Abstract
Background and Aims
Rapid onsite evaluation (ROSE) has been demonstrated to correlate with final cytologic interpretations and improves the diagnostic yield of EUS-FNA, however, its availability is variable across centers. The aim of this prospective study was to evaluate whether remote telecytology can substitute for ROSE.
Methods
Consecutive patients who underwent EUS-FNA for diverse indications at a high volume referral center were enrolled All samples were prospectively evaluated by three methods. ROSE was performed by a cytopathologist in the procedure room; simultaneously dynamic telecytology was done by a different cytopathologist in a remote location at our institution. The third method, final cytologic interpretation in the laboratory, was the gold standard. Telecytology was performed using an Olympus microscope system (BX) which broadcasts live images over the internet. Accuracy of telecytology and agreement with other methods were the principle outcome measurements.
Results
Twenty-five consecutive samples were obtained from participants 40–87 years (median age =63, 48% male). There was 88% agreement between telecytology and final cytology (p < 0.001) and 92% agreement between ROSE and final cytology (p <0.001). There was consistency between telecytology and ROSE (p-value for McNemar’s χ2 = 1.0). Cohen’s kappa for agreement for telecytology and ROSE was 0.80 (SE = 0.11), confirming favorable correlation.
Conclusion
Dynamic telecytology compares favorably to ROSE in the assessment of EUS acquired fine needle aspirates. If confirmed by larger trials, this system might obviate the need for onsite interpretation of EUS-FNA specimens.
Keywords: Endoscopic ultrasonography, Cytology, Telepathology, Fine needle aspirations, Fine needle biopsies, Endoscopy, ultrasonic
INTRODUCTION
Endoscopic ultrasound (EUS) guided fine needle aspiration (FNA) is a highly accurate method to sample lesions in the abdomen and posterior mediastinum [1,2]. Nonetheless, sampling error can diminish its accuracy and the procedure is associated with a small but significant risk of bleeding, pancreatitis, and infection [3].
It has been demonstrated that the benefit of EUS-FNA is maximized if a cytologist is present during the case to evaluate the specimens as they are acquired [4]. Nonetheless, this mandates a significant time requirement from the supporting cytologist and from a financial standpoint is inadequately compensated [5]. Thus, the availability of rapid onsite evaluation is limited across centers in Europe and the United States where most EUS-FNA are performed worldwide.
Telepathology, in which images are digitized and transmitted to an interpreting pathologist in a remote location has been demonstrated to be helpful, particularly in the evaluation of frozen sections [6]. We previously demonstrated that telecytology is a feasible method to assess archived EUS-FNA samples [7]. In this study our aim was to prospectively assess whether dynamic telecytology was comparable to Rapid onsite evaluation (ROSE) in the evaluation of EUS acquired FNA specimens from a variety of sites.
METHODS
Telecytology was performed using the Olympus microscope system (BX) (Olympus America, Center Valley, PA) coupled to a DP71-12.8 mega pixel cooled digital color camera (Olympus) [Figure 1A]. Using MicroSuite5 software with the NetCam feature (Olympus) streaming live images were broadcasted over the internet from a static IP address [Figure 1B]. The images were transmitted in real time with no detectable delay in transmission. The resolution of the transmitted images was 800 × 600 pixels. The approximate cost of the telecytology sytem was $24,000. Upon obtaining Institutional Review Board approval patients undergoing EUS-FNA for standard clinical indications were enrolled. The procedures were performed using the curvilinear echoendoscope (UC 30 P or UCT 140, Olympus America, Center Valley, PA). Fine needle aspiration was performed using 22 and 25 gauge needles (Wilson Cook, Winston Salem, NC) as previously described [2]. In order to obtain a representative distribution of lesions, cytology from consecutive cases were obtained.
Figure 1.
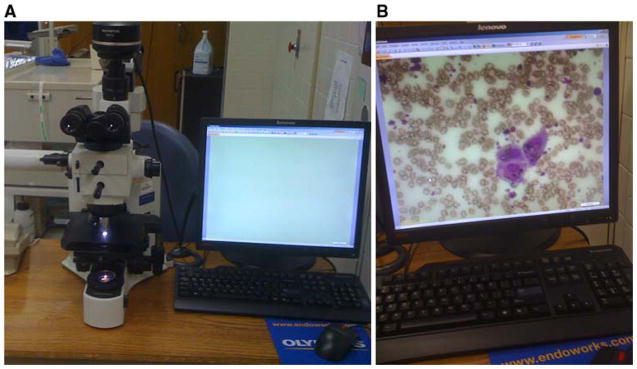
1A). Telecytology apparatus comprised of BX microscope coupled to a DP71-12.8 mega pixel cooled digital color camera interfaced with desktop computer using NetCam software. 1B) Live streaming images used for telecytology interpretation with resolution of 800 × 600 pixels.
