) high-fat diet.
Taken together, these data indicated that altered hepatic level of PPARα in L-FABP (−/−) mice fed control diet ad libitum did not significantly contribute to reduced expression of PPARα regulated enzymes rate limiting in LCFA β-oxidation (i.e. CPT1A, ACOX1). Likewise, since the hepatic level of PPARα was in increased in L-FABP (−/−) mice fed control diet ad libitum, this again did not significantly contribute to reduced expression of PPARα regulated enzymes rate limiting in LCFA β-oxidation (i.e. CPT1A, HMG-CoA synthase, ACOX1). These data suggested that, under ad libitum feeding regimen, reduced expression of key LCFA β-oxidative enzymes and lower LCFA β-oxidation was associated with loss of L-FABP’s ability to activate PPARα rather than with reduced hepatic level of PPARα in L-FABP (−/−) mice.
DISCUSSION
L-FABP’s involvement in hepatic fatty acid oxidation in vivo has been shown by studies using L-FABP gene-ablated mice. Regardless of whether back-crossed to C57Bl/6NCr background as described herein or C57Bl/6J background as described by others, L-FABP (−/−) mice fed control-chow ad libitum exhibited decreased hepatic LCFA uptake, cytosolic LCFA binding capacity, LCFA β-oxidation (mitochondrial, peroxisomal), and peroxisomal branched-chain LCFA oxidation (1, 30, 38, 40, 41). In contrast, L-FABP gene ablation did not diminish either intestinal LCFA uptake or muscle utilization of LCFA (rev. in (1, 63, 64). To control for LCFA utilization by other tissues, organ-specific cross talk, or endocrine influences (65), when the above experiments were also performed with cultured primary mouse hepatocytes from L-FABP (−/−) mice on the C57Bl/6NCr background the loss of L-FABP recapitulated the in vivo described decrease in LCFA uptake (both straight and branched chain LCFA), intracellular LCFA transport, straight chain LCFA β-oxidation, and branched-chain LCFA oxidation (33, 36, 58). Further, LCFA nuclear targeting and expression of PPARα-regulated proteins in LCFA β oxidation was decreased in cultured primary hepatocytes L-FABP (−/−) hepatocytes from L-FABP (−/−) mice on the C57Bl/6NCr background (13, 37, 52, 58). Taken together, these findings suggested that regardless of the background strain the loss of L-FABP would result in diversion of LCFA from liver to adipose tissue for increased FTM and weight gain—especially in mice fed high-fat diet ad libitum—thereby resulting in diet-induced obesity. While this expectation was borne out in L-FABP (−/−) mice on the C57Bl/6NCr background pair-fed high fat diet, the opposite observation was obtained with L-FABP (−/−) mice on the C57Bl/6J background ad libitum fed high-fat diet or C57Bl/6J mice treated with L-FABP antisense (rev. in (1, 66). The results presented herein addressed the possibility that the opposite phenotypes of the L-FABP (−/−) mice might be due at least in part to differences in diet-type (control-chow versus high fat diet) and dietary regimen (pair-fed versus ad libitum fed). The data contributed the following new insights.
First, L-FABP gene-ablation decreased fatty acid oxidation in mice on the C57BL/6NCr background. L-FABP gene ablation decreased LCFA β-oxidation regardless of whether the mice were ad libitum fed as described herein or pair-fed (42), especially when fed high-fat diet. The decreased LCFA β-oxidation was not due to a reduction of PPARα level, but rather was associated with reduced expression of PPARα regulated genes, especially those in LCFA β oxidation. This was consistent with the fact that CPT1A, CPT2, HMG-CoA synthase, and ACO1 are all enzyme regulated by PPARα itself (16–19) and L-FABP in turn interacts with and facilitates ligand-mediated PPARα transcriptional activity (2, 13–15). The largest decreases in levels of hepatic β-oxidative enzymes (CPT1A, HMG-CoA synthase, ACOX1) upon L-FABP gene-ablation were in mice fed high-fat diet—regardless whether fed ad libitum as shown herein or pair-fed (42). Although expression of CPT2 was unaltered in ad libitum fed L-FABP (−/−) mice (shown herein), it was increased upon pair feeding (42). However, this protein is not rate limiting in mitochondrial LCFA β-oxidation. Taken together, these findings suggested that reduced LCFA β-oxidation would divert LCFA to pathways leading to increased fat storage and weight gain.