During the case preparation of the FNA samples was done by the cytology fellow or resident in the procedure room. Thereafter, each specimen was analyzed by ROSE by an attending cytopathologist. After each slide was viewed by the ROSE cytologist, the cytology fellow would transmit streaming images of the slide to a different attending cytologist at a remote location in the medical center. The telecytologist was blinded to the ROSE cytologist’s interpretation and vice versa. An internet based instant messaging system (Yahoo, Redwood City, CA) was used to allow the attending telecytologist to instruct the cytology fellow to make adjustments in the focus, stage, objective, and brightness of the microscope. The telecytologist also prospectively noted adequacy, diagnosis, and whether there were any image and sample deficiencies for each specimen. The two senior cytology fellows who operated the microscope alternated evenly between cases. Four cytopathologists, each with greater than ten years of experience, performed alternate roles in the study.
The prospective interpretations of the ROSE and telecytologists were entered into a database. The final diagnosis was rendered by the pathologist who performed the initial rapid on site evaluation upon complete evaluation of all fixed stains, cell block, immunohistochemical, and other special stains. When the final cytologic interpretation became available, generally several days after the procedure, this information was entered in the database as the final diagnosis. Consistency of dynamic telecytology with other methods was assessed by calculating McNemar’s χ2to examine if diagnosis (benign, malignant, atypical) were significantly different than standard methods. Additionally, Cohen’s kappa was computed to assess the level of agreement across methods. Statistical analysis was performed using the SPSS Version 18 program (SPSS, Chicago, IL); α = 0.05.
RESULTS
Twenty-five samples were acquired from twenty-four participants. The median age was 63 (range 40–87), there were 11 men and 13 women. Most of the patients were Caucasian (n=21), three were African American and one was Hispanic. There was a wide anatomic variation in the FNA targets, though the pancreas was the site in the majority of patients [Table 1]. Nine of the lesions were pancreatic masses, three were pancreatic cysts, six were lymph nodes, and there were six “other” lesions including two adrenal nodules, a mediastianal mass, a bile duct tumor, a gastric stromal tumor, and a rectal cancer. EUS was performed by two endoscopists (M.E., S.V.) who have each performed more than 5000 endosonographic procedures. Adequate tissue was obtained in all cases with the median number of passes being 3 (range 2–4). The median size of the lesions was 2.5cm (range 1.5–6.8).
Table 1.
| FNA Target | N |
|---|---|
| Pancreas Mass | 10 |
| Pancreas Cyst | 3 |
| Mediastinal Lymph Node | 4 |
| Abdominal Lymph Node | 2 |
| Adrenal Gland | 2 |
| Bile Duct Mass | 1 |
| Mediastinal Mass | 1 |
| Rectal Mass | 1 |
| Subepithelial Gastric Mass | 1 |
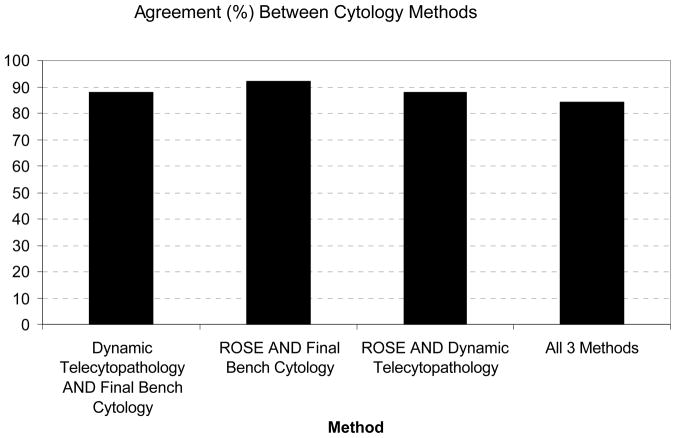
Diagnosis based on dynamic telecytology was not significantly different than either bench cytology (p = 0.56) or ROSE (p = 0.56); additionally, there was no significant difference in agreement between bench telecytology and ROSE (p = 0.37). This consistency across methods is reflected in Cohen’s kappa for agreement, reported in Table 2. In 84% of cases there was agreement between all three methods (Figure 2). Overall the sensitivity of ROSE and telecytology was the same at 89%, though the specificity of ROSE was 93%, whereas the specificity of telecytology was 87%.
Table 2.
| Comparison of Methods | Kappa | Standard Error | 95% Confidence Interval |
|---|---|---|---|
| ROSE versus Final Bench Cytology | 0.85 | 0.09 | (0.67, 1.00) |
| Telecytology versus Final Bench Cytology | 0.79 | 0.11 | (0.58, 1.00) |
| ROSE versus Telecytology | 0.80 | 0.11 | (0.58, 1.00) |
Figure 2.
No difference in agreement between telecytology versus ROSE cytology compared to the final cytologic evaluation. Agreement between telecytology, ROSE, and the final bench cytology was seen in most cases.
When stratified by lesion type, there remained considerable consistency across methods. For the six “other” lesions there was perfect agreement across the methods. In eight of ten pancreas masses there was complete agreement between the methods, in the other two cases cells were interpreted to be atypical or suspicious by telecytology but malignant by ROSE and final bench cytology. For lymph nodes, five of six samples were rated identically across the methods; one mediastinal lymph node was suspicious by ROSE but malignant by telecytology and final bench cytology. For pancreas cysts there was agreement in two cases but the third was interpreted as atypical by ROSE and telecytology but benign on final bench analysis. Kappa coefficients for these comparisons stratified by lesion type range from 0.4–1.0, However the low kappa, 0.4, was for pancreas cysts and it has little power given that it was based on a sample size of three.
All three methods confirmed sample adequacy in 100% of cases. In two cases there were image deficiencies. In one of these cases pixilation was prospectively noted and in the other extensive blood and gastrointestinal cell contaminants limited assessment by the telecytologist. Of importance these two cases corresponded to the two pancreas mass samples which were interpreted as atypical and suspicious by telecytology but malignant by the other methods
DISCUSSION
EUS with FNA has an accuracy which exceeds 90% for sampling of lesions in the abdomen and posterior mediastinum [1,2]. Nonetheless, optimal results are achieved with rapid on-site cytologic evaluation [4]. Unfortunately, the availability of ROSE is variable in different centers. We present the first prospective study evaluating the role of dynamic telecytology to support EUS guided FNA. Our results suggest that telecytology represents a promising alternative, particularly when on-site support is not available. Larger studies, optimized technology, and experience with this method are needed to further advance the telecytology approach.
Klapman et al demonstrated that definitive answer could be achieved in 78% of cases when EUS guided FNA was supported by ROSE versus 52.5% of cases where it was not [4]. Iglesias-Garcia et al recently reported that EUS-FNA without ROSE is less accurate and results in more needle passes and complications in the evaluation of pancreatic masses than procedures supported by an on-site pathologist [8]. Unfortunately, inconclusive results necessitate repeat procedures and consequently result in additional complications and cost [3]. Nasuti et al report that on-site FNA evaluation at the University of Pennsylvania reduced non diagnostic aspiration rates from 20% to <1%, the cost saved from avoiding repeat procedures was greater than four hundred thousand dollars annually [9]. Nonetheless, while bedside cytology minimizes overall system costs it has an adverse financial impact on cytology services. Layfield et al performed a cost and compensation analysis for providing immediate on-site cytologic evaluations based on Medicare payments and demonstrated that the reimbursement for on-site cytologic evaluation was totally inadequate to reimburse the staff cytopathologist assisting with the case [5]. As a consequence of limited staff and resources ROSE availability is variable across centers.
In order to improve efficiency pathologists have used a variety of telepathology systems to review specimens remotely. In several large series the accuracy of telepathology for frozen section analysis exceeded 90% [6]. Initial reception of remote viewing of cytologic specimens, telecytology, was mixed with initial reports suggesting a diminished ability to render a definitive diagnosis [10–11]. However, dynamic telecytology in which a continuous image is obtained has been associated with results comparable to ROSE [12]. In dynamic telecytology changes in the depth of focus allows the viewer to scroll through image planes along an axis perpendicular to the slide, the Z axis, which enhances evaluation [13–14]. While continuous communication between the operator of the microscope and the telecytologist was achieved using instant messaging in our study an IP streaming protocol or other method of real time communication could likely be used with comparable efficacy.