Second, L-FABP (−/−) mice on the C57Bl/6NCr background exhibited higher FTM when fed high-fat, but not control, diet. L-FABP gene ablation increased FTM in both ad libitum fed as described herein and pair-fed (42) high-fat diet regimens. Thus, rather than protecting mice from high-fat diet-induced increases in FTM, loss of L-FABP in mice on the C57Bl/6J background significantly increased the FTM and FTM/energy intake with the extent depending on both diet type and dietary regimen. It is important to note that L-FABP gene ablation did not diminish either intestinal LCFA uptake or muscle utilization of LCFA (rev. in (1, 63, 64). Thus, the increased FTM is consistent with the fact that L-FABP gene ablation reduced hepatic LCFA β oxidation as shown herein and earlier by our and other labs (1, 30, 38, 40, 41). Taken together these data suggest that the increased FTM in L-FABP (−/−) mice fed in high-fat diet would lead to increased weight gain/obesity.
Third, L-FABP (−/−) mice on the C57BL/6NCr background exhibited high-fat diet-induced obesity. L-FABP gene ablation resulted in greater weight gain and adiposity in both ad libitum fed as described herein and pair-fed (42) high-fat dietary regimens. Thus, high-fat diet fed L-FABP (−/−) mice on the C57Bl/6NCr background exhibited increased weight gain/obesity regardless of ad libitum or pair-fed dietary regimen.
These data with L-FABP (−/−) mice on the C57Bl/6NCr background were consistent with the known biochemistry and cell biology of L-FABP which suggested that decreased hepatic LCFA β-oxidation would lead to increased weight gain and FTM in response to high-fat diet. This was not due to ad libitum dietary feeding regimen since similar findings were obtained when the L-FABP (−/−) mice were pair-fed high-fat diet (42). In contrast, despite similarly decreased hepatic LCFA uptake and decreased hepatic LCFA β-oxidation, L-FABP (−/−) mice on the C57Bl/6J background were protected from diet-induced weight gain (rev. in (1). Several other factors have been considered as potentially contributing to these phenotypic differences between L-FABP (−/−) mice on the C57BL6NCr versus C57BL6J backgrounds: i) Strain differences are a likely and important contributor to the opposite whole body phenotypes noted between the two L-FABP (−/−) mouse models. For example, the wild-type C57BL/6J (Jackson Labs) mice are more susceptible to high fat diet induced obesity than the C57Bl/6NCr mice (from the National Cancer Institute, Frederick Cancer Research and Developmental Center, Maryland) used herein (JAX NOTES, Issue 511, Fall, 2008, http://jaxmice.jax.org/jaxnotes/511/511n.html) (67–69). The C57BL/6J mouse strain exhibits a nicotinamide nucleotide transhydrogenase (Nnt) gene mutation removing 5 exons and reported as leading to higher weight gain and higher non-fasting plasma glucose when fed a high-fat diet (JAX NOTES, Issue 511, Fall, 2008, http://jaxmice.jax.org/jaxnotes/511/511n.html). Other genetic polymorphisms among the C57BL/6 substrains have also been reported (70, 71) and a panel of 95 markers of which 93 distinguish C57BL/6J from C57BL/6NCrl has been developed to solely distinguish C57BL/6 substrains. [http://www.taconic.com/user-assets/Documents/Library/B6SNPs_AALAS2010.pdf; (72)]. Behavioral differences potentially related to these genetic variations have also been indicated among the different C57BL/6 substrains (73). ii) Differences in design of constructs used to ablate the L-FABP gene have been considered but are less likely to contribute to the opposite phenotypes. For example, the L-FABP null mice on C57BL/6J background were created using a vector that resulted in overexpression of a green fluorescent protein (GFP), but normal wild-type C57BL/6J mice not overexpressing GFP were used as controls (41). GFP has been observed in vitro to be an electron donor to acceptors like nicotinamide adenine dinucleotide, flavin adenine dinucleotide, and cytochrome c as well as flavin mononucleotide (74). These electron acceptors are intimately involved in LCFA metabolism (rev. (1). Despite these differences, however, a similar phenotypes were observed when L-FABP was downregulated by L-FABP antisense treatment of C57Bl/6J mice as compared to L-FABP gene ablation in C57Bl/6J mice (63, 66, 66, 75); iii) Differences in back-cross generation number did not account the phenotypic differences between the two L-FABP KO mouse models (76); iv) While differences in intestinal microbiota have also been suggested as a potential contributor to the divergent whole body phenotypes of L-FABP (−/−) mice on the two genetic backgrounds, there is as yet no evidence addressing this point (rev. in (1). Taken together these findings indicated the potential differences in background strain as the most likely contributor to the differences in whole body phenotypes noted between L-FABP (−/−) mice on the C57Bl/6NCr versus C57Bl/6J backgrounds. Since other investigators have now backcrossed our L-FABP (−/−) mice on the C57Bl/6NCr background to the C57Bl/6J background for 10 generations (64), future studies beyond the scope of the present investigation may allow investigators to further address this issue by identifying additional gene(s) interacting with the L-FABP gene to impact whole body phenotype. While differences in background strains may complicate the comparison and interpretation of the results between the two L-FABP KO models under various dietary stresses, they offer the opportunity to further explore the roles of subtle genetic differences that contribute to diet-induced weight gain and obesity.