In the only previous study of telecytology used in EUS-FNA acquired specimens, our group used remotely operated telecytology to evaluate selected slides from 40 archived cases [7]. The telecytologist’s interpretation was compared to the original on-site evaluation. The Kappa statistic for telecytology evaluation compared to ROSE was 0.65 (95% CI; 0.41–0.88) suggesting that this is a comparable method for EUS-FNA acquired specimen evaluation. However, while not statistically significant the Kappa statistic for telecytology and final diagnosis 0.61 (0.37–0.85) was less than the Kappa statistic for ROSE and the final diagnosis 0.79 (0.61–0.98).
Our current prospective study indicates that dynamic telecytology is a promising method to interpret EUS-FNA specimens. Several particular strengths of this project were that all samples were prospectively evaluated by ROSE and telecytology by cytologists blinded to one another’s interpretation. This design better reflects the potential clinical use of telecytology and obviates potential bias which would be introduced if the methods were used to assess different groups of samples. We did note a better correlation between telecytology and ROSE, the kappa was 0.8 (95% CI 0.58–1.00) compared to our prior retrospective study, 0.65 (95% CI 0.41–0.88) as well as correlation with telecytology and final bench cytology [7].
However, there were several important limitations of our study. The most important was that sample size was relatively small. While our results were promising, limitations in sample variability may have led to attrition in the difference which could be detected. Another important problem is that the small number limited our ability to stratify results by type of lesion. While limited results in this area were encouraging it would be clinically helpful and technically important to know if the technology performs as well for pancreatic masses, for example, as other lesions.
Additionally, there were two cases of pancreas malignancy which were confirmed by ROSE but were only found to be suspicious or atypical by telecytology. In both cases problems with image and sample quality were noted prospectively by the telecytologist. In the first case the sample was recorded to be bloody and in the second pixilation was noted to compromise the evaluation. A larger prospective study is needed to glean whether problems with sample preparation and pixilation represent a limitation of the telecytology method or whether it is related to the development of the technique and relatively small sample size in this pilot project. It is critical that sample handling be optimized. Shorter telecytology evaluation times and better correlation with final results have been demonstrated when more experienced operators performed the sample preparation and analysis [10,15]. Additionally, efforts to improve the technology will be imperative. When problems with pixilation and other problems are addressed it is possible that this approach may eventually yield technical advantages given its ability to efficiently process, magnify, and reevaluate images [14].
Another limitation is that in each case only one on-site cytologist and one telecytologist evaluated the sample. Alli et al report that there was greater inter-observer variability for telecytology than was seen for bench evaluations [16]. Furthermore, the final diagnosis was rendered by the ROSE cytologist which may introduce bias. In larger prospective studies multiple pathologists, all randomized to different roles should evaluate the samples to optimize results.
Additionally, the time required to perform telecytology as compared to ROSE was not recorded. Though not formally measured, the use of the telecytology method did not appear to appreciably prolong procedure time or differ from the time required to perform ROSE. Additionally, specimen preparation was performed by a cytology resident and the telecytologist was involved only during the high-yield evaluation period. In many centers cytotechnologists are used to support EUS procedures. While cytotechnologists may help to assess specimen adequacy they cannot offer a formal interpretion and consequently the frequency of indeterminate results is greater than when staff pathologists perform ROSE [17]. Telecytology is likely to be cost effective for health care systems as both the likelihood of expensive repeat procedures to obtain definitive diagoses as well as the time requirement for staff pathologists are minimized. The endoscopy team may also be trained to prepare the samples for the remote telecytologist which saves the cost of having a cytotechnologist present during the procedure and further optimizes resource use. This would likely not add significant time to the procedure as slide preparation may be done while the endoscopist is performing additional sonographic evaluation. Careful cost-analysis studies are needed to compare the resources saved by optimizing the cytopathologists’s time versus the cost of the telecytology system.
EUS-FNA has emerged as a critical method to sample abdominal and posterior mediastinal lesions. Evidence suggests that the best results occur when an on site pathologist provides real time interpretation. However, ROSE is not cost effective for the pathologist interpreting the samples. Gross or microscopic inspection of samples by endosonographers has been demonstrated to have limited utility [18]. In this prospective pilot study of the performance of telecytology, specimen adequacy was confirmed in all cases and interpretation compared favorably to ROSE. Telecytology represents a promising alternative to ROSE, particularly in situations where on site evaluation is unavailable to endosonographers. Further studies will be needed to evaluate telecytology as experience with this method grows and technology continues to evolve.