In summary, the diet-induced increased weight-gain, weight gain/energy intake, and FTM of L-FABP (−/−) mice on the C57Bl/6NCr background were consistent with a wealth of evidence of L-FABP’s role with LCFA uptake, intracellular transport, oxidation, and regulation obtained in vitro, in cultured cells overexpressing L-FABP, in cultured primary hepatocytes, and in vivo. Furthermore, the results herein were consistent with the reported higher weight gains involving adipocyte fatty acid binding protein (A-FABP) and intestinal fatty acid binding protein (I-FABP) gene-ablated mice (77, 78). Such studies help underscore the role of L-FABP and other FABPs with dietary fat intake and the potential for profound effects resulted from protein modifications.
Supplementary Material
Acknowledgments
This work was supported in part by the United States Public Health Service National Institutes of Health grants DK41402 (FS and ABK), GM31651 (FS and ABK), and DK70965 (BPA).
Abbreviations
- L-FABP
liver type fatty acid binding protein
- SCP-2
sterol carrier protein-2
- SCP-x
sterol carrier protein-x
- ACBP
acyl CoA binding protein
- PPARα
peroxisome proliferator-activated receptor α
- ACOX1
peroxisomal acyl-coenzyme A oxidase 1
- FATP-2
fatty acid transporter-2
- FATP-4
fatty acid transporter-4
- FATP-5
fatty acid transporter 5
- GOT
glutamic oxaloacetic aminotransferase
- CPT1
carnitine palmitoyltransferase I
- CPT2
Carnitine palmitoyltransferase II
- β-OH butyrate
β-hydroxybutyrate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- COX IV
cytochrome c oxidase IV
- FTM
fat tissue mass
- DEXA
dual emission X-ray absorptiometry
- LTM
lean tissue mass
- WT
wild-type
- L-FABP (+/+)
L-FABP gene ablated (L-FABP (−/−)), null
Footnotes
Conflict of interest—The authors have no conflict of interest to report.
REFERENCE LIST
- 1.Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and Dietary Obesity. Journal of Nutritional Biochemisty. 2010;21:1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids. 2008;43:1–17. doi: 10.1007/s11745-007-3111-z. [DOI] [PubMed] [Google Scholar]
- 3.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 4.Jolly CA, Wilton DA, Schroeder F. Microsomal fatty acyl CoA transacylation and hydrolysis: fatty acyl CoA species dependent modulation by liver fatty acyl CoA binding proteins. Biochim Biophys Acta. 2000;1483:185–197. doi: 10.1016/s1388-1981(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids. 1998;92:1–25. doi: 10.1016/s0009-3084(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 6.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Nemecz G, Schroeder F. Selective binding of cholesterol by recombinant fatty acid-binding proteins. J Biol Chem. 1991;266:17180–17186. [PubMed] [Google Scholar]
- 8.Hostetler HA, Lupas D, Tan Y, Dai J, Kelzer MS, Martin GG, Woldegiorgis G, Kier AB, Schroeder F. Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyltransferase I. Mol Cell Biochem. 2011;355:135–148. doi: 10.1007/s11010-011-0847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woldegiorgis G, Bremer J, Shrago E. Substrate inhibition of carnitine palmitoyltransferase by palmitoyl-CoA and activation by phospholipids and proteins. Biochim Biophys Acta. 1985;837:135–140. doi: 10.1016/0005-2760(85)90236-x. [DOI] [PubMed] [Google Scholar]
- 10.Bhuiyan AKMJ, Pande SV. Carnitine palmitoyltransferase activities: effects of serum albumin, acyl-CoA binding protein and fatty acid binding protein. Mol Cell Biochem. 1994;139:109–116. doi: 10.1007/BF01081733. [DOI] [PubMed] [Google Scholar]
- 11.Reubsaet FA, Veerkamp JH, Bruckwilder ML, Trijbels JM, Monnens LA. The involvement of fatty acid-binding protein in peroxisomal fatty acid oxidation. FEBS Lett. 1990;267:229–230. doi: 10.1016/0014-5793(90)80931-8. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence JW, Kroll DJ, Eacho PI. Ligand dependent interaction of hepatic fatty acid binding protein with the nucleus. J Lipid Res. 2000;41:1390–1401. [PubMed] [Google Scholar]
- 13.Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, Schroeder F. Liver type Fatty Acid Binding Protein (L-FABP) interacts with peroxisome proliferator activated receptor-α in cultured primary hepatocytes. J Lipid Res. 2009;50:1663–1675. doi: 10.1194/jlr.M900058-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and PPARα. J Lipid Res. 2010;51:3103–3116. doi: 10.1194/jlr.M005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velkov T. Interactions between human liver fatty acid binding protein and peroxisome proliferator activated receptor drugs. PPAR Research. 2012 doi: 10.1155/2013/938401. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oosterveer MH, Grefhourst A, van Dijk TH, Havinga R, Staels B, Kuipers F, Groen AK, Reijngoud DJ. Fenofibrate simultaneously induces hepatic fatty acid oxidation, synthesis, and elongation in mice. J Biol Chem. 2009;284:34036–34044. doi: 10.1074/jbc.M109.051052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekiya M, Yhagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga JI, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Froyland L, Madsen L, Vaagenes H, Totland GK, Auwerx J, Kryvi H, Staels B, Berge RK. Mitochondrion is the principal target for nutritional and pharmacological control of triglyceride metabolism. J Lipid Res. 1997;38:1851–1858. [PubMed] [Google Scholar]
- 19.Bijland S, Pieterman EJ, Maas ACE, van der Hoorn JWA, van Erk MJ, van Klinken JB, Havekes LM, van Dijk KW, Princen HM, Rensen PCN. Fenofibrate increases VLDL triglyceride production despite reducing plasma triglyceride levels in APOE3-Leiden. CETP mice. J Biol Chem. 2010;285:25168–25175. doi: 10.1074/jbc.M110.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J Biol Chem. 2010;285:18693–18708. doi: 10.1074/jbc.M109.079897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid binding protein targets fatty acids to the nucleus: real-time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem. 2002;277:29139–29151. doi: 10.1074/jbc.M202923200. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, Schroeder F. Liver fatty acid binding protein colocalizes with peroxisome proliferator receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry. 2004;43:2484–2500. doi: 10.1021/bi0352318. [DOI] [PubMed] [Google Scholar]
- 23.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res. 1999;40:1371–1383. [PubMed] [Google Scholar]
- 24.Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 25.Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]
- 26.Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- 27.Wolfrum C, Buhlman C, Rolf B, Borchers T, Spener F. Variation of liver fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim Biophys Acta. 1999;1437:194–201. doi: 10.1016/s1388-1981(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Qu X, Yan J, Huang Q, Yuan HY, Ouyang DS. L-FABP T94A decreased fatty acid uptake and altered hepatic triglyceride and cholesterol accumulation in Chang liver cells stably transfected with L-FABP. Mol Cell Biochem. 2010;345:207–214. doi: 10.1007/s11010-010-0574-7. [DOI] [PubMed] [Google Scholar]
- 29.Atshaves BP, Storey SM, Petrescu AD, Greenberg CC, Lyuksyutova OI, Smith R, Schroeder F. Expression of fatty acid binding proteins inhibits lipid accumulation and alters toxicity in L-cell fibroblasts. Am J Physiol. 2002;283:C688–C703. doi: 10.1152/ajpcell.00586.2001. [DOI] [PubMed] [Google Scholar]
- 30.Atshaves BP, Storey S, Huang H, Schroeder F. Liver fatty acid binding protein expression enhances branched-chain fatty acid metabolism. Mol Cell Biochem. 2004;259:115–129. doi: 10.1023/b:mcbi.0000021357.97765.f2. [DOI] [PubMed] [Google Scholar]
- 31.Jefferson JR, Slotte JP, Nemecz G, Pastuszyn A, Scallen TJ, Schroeder F. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid binding protein. J Biol Chem. 1991;266:5486–5496. [PubMed] [Google Scholar]