Acknowledgments
We wish to thank Olympus America for providing the necessary equipments to make this project feasible. This project was supported by NIH/NCRR SC-CTSI Grant Number UL1 RR031986. This work was presented as an abstract at the American Society for Gastrointestinal Endoscopy Annual Meeting in May 2011, Chicago, Illinois, USA
References
- 1.Gress FG, Savides TJ, Sandler A, et al. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: a comparison study. Ann Intern Med. 1997;127:604–612. doi: 10.7326/0003-4819-127-8_part_1-199710150-00004. [DOI] [PubMed] [Google Scholar]; Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]; Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–249. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis. 2008;26:356–363. doi: 10.1159/000177022. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622–629. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–1294. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 5.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93:319–322. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 6.Frierson HF, Jr, Galgano MT. Frozen-section diagnosis by wireless telepathology and ultra portable computer: use in pathology resident/faculty consultation. Hum Pathol. 2007;38:1330–1334. doi: 10.1016/j.humpath.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Kim B, Chhieng DC, Crowe DR, et al. Dynamic telecytopathology of on site rapid cytology diagnoses for pancreatic carcinoma. Cytojournal. 2006;3:27. doi: 10.1186/1742-6413-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 9.Nasuti JF, Gupta PK, Baloch ZW. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol. 2002;27:1–4. doi: 10.1002/dc.10065. [DOI] [PubMed] [Google Scholar]
- 10.Briscoe D, Adair CF, Thompson LD, et al. Telecytologic diagnosis of breast fine needle aspiration biopsies. Intraobserver concordance. Acta Cytol. 2000;44:175–180. doi: 10.1159/000326357. [DOI] [PubMed] [Google Scholar]
- 11.Mairinger T, Gschwendtner A. Telecytology using preselected fields of view: the future of cytodiagnosis or a dead end? Am J Clin Pathol. 1997;107:620–621. doi: 10.1093/ajcp/107.5.620a. [DOI] [PubMed] [Google Scholar]
- 12.Alsharif M, Carlo-Demovich J, Massey C, et al. Telecytopathology for immediate evaluation of fine-needle aspiration specimens. Cancer Cytopathol. 2010;118:119–126. doi: 10.1002/cncy.20074. [DOI] [PubMed] [Google Scholar]
- 13.Yamashiro K, Taira K, Matsubayashi S, et al. Comparison between a traditional single still image and a multiframe video image along the z-axis of the same microscopic field of interest in cytology: Which does contribute to telecytology? Diagn Cytopathol. 2009;37:727–731. doi: 10.1002/dc.21078. [DOI] [PubMed] [Google Scholar]
- 14.Marchevsky AM, Nelson V, Martin SE, et al. Telecytology of fine-needle aspiration biopsies of the pancreas: a study of well-differentiated adenocarcinoma and chronic pancreatitis with atypical epithelial repair changes. Diagn Cytopathol. 2003;28:147–152. doi: 10.1002/dc.10247. [DOI] [PubMed] [Google Scholar]
- 15.Kerr SE, Bellizzi AM, Stelow EB, Frierson HF, Jr, Policarpio-Nicolas ML. Initial assessment of fine-needle aspiration specimens by telepathology: validation for use in pathology resident-faculty consultations. Am J Clin Pathol. 2008;130:409–413. doi: 10.1309/NA7Y7THPTBF112A0. [DOI] [PubMed] [Google Scholar]
- 16.Alli PM, Ollayos CW, Thompson LD, et al. Telecytology: intraobserver and interobserver reproducibility in the diagnosis of cervical-vaginal smears. Hum Pathol. 2001;32:1318–1322. doi: 10.1053/hupa.2001.29651. [DOI] [PubMed] [Google Scholar]
- 17.Yan AVK, Lane C, et al. The Performance of Pathology Trainees Compared to Non-Physician Cytotechnologists in the Assessment of EUS-FNA Specimen Adequacy. Gastrointest Endosc. 2012;75:AB447. [Google Scholar]
- 18.Savoy AD, Raimondo M, Woodward TA, et al. Can endosonographers evaluate on-site cytologic adequacy? A comparison with cytotechnologists. Gastrointest Endosc. 2007;65:953–957. doi: 10.1016/j.gie.2006.11.014. [DOI] [PubMed] [Google Scholar]; Nguyen YP, Maple JT, Zhang Q, et al. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264–1270. doi: 10.1016/j.gie.2008.08.030. [DOI] [PubMed] [Google Scholar]