- 32.Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol. 2010;299:244–254. doi: 10.1152/ajpgi.00446.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012;302:G824–G839. doi: 10.1152/ajpgi.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2012;303:G837–G850. doi: 10.1152/ajpgi.00489.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moya M, Gomez-Lechon MJ, Castell JV, Jover R. Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. Chem Biol Interactions. 2010;184:376–387. doi: 10.1016/j.cbi.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 37.McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-alpha activity in cultured primary hepatocytes. Arch Biochem Biophys. 2009;485:160–173. doi: 10.1016/j.abb.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 39.Martin GG, Huang H, Atshaves BP, Binas B, Schroeder F. Ablation of the liver fatty acid binding protein gene decreases fatty acyl CoA binding capacity and alters fatty acyl CoA pool distribution in mouse liver. Biochem. 2003;42:11520–11532. doi: 10.1021/bi0346749. [DOI] [PubMed] [Google Scholar]
- 40.Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid-binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPAR-a in fasting mice. FASEB J. 2004;18:347–349. doi: 10.1096/fj.03-0330fje. [DOI] [PubMed] [Google Scholar]
- 41.Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 42.Atshaves BP, McIntosh AL, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene ablated mice. Lipids. 2010;45:97–110. doi: 10.1007/s11745-009-3379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Bandichhor R, Petrescu AD, Vespa A, Kier AB, Schroeder F, Burgess K. Water Soluble through bond energy transfer cassettes in intracellular imaging. Bioconjugate Journal. 2006;17:1219–1225. doi: 10.1021/ja063784a. [DOI] [PubMed] [Google Scholar]
- 45.Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochem. 2008;47:5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- 47.Watkins PA. Very long chain acyl-CoA synthetases. J Biol Chem. 2008;283:1773–1777. doi: 10.1074/jbc.R700037200. [DOI] [PubMed] [Google Scholar]
- 48.Bradbury MW, Stump D, Guarnieri F, Berk PD. Molecular modeling and functional confirmation of a predicted fatty acid binding site of mitochondrial asparatate aminotransferase. J Mol Biol. 2011;412:412–422. doi: 10.1016/j.jmb.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berk PD, Wada H, Horio Y, Potter BJ, Sorrentino D, Zhou SL, Isola LM, Stump D, Kiang CL, Thung S. Plasma membrane fatty acid binding protein and mitochondrial glutamic-oxaloacetic transaminase of rat liver are related. Proc Natl Acad Sci U S A. 1990;87:3484–3488. doi: 10.1073/pnas.87.9.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stump DD, Zhou SL, Berk PD. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol. 1993;265:G894–902. doi: 10.1152/ajpgi.1993.265.5.G894. [DOI] [PubMed] [Google Scholar]
- 51.Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart, and liver measured with the flourscent probe ADIFAB. J Biol Chem. 1994;269:23918–23930. [PubMed] [Google Scholar]
- 52.McIntosh AL, Atshaves BP, Wellberg EKDV, Smith WL, Schroeder F. Uptake kinetics of fluorescent long chain n-3 and n-6 fatty acids in intact cells. FASEB J. 2005;19:A292. [Google Scholar]
- 53.Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- 54.Frolov AA, Schroeder F. Acyl coenzyme A binding protein: conformational sensitivity to long chain fatty acyl-CoA. J Biol Chem. 1998;273:11049–11055. doi: 10.1074/jbc.273.18.11049. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Atshaves BP, Frolov A, Kier AB, Schroeder F. Acyl-coenzyme A binding protein expression alters liver fatty acyl coenzyme A metabolism. Biochemistry. 2005;44:10282–10297. doi: 10.1021/bi0477891. [DOI] [PubMed] [Google Scholar]
- 56.Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J. 1997;323:1–12. doi: 10.1042/bj3230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knudsen J, Jensen MV, Hansen JK, Faergeman NJ, Neergard T, Gaigg B. Role of acyl CoA binding protein in acyl CoA transport, metabolism, and cell signaling. Mol Cell Biochem. 1999;192:95–103. doi: 10.1007/978-1-4615-4929-1_11. [DOI] [PubMed] [Google Scholar]
- 58.Petrescu AD, Huang H, Martin GG, McIntosh AL, Storey SM, Landrock D, Kier AB, Schroeder F. Impact of L-FABP and glucose on polyunsaturated fatty acid induction of PPARa regulated b-oxidative enzymes. Am J Physiol Gastrointest and Liver Phys. 2012;304:G241–G256. doi: 10.1152/ajpgi.00334.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate PPARalpha and PPARgamma gene expresion via L-FABP: a signaling path to the nucleus. Proc Natl Acad Sci. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gyamfi MA, He L, French SW, Damjanov I, Wan YJY. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J Pharm Exp Ther. 2008;324:443–453. doi: 10.1124/jpet.107.132258. [DOI] [PubMed] [Google Scholar]
- 61.Madsen L, Rustan AC, Vaagenes H, Berge K, Dyroy E, Berge RK. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids. 1999;34:951–963. doi: 10.1007/s11745-999-0445-x. [DOI] [PubMed] [Google Scholar]
- 62.Desvergne B, Wahli W. Peroxisome proliferator activated receptors: nuclear control of metabolism. Endocrine Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 63.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against western diet-induced obesity and hepatic steatosis in liver fatty acid binding protein knockout mice. Hepatology. 2006;44:1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 64.Lagakos WS, Gajda AM, Agellon LB, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of intestinal and liver-type fatty acid binding proteins in intestine and in whole body energy homeostasis. Am J Physiol Gastrointest and Liver Phys. 2011;300:G803–G814. doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savage DB. Cur Opin Lipidology. 2010;21:329–336. doi: 10.1097/MOL.0b013e32833b7782. [DOI] [PubMed] [Google Scholar]
- 66.Newberry EP, Kennedy SM, Xie Y, Luo J, Crooke RM, Graham MJ, Fu J, Piomelli D, Davidson NO. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-FABP −/− mice. Journal of Lipid Research. 2012;53:744–754. doi: 10.1194/jlr.M020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tallman DL, Noto AD, Taylor CG. Low and high fat diets inconsistently induce obesity in C57BL/6J mice and obesity compromises n-3 fatty acid status. Lipids. 2009;44:577–580. doi: 10.1007/s11745-009-3312-8. [DOI] [PubMed] [Google Scholar]
- 68.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, Skaf J, Kozak LP. Changes in gene expression foreshdow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2:e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 70.Zurita E, Chagoyen M, Cantero M, Alonso R, Gonzalez-Neira A, Lopez-Jimenez A, Lopez-Moreno JA, Landel CP, Benitez J, Pazos F, Montoliu L. Genetic polymorphisms among C57Bl/6 mouse inbred strains. Transgenic Res. 2011;20:481–489. doi: 10.1007/s11248-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 71.Mekada K, Abe K, Murakami A, Nakamura S, Nakata H, Moriwaki K, Obata Y, Yoshiki A. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 72.Perez AV, Gray JL, rusconi JC, Bothe GWM. A panel of 95 markers that differentiate C57Bl/6 substrains. AALAS National Meeting; Atlanta, GA. 2010. p. 159. [Google Scholar]
- 73.Bryant CD, Zhang NN, Sokoloff G, Fanselow MS, Ennes HS, Palmer AA, McRoberts JA. Behavioral differences among C57Bl/6 substrains: implications for transgenic and knockout studies. J Neurogenetics. 2008;22:315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bogdanov AM, Mishin AS, Yampolsky IV, Belousov VV, Chudakov DM, Subach FV, Verkhusha VV, Lukyanov S, Lukyonov KA. Green fluorescent proteins are light-induced electron donors. Nature Chemical Biology. 2009 doi: 10.1038/nchembio.174. Published online April 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-FABP−/− mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest and Liver Phys. 2008;294:G307–G314. doi: 10.1152/ajpgi.00377.2007. [DOI] [PubMed] [Google Scholar]
- 76.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hotamisligl GS, Johnson RS, Distel RJ, Ellis RF, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 78.Vassileva G, Huwyler L, Poirer K, Agellon LB, Toth MJ. The intestinal fatty acid binding protein is not essential for dietary fat absorption in mice. FASEB J. 2000;14:2040–2046. doi: 10.1096/fj.99-0959com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.